Introduction
Vaginal malignant melanoma (VMM), a type of primary
malignant melanoma (MM), is an aggressive and rare gynecological
malignancy, accounting for 3% of all vaginal malignancies (1) and 0.3-0.8% of all MMs (2). VMM has been shown to arise from the
melanocytes present in the basal layer of the vaginal epithelium
(3). Although MM might arise
anywhere in the vagina, the anterior wall of the lower third
portion is the most common site (4). The most common symptom of VMM is
vaginal bleeding (5). Despite
multiple treatment options, the prognosis is poor, with a 5-year
survival rate of 5–25% (1). One
reason for this poor prognosis is that patients are often at an
advanced stage when first diagnosed.
VMM is usually treated by means of radiotherapy (RT)
combined with chemotherapy after surgery. The surgical strategies
include radical local resection of the primary tumor and lymph node
dissection. For cases of advanced disease, especially those with
distant metastases, the prognosis is poor (6). Since the disease is rare, there is a
lack of systematic and effective guidelines. The present study
reports a case in which a patient with advanced stage primary VMM
benefitted from RT combined with anti-angiogenic therapy and immune
checkpoint inhibitors (ICIs). This reported case, together with
other successful cases reported in the literature, provide useful
references for the application of combination therapy to treat
MM.
Case report
A 55-year-old female patient was admitted to
Affiliated Hospital of Weifang Medical University (Weifang, China)
in June 2016 with a mass on the left side of the vaginal opening.
For personal reasons, the surgery for complete resection was
postponed to September 2016 and performed at another hospital. Data
from another hospital showed that the postoperative pathology was
MM with BRAF V600F gene mutation. For 45 months after the
operation, the patient was treated in other hospitals. The patient
received high-dose interferon α-2b (20 MIU/m2; 5 times a
week) for 3 months. Then a low-dose interferon α-2b (10
MIU/m2; 3 times a week) maintenance therapy was
performed for 1 month. After 1 month of low-dose interferon
maintenance therapy, the bilateral inguinal lymph nodes developed
metastases. The patient experienced recurrence of the primary tumor
in September 2017, May 2018 and October 2018. Both the December
2018 and February 2019 follow-up showed that the vulvar mass was
enlarged. The tumor invaded the vagina, cervix and adjacent muscles
in September 2019. In January 2020, the patient's re-examination
revealed left adrenal metastases. When the above condition
progressed, the patient received irregular treatments, such as
surgery, interferon therapy, chemotherapy and targeted therapy in
other hospitals. The specific treatment plans used by the other
hospitals are unknown. The last tumor assessment in other hospitals
was stable disease (SD).
Until April 2020, the patient was admitted to
Affiliated Hospital of Weifang Medical University (Weifang, China)
again due to local tumor recurrence and progression. A physical
examination revealed structural changes in the vulva and swelling
of the left vulva. The pudendal mass was ~8×5 cm in size, with
convex growth, ulceration and bleeding. The mass was hard and
poorly mobile. The patient was treated with apatinib for 15 days
[500 mg every day (qd)]. After taking Apatinib, the tumor was
slightly smaller than before, but typical symptoms of nephrotic
syndrome occurred, so the drug was discontinued. For personal
reasons, the patient underwent blood genetic testing at another
hospital (Weifang People's Hospital, Weifang, China). Blood genetic
tests 1 week after presentation revealed BRAF (V600 wild-type). The
patient was resistant to dabrafenib and vemurafenib. After 8 days,
the whole abdomen was imaged. This was compared with the
examination report from the visit to the other hospitals 3 months
before. The local lesions and metastases were enlarged. The overall
tumor assessment indicated progressive disease (PD). The next day,
the patient started four cycles of intravenous chemotherapy with
temozolomide [200 mg/m2 on days 1–5, every 4 weeks
(q4w)]. After 1 month, the magnetic resonance imaging was reviewed
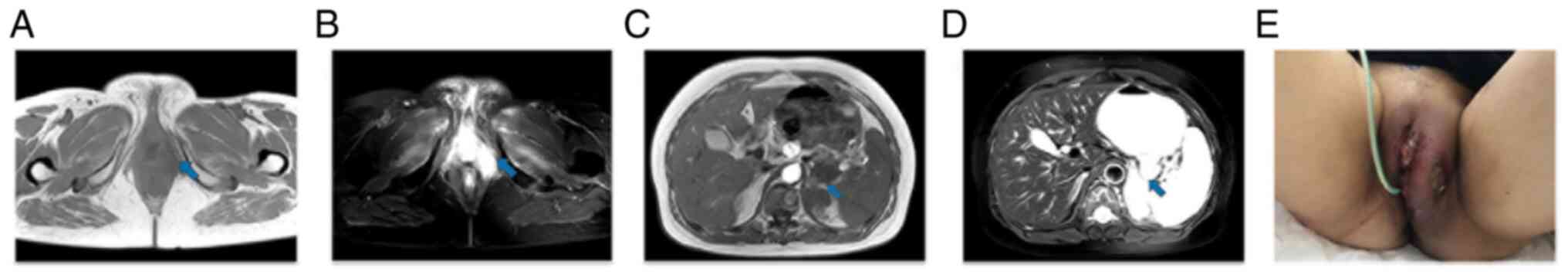
and the primary tumor was found to be larger than before (Fig. 1A and B), with new metastases. The
tumor status was assessed as PD (Fig.
1). The patient was administered primary tumor RT (2 GY each
fraction for 35 fractions) combined with 4 cycles of temozolomide
(200 mg/m2 on days 1–5, q4w), 6 cycles of anlotinib (8
mg on days 1–14, q3w), and 8 cycles of toripalimab (240 mg q2w)
(Fig. 2 shows the usage time of
various drugs). Over 3 months later, magnetic resonance imaging
showed that the primary tumor was smaller than before (Fig. 3A and B); however, the left adrenal
metastases were larger than before (Fig. 3C and D) and new metastases had
appeared in multiple places (Fig.
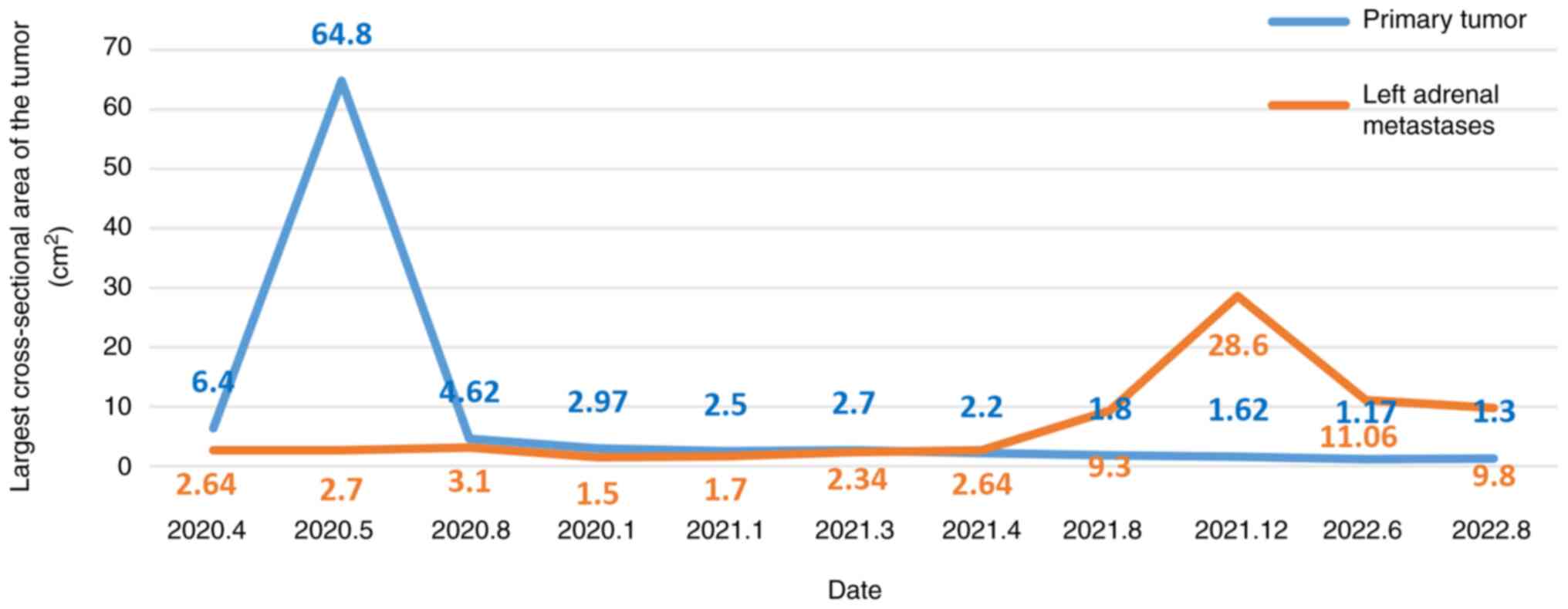
2). The tumor status was assessed as PD (Fig. 3). The antitumor regimen was changed
to lenvatinib (12 mg qd) combined with toripalimab (240 mg q2w).
The patient was followed up after 4 cycles of combination therapy,
and partial response (PR) was found in tumor evaluation (Fig. 4). Both the primary tumor (Fig. 4A and B) and metastases (Fig. 4C and D) were smaller than before.
Afterwards, the patient was regularly reviewed every 3 months, and
SD was assessed for each tumor status. However, tumor status was
assessed as PR at a review in August 2021. In December 2021, the
patient was re-examined (Fig. 5)
and the left adrenal metastases were significantly larger than
before (Fig. 5C and D). The tumor
status was evaluated as PD. From mid-December 2021 to mid-January
2022, RT was added for the left adrenal metastatic tumor area (2.8
GY each fraction for 15 fractions). After RT, the patient underwent
blood sample analysis at another hospital (Weifang People's
Hospital, Weifang, China), which showed elevated CD4/CD8 values in
lymphocytes (2.24; normal range, 0.98-1.94). The patient's symptoms
improved and the tumor status was evaluated as PR. The patient was
scheduled to receive lenvatinib (12 mg qd) in combination with 10
cycles of toripalimab (240 mg q2w) for 5 months. At 6 months
post-RT, both the primary tumor (Fig.
6A and B) and the left adrenal metastases (Fig. 6C and D) were smaller than before on
magnetic resonance imaging assessment. The tumor status was
assessed as PR (Fig. 6). In August
2022, the patient was readmitted to Affiliated Hospital of Weifang
Medical University (Weifang, China) for review. The patient's
condition was stable, and the tumor status was evaluated as SD
(Fig. 2). The patient had no
obvious discomfort and had a good prognosis. The patient was asked
to have a follow up every 3 months thereafter.
 | Figure 2.Diagnosis and treatment process. Red,
PD; yellow, SD; green, PR; blue, CR; grey, new metastatic lesions.
RT, radiotherapy; PD, progressive disease; SD, stable disease; PR,
partial response; CR, complete response. |
Discussion
VMM is an aggressive tumor, with a 5-year survival
rate of 5–25% and a high recurrence rate; it easily spreads to the
local lymph nodes and develops distant metastasis (7). At present, there are a variety of
treatments for VMM (2). Early VMM
can be treated by complete surgical resection. However, distant
metastatic vaginal melanoma has a poor prognosis. For distant
metastatic vaginal melanoma, more emphasis is placed on combination
therapy.
Due to the highly immunogenic nature of MM,
immunotherapy is an effective treatment strategy for primary VMM
(8). Studies have shown that RT
and anti-angiogenic therapy combined with immunotherapy can enhance
the immune efficacy in melanoma (9–12).
In addition to normalizing blood vessels and improving the hypoxic
environment of tumors, anti-angiogenic drugs have been shown to
promote tumor infiltration of CD8+ T lymphocytes and
enhance cancer immunotherapy (12). In this way, the combination of
targeted drugs and ICIs has a synergistic effect. Anti-angiogenic
agents in combination with programmed cell death protein 1
(PD-1)/programmed death-ligand 1 (PD-L1) antibodies have now become
the standard first-line therapy for non-small cell lung cancer
(NSCLC), renal cell carcinoma, endometrial cancer and
hepatocellular carcinoma (13).
Clinical studies have shown that RT can promote and
activate the immune system, showing that immunotherapy combined
with RT can obtain better clinical efficacy. RT and immunotherapy
synergistically inhibit tumor growth and exceed the additive effect
of the two, which is beneficial to patients (14). Both previous studies (15,16)
and the present report found that after local RT, the tumor at the
RT site was reduced, and multiple metastases at the non-RT site
were also reduced, which was considered to be the abscopal effect
of tumor treatment-RT to one site resulting in regression of
distant non-irradiated metastatic cancer (17). Targeted ionizing radiation has long
been known to lead to direct local cell death. However, it is
increasingly recognized that radiation is able to induce tumor
regression at distant tumor sites that are not irradiated. For
example, Stamell et al (18) reported that patients with
metastatic melanoma who received primary palliative RT also
experienced regression of non-irradiated metastases. Despite a
growing number of trials and cases reporting the absolute efficacy
of RT alone, the overall incidence of the abscopal effect remains
relatively low (19,20). Considering that immunotherapy
decreases the immunotolerance of the host to tumors, combining RT
with immunotherapy may enhance the antitumor immune response, thus
increasing the chance of an abscopal effect (21–23).
In a study using a mouse model of melanoma, administration of
radiation prior to or concurrent with cytotoxic
T-lymphocyte-associated antigen 4 (CTLA-4) blockade delayed tumor
growth and prolonged survival time compared with that in mice
treated with anti-CTLA-4 alone (24). Another important rationale for
combining RT and immunotherapy is that RT has the potential to
overcome resistance to immune checkpoint blockade. Early evidence
suggests the clinical feasibility of combining RT and immunotherapy
in NSCLC. Yuan et al (25)
described the case of a patient with PD-L1-negative metastatic
scaly NSCLC refractory to nivolumab. After palliative RT, the
patient showed near total regression of the irradiated primary
tumor and marked regression of the metastases in the RT field,
consistent with an abscopal effect. Komatsu et al (26) reported a case of enlarged liver
metastases in a patient with metastatic NSCLC after treatment with
nivolumab. The patient subsequently underwent RT, which resulted in
shrinkage of both irradiated liver metastases and non-irradiated
lung metastases. The lung metastases shrank without RT, in line
with the abstract effect. These case reports point to a potential
role for RT in overcoming immune resistance mechanisms in patients
with cancer when RT is performed after the initial failure of
immunotherapy. However, a recent retrospective study of 225
patients did not find a prolonged survival benefit of combined ICIs
and RT in patients with advanced mucosal melanoma, although it
could improve the local symptoms at the irradiation site and
improve the quality of life of affected patients (27).
The abscopal effect of ipilimumab in combination
with stereotactic RT in patients with melanoma was reported by
Chicas-Sett et al (28).
Another study reported the more favorable benefit-risk ratio of
anti-PD-1 therapy compared with that of other adjuvant therapies,
and indicated that the treatment should be used preferentially
(29). A further study reported
that anti-PD-1 therapy exhibited improved outcomes compared with
immunotherapy in the treatment of female lower genital tract
melanoma (30). Therefore,
promising clinical immunological agents, such as PD-1/PD-L1
blockers, should also be explored in combination with RT (31). In the study by Deng et al
(32), PD-L1 expression was
upregulated in the tumor microenvironment after RT, and anti-PD-L1
enhanced the efficacy of RT through cytotoxic T cells. It was
demonstrated that RT and anti-PD-L1 treatment synergistically
promoted antitumor immunity (32).
A prospective study demonstrated that immune checkpoint blockade
produces a response even in the absence of PD-L1 expression
(2). This suggests that more
people may benefit from ICIs (2).
ICIs cause the release of T cells to attack tumor
cells, while also regulating the tumor microenvironment by the
normalization of the tumor blood vessels. This normalization
enhances the infiltration of lymphocytes, and increased numbers and
activation of CD4+ T lymphocytes in turn promote the
normalization of the blood vessels, in a cycle of mutual
regulation. Tumor blood vessel normalization by ICIs not only
creates a feedback loop, reprograms the tumor immune
microenvironment and enhances the immunotherapeutic effect, it also
improves tumor hypoxia, decreases HIF-1 expression, decreases
reactive oxygen species inactivation and sensitizes tumors to
radiation. Most anti-angiogenic drugs also increase
radiosensitivity and are combined with RT (33). In the present report, a patient
with advanced primary VMM underwent surgery, interferon therapy,
chemotherapy and targeted therapy, and the patient's disease
progressed. The patient was transferred to Affiliated Hospital of
Weifang Medical University (Weifang, China) for RT combined with
anti-angiogenesis therapy and ICIs, and their condition improved.
After 19 months of anti-angiogenic therapy combined with ICI
maintenance therapy, the patient's tumor progressed. After the
patient underwent RT combined with anti-angiogenic therapy and ICIs
again, the tumor status was evaluated as PR (Fig. 7).
In conclusion, primary VMM is an aggressive and rare
malignancy that is usually diagnosed at an advanced stage, has a
high rate of recurrence and metastasis, and has no consistent
guidelines in terms of treatment. Based on aforementioned
interactions and synergy, as well as the efficacy in the patient
reported in the present case, a trimodal approach combining RT with
anti-angiogenic therapy and immunotherapy is a promising treatment
strategy. To the best of our knowledge, no clinical trials
combining all three treatment modalities have been published. RT
with either anti-angiogenic therapy or immunotherapy appears
feasible, but presents clinicians and researchers with numerous
challenges.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PY, XLM, YFZ, YS, YTW and ZL contributed to the
study conception and design. Material preparation, and data
collection and analysis were performed by ZL, XLM, YFZ, YS and YTW.
The first draft of the manuscript was written by PY and all authors
commented on previous versions of the manuscript. ZL and XLM
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study involving human participants was reviewed
and approved by the Affiliated Hospital of Weifang Medical
University Ethics Committee (approval no. wyfy-2021-ky-072).
Informed consent was obtained from all individual participants
included in the study.
Patient consent for publication
The patient provided written informed consent for
the publication of the study and the associated images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Puri S and Asotra S: Primary vaginal
malignant melanoma: A rare entity with review of literature. J
Cancer Res Ther. 15:1392–1394. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang HY, Wu XY, Zhang X, Yang XH, Long YK,
Feng YF and Wang F: Prevalence of NRAS mutation, PD-L1 expression
and amplification, and overall survival analysis in 36 primary
vaginal melanomas. Oncologist. 25:e291–e301. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta D, Malpica A, Deavers MT and Silva
EG: Vaginal melanoma: A clinicopathologic and immunohistochemical
study of 26 cases. Am J Surg Pathol. 26:1450–1457. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piura B: Management of primary melanoma of
the female urogenital tract. Lancet Oncol. 9:973–981. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Terzakis E, Androutsopoulos G, Adonakis G,
Zygouris D, Grigoriadis C and Decavalas G: Vaginal primary
malignant melanoma: Report of four cases and review of the
literature. Eur J Gynaecol Oncol. 32:122–124. 2011.PubMed/NCBI
|
|
6
|
Guo N and Zhang J: Primary vaginal
malignant melanoma: A rare case report of successful treatment with
nivolumab. Medicine (Baltimore). 100:e256912021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Frumovitz M, Etchepareborda M, Sun CC,
Soliman PT, Eifel PJ, Levenback CF and Ramirez PT: Primary
malignant melanoma of the vagina. Obstet Gynecol. 116:1358–1365.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Takahashi J and Nagasawa S:
Immunostimulatory effects of radiotherapy for local and systemic
control of melanoma: A review. Int J Mol Sci. 21:93242020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fukumura D, Kloepper J, Amoozgar Z, Duda
DG and Jain RK: Enhancing cancer immunotherapy using
antiangiogenics: Opportunities and challenges. Nat Rev Clin Oncol.
15:325–340. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie Y, Chen Z, Zhong Q, Chen Y, Shangguan
W and Xie W: Efficacy and safety of immunological checkpoint
inhibitors combined with anti-angiogenic drugs in first-line
treatment of metastatic renal cell carcinoma: A systematic review
and meta-analysis. Transl Androl Urol. 10:300–309. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee WS, Yang H, Chon HJ and Kim C:
Combination of anti-angiogenic therapy and immune checkpoint
blockade normalizes vascular-immune crosstalk to potentiate cancer
immunity. Exp Mol Med. 52:1475–1485. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goedegebuure RSA, de Klerk LK, Bass AJ,
Derks S and Thijssen VLJL: Combining radiotherapy with
anti-angiogenic therapy and immunotherapy; a therapeutic triad for
cancer? Front Immunol. 9:31072019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hack SP, Zhu AX and Wang Y: Augmenting
anticancer immunity through combined targeting of angiogenic and
PD-1/PD-L1 pathways: Challenges and opportunities. Front Immunol.
11:5988772020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang B, Yan X, Sheng X, Si L, Cui C, Kong
Y, Mao L, Lian B, Bai X, Wang X, et al: Safety and clinical
activity with an anti-PD-1 antibody JS001 in advanced melanoma or
urologic cancer patients. J Hematol Oncol. 12:72019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu Y, Dong Y, Kong L, Shi F, Zhu H and Yu
J: Abscopal effect of radiotherapy combined with immune checkpoint
inhibitors. J Hematol Oncol. 11:1042018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhao X and Shao C: Radiotherapy-mediated
immunomodulation and anti-tumor abscopal effect combining immune
checkpoint blockade. Cancers (Basel). 12:27622020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ngwa W, Irabor OC, Schoenfeld JD, Hesser
J, Demaria S and Formenti SC: Using immunotherapy to boost the
abscopal effect. Nat Rev Cancer. 18:313–322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Stamell EF, Wolchok JD, Gnjatic S, Lee NY
and Brownell I: The abscopal effect associated with a systemic
anti-melanoma immune response. Int J Radiat Oncol Biol Phys.
85:293–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rogers SJ, Puric E, Eberle B, Datta NR and
Bodis SB: Radiotherapy for melanoma: More than DNA damage. Dermatol
Res Pract. 2019:94353892019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hendrickx A, Cozzio A, Plasswilm L and
Panje CM: Radiotherapy for lentigo maligna and lentigo maligna
melanoma-a systematic review. Radiat Oncol. 15:1742020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hodge JW, Sharp HJ and Gameiro SR:
Abscopal regression of antigen disparate tumors by antigen cascade
after systemic tumor vaccination in combination with local tumor
radiation. Cancer Biother Radiopharm. 27:12–22. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Demaria S, Kawashima N, Yang AM, Devitt
ML, Babb JS, Allison JP and Formenti SC: Immune-mediated inhibition
of metastases after treatment with local radiation and CTLA-4
blockade in a mouse model of breast cancer. Clin Cancer Res.
11:728–734. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vatner RE, Cooper BT, Vanpouille-Box C,
Demaria S and Formenti SC: Combinations of immunotherapy and
radiation in cancer therapy. Front Oncol. 4:3252014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Twyman-Saint Victor C, Rech AJ, Maity A,
Rengan R, Pauken KE, Stelekati E, Benci JL, Xu B, Dada H, Odorizzi
PM, et al: Radiation and dual checkpoint blockade activate
non-redundant immune mechanisms in cancer. Nature. 520:373–377.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yuan Z, Fromm A, Ahmed KA, Grass GD, Yang
GQ, Oliver DE, Dilling TJ, Antonia SJ and Perez BA: Radiotherapy
rescue of a nivolumab-refractory immune response in a patient with
PD-L1-negative metastatic squamous cell carcinoma of the lung. J
Thorac Oncol. 12:e135–e136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Komatsu T, Nakamura K and Kawase A:
Abscopal effect of nivolumab in a patient with primary lung cancer.
J Thorac Oncol. 12:e143–e144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Umeda Y, Yoshikawa S, Kiniwa Y, Maekawa T,
Yamasaki O, Isei T, Matsushita S, Nomura M, Nakai Y, Fukushima S,
et al: Real-world efficacy of anti-PD-1 antibody or combined
anti-PD-1 plus anti-CTLA-4 antibodies, with or without
radiotherapy, in advanced mucosal melanoma patients: A
retrospective, multicenter study. Eur J Cancer. 157:361–372. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chicas-Sett R, Morales-Orue I,
Rodriguez-Abreu D and Lara-Jimenez P: Combining radiotherapy and
ipilimumab induces clinically relevant radiation-induced abscopal
effects in metastatic melanoma patients: A systematic review. Clin
Transl Radiat Oncol. 9:5–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Moya-Plana A, Herrera Gómez RG, Rossoni C,
Dercle L, Ammari S, Girault I, Roy S, Scoazec JY, Vagner S, Janot
F, et al: Evaluation of the efficacy of immunotherapy for
non-resectable mucosal melanoma. Cancer Immunol Immunother.
68:1171–1178. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Indini A, Di Guardo L, Cimminiello C,
Lorusso D, Raspagliesi F and Del Vecchio M: Investigating the role
of immunotherapy in advanced/recurrent female genital tract
melanoma: A preliminary experience. J Gynecol Oncol. 30:e942019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reynders K, Illidge T, Siva S, Chang JY
and De Ruysscher D: The abscopal effect of local radiotherapy:
Using immunotherapy to make a rare event clinically relevant.
Cancer Treat Rev. 41:503–510. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deng L, Liang H, Burnette B, Beckett M,
Darga T, Weichselbaum RR and Fu YX: Irradiation and anti-PD-L1
treatment synergistically promote antitumor immunity in mice. J
Clin Invest. 124:687–695. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Zhao Q, Zheng Z, Liu S, Meng L,
Dong L and Jiang X: Vascular normalization in immunotherapy: A
promising mechanisms combined with radiotherapy. Biomed
Pharmacother. 139:1116072021. View Article : Google Scholar : PubMed/NCBI
|