Introduction
Small cell lung cancer (SCLC) is a poorly
differentiated pulmonary neuroendocrine carcinoma that is
characterized by its rapid doubling time, high growth fraction,
early development of widespread metastases, and high sensitivity to
initial chemotherapy and radiotherapy (1). Approximately two-thirds of patients
present with extensive stage disease (ES-SCLC), which has been
classically defined as tumour or nodal volume that cannot be safely
encompassed within a single radiation field. Even with treatment,
ES-SCLC has a poor prognosis with a median survival of 8 to 13
months and a 5-year survival of 3% relative to the overall
population (2–4).
Brain metastases, in particular, are found in 20% of
patients at the time of diagnosis (5,6).
Notably, whole brain radiotherapy (WBRT) has historically been
preferred over stereotactic approaches in SCLC due to the frequent
occurrence of multiple metastases and high likelihood of occult
disease, as demonstrated by often rapid and diffuse central nervous
system (CNS) progression (7).
Despite its radiosensitivity, intracranial recurrence after cranial
irradiation can occur in up to 30% of patients, and this risk
increases with prolonged survival (8–10).
In the literature, the rates of intracranial recurrence are
reported primarily after prophylactic cranial irradiation (PCI) and
usually occur between 4 and 27 months post-PCI (6,10–13).
Treatment decisions for intracranial recurrence are
often complex and should account for a variety of factors including
volume of disease, extent of extracranial disease, symptom burden,
previous therapy, performance status, and patient preference
(10). For patients previously
treated with WBRT, stereotactic approaches including stereotactic
radiosurgery are often advantageous in providing local control
while reducing the volume of brain re-irradiated and thus overall
toxicity. In this article, we present a case of a patient with
ES-SCLC experiencing long-term survival following initial WBRT and
subsequent salvage stereotactic radiation for multiple intracranial
recurrences.
Case report
A 62-year-old man with a 40-pack-year smoking
history presented to the Emergency Department in 2015 with
acute-on-chronic dyspnea and a 5-week history of right-sided
sensory changes in both the upper and lower extremities. Physical
exam findings confirmed right-sided focal neurological deficits but
preserved cranial nerve and cerebellar function. His Eastern
Cooperative Oncology Group (ECOG) performance status score was
1.
MRI of the head revealed 7 intracranial metastases
within the left cerebral hemisphere, the largest of which measured
2 cm in diameter and was located in the left postcentral gyrus
(Fig. 1A). Staging investigations
demonstrated an 8.1 cm mass in the right apex of the lung with
involvement of the chest wall and mediastinum, with no further
distant metastases. Pathology from a CT-guided core biopsy of the
lung mass revealed small cell carcinoma with positive
immunohistochemical staining for synaptophysin, thyroid
transcription factor 1, and cytokeratin AE1/AE3, with a perinuclear
dot staining pattern. The malignant cells were negative for p63 and
cytokeratin 5/6. Therefore, the patient was diagnosed with
ES-SCLC.
In the context of numerous symptomatic brain
metastases, palliative WBRT was delivered to a total dose of 20 Gy
in 5 fractions. The patient then completed 6 cycles of palliative
chemotherapy with cisplatin and etoposide. He endorsed overall
improvement in his symptoms and functional abilities with
treatment. Subsequent imaging showed interval reduction in the size
of the right lung mass with no evidence of brain metastases on CT
head (Fig. 1B). The patient then
completed consolidative thoracic radiotherapy to a total dose of 30
Gy in 10 fractions. Regular follow-up imaging over the next 40
months revealed no evidence of disease progression in the thorax or
CNS.
In mid-2019, approximately 3 years post-treatment,
the patient began to notice a persistent, left-sided headache
accompanied by short-term memory deficits. Physical examination
demonstrated left-sided cerebellar dysfunction with nystagmus and
clumsiness on heel-to-toe walking. Restaging investigations
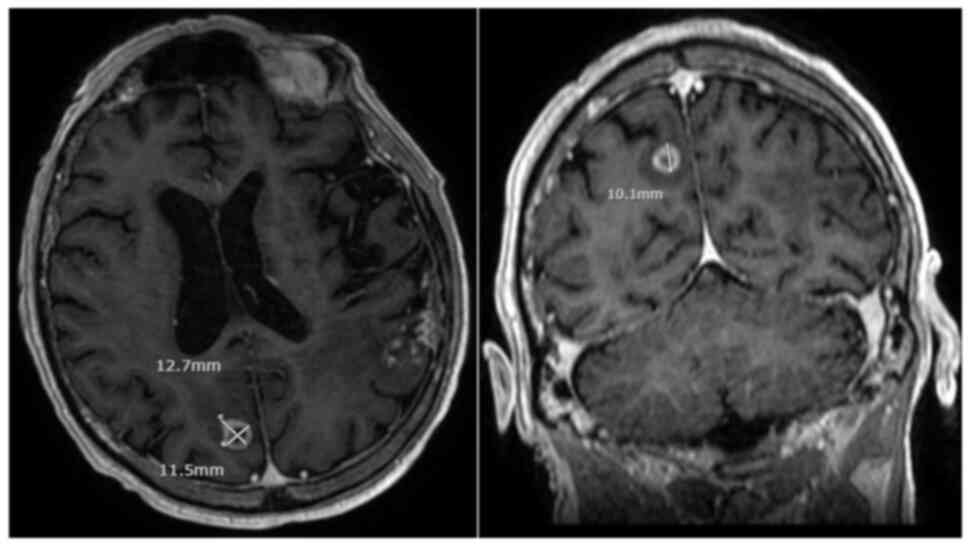
identified a new solitary mass measuring 3.6 cm with a broad dural
base overlying the lateral left temporal and occipital lobe
(Fig. 2A). No other evidence of
intrathoracic or intraabdominal disease was appreciated.
Neurosurgery was consulted but did not recommend surgical resection
given the high risk of morbidity, particularly Wernicke's aphasia.
The patient thus underwent stereotactic radiation to the solitary
brain metastasis, 30 Gy in 5 fractions (Table SI). Follow-up MRI imaging
demonstrated positive response to treatment with a gradual decrease
in the size of the mass. However, there was also subsequent
evidence of evolving radiation necrosis in the treated area,
associated with mild memory loss and word-finding difficulties
(Fig. 2B). As these symptoms were
overall quite minor and improved spontaneously with time, no
specific treatments for radionecrosis were implemented.
In late 2021, almost 6 years from his initial
diagnosis, the patient began to experience episodes of transient
aphasia. Repeat imaging of the head revealed a new enhancing mass
in the right parietooccipital lobe measuring 1.1 cm in diameter,
with no evidence of residual metastasis in the left hemisphere
(Fig. 3). Further staging
investigations did not identify any extracranial disease
progression. Notably, the previous area of radionecrosis remained
stable over time and the patient continued to deny any significant
neurologic symptoms that would necessitate intervention. He went on
to receive a second course of stereotactic radiotherapy, 30 Gy in 5
fractions, and will be monitored for treatment response. At the
present time, the patient is functionally independent, ambulatory,
and continues to participate within his local community by teaching
firearm safety. His ECOG performance status remains unchanged. The
timeline of the present case is shown in Fig. 4.
Discussion
Following initial WBRT in ES-SCLC, repeat WBRT has
been a conventional consideration for intracranial recurrence given
the high likelihood of occult disease. However, the life expectancy
for such patients is already quite poor, and even with salvage
WBRT, median survival ranges from 2 to 5 months (14–16).
Furthermore, re-irradiation of the whole brain raises concerns
regarding cumulative tissue toxicity impacting cognition and
quality of life (17).
In contrast, stereotactic treatments deliver high
dose and precisely targeted radiation to attain local control while
limiting dose to surrounding normal structures (18). It has become increasingly popular
in the treatment of limited intracranial disease for non-small cell
histologies, although the literature supporting its use in SCLC is
sparse. Retrospective studies, however, suggest local control rates
upwards of 70% and minimal toxicity even after prior WBRT. Yet,
median overall survival remains poor, ranging from 3 to 14 months
following salvage stereotactic radiotherapy (8,18–25).
Furthermore, despite the decent local control rates, distant brain
failure occurs in the majority of patients. In these instances,
further retreatment with stereotactic radiation is often feasible
and represents an additional advantage of stereotactic approaches
over repeat WBRT.
One of the largest retrospective studies
investigating outcomes of re-irradiation for intracranial
recurrence in the setting of SCLC comes from the MD Anderson Cancer
Center (9). Salvage stereotactic
radiation was associated with a significant overall survival
benefit at 6 months compared to salvage WBRT (58 vs. 21%;
P<0.001), although this is likely confounded by selection bias
(9). On multivariate analysis,
poor performance status and uncontrolled extracranial disease were
associated with worse overall survival. Other important prognostic
factors include the receipt of chemotherapy prior to intracranial
recurrence, tumour volume, and time between initial WBRT and
salvage therapy (9,18,21,23).
The patient presented in this case report had many positive
prognostic factors, highlighting the importance of not only control
of systemic disease, but also aggressive management of intracranial
recurrence in maximizing CNS control, overall survival, and quality
of life (10,23).
While salvage stereotactic radiation can reduce the
cumulative radiation dose to the entire brain compared to repeat
WBRT, stereotactic approaches do still carry a risk of neurologic
morbidity from radiation necrosis (10,26).
The patient presented here was no exception. Radionecrosis rates
following salvage stereotactic radiation after previous WBRT or PCI
range from 0 to 12.5% and can lead to adverse symptoms such as
hemiparesis, imbalance, aphasia, and loss of vision (21,23,25,27).
However, fractionated stereotactic regimens can reduce the risk of
radionecrosis particularly for larger brain metastases (28). For neuroendocrine tumours that are
inherently radiosensitive, using a lower stereotactic dose may
further minimize late effects while still providing reasonable
disease control. The specific dose and fractionation used in this
case report, 30 Gy in 5 fractions, was selected with this in mind,
and is in fact one of several standard regimens for intact brain
metastases at the institution where this patient was treated.
Ultimately, optimizing radiation dose and fractionation is
necessary to balance local control with treatment-related toxicity,
though more data is still required to better understand the
long-term effects of stereotactic radiation.
In conclusion, we present a case of a patient with
ES-SCLC who has survived over 6 years following initial WBRT and
multiple courses of salvage stereotactic radiation for separate
intracranial recurrences. This case challenges commonly held
notions that intracranial recurrence in SCLC is always diffuse and
associated with simultaneous systemic disease progression or
clinical deterioration. Amongst patients with isolated intracranial
recurrence, it is important to identify appropriate candidates for
salvage stereotactic radiation in order to maintain quality of
life, expand re-treatment options, and ultimately prolong
survival.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to acknowledge Mr. Gabriel
Boldt, clinical research librarian at London Health Sciences Centre
(London, ON, Canada), for his assistance in performing the initial
literature search.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AV acquired and collected patient data, performed
the literature review and data interpretation, and drafted the
manuscript. BA conceived and designed the present study, revised
the manuscript, and was responsible for the treatment of the
patient. TT contributed to the conceptualization of the case report
and manuscript drafting, and provided critical revisions on the
intellectual content. AV, BA and TT confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was provided by the patient
for publication of the case report in all formats, including
publication of all clinical details and diagnostic images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CNS
|
central nervous system
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
ES
|
extensive-stage
|
|
PCI
|
prophylactic cranial irradiation
|
|
SCLC
|
small cell lung cancer
|
|
WBRT
|
whole brain radiotherapy
|
References
|
1
|
Elias AD: Small cell lung cancer:
State-of-the-art therapy in 1996. Chest. 112 (Suppl 4):251S–258S.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jett JR, Schild SE, Kesler KA and
Kalemkerian GP: Treatment of small cell lung cancer: Diagnosis and
management of lung cancer, 3rd ed: American college of chest
physicians evidence-based clinical practice guidelines. Chest. 143
(Suppl 5):e400S–e419S. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demedts IK, Vermaelen KY and van Meerbeeck
JP: Treatment of extensive-stage small cell lung carcinoma: Current
status and future prospects. Eur Respir J. 35:202–215. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Howlader N NA, Krapcho M, Miller D, Brest
A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer
EJ and Cronin KA: SEER cancer statistics review. 5-Year Survival
Rates National Cancer Institute; Bethesda: MD1975. 2017
|
|
5
|
Seute T, Leffers P, ten Velde GP and
Twijnstra A: Neurologic disorders in 432 consecutive patients with
small cell lung carcinoma. Cancer. 100:801–806. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Slotman B, Faivre-Finn C, Kramer G, Rankin
E, Snee M, Hatton M, Postmus P, Collette L, Musat E and Senan S;
EORTC Radiation Oncology Group and Lung Cancer Group, :
Prophylactic cranial irradiation in extensive small-cell lung
cancer. N Engl J Med. 357:664–672. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsao MN, Lloyd N, Wong RK, Chow E,
Rakovitch E, Laperriere N, Xu W and Sahgal A: Whole brain
radiotherapy for the treatment of newly diagnosed multiple brain
metastases. Cochrane Database Syst Rev.
2012:CD0038692012.PubMed/NCBI
|
|
8
|
Bernhardt D, Adeberg S, Bozorgmehr F,
Opfermann N, Hoerner-Rieber J, König L, Kappes J, Thomas M, Herth
F, Heußel CP, et al: Outcome and prognostic factors in patients
with brain metastases from small-cell lung cancer treated with
whole brain radiotherapy. J Neurooncol. 134:205–212. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Suzuki R, Wei X, Allen PK, Welsh JW, Cox
JD, Komaki R and Lin SH: Outcomes of re-irradiation for brain
recurrence after prophylactic or therapeutic whole-brain
irradiation for small cell lung Cancer: A retrospective analysis.
Radiat Oncol. 13:2582018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fairchild A, Guest N, Letcher A, Mazure B,
Ghosh S, Gabos Z, Chu KP, Debenham B, Nijjar T, Severin D, et al:
Should stereotactic radiosurgery be considered for salvage of
intracranial recurrence after prophylactic cranial irradiation or
whole brain radiotherapy in small cell lung cancer? A
population-based analysis and literature review. J Med Imaging
Radiat Sci. 51:75–87.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arriagada R, Le Chevalier T, Rivière A,
Chomy P, Monnet I, Bardet E, Santos-Miranda JA, Péhoux CL, Tarayre
M, Benhamou S and Laplanche A: Patterns of failure after
prophylactic cranial irradiation in small-cell lung cancer:
Analysis of 505 randomized patients. Ann Oncol. 13:748–754. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ramlov A, Tietze A, Khalil AA and Knap MM:
Prophylactic cranial irradiation in patients with small cell lung
cancer. A retrospective study of recurrence, survival and
morbidity. Lung Cancer. 77:561–566. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Aupérin A, Arriagada R, Pignon JP, Péchoux
CL, Gregor A, Stephens RJ, Kristjansen PE, Johnson BE, Ueoka H,
Wagner H and Aisner J: Prophylactic cranial irradiation for
patients with small-cell lung cancer in complete remission. New
Engl J Med. 341:476–484. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Postmus PE, Haaxma-Reiche H, Gregor A,
Groen HJ, Lewinski T, Scolard T, Kirkpatrick A, Curran D, Sahmoud T
and Giaccone G: Brain-only metastases of small cell lung cancer;
efficacy of whole brain radiotherapy. An EORTC phase II study.
Radiother Oncol. 46:29–32. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bernhardt D, Adeberg S, Bozorgmehr F,
Opfermann N, Hörner-Rieber J, König L, Kappes J, Thomas M,
Unterberg A, Herth F, et al: Outcome and prognostic factors in
single brain metastases from small-cell lung cancer. Strahlenther
Onkol. 194:98–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bernhardt D, Bozorgmehr F, Adeberg S,
Opfermann N, von Eiff D, Rieber J, Kappes J, Foerster R, König L,
Thomas M, et al: Outcome in patients with small cell lung cancer
re-irradiated for brain metastases after prior prophylactic cranial
irradiation. Lung Cancer. 101:76–81. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Son CH, Jimenez R, Niemierko A, Loeffler
JS, Oh KS and Shih HA: Outcomes after whole brain reirradiation in
patients with brain metastases. Int J Radiat Oncol Biol Phys.
82:e167–e172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sheehan J, Kondziolka D, Flickinger J and
Lunsford LD: Radiosurgery for patients with recurrent small cell
lung carcinoma metastatic to the brain: Outcomes and prognostic
factors. J Neurosurg. 102:247–254. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wegner RE, Olson AC, Kondziolka D,
Niranjan A, Lundsford LD and Flickinger JC: Stereotactic
radiosurgery for patients with brain metastases from small cell
lung cancer. Int J Radiat Oncol Biol Phys. 81:e21–e27. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakazaki K, Higuchi Y, Nagano O and
Serizawa T: Efficacy and limitations of salvage gamma knife
radiosurgery for brain metastases of small-cell lung cancer after
whole-brain radiotherapy. Acta Neurochir (Wien). 155:107–114. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris S, Chan MD, Lovato JF, Ellis TL,
Tatter SB, Bourland JD, Munley MT, deGuzman AF, Shaw EG, Urbanic JJ
and McMullen KP: Gamma knife stereotactic radiosurgery as salvage
therapy after failure of whole-brain radiotherapy in patients with
small-cell lung cancer. Int J Radiat Oncol Biol Phys. 83:e53–59.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakazaki K, Yomo S, Kondoh T, Serizawa T,
Kenai H, Kawagishi J, Sato S, Nagano O, Aiyama H, Kawai H, et al:
Salvage gamma knife radiosurgery for active brain metastases from
small-cell lung cancer after whole-brain radiation therapy: A
retrospective multi-institutional study (JLGK1701). J Neurooncol.
147:67–76. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rava P, Sioshansi S, DiPetrillo T,
Cosgrove R, Melhus C, Wu J, Mignano J, Wazer DE and Hepel JT: Local
recurrence and survival following stereotactic radiosurgery for
brain metastases from small cell lung cancer. Pract Radiat Oncol.
5:e37–e44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li XP, Xiao JP, Chen XJ, Jiang XS, Zhang Y
and Xu YJ: Fractionated stereotactic radiotherapy for small-cell
lung cancer patients with brain metastases. J Cancer Res Ther.
10:597–602. 2014.PubMed/NCBI
|
|
25
|
Olson AC, Wegner RE, Rwigema JC, Heron DE,
Burton SA and Mintz AH: Clinical outcomes of reirradiation of brain
metastases from small cell lung cancer with Cyberknife stereotactic
radiosurgery. J Cancer Res Ther. 8:411–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blonigen BJ, Steinmetz RD, Levin L, Lamba
MA, Warnick RE and Breneman JC: Irradiated volume as a predictor of
brain radionecrosis after linear accelerator stereotactic
radiosurgery. Int J Radiat Oncol Biol Phys. 77:996–1001. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Faramand A, Niranjan A, Kano H, Flickinger
J and Lunsford LD: Primary or salvage stereotactic radiosurgery for
brain metastatic small cell lung cancer. J Neurooncol. 144:217–225.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Minniti G, D'Angelillo RM, Scaringi C,
Trodella LE, Clarke E, Matteucci P, Osti MF, Ramella S, Enrici RM
and Trodella L: Fractionated stereotactic radiosurgery for patients
with brain metastases. J Neurooncol. 117:295–301. 2014. View Article : Google Scholar : PubMed/NCBI
|