Introduction
Gastric cancer (GC), one of the most common
gastrointestinal malignancies worldwide (1), has a high incidence rate in East Asia
(2,3). The detection rate of early GC (EGC)
has increased due to introduction of the national cancer screening
system in the Republic of Korea. Endoscopic submucosal dissection
(ESD) was recently accepted as an early treatment modality for EGC
in several countries (2–8). ESD is considered a less invasive and
more accessible treatment strategy for EGC compared with surgery
(9–11). The extended criteria for the
application of ESD for EGC are based on large-scale Japanese data
(12). If performed accurately,
recovery time and the return to normal life are notably faster for
patients who undergo ESD compared with surgery, including open and
laparoscopic surgery (13). Thus,
ESD is a more desirable treatment strategy considering the
perioperative risk in patients with comorbidities.
Liver cirrhosis (LC) is also prevalent in East Asia,
owing to the high prevalence of hepatitis B and C viral infections
(14–16). Several studies have demonstrated
the association between LC and GC. A cohort study in Denmark
reported an increased standardized incidence ratio of GC in
patients with LC [a standardized incidence ratio of 1.9; 95%
confidence interval (CI), 1.3-2.6] (17). Furthermore, a study on 1,379
patients with LC reported a 2.6-fold higher incidence of GC in
cirrhotic patients compared with that in the general population
(18).
Several studies have reported the effectiveness of
ESD in patients with LC and EGC. A Korean study reported en bloc
and curative resection rates of 96.8 and 89.9%, respectively
(19). A Japanese study reported
en bloc and R0 resection rates of 88.9 and 77.8%, respectively, in
patients with EGC and LC (20).
Due to the high incidence of GC and LC in East Asia,
and the favorable outcomes of endoscopic therapy, the prognostic
expectations following ESD for EGC in patients with LC include
improving the quality of life and compliance of the patients.
However, this issue is yet to be investigated.
The present study aimed to compare the long-term
clinical outcomes and prognosis of EGC following ESD in patients
with and without LC.
Patients and methods
Patient enrollment
The present study retrospectively collected data on
ESD procedures performed between March 2007 and March 2016.
Patients were diagnosed according to the International
Classification of Diseases, 10th edition (21), and the lesions were classified into
lower third (C16.0, 16.1), middle third (C16.2) and upper third
(C16.3, 16.4), according to their location. The total number of ESD
procedures performed during the study period was 688. Patients
diagnosed with LC, who underwent endoscopic treatment for EGC at
Korea University Guro Hospital (Seoul, South Korea), were enrolled
in the present study. The male to female ratio was 88.2:11.8, and
the mean age was 62.64±7.85 years (age range, 51–79 years). All
patients were Korean. The inclusion criteria were as follows: i)
Presence of LC in imaging tests; ii) histologically diagnosed
gastric cancer and iii) lesions localized in the mucosal layer. The
exclusion criteria were as follows: i) Absence of LC in imaging
tests; ii) incomplete clinical or blood test results, namely
prothrombin time/international normalized ratio, presence of
ascites, bilirubin level, albumin level or presence of
encephalopathy, for calculating the Child-Pugh score (22); iii) history of receiving a liver
transplant; and iv) biopsy results indicating a benign lesion. LC
was diagnosed based on radiological examination, clinical data and
medical history. Liver function was assessed using the Child-Pugh
scoring system. To determine the therapeutic effect of ESD and the
prognosis of EGC, propensity score-matched patients with EGC but
without LC (n=51) were used as the controls. The present study was
reviewed and approved by the Korea University Guro Hospital
Institutional Review Board (approval no. 2019GR0467) and written
informed consent was provided by all participants prior to the
study start.
Endoscopic procedure
Experienced endoscopists (n=2) used a GIF-H260
endoscope (Olympus Corporation) to perform the endoscopies in the
present study. ESD was performed under standard conscious sedation
induced by propofol (Daewon Pharm. Co., Ltd., https://www.daewonpharm.com/main/index.jsp, 1.5-2.5
mg/kg) and/or midazolam (Bukwang Pharm Co., Ltd., https://www.bukwang.co.kr, 2–2.5 mg) in patients
placed in the left decubitus or prone position. Blood pressure and
oxygen saturation were carefully monitored during endoscopy.
Standard ESD procedures were performed, including lesion marking,
mucosal dissection, submucosal dissection and hemostasis. Markings
were made around the lesion via needle coagulation, and normal
saline solution with epinephrine (Daihan Pharm Co., Ltd.,
http://www.daihan.com/main/main.php)
1:10,000 and indigo carmine (Korea United Pharm, Inc., http://www.kup.co.kr) was injected into the submucosa.
After making a circumferential incision around the lesion, ESD was
performed using an insulation-tipped electrosurgical knife (IT
knife, KD-610L); until complete resection was achieved. When
bleeding occurred, endoscopic hemostasis was performed using
hemostatic forceps and an endoscope clip. Following endoscopic
resection, all visible vessels were coagulated. All ESD procedures
were performed the same in the LC and control groups. Patients were
advised to drink water on the day of the procedure after a review
of their blood test results and radiographs.
Definitions
En bloc resection was defined as the removal of the
tumor lesion without its fragmentation. Complete resection was
defined as resection with tumor-free lateral and deep resection
margins following lesion removal. Procedure time was defined as the
time from tumor marking to hemostasis completion and specimen
retrieval. Bleeding was defined as the occurrence of bleeding
requiring endoscopic hemostasis during second-look endoscopy, with
clinical manifestations, such as melena or hematemesis. Perforation
was confirmed based on direct observation during endoscopy or from
abdominal imaging.
Patient follow-up
Patients were followed up until they were lost to
follow-up or died. Following ESD, complete blood cell count
determination and chest radiography were performed. Second-look
endoscopy was performed a day after ESD to check for post-ESD
ulcers. If no complications, such as bleeding or perforation, were
observed, patients started an oral diet. An intravenous proton pump
inhibitor was started on the morning of the procedure and replaced
with an oral proton pump inhibitor following discharge. An
endoscopy was performed 3, 6 and 12 months after endoscopic
resection. Abdominal computed tomography was performed every 6
months for the first year and annually thereafter. Patient data
were acquired from endoscopic results, radiological reports and
clinical records.
Statistical analysis
Statistical analysis was performed using SPSS
version 20.0 software (IBM Corp.). Endoscopy results were analyzed
using an independent sample t-test and Fisher's exact test to
compare the variables. Propensity score matching was used to match
the LC-EGC group with the non-LC-EGC group in a 1:3 ratio,
according to age, sex, lesion location, tumor histology and tumor
size. Cox regression analysis was performed to assess the
association between predictive factors and cancer recurrence or
bleeding risk following ESD. P-values were derived from two-tailed
tests and P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient population
Of the 688 patients enrolled in the present study,
73% were men and 27% were women (mean age, 64.36±14.43 years, range
46–81). No significant differences in variables were observed
between the LC-EGC and non-LC-EGC groups (Table I). The average age of patients with
EGC and LC was 64.4 years. Alcohol consumption was the main cause
of LC in ~70% of all patients, followed by hepatitis B in 14% of
patients. In the LC group, esophageal varices were present in 71%
of all patients, gastric varices in 21% and ascites in 50%.
 | Table I.Baseline characteristics of patients
with EGC, with and without LC. |
Table I.
Baseline characteristics of patients
with EGC, with and without LC.
|
| Total population | Propensity
score-matched population |
|---|
|
|
|
|
|---|
| Characteristic | LC-EGC group
(n=17) | Non-LC-ECG group
(n=671) | P-value | LC-ECG group
(n=17) | Non-LC-ECG group
(n=51) | P-value |
|---|
| Age, years | 62.64±7.85 | 64.25±13.82 | 0.96 | 62.64±7.85 | 64.51±9.40 | 0.96 |
| Sex |
|
| 0.16 |
|
| 0.18 |
|
Male | 15 (88.2) | 490 (73.0) |
| 15 (88.2) | 37 (72.5) |
|
|
Female | 2 (11.8) | 181 (27.0) |
| 2 (11.8) | 14 (27.5) |
|
| Smoking | 5 (29.4) | 116 (17.3) | 0.27 | 5 (29.4) | 9 (17.6) | 0.14 |
| Comorbidity |
|
|
|
|
|
|
|
Hypertension | 4 (23.5) | 258 (38.5) | 0.21 | 4 (23.5) | 23 (45.1) | 0.11 |
|
Diabetes | 5 (29.4) | 110 (16.4) | 0.15 | 5 (29.4) | 11 (21.6) | 0.50 |
| Cardiovascular
disease | 1 (5.9) | 36 (5.4) | 0.92 | 1 (5.9) | 6 (11.8) | 0.48 |
| Hx of other
cancers | 2 (11.8) | 25 (3.7) | 0.09 | 2 (11.8) | 1 (2.0) | 0.08 |
| Medication |
|
|
|
|
|
|
|
Aspirin | 1 (5.9) | 59 (8.8) | 0.90 | 1 (5.9) | 6 (11.8) | 0.65 |
|
Antithrombotic agent | 0 (0.0) | 25 (3.7) | 0.41 | 0 (0.0) | 5 (9.8) | 0.18 |
Most of the patients in both groups were men. No
intergroup differences were observed in the rates of comorbidities,
such as hypertension and diabetes mellitus. In addition, no
intergroup differences in dosage associated with bleeding risk were
observed. The propensity score matching of all patients produced
matched pairs in a 1:3 ratio (LC-EGC group: non-LC-EGC group).
Clinical outcomes
The en bloc and complete resection rates were
comparable between the LC-EGC and non-LC-EGC groups (Table II). The histological type was
mainly differentiated cancer, with no significant intergroup
differences. In the mean follow-up period of 33.15±26.58 months,
four and three cases of recurrence occurred in the LC-EGC and
non-LC-EGC groups, respectively. Surgery was performed in four
cases, whereas repeat ESD was performed in the other three cases.
During follow-up, one death occurred that was not associated with
cancer or the procedure.
 | Table II.Clinical characteristics and outcomes
of endoscopic submucosal dissection in patients with and without
LC. |
Table II.
Clinical characteristics and outcomes
of endoscopic submucosal dissection in patients with and without
LC.
| Characteristic | LC-ECG group
(n=17) | Non-LC-ECG group
(n=51) | P-value |
|---|
| Location |
|
| 0.821 |
| Lower
third (C16.0, 16.1) | 9 (52.9) | 27 (52.9) |
|
| Middle
third (C16.2) | 7 (41.2) | 22 (43.2) |
|
| Upper
third (C16.3, 16.4) | 1 (5.9) | 2 (3.9) |
|
| Gross type |
|
| 0.672 |
|
Elevated | 8 (47.1) | 22 (43.1) |
|
|
Flat | 7 (41.2) | 18 (35.3) |
|
|
Depressed | 2 (11.8) | 11 (21.6) |
|
| Presence of
ulcer | 2 (11.8) | 12 (23.5) | 0.299 |
| Procedure type
(:) |
|
| 0.622 |
|
ESD | 15 (88.2) | 47 (92.2) |
|
|
EMR | 2 (11.8) | 4 (7.8) |
|
| Procedure time,
min | 45.9±27.7 | 45.25±27.47 | 0.935 |
| Complication |
|
|
|
|
Bleeding | 2 (11.8) | 11 (21.6) | 0.373 |
|
Perforation | 0 (0.0) | 2 (3.9) | 0.561 |
| En bloc
resection rate | 16 (94.1) | 49 (96.1) | 0.733 |
| Tumor size, mm | 15.3±6.9 | 17.22±13.44 | 0.462 |
| Histopathology |
|
| 0.561 |
|
Differentiated type | 14 (82.4) | 48 (93.5) |
|
|
Undifferentiated type | 3 (17.6) | 3 (6.5) |
|
| Invasion depth |
|
| 0.399 |
|
Mucosa | 12 (70.6) | 41 (80.4) |
|
|
Submucosa | 5 (29.4) | 10 (19.6) |
|
| Complete resection
rate | 100.0 | 43 (84.3) | 0.554 |
| Follow-up duration,
months | 23.71±20.21 | 36.29±27.84 | 0.091 |
| Recurrence
rate | 4 (23.5) | 3 (5.9) | 0.038a |
| Death | 1 (5.8) | 0 (0.0) |
<0.001b |
|
Cancer-associated | 0 (0.0) | 0 (0.0) | 1.000 |
|
Procedure-associated | 0 (0.0) | 0 (0.0) | 1.000 |
Factors associated with adverse events
and recurrence
Cox regression analysis was performed to identify
the factors associated with procedure-related bleeding and cancer
recurrence (Tables III and
IV). Multivariate analysis
demonstrated that procedure time was an independent predictive
factor for bleeding following ESD of EGC [adjusted hazard ratio
(HR), 1.017; 95% CI, 1.001-1.032; P=0.033]. Furthermore, LC was
significantly associated with cancer recurrence following ESD of
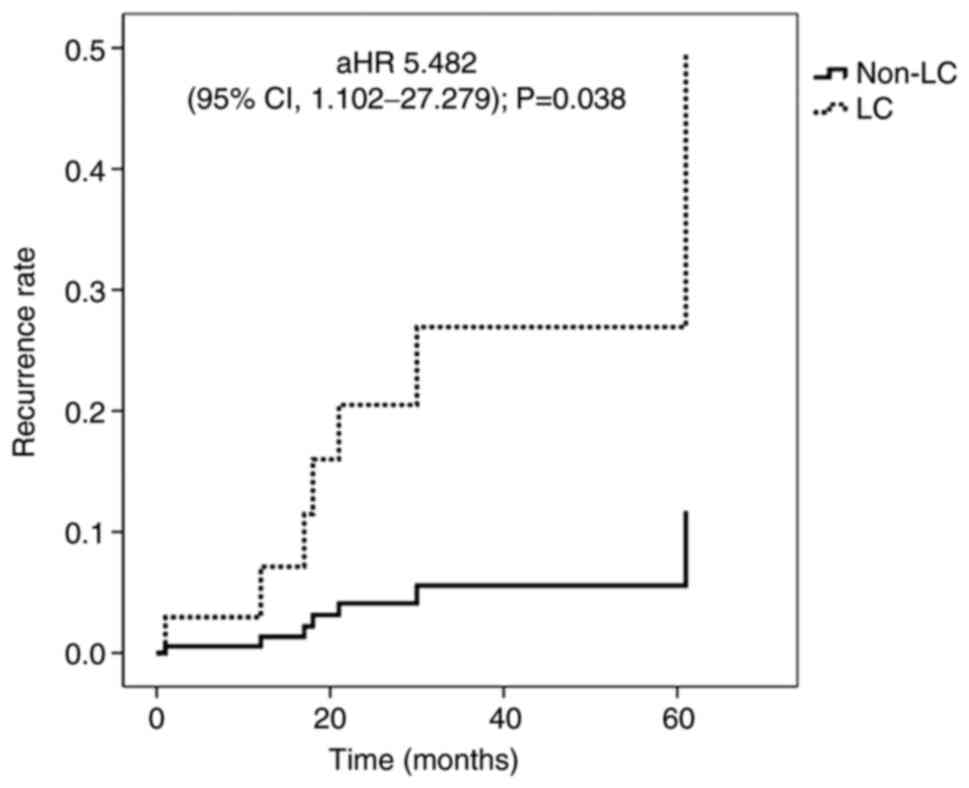
EGC (adjusted HR, 5.482; 95% CI, 1.102-27.279; P=0.038; Fig. 1). In one patient from the LC-EGC
group, cancer recurrence occurred 2 years after endoscopy. Repeat
ESD and complete resection were performed (Fig. 2).
 | Table III.Cox proportional hazard analysis of
the effect of factors on bleeding after endoscopic submucosal
dissection. |
Table III.
Cox proportional hazard analysis of
the effect of factors on bleeding after endoscopic submucosal
dissection.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Diabetes | 2.610 | 0.772-8.829 | 0.123 | 1.642 | 0.411-6.568 | 0.483 |
| Hypertension | 1.541 | 0.515-4.612 | 0.439 | - | - | - |
| Liver
cirrhosis | 1.002 | 0.215-4.664 | 0.998 | - | - | - |
| Aspirin | 2.233 | 0.477-10.461 | 0.308 | 0.881 | 0.044-17.490 | 0.934 |
| Other thrombotic
agent | 2.974 | 0.638-13.868 | 0.165 | 2.898 | 0.146-57.652 | 0.486 |
| Procedure time,
min | 1.019 | 1.006-1.003 | 0.006b | 1.017 | 1.001-1.032 | 0.033a |
 | Table IV.Cox proportional hazard analysis of
the effect of factors on recurrence after endoscopic submucosal
dissection. |
Table IV.
Cox proportional hazard analysis of
the effect of factors on recurrence after endoscopic submucosal
dissection.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age, years | 1.003 | 0.925-1.088 | 0.936 | - | - | - |
| Sex | 1.815 | 0.216-15.278 | 0.583 | - | - | - |
| Diabetes | 0.736 | 0.088-6.173 | 0.778 | - | - | - |
| Hypertension | 0.276 | 0.033-2.294 | 0.234 | 0.269 | 0.032-2.288 | 0.229 |
| Liver
cirrhosis | 5.172 | 1.123-23.822 | 0.035a | 5.482 | 1.102-27.279 | 0.038a |
| History of other
cancer | 0.044 |
0.000-18,963.051 | 0.638 | - | - | - |
| Procedure type | 0.038 | 0.000-423.832 | 0.491 | - | - | - |
| Invasion depth | 0.603 | 0.073-5.014 | 0.640 | - | - | - |
Discussion
The present study compared the long-term prognosis
and effects of the endoscopic treatment of EGC in patients with and
without LC. No significant differences were observed in the en bloc
resection and complete resection rates between the LC-EGC and
non-LC-EGC groups. The recurrence rate following ESD of EGC was
higher in the LC-EGC group compared with that in the non-LC-EGC
group (23.5 vs. 5.9%). No intergroup differences were observed in
the incidence of periprocedural complications, such as bleeding.
However, a higher risk of periprocedural bleeding was observed with
longer procedure times.
ESD is a common treatment for EGC and is more
effective than conventional endoscopic mucosal resection (23). Previous studies have reported that
the incidence of gastrointestinal lesions, including GC, is high in
patients with LC (17,18,24,25).
However, surgical treatment in patients with LC is associated with
high morbidity and mortality rates (26). Surgical resection in patients with
LC is associated with several complications, including pneumonia,
ascites, bleeding, infection and hepatic coma (27). Several studies have reported the
safety and efficacy of ESD in cirrhotic patients (19,28).
ESD is feasible in patients with early cirrhosis and is effective
in patients with Child-Pugh class B cirrhosis (28). Bleeding complications tend to
frequently occur following ESD procedures, and patients with LC
have a higher risk of bleeding due to coagulation disorders and low
platelet count (29). Thus,
hemorrhagic complications associated with ESD procedures should be
considered. However, in the present study, no significant
differences in the incidence of bleeding complications following
ESD were observed between the LC-EGC and non-LC-EGC groups, which
is consistent with previous findings (19,30).
In the present study, LC was significantly
associated with cancer recurrence following ESD of EGC. Previous
epidemiological studies have also reported a high incidence of GC
in patients with cirrhosis (17,18).
The association between LC and GC development remains ambiguous.
However, increased gastric epithelial cell proliferation is known
to play an important role in the development of GC (31). A previous study reported that the
presence of congestive gastropathy significantly increases the
proliferation of epithelial cells in the gastric mucosa of patients
with LC, and is further activated by Helicobacter pylori
infection (24). Patients with LC
have also been suggested to have zinc deficiency, which may play a
role in epithelial carcinogenesis of the esophagus (32). Furthermore, LC may affect the
metabolism of carcinogens and hormones, and increase the risk of
cancer (33). It is important to
consider the potential changes that occur in the body's immune
system and the risk of infection in patients with LC, as liver
dysfunction can affect the metabolism of lipids and water-soluble
drugs (34). As LC is an
underlying disease accompanying GC regardless of early treatment,
patients with LC are still at an increased risk of carcinogenesis
even after undergoing ESD for EGC (33).
The present study is not without limitations. Given
the retrospective design and limited sample size, the present study
is susceptible to various biases and unmeasured confounding
factors. However, an attempt was made to balance the variables
through propensity score matching between the LC-EGC and non-LC-EGC
groups.
Only a few studies to date have investigated ECG
recurrence following endoscopic treatment in patients with LC. The
results presented in the current study may aid the decision-making
process for patients with and without LC and EGC, and may encourage
patient compliance. However, large-scale studies are required to
confirm these results.
The recurrence rate was high in patients with LC.
However, it was not associated with higher overall mortality. As
the prognosis was good even in cases of relapse, repeat ESD can be
recommended if the condition of the patient and the laboratory
findings are satisfactory.
In conclusion, the present study demonstrated that
the endoscopic resection of EGC in patients with LC is safe and
effective, with high en bloc resection and complete resection
rates. Thus, ESD can be actively performed in selected patients
with LC. However, attention should be paid to potential cancer
recurrence during clinical follow-up after ESD.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Research
Foundation of Korea (NRF) grant funded by the Korean Government
(grant no. NRF-2019R1C1C1009819) and Korea University, Seoul,
Republic of Korea (grant no. K1924971). Funding support was
provided to Korea University Guro Hospital for data collection and
analysis.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
SHK, MKJ and JJP designed the present study and
drafted the initial manuscript. SHK and JJP critically revised the
manuscript for important intellectual content. AYY, SMK, WSK and
BJL acquired and analyzed the data. SHK, SWL and HJC analyzed the
data. SHK and MKJ confirmed the authenticity of all the raw data
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
Korea University Guro Hospital Institutional Review Board (Seoul,
South Korea; approval no. 2019GR0467). Written informed consent was
provided by all participants prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
LC
|
liver cirrhosis
|
|
ESD
|
endoscopic submucosal dissection
|
|
EGC
|
early gastric cancer
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Machlowska J, Baj J, Sitarz M, Maciejewski
R and Sitarz R: Gastric cancer: Epidemiology, risk factors,
classification, genomic characteristics and treatment strategies.
Int J Mol Sci. 21:40122020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sugano K: Screening of gastric cancer in
Asia. Best Pract Res Clin Gastroenterol. 29:895–905. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jun JK, Choi KS, Lee HY, Suh M, Park B,
Song SH, Jung KW, Lee CW, Choi IJ, Park EC and Lee D: Effectiveness
of the Korean national cancer screening program in reducing gastric
cancer mortality. Gastroenterology. 152:1319–1328.e1317. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gotoda T, Ho KY, Soetikno R, Kaltenbach T
and Draganov P: Gastric ES: Current status and future directions of
devices and training. Gastrointest Endosc Clin N Am. 24:213–233.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chu YN, Yu YN, Jing X, Mao T, Chen YQ,
Zhou XB, Song W, Zhao XZ and Tian ZB: Feasibility of endoscopic
treatment and predictors of lymph node metastasis in early gastric
cancer. World J Gastroenterol. 25:5344–5355. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tate DJ, Klein A, Sidhu M, Desomer L,
Awadie H, Lee EY, Mahajan H, McLeod D and Bourke MJ: Endoscopic
submucosal dissection for suspected early gastric cancer: Absolute
versus expanded criteria in a large Western cohort (with video).
Gastrointest Endosc. 90:467–479. e4642019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee J, Kim BW, Huh CW, Kim JS and Maeng
LS: Endoscopic factors that can predict histological ulcerations in
early gastric cancers. Clin Endosc. 53:328–333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee SH, Kim MC, Jeon SW, Lee KN, Park JJ
and Hong SJ: Risk factors and clinical outcomes of non-curative
resection in patients with early gastric cancer treated with
endoscopic submucosal dissection: A retrospective multicenter study
in Korea. Clin Endosc. 53:196–205. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gotoda T, Yamamoto H and Soetikno RM:
Endoscopic submucosal dissection of early gastric cancer. J
Gastroenterol. 41:929–942. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Isomoto H, Shikuwa S, Yamaguchi N, Fukuda
E, Ikeda K, Nishiyama H, Ohnita K, Mizuta Y, Shiozawa J and Kohno
S: Endoscopic submucosal dissection for early gastric cancer: A
large-scale feasibility study. Gut. 58:331–336. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang D, Kotzev AI and Draganov PV:
Endoscopic submucosal dissection for early gastric cancer in the
West: The absolute but not final word. Gastrointest Endosc.
90:480–482. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gotoda T, Yanagisawa A, Sasako M, Ono H,
Nakanishi Y, Shimoda T and Kato Y: Incidence of lymph node
metastasis from early gastric cancer: Estimation with a large
number of cases at two large centers. Gastric Cancer. 3:219–225.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gotoda T: Endoscopic resection of early
gastric cancer. Gastric Cancer. 10:1–11. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Goh LY, Leow AH and Goh KL: Observations
on the epidemiology of gastrointestinal and liver cancers in the
Asia-Pacific region. J Dig Dis. 15:463–468. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chung W: The cost of liver disease in
Korea: Methodology, data, and evidence. Clin Mol Hepatol. 21:14–21.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sarin SK, Kumar M, Eslam M, George J, Al
Mahtab M, Akbar SM, Jia J, Tian Q, Aggarwal R, Muljono DH, et al:
Liver diseases in the Asia-Pacific region: A lancet
gastroenterology & hepatology commission. Lancet Gastroenterol
Hepatol. 5:167–228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sorensen HT, Friis S, Olsen JH, Thulstrup
AM, Mellemkjaer L, Linet M, Trichopoulos D, Vilstrup H and Olsen J:
Risk of liver and other types of cancer in patients with cirrhosis:
A nationwide cohort study in Denmark. Hepatology. 28:921–925. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zullo A, Romiti A, Tomao S, Hassan C,
Rinaldi V, Giustini M, Morini S and Taggi F: Gastric cancer
prevalence in patients with liver cirrhosis. Eur J Cancer Prev.
12:179–182. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi YK, Ahn JY, Kim DH, Jung KW, Na HK,
Choi KD, Lee JH, Song HJ, Lee GH and Jung HY: Efficacy and safety
of endoscopic submucosal dissection for gastric neoplasms in
patients with compensated liver cirrhosis: A propensity
score-matched case-control study. Gastrointest Endosc.
87:1423–1431. e14232018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ogura K, Okamoto M, Sugimoto T, Yahagi N,
Fujishiro M, Kakushima N, Kodashima S, Kawabe T and Omata M:
Efficacy and safety of endoscopic submucosal dissection for gastric
cancer in patients with liver cirrhosis. Endoscopy. 40:443–445.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eom BW, Jung KW, Won YJ, Yang H and Kim
YW: Trends in gastric cancer incidence according to the
clinicopathological characteristics in Korea, 1999–2014. Cancer Res
Treat. 50:1343–1350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsoris A and Marlar CA: Use of the child
pugh score in liver disease. StatPearls. StatPearls Publishing.
(Treasure Island, FL). 2021.
|
|
23
|
Oka S, Tanaka S, Kaneko I, Mouri R, Hirata
M, Kawamura T, Yoshihara M and Chayama K: Advantage of endoscopic
submucosal dissection compared with EMR for early gastric cancer.
Gastrointest Endosc. 64:877–883. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zullo A, Hassan C and Morini S:
Helicobacter pylori infection in patients with liver cirrhosis:
Facts and fictions. Dig Liver Dis. 35:197–205. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sonnenberg A and Wasserman IH:
Associations of peptic-ulcer and gastric-cancer with other diseases
in us veterans. Am J Public Health. 85:1252–1255. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jang HJ, Kim JH, Song HH, Woo KH, Kim M,
Kae SH, Lee J, Cho JW, Kang JH, Lee SI, et al: Clinical outcomes of
patients with liver cirrhosis who underwent curative surgery for
gastric cancer: A retrospective multi-center study. Dig Dis Sci.
53:399–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nicoll A: Surgical risk in patients with
cirrhosis. J Gastroenterol Hepatol. 27:1569–1575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Choe WH, Kim JH, Park JH, Kim HU, Cho DH,
Lee SP, Lee TY, Lee SY, Sung IK, Park HS and Shim CS: Endoscopic
submucosal dissection of early gastric cancer in patients with
liver cirrhosis. Dig Dis Sci. 63:466–473. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aytac S, Turkay C, Bavbek N and Kosar A:
Hemostasis and global fibrinolytic capacity in chronic liver
disease. Blood Coagul Fibrinolysis. 18:623–626. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kato M, Nishida T, Hamasaki T, Kawai N,
Yoshio T, Egawa S, Yamamoto K, Ogiyama H, Komori M, Nakahara M, et
al: Outcomes of ESD for patients with early gastric cancer and
comorbid liver cirrhosis: A propensity score analysis. Surg Endosc.
29:1560–1566. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Anti M, Armuzzi A and Gasbarrini G:
Epithelial cell turnover and apoptosis. Ital J Gastroenterol. 30
(Suppl 3):S276–S278. 1998.PubMed/NCBI
|
|
32
|
Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM,
Taylor PR, Dong ZW, Mark SD and Dawsey SM: Zinc concentration in
esophageal biopsy specimens measured by x-ray fluorescence and
esophageal cancer risk. J Natl Cancer Inst. 97:301–306. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Becker U, Almdal T, Christensen E, Gluud
C, Farholt S, Bennett P, Svenstrup B and Hardt F: Sex-Hormones in
postmenopausal women with primary biliary-cirrhosis. Hepatology.
13:865–869. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hara K, Kohno S, Koga H, Kaku M, Tomono K,
Sakata S, Komatsu K and Omagari K: Infections in patients with
liver-cirrhosis and hepatocellular-carcinoma. Internal Med.
34:491–495. 1995. View Article : Google Scholar : PubMed/NCBI
|