Introduction
CIC-rearranged sarcoma (CRS) is the most common and
characteristic undifferentiated round cell sarcoma type in the
Ewing sarcoma family (1), with
molecular CIC gene rearrangement. CRS mostly occurs in the trunk
and limb soft tissues of young individuals (total range, 0.5–83
years; mean, 27–37 years; median, 24.5–33 years), and less in bone
and viscera. Histologically, the tumor is composed of diffuse and
juvenile round cells, with obvious nuclear mitosis, obvious
nucleoli, obvious necrosis and a small number of chrysanthemum
groups. Immunohistochemistry for weak or focal expression of CD99,
unlike diffuse and strong positive Ewing's sarcoma, is of certain
suggestive value for high expression of Wilms tumor gene 1 (WT1)
and diffuse nuclear positive for the diagnosis of CRS (2). Retroperitoneal disease is rare, with
a total of no more than 10 cases reported (2,3). Le
Guellec et al (3) reported
in 2016 on a 57-year-old male with undifferentiated round-cell
sarcoma that occurred retroperitoneally with CIC rearrangement. The
histological morphology included necrosis and mitosis 11/10
high-power field (HPF), and immunohistochemistry indicated negative
CD99. WT1 and E-twentysix translocation variant 4 (ETV4) were
diffusively positive. In 2017, Antonescu et al (2) reported a total of 6 cases of
retroperitoneal/perineal/pelvic sarcoma accompanied by CIC, without
specific content. Patients with Ewing's sarcoma was referred for
treatment, but 70% of CRS cases had poor response to chemotherapy
and the 5-year survival rate was 44%, significantly lower than that
of ES (2). The present study
reported a case of CIC rearrangement sarcoma in retroperitoneal
perirenal tissues and analyzed the clinicopathological and
molecular characteristics in comparison with the literature.
Case report
Case presentation
A 69-year-old male patient was admitted to the First
People's Hospital of Xiaoshan District (Hangzhou, China) in May
2021 due to left lower abdominal mass persisting for one month. One
month previously, the patient had noticed a painless mass in the
left lower abdomen and slight abdominal distention had been present
but no hematuria. On examination, the abdomen was soft and a huge
mass was palpitated in the left lower abdomen, but there was no
tenderness and no migratory dullness. The serum levels of tumor
markers were as follows: Carcinoembryonic antigen (CEA), 2.51 µg/l
(reference range, 0.00-5.00 µg/l); CA125, 15.9 kU/l (normal, <35
kU/l); prostate-specific antigen (PSA), 0.735 ng/ml (normal, <4
ng/ml); alpha-fetoprotein (3rd generation), 1.85 µg/l (normal,
<10 µg/l). Routine urinalysis was normal. Computed tomography
(CT) scan of the urinary system indicated a large irregular mass of
soft tissue shadow in the left retroperitoneal perirenal area,
~15.0×10.0×9.5 cm in size (Fig.
1). The tumor was wrapped around and squeezed the kidney and
changed its appearance, and it was closely related to the left
adrenal gland, psoas major muscle and the surrounding intestine.
Multiple lymph node metastases were noticed around the mass and
abdominal aorta, and soft tissue shadow was observed in the left
middle and upper ureter traveling area. Ultrasound-guided needle
biopsy of the retroperitoneal tumor was performed two days after
presentation.
Pathological findings
Macro-examination
Four grayish white strip puncture tissues were
obtained, measuring 2.0×0.3×0.2 cm. The tissue was fixed with 4%
neutral formalin and embedded in paraffin, and 4-µm serial sections
were prepared that were subjected to H&E staining and envision
immunohistochemical staining and specific staining and fluorescence
in situ hybridization (FISH) examination.
Microscopic observation
After conventional preparation of paraffin sections,
H&E staining and microscopic observation, histological analysis
indicated that the tumor was composed of small- to medium-sized
juvenile blue round cells diffused into nests (Fig. 2) with hyperchromatic nuclei and
prominent nucleoli (Fig. 3);
mitotic images were occasional, with little or no cytoplasm and a
high nucleo-plasma ratio. There were scattered apoptotic bodies
between tumor cells, clusters of eosinophils in areas and scattered
lymphocytes. Homogeneous eosinophilic stroma was seen around the
nestlike tumor cells. The tumor involved adipose tissue with no
obvious hemorrhagic necrotic foci.
Immunohistochemical staining with antibodies from
EnVision Systems (Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd. and Fuzhou Maixin Biotechnology Development Co., Ltd) and
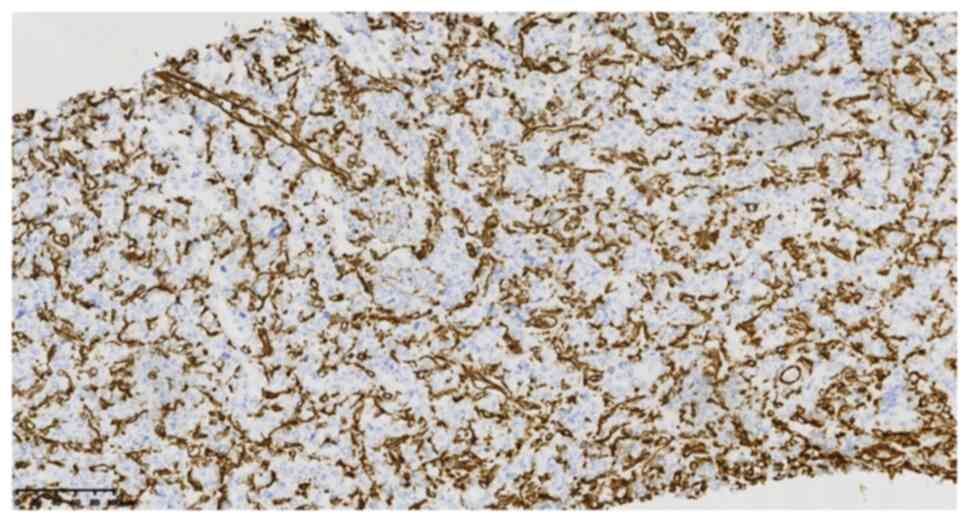
specific staining provided the following results: CD99 (cat. no.
2009090059d; scattered +) (Fig.
4), integrase interactor 1 (cat. no. 20122832; +), TLE1 (cat.
no. 170515686A; partial +), FLI-1 (cat. no. 19101721; partial +),
WT1 (cat. no. 1905220678b; -), cytokeratin (cat. no. 21061509; -)
(Fig. 5), epithelial membrane
antigen (cat. no. 21020730; -), vimentin (cat. no. 21031351, -)
(Fig. 6), calretinin (cat. no.
2105260716c; -), CD34 (cat. no. 2005270034b; -), CD56 (cat. no.
21082702; -), S-100 (cat. no. 2012240585C8; -), synaptophysin (cat.
no. 2105130742c; -), chromogranin A (cat. no. 21060816; -), CD10
(cat. no. 20090802; -), Melan-A (cat. no. 2106160275b; -),
leukocyte common antigen (cat. no. 0385; -), mesothelial cell (cat.
no. 19122684; -), desmin (cat. no. 20092713; -), myeloperoxidase
(cat. no. 2101140379a; -), spalt-like transcription factor 4 (cat.
no. 2018052501; -), PSA (cat. no. 2012160146f; -), P504s (cat. no.
2101200546a; -), terminal deoxynucleotidyl transferase (cat. no.
19082326; -), Ki-67 (cat. no. 21030436; 60%+) (Fig. 7) and periodic acid-Schiff staining
(−). FISH examination: CIC gene-related translocation was detected
by a specific two-color separation fracture probe. The CIC probe
was purchased from Guangzhou Ambiping Co., Ltd. and specific
operational steps followed the kit instructions. The red and green
dots in the nucleus represent gene rearrangement and the yellow
dots represent no rearrangement, accounting for >80%. FISH
examination revealed CIC gene rupture and translocation (Fig. 8).
Pathological diagnosis
The patient was diagnosed with primary
retroperitoneal perirenal CRS.
Treatment and follow-up
The patient received the vincristine sulfate +
doxorubicin liposome + cyclophosphamide regimen as chemotherapy (1
cycle), antirotinib targeted therapy (2 weeks) and enhanced immune
function, then died of systemic failure due to advanced tumor after
4 months.
Discussion
CRS is a type of undifferentiated round-cell sarcoma
with molecular CIC gene rearrangement. The morphology and
immunohistochemistry findings are similar to those of Ewing sarcoma
and such lesions have been diagnosed as Ewing-like sarcoma or
undifferentiated round-cell sarcoma in the past. In 1996, Richkind
et al (4) proposed t(4;
19)(q35;q13.1) changes, and in 2006, scholars began to report
t(4;19)(q35;q13) and t(10;19)(q26;q13), resulting in a fusion gene
dominated by CIC-DUX or CIC-DUX4L (5). The most common pairing gene for CIC
gene fusion is DUX4, which is located at 4q35 or 10q26.3. CIC gene
and non-DUX4 gene fusion were detected in only ~5% of cases. These
fusion genes include FOXO4, LEUTX, NUTM1 and NUTM2A, and recently,
CIC-NUTM1 rearrangement variants have been reported to be more
common in the central nervous system (6). At the same time, differences in
clinicopathological morphology, immunohistochemistry, molecular
genes and therapeutic prognosis were observed between these
diseases and Ewing sarcoma, which was introduced by the World
Health Organization as a new chapter of undifferentiated small
round-cell sarcoma of bone and soft tissue in 2020 (7).
Regarding the clinical features, CRS tends to occur
in young individuals, with an age range of 0.5–83 years (3,8) and
an average age of 27–37 years (median age, 24.5–33 years) and a
slight male prevalence. According to mass case reports, CRS mainly
occurred in soft tissues in 85.5% of cases, parenchymal viscera in
11.7% and bone tissue in 28% of cases, among which location in the
soft tissues of the trunk and limbs was most common (2) and tumors located in the
retroperitoneum were reported in <10 cases (2,3). In
2016, Le Guellec et al (3)
reported on a 57-year-old male with undifferentiated round-cell
sarcoma with CIC rearrangement in the retroperitoneum. The
histological morphology included necrosis and mitosis 11/10 HPF,
and immunohistochemistry indicated negative CD99 and diffuse strong
positive WT1 and ETV4. In 2017, Antonescu et al (2) reported a total of 6 cases of
retroperitoneal/perineum/pelvis sarcoma accompanied by CIC, without
specific content. Tumors located in the gastrointestinal tract
caused retroperitoneal symptoms/signs, including abdominal mass,
abdominal distension and abdominal pain, and tumors occurring on
the surface of the body as rapidly growing, painless masses that
may be accompanied by ulceration; Tumors occurring in the urinary
system may exhibit changes such as hematuria and abdominal pain,
while intracranial tumors may cause symptoms/complaints such as
headache and vomiting. Imaging examination typically indicates the
following: Ultrasound displayed irregular mass shadows with low
echo. CT revealed low-density or isodensity masses and MRI and
18F-fluorodeoxyglucose positron emission tomography
frequently indicated necrotizing, high-metabolic soft-tissue masses
(9).
The pathological features are as follows:
Microscopically, CRS consists of small- to medium-sized round or
oval juvenile cells and the tumor cells are nodular, lobulated or
have a flake-like distribution. The tumor cells have bare nuclei or
sparse cytoplasm, the nucleo-cytoplasm ratio is high and it is
common to observe irregular nucleolar shapes, coarse chromatin or
vacuolar changes; furthermore, nucleoli are obvious and mitosis is
easy to observe. Spindle-cell changes may occur in 10% and
epithelioid or striated muscle changes are rare. Extensive map-like
hemorrhagic necrosis or mucinous changes of varying degrees may be
observed in the tumor, while chrysanthemum-shaped cluster
structures are rarely seen (10).
Regarding immunohistochemistry, the expression degree of CD99
varies, with focal, multi-focal weak positive or no expression.
Studies suggested that CD99 was focal positive in ~85% of cases,
which was different from the strong diffuse positivity in Ewing
sarcoma. Diffuse nuclear positivity with 70–95% WT1 expression had
suggestive value in the diagnosis of CRS (2,11).
In the study by Siegele et al (12), DUX4 was diffuse nuclear positive in
CIC-DUX4 sarcoma, with a sensitivity and specificity of 100%. CRS
was also reported to have the following features:
E-twentysix-related gene (ERG), TLE1 and CD56 positive, and
occasionally desmin, S-100, MUC4, EMA, CK (AE1/AE3) and calretinin
local positive (1,3).
CRS should be differentiated from the following
diseases: i) Retroperitoneal malignant lymphoma-the round tumor
cells are diffusely arranged in a sheet-like structure, but may be
distinguished by immunohistochemical expression of
lymphoma-associated antigens; ii) Ewing's sarcoma or other sarcomas
of the Ewing family-the morphology and immunohistochemistry overlap
with CRS, but based on EWSR1-FLI1, EWSR1-ERG, BCOR and the
expression of other related genes, it may be differentiated from
CRS; iii) leiomyosarcoma–histologically, the well-differentiated
tumor cells are fusiform and the nuclei are large and
hyperchromatic when poorly differentiated, with coexistence of
multinucleation and pleomorphism. Immunohistochemical expression of
SMA, desmin and H-caldesmon is positive; iv) high-grade myxoid
liposarcoma-it is composed of small round proliferative cells with
uniform morphology, occasionally with multiple vesicular
adipocytes. Tumor cells expressed S-100 and the Ki-67 index was
higher. DDIT3 gene-related translocations were detected by FISH; v)
Malignant mesothelioma-in small round-cell mesothelioma, the cell
morphology is juvenile. However, at least two positive markers of
mesothelioma were expressed among calretinin, CK5/6, vimentin
antibody and mesothelioma antibody, while CEA, CD15 and CD117 were
negative; and vi) poorly differentiated/neuroendocrine
carcinoma-poorly differentiated epithelial cells have a nest-like
structure but are positive for epithelial/neuroendocrine markers
and do not exhibit CIC rearrangements.
The current treatment of CRS is based on Ewing
sarcoma, which is mostly treated with surgery, chemotherapy,
radiotherapy and targeted therapy (13), but 70% of CRS cases respond poorly
to chemotherapy (2) and the 5-year
survival rate is 44%.
In conclusion, the present study reported another
rare case of retroperitoneal perirenal CRS in which the tumor was
composed of small- to medium-sized blue rounded immature cells in
nests with prominent nucleoli. On immunohistochemistry, the marker
CD99 was scattered positive and FISH examination revealed CIC gene
rupture and translocation. The clinicopathological features,
diagnosis and differential diagnosis, biological behavior and
prognosis of these tumors were discussed.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BH and JY drafted the manuscript and conceived the
study. HL was responsible for the collection and analysis of case
data and literature. BH revised the manuscript and interpreted the
data. BH and HL confirm the authenticity of all the raw data. All
authors agreed on the journal to which the article has been
submitted and agreed to be accountable for all aspects of the work.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the case study to be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Italiano A, Sung YS, Zhang L, Singer S,
Maki RG, Coindre JM and Antonescu CR: High prevalence of CIC fusion
with double-homeobox (DUX4) transcription factors in EWSR1-negative
undifferentiated small blue round cell sarcomas. Genes Chromosomes
Cancer. 51:207–218. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antonescu CR, Owosho AA, Zhang L, Chen S,
Deniz K, Huryn JM, Kao YC, Huang SC, Singer S, Tap W, et al:
Sarcomas with CIC-rearrangements are a distinct pathologic entity
with aggressive outcome: A clinicopathologic and molecular study of
115 cases. Am J Surg Pathol. 41:941–949. 2017. View Article : Google Scholar
|
|
3
|
Le Guellec S, Velasco V, Pérot G, Watson
S, Tirode F and Coindre JM: ETV4 is a useful marker for the
diagnosis of CIC-rearranged undifferentiated round-cell sarcomas: A
study of 127 cases including mimicking lesions. Mod Pathol.
29:1523–1531. 2016. View Article : Google Scholar
|
|
4
|
Richkind KE, Romansky SG and Finklestein
JZ: t(4;19)(q35;q13.1): A recurrent change in primitive mesenchymal
tumors? Cancer Genet Cytogenet. 87:71–74. 1996. View Article : Google Scholar
|
|
5
|
Haidar A, Arekapudi S, DeMattia F, Abu-Isa
E and Kraut M: High-grade undifferentiated small round cell sarcoma
with t(4;19)(q35;q13.1) CIC-DUX4 fusion: Emerging entities of soft
tissue tumors with unique histopathologic features-A case report
and literature review. Am J Case Rep. 16:87–94. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Loarer FL, Pissaloux D, Watson S,
Godfraind C, Galmiche-Rolland L, Silva K, Mayeur L, Italiano A,
Michot A, Pierron G, et al: Clinicopathologic features of CIC-NUTM1
sarcomas, a new molecular variant of the family of CIC-fused
sarcomas. Am J Surg Pathol. 43:268–276. 2019. View Article : Google Scholar
|
|
7
|
Lokuhetty D, White VA and Cree IA: World
Health Organization Classification of Tumours: soft Tissue and Bone
Tumours. 5th edition. WHO Classification of Tumours Editorial
Board. International Agency for Research on Cancer (IARC); Argonay:
2020
|
|
8
|
Zhao L, Sun M, Lliu QY, Yu L and Wang J:
Clinicopathological analysis of 10 cases of CIC reordering sarcoma.
Chin J Pathol. 48:515–521. 2019.(In Chinese).
|
|
9
|
Brady EJ, Hameed M, Tap WD and Hwang S:
Imaging features and clinical course of undifferentiated round cell
sarcomas with CIC-DUX4 and BCOR-CCNB3 translocations. Skeletal
Radiol. 50:521–529. 2021. View Article : Google Scholar
|
|
10
|
Loarer FL, Pissaloux D, Coindre JM, Tirode
F and Vince DR: Update on families of round cell sarcomas other
than classical Ewing sarcomas. Surg Pathol Clin. 10:587–620. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yoshida A, Goto K, Kodaira M, Kobayashi E,
Kawamoto H, Mori T, Yoshimoto S, Endo O, Kodama N, Kushima R, et
al: VCIC-rearranged sarcomas: A study of 20 cases and comparisons
with Ewing sarcomas. Am J Surg Pathol. 40:313–323. 2016. View Article : Google Scholar
|
|
12
|
Siegele B, Roberts J, Black JO, Rudzinski
E, Vargas SO and Galambos C: DUX4 immunohistochemistry is a highly
sensitive and specific marker for CIC-DUX4 fusion-positive round
cell tumor. Am J Surg Pathol. 41:423–429. 2017. View Article : Google Scholar
|
|
13
|
Oyama R, Takahashi M, Yoshida A, Sakumoto
M, Takai Y, Kito F, Shiozawa K, Qiao Z, Arai Y, Shibata T, et al:
Generation of novel patient-derived CIC-DUX4 sarcoma xenografts and
cell lines. Sci Rep. 7:47122017. View Article : Google Scholar : PubMed/NCBI
|