Introduction
Colorectal oncosurgery requires narrow-field pelvic
manipulation and nerve preservation; therefore, surgical robotic
systems have been introduced so that precise surgery can be
performed using articulated forceps and three-dimensional images
(1). Although a definitive
conclusion regarding robotic surgery's utility and long-term
results in comparison to those of laparoscopic surgery remains
unclear, robotic surgery has advantages in terms of short-term
results, detailed anatomical understanding, and completion of total
mesorectal excision (TME) (2,3).
Robotic surgery is also expected to be effective in colon cancer
due to the concept of complete mesocolic excision (CME) (4,5).
Moreover, the number of robotic surgeries performed is expected to
also increase in Japan as the procedure is covered by public
insurance as of April 2022.
The right-sided colon has a more complex and
variable vascular supply than the left-sided colon (6). CME of right-sided colorectal cancer
can be performed using a minimally invasive robotic approach. We
performed robot-assisted TME and lateral lymph node dissection for
rectal cancer using the da Vinci® Si™ system (Intuitive
Surgical Inc., Sunnyvale, CA, USA). Despite multiple reports on
right-sided colorectal cancer surgery using the da Vinci Xi™/X™
systems (Intuitive Surgical, Inc.) (4,5,7),
data are lacking on the use of the Si system, which requires more
ingenuity regarding port placement and cart insertion angle because
it has more arm interference than the Xi system (8). Herein, we describe a case of CME
using the Si system in combination with a rotation technique of the
port in a patient with cancer of the ascending colon with bulky
lymph node metastasis to the anterior pancreas.
Case report
A 63-year-old woman was admitted to Minoh City
Hospital (Minoh, Japan) in April 2022 because of anemia (blood
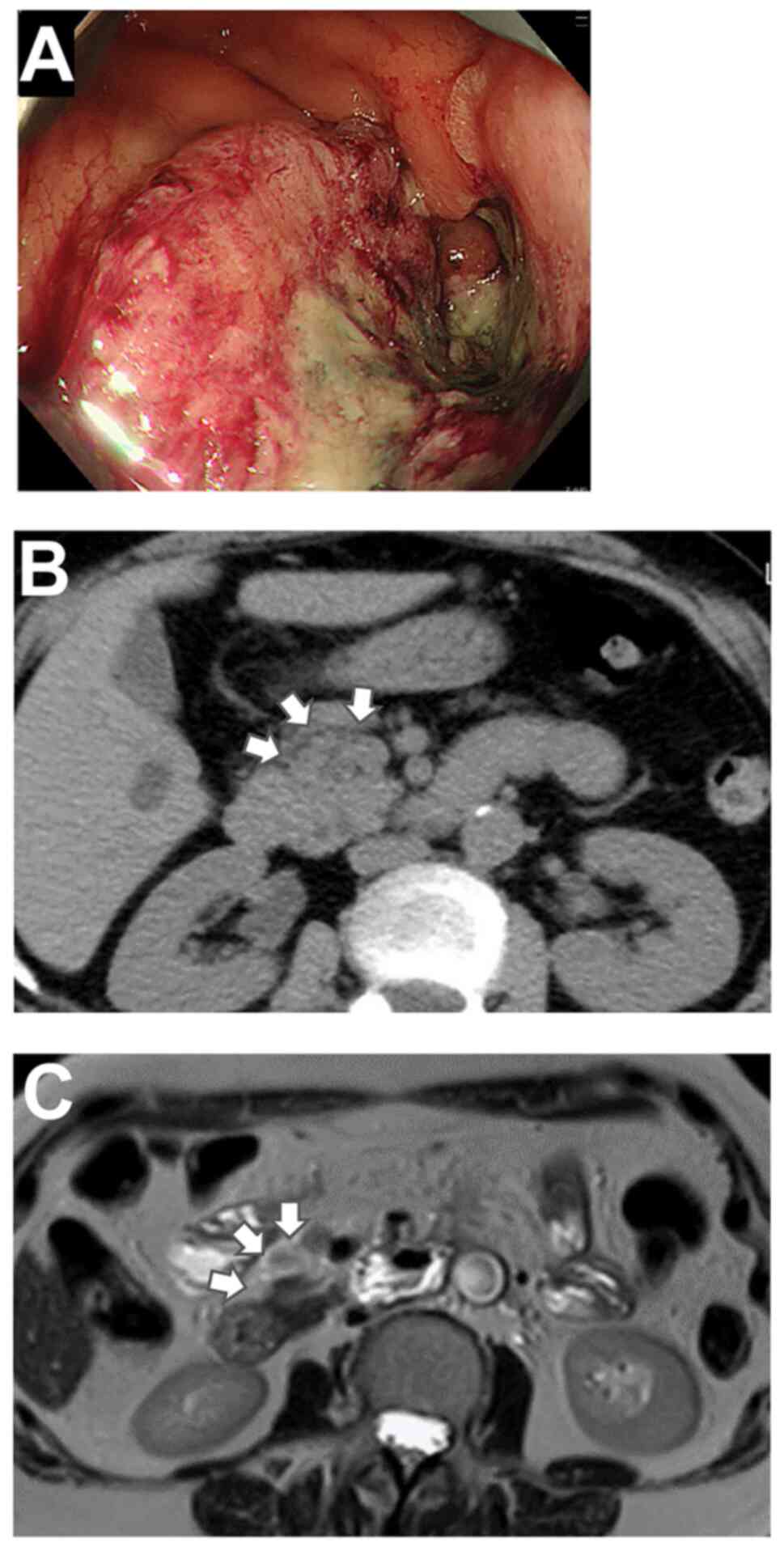
hemoglobin: 7.7 g/dl). Colonoscopy revealed a 2/3 circumscribed
type 2 tumor (Fig. 1A) in the
ascending colon that was diagnosed as colorectal cancer following
biopsy. Contrast-enhanced computed tomography (CT) could not be
performed due to pre-existing bronchial asthma. Non-contrast CT
revealed multiple lymph node metastases extending to the anterior
pancreas and no distant metastases (Fig. 1B). Magnetic resonance imaging (MRI)
revealed no obvious involvement of the pancreatic lymph nodes
(Fig. 1C). Robot-assisted right
hemicolectomy was planned for the patient with TNM stage of
T4aT2bM0, stage IIIc (9). All
other preoperative tests were unremarkable, and there were no other
pre-existing medical conditions.
Intraoperatively, the patient was placed in the
lithotomy position with the head low at 5° right superior at 5°
under general anesthesia. A longitudinal incision of 4 cm was made
at the umbilicus, and the abdomen was opened. A lap protector
(Hakko Co. Ltd., Nagano, Japan) was inserted into the abdomen. The
EZ access (Hakko) was adjusted, and the da Vinci camera port was
placed on it. The camera port was placed off-center to facilitate
its rotation to reduce interference with the da Vinci arm (Fig. 2A). Pneumoperitoneum was achieved
using 10 mmHg. The camera was inserted to identify the hepatic
curvature, and a straight line was drawn to connect the camera port
with the hepatic curvature. To ensure safety, parallel lines were
drawn at least 2 cm away from the rib arch, and two lines
(effectively passing through the camera) were drawn cephalad from
the line; an 8 mm port was placed on each of these two lines. In
our patient, the distance between the parallel lines was 7 cm; this
distance generally depends on the effective abdominal wall length.
One of the parallel lines was drawn on the foot side, and an 8 mm
port was placed on it. A 12 mm assistant port was placed away from
these lines (Fig. 2B and C).
The robotic cart was then placed on the patient's
right side. Central vascular dissection was performed using this
arrangement. During passive colonization of the hepatic curvature,
a rotation technique was used to rotate the EZ access
counterclockwise and move the camera port outward to the left,
thereby avoiding interference between the first robot arm (R1) and
the third robot arm (R3) (Fig.
2D). Monopolar curved scissors were placed in the first robot
arm (R1), fenestrated bipolar forceps were placed in the second
robot arm (R2), and ProGrasp™ forceps were placed in the third
robot arm (R3). First, using a medial approach, the superior
mesenteric vein (SMV) was exposed (Fig. 3A). The ileocolic vein and artery
were transected at the root and dissection was continued upward
toward the right colic vessels. The bulky metastatic lymph node at
the pancreatic head was grasped and dissected from the pancreas.
The anterior superior pancreaticoduodenal vein from the SMV was
preserved, and the right colic vein was ligated (Fig. 3B). The right colic artery, a branch
of the superior mesenteric artery, was ligated (Fig. 3C), and D3 lymphadenectomy was
performed (Fig. 3D). The mesentery
of the colon was transferred medially from the ureter to the
ovarian artery. The rotation was calculated again to avoid
interference between R1 and R3. Adhesions of the transverse colon
and omentum were dissected, and the dissected colon was transferred
from the hepatic curvature toward the ascending colon. Finally, the
right colon was excised by incising the serosa of the ileum from
the root of the mesentery of the small intestine. The EZ access was
removed, the transferred colon was raised externally, and the
transverse colon and terminal ileum were transected using an
electrocautery scalpel. The transverse colon and ileum were then
anastomosed using a linear stapler for functional end-to-end
anastomosis. The anastomosis was returned to the abdomen, an
anti-adhesion film was placed under the umbilical incision, and all
the ports were closed.
The total operation time was 271 min, and the
console time was 140 min. The operation was completed without
intraoperative complications, and the volume of blood loss was 48
ml. The patient resumed eating on the third postoperative day and
was discharged on the eighth postoperative day. No postoperative
complications were observed. The pathological diagnosis included
T4a and N2b, and the final diagnosis was stage IIIc. Adjuvant
chemotherapy (XELOX) (10) was
initiated 1 month after the surgery, and the patient is currently
under outpatient observation.
Discussion
The key points that complicate surgeries of
right-sided colon cancer are the variations in vascular supply and
the inclusion of the surgical trunk (6), which itself has a complex vascular
orientation, in the area of lymph node dissection. We have always
been conscious of this densely vascular region and, therefore,
place a small laparotomy port above the umbilicus, even during
laparoscopic surgery (11). Its
advantages include easy access to the vessels through the umbilical
incision in emergencies, such as bleeding, and extension of the
incision for easy conversion to open surgery. Although the number
of laparotomies performed has declined due to the widespread use of
laparoscopic and robotic surgeries (3,12),
it is important to anticipate possible conversions to open surgery
(13).
Compared with surgery for rectal cancer, surgery for
right-sided colorectal cancer requires a wider range of surgical
manipulation, which may be difficult during robotic surgery. EZ
access is versatile and allows the port to be placed in any
position; therefore, we have found it to be a useful device in
single-incision laparoscopic surgery and reduced-port surgery
(11,14). In robotic surgery, the camera port
can be easily added by intentionally placing it off-center, and the
camera axis can be shifted by rotating the EZ access using a
rotation technique, thus changing the axis of the other arms and
avoiding potential interference. Notably, this technique is very
easy. If EZ access is being used, the only requirement to change
from the conventional method to this rotation method is to ‘unplug
the camera port once from EZ access and plug it back in at the
edge’. This method can be tried without the requirement for
additional supplies and labor. It has been reported that the da
Vinci Si system requires port placement in an arc around the left
side of the lower abdomen to maintain the proper range of motion of
the robotic instruments and to avoid collisions between instruments
(13,15,16).
Therefore, it is difficult to place the port on the surgical trunk,
which is an important dissection site during right colon resection
(6). However, by using EZ access
with a rotation technique and avoiding instrument collision, it is
possible to place the port on the surgical trunk. Additionally, we
have also performed left colectomy using this method. There is less
arm interference in left colectomy than that in right colectomy
and, therefore, the benefits of this method may be more in right
colectomy (data not shown). However, this method may be useful in
patients in whom the required distance between the ports cannot be
achieved. In colon cancer surgery, the rotation of the EZ access
allows ligation of the vessels and transfer of the bowel without
changing the patient's position. We have similarly used the EZ
access in midline incisions to place the camera port in rectal
cancer surgery. However, the present report has a limitation.
Studies or reports on this procedure being performed using the
conventional method are lacking; thus, the method described in this
report cannot be compared with the conventional method.
Herein, we reported a case of right-sided colon
cancer with bulky lymph node metastasis to the anterior pancreas.
The report highlights the advantages of robotic surgery using the
Si system in conjunction with a rotation technique of the EZ access
port. We believe that this technique may also be effective with
newer robotic surgery systems.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SF, KD, TT and SM performed the surgery. KY, MH and
KN acquired the patient data. TH and YO contributed to the
conception of the study and supervised this study. SF drafted the
manuscript. SF and KD edited the manuscript and confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki. It was approved by the Minoh City
Hospital Ethics Committee (approval no. R0311B64).
Patient consent for publication
Written informed consent for publication was
obtained from the participant.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CME
|
complete mesocolic excision
|
|
SMV
|
superior mesenteric vein
|
References
|
1
|
Achilli P, Grass F and Larson DW: Robotic
surgery for rectal cancer as a platform to build on: Review of
current evidence. Surg Today. 51:44–51. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Safiejko K, Tarkowski R, Koselak M,
Juchimiuk M, Tarasik A, Pruc M, Smereka J and Szarpak L:
Robotic-assisted vs. standard laparoscopic surgery for rectal
cancer resection: A systematic review and meta-analysis of 19,731
patients. Cancers (Basel). 14:1802021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baek SJ, Piozzi GN and Kim SH: Optimizing
outcomes of colorectal cancer surgery with robotic platforms. Surg
Oncol. 37:1015592021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siddiqi N, Stefan S, Jootun R, Mykoniatis
I, Flashman K, Beable R, David G and Khan J: Robotic complete
mesocolic excision (CME) is a safe and feasible option for right
colonic cancers: Short and midterm results from a single-centre
experience. Surg Endosc. 35:6873–6881. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baca B, Benlice C, Hamzaoglu I and
Karahasanoglu T: Step by step revisiting and standardizing the
robotic approach of complete mesocolic excision for right-sided
colon cancer. Tech Coloproctol. 26:677–679. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ohira G, Hayano K, Imanishi S, Tochigi T,
Isozaki T, Kurata Y, Miyauchi H, Maruyama M, Endo S, Maruyama T and
Matsubara H: Preoperative evaluation of vascular anatomy of right
colic vessels using enhanced computed tomographic colonography. Jpn
J Radiol. 46:607–612. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khan JS, Ahmad A, Odermatt M, Jayne DG,
Ahmad NZ, Kandala N and West NP: Robotic complete mesocolic
excision with central vascular ligation for right colonic tumours-a
propensity score-matching study comparing with standard
laparoscopy. BJS Open. 5:zrab0162021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aliyev V, Arslan NC, Goksoy B, Guven K,
Goksel S and Asoglu O: Is robotic da Vinci Xi® superior
to the da Vinci Si® for sphincter-preserving total
mesorectal excision? Outcomes in 150 mid-low rectal cancer
patients. J Robot Surg. 2:10.1007/s11701–021-01356-8. 2022.
|
|
9
|
Bruerley JD, Gospodarowicz MK and
Wittekind C: TNM classification of malignant tumors. 8th ed.
Oxford: Wiley-Blackwell; 2016
|
|
10
|
Yoshino T, Arnold D, Taniguchi H,
Pentheroudakis G, Yamazaki K, Xu RH, Kim TW, Ismail F, Tan IB, Yeh
KH, et al: Pan-asian adapted ESMO consensus guidelines for the
management of patients with metastatic colorectal cancer: A
JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann
Oncol. 29:44–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fujino S, Miyoshi N, Ohue M, Noura S,
Fujiwara Y, Higashiyama M and Yano M: Z skin incision in
reduced-port surgery for colorectal cancer. Mol Clin Oncol.
4:611–615. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Park JW, Kang SB, Hao J, Lim SB, Choi HS,
Kim DW, Chang HJ, Kim DY, Jung KH, Kim TY, et al: Open versus
laparoscopic surgery for mid or low rectal cancer after neoadjuvant
chemoradiotherapy (COREAN trial): 10-year follow-up of an
open-label, non-inferiority, randomised controlled trial. Lancet
Gastroenterol Hepatol. 6:569–577. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Phan K, Kahlaee HR, Kim SH and Toh JWT:
Laparoscopic vs. robotic rectal cancer surgery and the effect on
conversion rates: A meta-analysis of randomized controlled trials
and propensity-score-matched studies. Tech Coloproctol. 23:221–230.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujino S, Miyoshi N, Noura S, Shingai T,
Tomita Y, Ohue M and Yano M: Single-incision laparoscopic cecectomy
for low-grade appendiceal mucinous neoplasm after laparoscopic
rectectomy. World J Gastrointest Surg. 6:84–87. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gossedge G and Jayne D: Robotic technique
for right colectomy. Surgery for cancers of the gastrointestinal
tract. Kim J and Garcia-Aguilar J: Springer; New York, NY: pp.
187–194. 2015, View Article : Google Scholar
|
|
16
|
Spinoglio G, Marano A, Bianchi PP, Priora
F, Lenti LM, Ravazzoni F and Formisano G: Robotic right colectomy
with modified complete mesocolic excision: Long-term oncologic
outcomes. Ann Surg Oncol. 23:684–691. 2016. View Article : Google Scholar : PubMed/NCBI
|