Introduction
Isolinderalactone is a sesquiterpene that may be
isolated from extracts of Lindera aggregata root (1). The root extract of Lindera
aggregata, commonly known as Lindera, has been used in
traditional medicine to treat chest and abdominal pain and renal,
cystic and rheumatic diseases (2).
Lindera extract has also been used to treat diabetes and
inflammation (3,4). Studies have demonstrated that
isolinderalactone can inhibit tumor cell growth and induce
apoptosis. For example, isolinderalactone has been shown to induce
cell death in A549 non-small cell lung cancer cells by increasing
p21 expression and regulating the Fas receptor/Fas ligand-mediated
apoptosis pathway (5). In
addition, isolinderalactone has been demonstrated to induce
apoptosis in MDA-MB-231 breast cancer cells (6) and inhibit the migration and invasion
of A549 lung cancer cells by inhibiting MMP-2 and β-catenin
expression (7). Furthermore, our
previous studies reported the ability of isolinderalactone to
induce ovarian cancer cell death via the STAT3-mediated pathway
(8) and inhibit glioblastoma
growth and tumor angiogenesis (9,10).
Gliomas are the most frequently occurring primary
malignant tumors of the brain. Glioblastoma multiforme (GBM) is the
most aggressive brain tumor among gliomas and is characterized by
high recurrence and mortality rates (11). The standard protocol for the
treatment of GBM is maximal tumor resection in combination with
radiotherapy and chemotherapy using the alkylating agent
temozolomide (12,13). However, the prognosis of GBM
patients is very poor, as the median survival time is only 14–18
months and the 5-year survival rate is <5% (13,14).
A significant feature of glioblastomas is a high
degree of angiogenesis (11,15).
GBM is associated with various types of neovascularization,
including vasculogenesis, angiogenesis and intussusceptive
microvascular growth (16). Tumor
angiogenesis is the tumor-induced formation of new blood vessels
from pre-existing blood vessels, and most solid tumors require
sprouting angiogenesis for tumor progression (17). GBM vasculature shows structurally
and functionally abnormal characteristics, such as changes in the
association between endothelial cells and pericytes that lead to
hyperpermeability and vessel leakage, and subsequently poor vessel
perfusion and nutrient delivery (18,19).
These vascular characteristics aggravate tumor hypoxia, and the
secretion of hypoxia-mediated pro-angiogenic factors from
inflammatory or tumor cells enhances vascular abnormalities.
Glioblastoma cells crosstalk with their
microenvironment, and GBM can recruit healthy brain cells and
create a tumor microenvironment to support tumor progression
(20). GBM secretes cytokines such
as vascular endothelial growth factor (VEGF), which stimulates the
formation of blood vessels for tumor growth (21,22).
VEGF is highly expressed in GBM and is associated with the grade of
malignancy and the prognosis of patients with GBM (18,19).
In our previous study, isolinderalactone suppressed the growth of
glioblastoma xenograft tumors in vivo and induced apoptosis
in U-87 GBM cells (9). In another
of our previous studies, isolinderalactone was shown to inhibit
hypoxia-stimulated VEGF expression in U-87 glioblastoma cells and
strongly reduce VEGF-triggered angiogenesis in vitro and
in vivo (10). In the
present study, the direct angiogenic effect of GBM and the effect
of isolinderalactone on GBM tumor-triggered angiogenesis were
investigated. Tumor supernatants, specifically conditioned medium
(CM) from U-87 cell culture, were used to trigger angiogenesis
instead of VEGF. The CM was used to simulate a GBM tumor, and the
ability of isolinderalactone to inhibit tumor cell
supernatant-triggered angiogenesis in vitro and in
vivo was investigated.
Materials and methods
Cell culture, hypoxic condition and
reagents
The human U-87 MG (ATCC HTB-14) cell line, which is
derived from a glioblastoma of unknown origin, was purchased from
the American Type Culture Collection. The U-87 MG cells were
cultured at 37°C in Dulbecco's modified Eagle's medium (DMEM; cat.
no. 11965; Thermo Fisher Scientific, Inc.) supplemented with 10%
fetal bovine serum (FBS; cat. no. 16000; Thermo Fisher Scientific,
Inc.) and 1% penicillin/streptomycin (cat. no. 15140; Thermo Fisher
Scientific, Inc.). Human brain microvascular endothelial cells
(HBMECs; passages 7–9; ACBRI 376; Cell Systems) were cultured at
37°C in Endothelial Cell Growth Medium-2
BulletKit™-microvascular (EGM-2-MV; CC-3202; Lonza
Group, Ltd.). For the experiments under hypoxic conditions, cells
were incubated at 37°C in a hypoxic incubator (Forma™;
cat. no. 3131; Thermo Fisher Scientific, Inc.) filled with 1%
O2, 5% CO2 and balance N2.
Isolinderalactone (ALB-RS-6003) was obtained from ALB Technology,
Ltd. and dissolved in dimethyl sulfoxide (DMSO; Duchefa Biochemie
B.V.). Isolinderalctone was used to treat U-87 cells under hypoxia
(2.5 or 5 µg/ml) and HBMECs were treated with HCM (2 µg/ml).
Recombinant human basic fibroblast growth factor (BFGF; F0291) was
purchased from Sigma-Aldrich. Recombinant human VEGF (293-VE) was
purchased from R&D Systems, Inc. and reconstituted in
phosphate-buffered saline (PBS) containing 0.1% bovine serum
albumin (cat. no. 0332; VWR International, LLC.) according to the
manufacturer's protocol.
RNA extraction and reverse
transcription-PCR (RT-PCR)
Total RNA was isolated from U-87 cells using
TRIzol® Reagent (cat. no. 15596; Thermo Fisher
Scientific, Inc.). A PrimeScript™ 1st Strand cDNA
Synthesis Kit (cat. no. 6110; Takara Bio, Inc.) was used to
synthesize cDNA from 2 µg total RNA, according to the
manufacturer's protocol. End-point PCR for the measurement of VEGF
was performed using GAPDH as the reference gene for normalization
with the following primers: VEGF (23) forward,
5′-GAGAATTCGGCCTCCGAAACCATGAACTTTCTGCT-3′ (nucleotides plus
EcoRI adapter) and reverse,
5′-GAGCATGCCCTCCTGCCCGGCTCACCGC-3′ (nucleotides plus SphI
adapter) with a melting temperature of 65°C for 30 cycles; and
GAPDH forward, 5′-CGTGGAAGGACTCATGAC-3′ and reverse,
5′-CAAATTCGTTGTCATACCAG-3′ with a melting temperature of 55°C for
27 cycles. The PCR products were mixed with Ezstain DNA loading dye
(cat. no. B006M; Enzynomics, Inc.) and analyzed using a 1.2%
agarose gel.
Western blot assay
U-87 cells were lysed using RIPA buffer (cat. no.
9806; Cell Signaling Technology, Inc.) supplemented with a protease
inhibitor mixture (P3100; GenDEPOT) and a phosphatase inhibitor
mixture (P3200; GenDEPOT). Total protein content was determined by
Pierce™ BCA Protein Assay Kit (cat. no. #23225; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. Total protein (20–30 µg/lane) was separated on 12%
gels using SDS-PAGE and transferred onto a nitrocellulose membrane
(Amersham; Cytiva). After transfer, the membrane was incubated with
5% skimmed milk (cat. no. #232100; Becton, Dickinson and Company)
in PBS containing 0.1% Tween-20 (cat. no. TR1027-500-00; Biosesang)
for 1 h at room temperature. The membrane was immunoblotted using
an anti-VEGF antibody (1:1,000; sc-7269; Santa Cruz Biotechnology,
Inc.) for 16 h at 4°C. An α-tubulin antibody (1:3,000; T5168;
Sigma-Aldrich; Merck KGaA), as the internal control, was incubated
for 16 h at 4°C. For the incubation with secondary antibody,
HRP-conjugated anti-mouse antibody (1:10,000; #1031-05;
SouthernBiotech) was applied for 1 h at room temperature.
Chemiluminescence intensity was measured using an
ImageQuant™ LAS 4000 apparatus (Cytiva). The
quantification of band intensity was performed using ImageJ
software (V1.8.0; National Institutes of Health) and normalized to
the intensity of α-tubulin.
Preparation of CM from glioblastoma
cells
The CM from normoxic U-87 cells (NCM) and the CM
from hypoxic U-87 cells (HCM) were collected, concentrated and used
in further experiments.
In vitro angiogenesis assay
U-87 cells were seeded at 1.5×106
cells/100 mm dish and cultured for 2 days. The medium was replaced
with EGM-2-MV (without VEGF and bFGF) and incubated under normoxic
or hypoxic conditions. After 16 h, CM was harvested, filtered using
a Minisart® Syringe Filter (pore size 0.45 µm; Sartorius
AG), concentrated twice (for wounding migration assay) or 10 times
(for tube formation assay) with an Amicon® Ultra-15
Centrifugal Filter Unit (UFC901024; MilliporeSigma) and used to
treat HBMECs.
In vivo mouse Matrigel plug assay
The U-87 cell medium was replaced with DMEM
containing 0.5% FBS and incubated under normoxic or hypoxic
conditions for 16 h. CM was collected, filtered using a Minisart
Syringe Filter (pore size 0.45 µm), concentrated 100 times with an
Amicon Ultra-15 Centrifugal Filter Unit and mixed with Matrigel (50
µl concentrated CM + 500 µl Matrigel).
3D angiogenic sprouting assay
U-87 cells cultured in DMEM containing 10% FBS were
incubated under normoxic or hypoxic conditions for 16 h. CM was
collected, filtered using a Minisart Syringe Filter (pore size 0.45
µm), and mixed with EGM-2-MV (without VEGF and bFGF) at a 2:1 ratio
for use as interstitial fluid.
In vitro angiogenesis assay: Wounding
migration assay
The migration assay was performed as described
previously (24). Briefly, HBMECs
were seeded into 60-mm culture dishes and incubated until they
reached ~90% confluence. The monolayer was wounded with a razor and
washed with an endothelial basal medium to remove cell debris, and
the NCM (5 ml) or HCM (5 ml) from U-87 cells and 1 mM thymidine
(T1895; Sigma-Aldrich) were added. The HCM-treated cells were
incubated with or without isolinderalactone (2 µg/ml), and the
cells were incubated for 16 h. The cells were then fixed, stained
and imaged with an Olympus TH4-200 microscope (light microscope;
Olympus Corporation). The migration activity was quantified by
counting the number of cells that moved beyond the reference
line.
In vitro angiogenesis assay: Tube
formation assay
HBMECs (3×104 cells/well) were seeded
onto polymerized Matrigel (cat. no. 354230; BD Biosciences) in a
24-well plate, and a tube formation assay was performed as
previously described (24,25). HBMECs incubated with NCM (1 ml) or
HCM (1 ml), the latter with or without 2 µg/ml isolinderalactone,
were imaged with a TH4-200 microscope every hour for 10 h.
Capillary-like tube networks were observed and the tube length and
tube branching points (>3) were analyzed using iSolution full
image analysis software (Image & Microscope Technology).
3-Dimensional (3D) angiogenic
sprouting assay in a microfluidic device
3D chips (DAX01) were purchased from AIM Biotech
Pte. Ltd. and an angiogenic sprouting assay was performed as
previously described (10). AIM
connectors (LUC-1; AIM Biotech Pte. Ltd.) and a 1-cc syringe (Jung
Rim Medical Industrial Co., Ltd.) were used to apply interstitial
flow. Briefly, the central gel channel was filled with 2.5 mg/ml
collagen gel solution (pH 7.4; 10 µl) prepared using Collagen I,
rat tail (cat. no. 354236; Corning, Inc.), 10X PBS (cat. no.
70011-044; Thermo Fisher Scientific, Inc.) and 0.5 M NaOH (cat. no.
221465; Sigma-Aldrich), following the AIM Biotech protocol. The
gel-filled device was polymerized for 30 min at 37°C, the lateral
media channels were hydrated using growth medium (EGM-2-MV, without
VEGF or bFGF), and the devices were kept in a humidified
CO2 incubator until cell seeding. HBMECs were suspended
in growth medium at a concentration of 1.5×106 cells/ml,
and the cell suspension (40 µl) was seeded onto one lateral channel
of the 3D chip. After seeding the cells, the chips were tilted 90°
to allow the cells to adhere to the gel surface and incubated at
37°C for 15 min. The chips were then returned to their original
orientation and incubated for 3 h. Then, the medium was replaced
with fresh growth medium to remove any unattached cells. The next
day, the AIM connectors and 1-cc syringe were connected.
Subsequently, 200 µl growth medium was supplied to the cell seeding
channel, and 1,000 µl of a mixture of NCM or HCM with growth medium
and bFGF (10 ng/ml) was supplied to the medium channel. DMSO (0.1%)
or isolinderalactone (2 µg/ml) was added to the medium channel
containing the HCM mixture 6 days later. The medium was replaced
daily, and sprouting was monitored using a TH4-200 phase-contrast
microscope.
Immunofluorescence staining and
quantification of sprouting
After 8 days of culture on the chip,
immunofluorescence staining was performed according to the
manufacturer's protocol. Briefly, HBMECs were washed, fixed with 4%
paraformaldehyde for 15 min at room temperature, and permeabilized
with 0.1% Triton X-100 for 30 min at room temperature. The cells
were incubated with blocking buffer comprising 10% normal goat
serum (S-1000; Vector Laboratories, Inc.) in 1X PBS for 2 h at room
temperature, and then incubated with anti-VE-cadherin antibody
(1:100 in 1X PBS; sc-9989; Santa Cruz Biotechnology, Inc.)
overnight at 4°C. The next day, the cells were washed with PBS and
incubated with Alexa Fluor 488-conjugated secondary antibody
(1:200; A-11001; Thermo Fisher Scientific, Inc.) and
rhodamine-phalloidin for F-actin (5 U/ml; R415; Thermo Fisher
Scientific, Inc.) for 1 h at room temperature. Nuclei were stained
with DAPI (D1306; Molecular Probes; Thermo Fisher Scientific,
Inc.). The z-stack images of angiogenic sprouting were captured
using a Zeiss LSM 700 laser scanning confocal microscope (Carl
Zeiss AG). The sprout diameter was analyzed using images captured
from orthographic views of the middle of the sprouts, as previously
reported (26). Sprout length was
measured starting from the point at which the sprouts began to
extend. All analyses were performed using iSolution full image
analysis software.
In vivo mouse Matrigel plug assay
A total of 16 C57BL/6 mice (6-week-old males; 20–25
g) were purchased from Nara Biotechnology. The mice were housed
under a 12-h light/dark cycle at 23±1°C with 40–60% humidity and
allowed ad libitum access to food and water. The mice were
adapted to these conditions for at least 7 days prior to the
experiments. The mice were maintained in accordance with the Pusan
National University Guidelines for the Care and Use of Laboratory
Animals and the experiments using the mice were approved by the
Institutional Review Board of Pusan National University
(PNU-2019-2361). Humane endpoints for euthanasia were considered to
be the prolonged observation of a 40% reduction in food and water
intake and/or unstable breathing. The feeding and condition of the
mice were assessed and monitored daily post-surgery for any signs
of decreased activity and a reduction in food intake. However, no
signs of these conditions were observed. The following groups were
established: NCM (n=4), HCM + DMSO (n=6) and HCM + iso (n=6).
Growth factor-reduced Matrigel (500 µl; cat. no. 354230) containing
NCM or HCM (concentrated 100 times) was injected subcutaneously
under anesthesia. The mice were anesthetized with isoflurane
delivered using a face mask (3% for induction and 1.5% for
maintenance, in 80% N2O and 20% O2). The next
day, the treatment of the mice in the HCM + iso group was initiated
with an intraperitoneal injection of 5 mg/kg/day isolinderalactone
for 6 days. The mice in the HCM + DMSO group were treated with DMSO
at the same time points. On day 8, the mice were sacrificed by
exposure to 70% carbon dioxide following deep anesthesia with 50
mg/kg sodium thiopental (IP), and the Matrigel plugs were
harvested. The hemoglobin content of the Matrigel plugs was
measured using a hemoglobin assay kit (MAK115; Sigma-Aldrich) to
evaluate blood vessel formation.
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. The differences between groups were analyzed for
statistical significance using one-way ANOVA followed by Tukey's
post hoc test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Direct angiogenic effect of GBM CM is
suppressed by isolinderalactone
To examine the direct angiogenic effect of GBM, CM
from U-87 tumor cells was used. The CM was obtained from U-87 cells
under normoxic or hypoxic conditions. Before collecting the CM,
western blotting and RT-PCR analyses confirmed that VEGF expression
was stimulated in U-87 cells under hypoxic conditions (Fig. 1). Hypoxia significantly increased
VEGF protein and mRNA levels in the U-87 cells compared with those
in U-87 cells cultured under normoxic conditions, and these
increases in VEGF levels were significantly decreased by treatment
with isolinderalactone. Culture supernatants were obtained from
normoxic and hypoxic U-87 cells and used to perform in vitro
and in vivo assays (Fig.
2A).
First, whether HCM affects angiogenesis was
investigated using in vitro angiogenesis assays, in which
HCM was used to simulate the angiogenesis-stimulating effects of
glioblastoma on neighboring endothelial cells. The effect of HCM on
endothelial migration, a critical feature in the formation of new
blood vessels, was evaluated (27). HCM significantly stimulated the
migration of HBMECs, whereas isolinderalactone treatment
significantly attenuated the HCM-induced endothelial migration
(Fig. 2B). The effect of HCM on
capillary-like tube structure stabilization was then examined. HCM
clearly increased capillary-like tube formation, whereas
isolinderalactone suppressed the HCM-induced formation of tube
structures (Fig. 3A).
Quantification of the tube length and branching points revealed the
significant suppression of HCM-mediated tube formation by
isolinderalactone (Fig. 3B and
C).
Isolinderalactone inhibits 3D
angiogenic sprouting of tumor cell supernatants
The anti-angiogenic effect of isolinderalactone was
further examined in a 3D microfluidic device after the application
of interstitial flow using glioblastoma CM (Fig. 4A). Angiogenic sprout growth was
observed following HCM stimulation, and the addition of
isolinderalactone to the cell culture inhibited sprout formation
(Fig. 4B). Immunofluorescence
staining of the HBMECs with anti-VE-cadherin (green) and
anti-F-actin (red) antibodies showed that sprout length was
significantly increased in the HCM-treated group, and the
HCM-induced increase in sprout length was significantly decreased
by isolinderalactone (Fig. 4C and
D). However, sprout diameter did not differ among the groups
(Fig. 4E).
Isolinderalactone suppresses tumor
cell supernatant-mediated in vivo angiogenesis
To evaluate whether U-87 cell CM induces in
vivo angiogenesis, which is inhibited by isolinderalactone, an
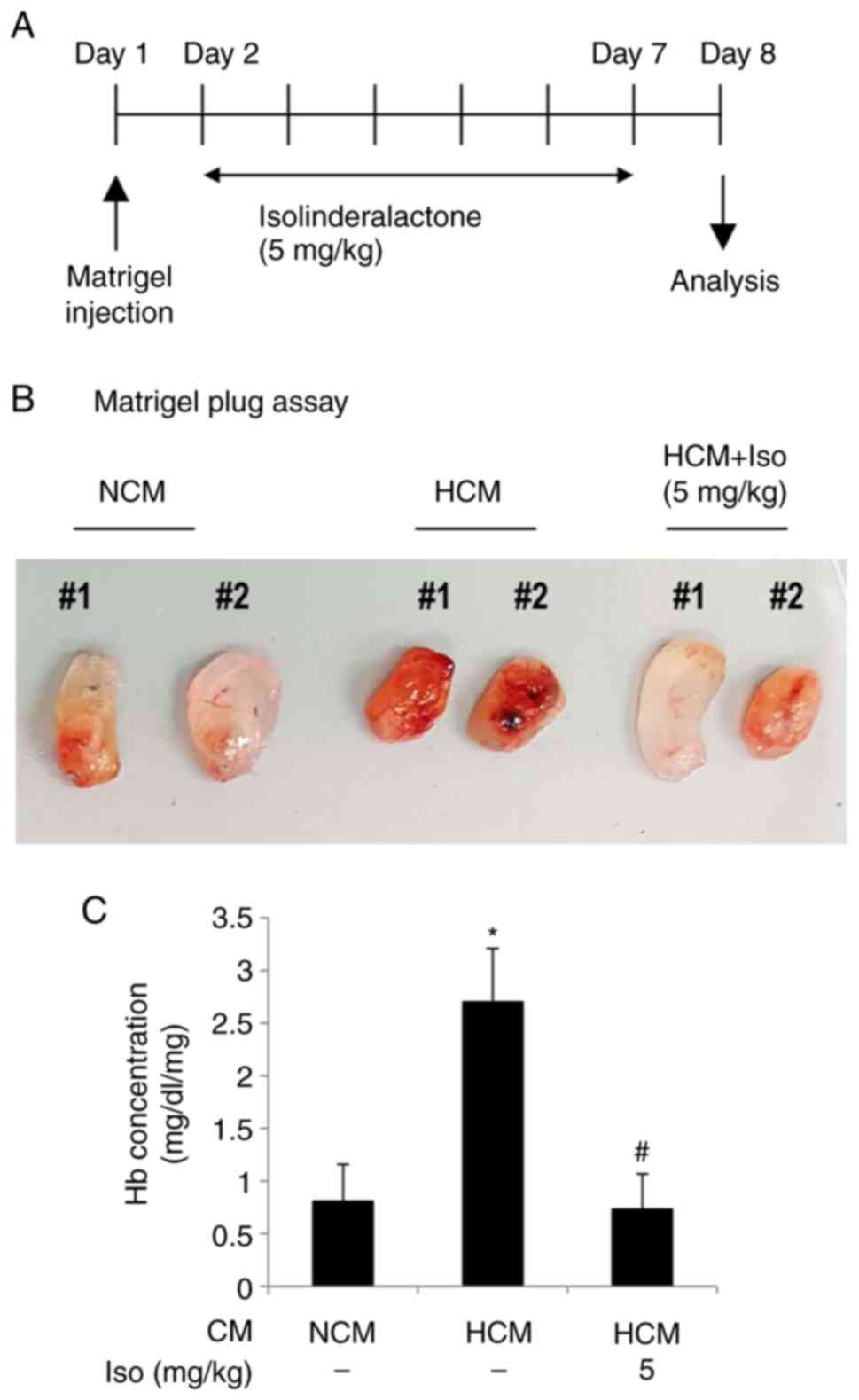
in vivo mouse Matrigel plug assay was performed (Fig. 5). Mice were subcutaneously injected
with Matrigel containing either NCM or HCM. Isolinderalactone
treatment of mice with HCM was initiated after 24 h (Fig. 5A). HCM strongly increased
angiogenic activity in the Matrigel plug, which was notably
suppressed by isolinderalactone (Fig.
5B). The Matrigel with HCM had a significantly increased
hemoglobin concentration compared with that in the Matrigel with
NCM, and the hemoglobin concentration in the
isolinderalactone-injected HCM group was significantly lower
compared with that in the untreated HCM group (Fig. 5C).
Discussion
GBM is the most frequently occurring malignant
primary brain tumor in adults (11). It is categorized as a grade IV
astrocytoma in the World Health Organization classification and is
the most aggressive tumor of the central nervous system (28). A common characteristic of GBMs is
the high degree of tumor vascularization with extensive
angiogenesis and abnormal vessels. GBMs show remarkable endothelial
proliferation, convoluted vessels with a variable diameter, reduced
pericyte coverage and thickened basement membranes (18,19).
Abnormal GBM vascularization is mainly due to the upregulation of
angiogenic factors, including VEGF, which is an essential growth
factor for vascular endothelial cells (18). Angiogenesis involves multiple
steps: Signaling from ischemic tissues or hypoxic solid tumors
activates quiescent endothelial cells, which degrade the basement
membrane and extracellular matrix. The endothelial cells
proliferate, migrate into the connective tissue and move toward the
source of angiogenic stimuli. The formation of lumen formation,
tubular sprouts and new basement membrane occurs, pericytes are
recruited and functional vascular networks are formed. These
angiogenic processes are tightly regulated by angiogenic and
anti-angiogenic factors (29).
Anti-angiogenic therapy using bevacizumab, a
humanized monoclonal antibody targeting VEGF, is the most studied
anti-angiogenic treatment and shows the most promising results
against tumor progression (30).
However, clinical trials of bevacizumab have not demonstrated any
extension of overall survival, although the drug appears to prolong
progression-free survival and improve the quality of life (30). It is hypothesized that the
development of resistance to anti-angiogenic therapy is based on
the upregulation of angiogenic or pro-angiogenic factors other than
VEGF. Angiogenic cytokines such as hepatocyte growth factor, FGF-2,
platelet-derived growth factor, angiopoietins and interleukin-8 are
upregulated in GBM (31–34). A study reported that the combined
blockade of VEGF, angiopoietin-2 (Ang-2) and programmed cell death
protein-1 increased the survival of GBM-bearing mice compared with
that of GCM-bearing mice in which only VEGF or Ang-2 was blocked
(35). Our previous study showed
that isolinderalactone inhibited the VEGF expression pathway in
GBMs under hypoxia and VEGF-induced angiogenesis (10). Moreover, the present study
demonstrated that isolinderalactone successfully decreased the
angiogenesis induced by hypoxic U-87 culture supernatant in
vitro and in vivo. Notably, isolinderalactone reduced
the sprout formation of HBMECs in a 3D microfluidic device after
the application of interstitial flow using CM obtained from hypoxic
U-87 cells. We hypothesize that the CM obtained from tumor cells
contains numerous pro-angiogenic factors secreted by the cells,
including VEGF, which thereby stimulate angiogenesis, and
isolinderalactone can efficiently suppress this process.
The present study has certain limitations. First,
the anti-angiogenic mechanism of isolinderalactone during
HCM-mediated angiogenesis was not explored. Although HCM was used
instead of recombinant VEGF, the activation of VEGFR2 in the HBMECs
may be the target of isolinderalactone, as observed in our previous
study (10). Further study is
required to elucidate the mechanism. In addition, the constituents
of the HCM were did not analyzed and the effective proteins or
components with angiogenic activity were not identified. In
addition to angiogenic cytokines, microRNA-containing extracellular
vesicles, and non-coding RNAs can contribute to tumor angiogenesis
(36,37). Thus, further research is necessary
to identify such proteins or components. Another limitation of the
present study is that only one GBM cell line was utilized.
Therefore, the findings may not extend to all types of GBM.
Isolinderalactone may have potential as an inhibitor of GBM
angiogenesis, but confirmation of its general effectiveness should
be explored in further investigations. In summary, the present
study demonstrated that the supernatants of GBM cells cultured
under hypoxic conditions stimulate angiogenesis, which is
successfully inhibited by isolinderalactone. These findings
indicate that isolinderalactone may have the potential to be
developed as an anti-angiogenic medicine for GBM treatment.
Acknowledgements
Not applicable.
Funding
The current study was supported by a National Research
Foundation of Korea grant funded by the Korea government (grant no.
2020R1A2C1012564).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SYL and HKS participated in the study design. JHP,
KHC and HK conducted the experiments. SYL performed the data
analysis. SYL and HKS wrote or contributed to the writing of the
manuscript. SYL and HKS confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kobayashi W, Miyase T, Sano M, Umehara K,
Warashina T and Noguchi H: Prolyl endopeptidase inhibitors from the
roots of Lindera strychnifolia F. Vill. Biol Pharm Bull.
25:1049–1052. 2002. View Article : Google Scholar
|
|
2
|
Gan LS, Zheng YL, Mo JX, Liu X, Li XH and
Zhou CX: Sesquiterpene lactones from the root tubers of Lindera
aggregata. J Nat Prod. 72:1497–1501. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ohno T, Takemura G, Murata I, Kagawa T,
Akao S, Minatoguchi S, Fujiwara T and Fujiwara H: Water extract of
the root of Lindera strychnifolia slows down the progression
of diabetic nephropathy in db/db mice. Life Sci. 77:1391–1403.
2005. View Article : Google Scholar
|
|
4
|
Wang F, Gao Y, Zhang L and Liu JK:
Bi-linderone, a highly modified methyl-linderone dimer from
Lindera aggregata with activity toward improvement of
insulin sensitivity in vitro. Org Lett. 12:2354–2357. 2010.
View Article : Google Scholar
|
|
5
|
Chang WA, Lin ES, Tsai MJ, Huang MS and
Kuo PL: Isolinderalactone inhibits proliferation of A549 human
non-small cell lung cancer cells by arresting the cell cycle at the
G0/G1 phase and inducing a Fas receptor and soluble Fas
ligand-mediated apoptotic pathway. Mol Med Rep. 9:1653–1659. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yen MC, Shih YC, Hsu YL, Lin ES, Lin YS,
Tsai EM, Ho YW, Hou MF and Kuo PL: Isolinderalactone enhances the
inhibition of SOCS3 on STAT3 activity by decreasing miR-30c in
breast cancer. Oncol Rep. 35:1356–1364. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chuang CH, Wang LY, Wong YM and Lin ES:
Anti-metastatic effects of isolinderalactone via the inhibition of
MMP-2 and up regulation of NM23-H1 expression in human lung cancer
A549 cells. Oncol Lett. 15:4690–4696. 2018.
|
|
8
|
Rajina S, Kim WJ, Shim JH, Chun KS, Joo
SH, Shin HK, Lee SY and Choi JS: Isolinderalactone induces cell
death via mitochondrial superoxide- and STAT3-mediated pathways in
human ovarian cancer cells. Int J Mol Sci. 21:75302020. View Article : Google Scholar
|
|
9
|
Hwang JY, Park JH, Kim MJ, Kim WJ, Ha KT,
Choi BT, Lee SY and Shin HK: Isolinderalactone regulates the
BCL-2/caspase-3/PARP pathway and suppresses tumor growth in a human
glioblastoma multiforme xenograft mouse model. Cancer Lett.
443:25–33. 2019. View Article : Google Scholar
|
|
10
|
Park JH, Kim MJ, Kim WJ, Kwon KD, Ha KT,
Choi BT, Lee SY and Shin HK: Isolinderalactone suppresses human
glioblastoma growth and angiogenic activity in 3D microfluidic chip
and in vivo mouse models. Cancer Lett. 478:71–81. 2020. View Article : Google Scholar
|
|
11
|
Camelo-Piragua S and Kesari S: Further
understanding of the pathology of glioma: Implications for the
clinic. Expert Rev Neurother. 16:1055–1065. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stupp R, Hegi ME, Mason WP, van den Bent
MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B,
Belanger K, et al: Effects of radiotherapy with concomitant and
adjuvant temozolomide versus radiotherapy alone on survival in
glioblastoma in a randomised phase III study: 5-Year analysis of
the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar
|
|
14
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Norden AD, Drappatz J and Wen PY:
Antiangiogenic therapies for high-grade glioma. Nat Rev Neurol.
5:610–620. 2009. View Article : Google Scholar
|
|
16
|
Ribatti D and Pezzella F: Vascular
co-option and other alternative modalities of growth of tumor
vasculature in glioblastoma. Front Oncol. 12:8745542022. View Article : Google Scholar
|
|
17
|
Hanahan D and Folkman J: Patterns and
emerging mechanisms of the angiogenic switch during tumorigenesis.
Cell. 86:353–364. 1996. View Article : Google Scholar
|
|
18
|
Plate KH and Mennel HD: Vascular
morphology and angiogenesis in glial tumors. Exp Toxicol Pathol.
47:89–94. 1995. View Article : Google Scholar
|
|
19
|
Chi AS, Sorensen AG, Jain RK and Batchelor
TT: Angiogenesis as a therapeutic target in malignant gliomas.
Oncologist. 14:621–636. 2009. View Article : Google Scholar
|
|
20
|
Sisakht AK, Malekan M, Ghobadinezhad F,
Firouzabadi SNM, Jafari A, Mirazimi SMA, Abadi B, Shafabakhsh R and
Mirzaei H: Cellular conversations in glioblastoma progression,
diagnosis and treatment. Cell Mol Neurobiol. Apr 11–2022.(Epub
ahead of print). View Article : Google Scholar
|
|
21
|
Plate KH, Breier G, Weich HA and Risau W:
Vascular endothelial growth factor is a potential tumour
angiogenesis factor in human gliomas in vivo. Nature. 359:845–848.
1992. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gilbertson RJ and Rich JN: Making a
tumour's bed: Glioblastoma stem cells and the vascular niche. Nat
Rev Cancer. 7:733–736. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nomura M, Yamagishi S, Harada S, Hayashi
Y, Yamashima T, Yamashita J and Yamamoto H: Possible participation
of autocrine and paracrine vascular endothelial growth factors in
hypoxia-induced proliferation of endothelial cells and pericytes. J
Biol Chem. 270:28316–28324. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang J, Kim WJ, Jun HO, Lee EJ, Lee KW,
Jeong JY and Lee SW: Hypoxia-induced fibroblast growth factor 11
stimulates capillary-like endothelial tube formation. Oncol Rep.
34:2745–2751. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee SW, Kim WJ, Choi YK, Song HS, Son MJ,
Gelman IH, Kim YJ and Kim KW: SSeCKS regulates angiogenesis and
tight junction formation in blood-brain barrier. Nat Med.
9:900–906. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
26
|
van Duinen V, Zhu D, Ramakers C, van
Zonneveld AJ, Vulto P and Hankemeier T: Perfused 3D angiogenic
sprouting in a high-throughput in vitro platform. Angiogenesis.
22:157–165. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Folkman J: Angiogenesis: An organizing
principle for drug discovery? Nat Rev Drug Discov. 6:273–286. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Louis DN, Perry A, Wesseling P, Brat DJ,
Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM,
Reifenberger G, et al: The 2021 WHO classification of tumors of the
central nervous system: A summary. Neuro Oncol. 23:1231–1251. 2021.
View Article : Google Scholar
|
|
29
|
Holash J, Wiegand SJ and Yancopoulos GD:
New model of tumor angiogenesis: Dynamic balance between vessel
regression and growth mediated by angiopoietins and VEGF. Oncogene.
18:5356–5362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Fine HA: Bevacizumab in glioblastoma-still
much to learn. N Engl J Med. 370:764–765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schmidt NO, Westphal M, Hagel C, Ergün S,
Stavrou D, Rosen EM and Lamszus K: Levels of vascular endothelial
growth factor, hepatocyte growth factor/scatter factor and basic
fibroblast growth factor in human gliomas and their relation to
angiogenesis. Int J Cancer. 84:10–18. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reiss Y, Machein MR and Plate KH: The role
of angiopoietins during angiogenesis in gliomas. Brain Pathol.
15:311–317. 2005. View Article : Google Scholar
|
|
33
|
Brat DJ, Bellail AC and Van Meir EG: The
role of interleukin-8 and its receptors in gliomagenesis and
tumoral angiogenesis. Neuro Oncol. 7:122–133. 2005. View Article : Google Scholar
|
|
34
|
Shih AH and Holland EC: Platelet-derived
growth factor (PDGF) and glial tumorigenesis. Cancer Lett.
232:139–147. 2006. View Article : Google Scholar
|
|
35
|
Di Tacchio M, Macas J, Weissenberger J,
Sommer K, Bähr O, Steinbach JP, Senft C, Seifert V, Glas M,
Herrlinger U, et al: Tumor vessel normalization, immunostimulatory
reprogramming, and improved survival in glioblastoma with combined
inhibition of PD-1, angiopoietin-2, and VEGF. Cancer Immunol Res.
7:1910–1927. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lucero R, Zappulli V, Sammarco A, Murillo
OD, Cheah PS, Srinivasan S, Tai E, Ting DT, Wei Z, Roth ME, et al:
Glioma-derived miRNA-containing extracellular vesicles induce
angiogenesis by reprogramming brain endothelial cells. Cell Rep.
30:2065–2074.e4. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li D, Zhang Z, Xia C, Niu C and Zhou W:
Non-coding RNAs in glioma microenvironment and angiogenesis. Front
Mol Neurosci. 14:7636102021. View Article : Google Scholar
|