Introduction
Multiple primary malignant neoplasms (MPMNs) are
multiple tumors with different pathogenetic origins that manifest
in one or more organs and tissues of the same patient; they may be
synchronous or metachronous (1).
The synchronous occurrence of MPMNs is very rare. It is usually
difficult to assess the staging of each neoplasm, and to determine
the optimal treatment according to the individual tumor risk
(2). Mantle cell lymphoma (MCL) is
an aggressive B-cell non-Hodgkin's lymphoma (NHL) with a
characteristic chromosomal translocation, t(11;14)(q13;q32), that
can lead to cyclin D1 overexpression (3). Small cell lung cancer (SCLC) is an
aggressive cancer type of neuroendocrine origin with a poor
prognosis, which is strongly associated with cigarette smoking
(4). Synchronous MCL bone marrow
involvement (MCLBMI) complicated with extensive-stage SCLC
(ES-SCLC) is extremely rare. The diagnosis of this condition and
its treatment requires a multidisciplinary medical team to ensure
an optimal clinical outcome. In the current study, a case report of
a male patient affected by primary synchronous tumors, including
MCLBMI and ES-SCLC, is presented. The reported information aims to
improve the awareness for the diagnosis of MCLBMI complicated with
ES-SCLC and provide a reference for appropriate diagnosis and
treatment.
Case report
Patient
A 59-year-old man was admitted to the Department of
Spinal Surgery of the College of Medicine, Lishui Hospital,
Zhejiang University (Lishui, China) in September 2020 due to lumbar
disc herniation. The patient had not presented with serious
illnesses in the past and was a heavy smoker. A physical
examination indicated an axillar lymph node (diameter, 2 cm), which
was hard and fixed. The blood cell count indicated thrombocytopenia
(platelet count, 52×109/l; reference range,
125–350×109/l). The whole-body positron emission
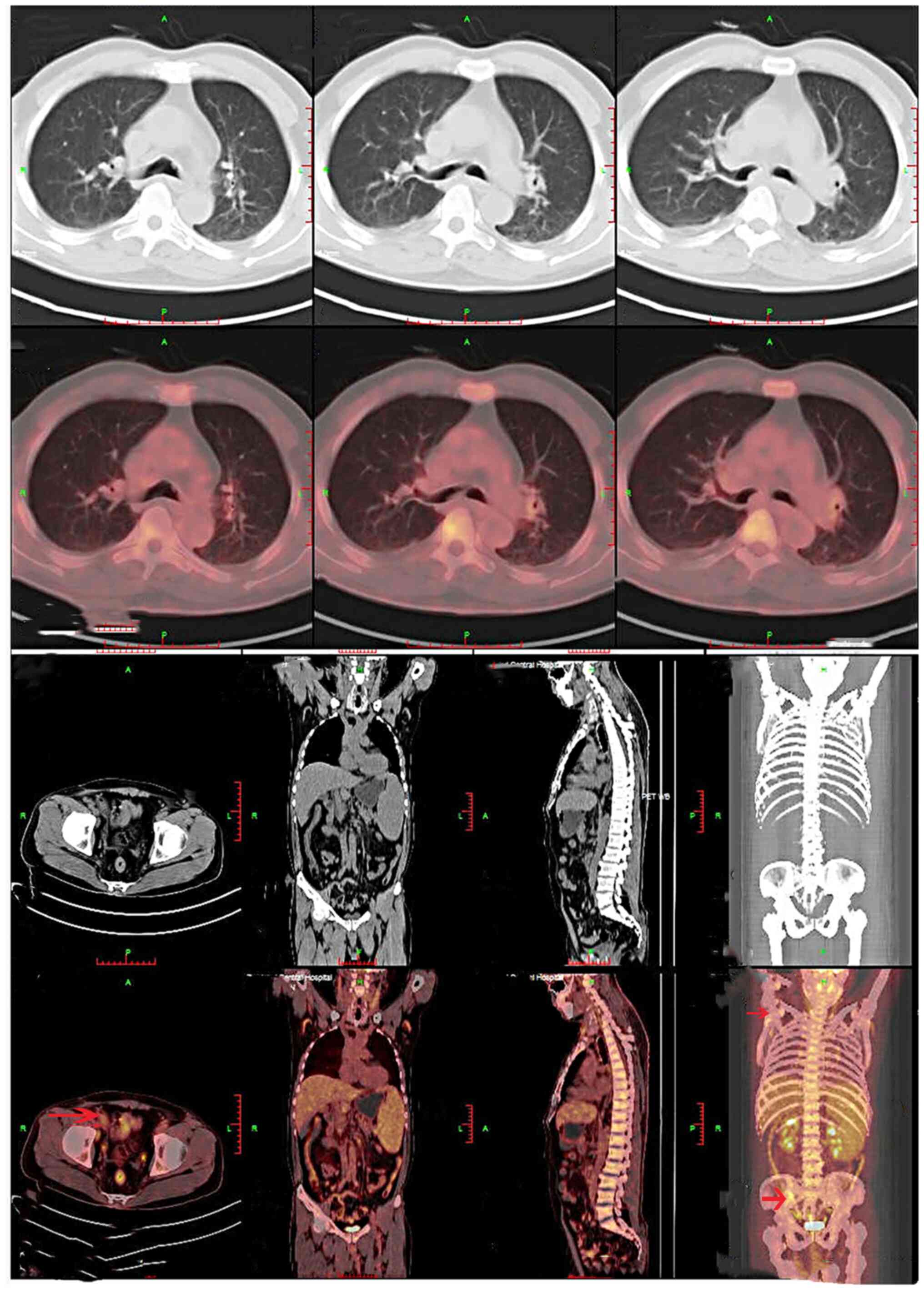
tomography (PET)/computed tomography (CT) scan, which was performed
on 7 days post-presentation, indicated radiotracer uptake in the
lymph nodes and systemic lymphadenopathy, including the presence of
cervical, submandibular, axillar, paraaortic, intrapelvic and
inguinal lymph nodes. Splenomegaly was also noted in the patient
(Fig. 1). A lymph node biopsy was
recommended; however, this was refused by the patient. In March
2021, the patient visited the hospital again due to the enlargement
of the right axillary lymph nodes. A right axillary lymph node
biopsy was performed, which revealed lymphoproliferative lesions,
and lymphoma was suspected. A bone marrow biopsy revealed a low
number of atypical lymphoid cells in small clusters, with scattered
infiltration in the bone marrow hematopoietic tissue. Therefore,
chronic lymphocytic leukemia or lymphoma was suspected. The patient
refused to undergo a further immunohistochemical examination of the
bone marrow and a lymph node biopsy for personal reasons. In July
2021, the patient visited a local hospital due to chest pain. Chest
CT revealed a solid lesion in the left upper lobe of the lung (~3
cm in diameter). Subsequently, the patient visited another hospital
(Shanghai Zhongshan Hospital; Shanghai, China) and underwent a
whole-body PET/CT scan. The scan revealed several soft-tissue
masses in the left lung near the hilum and posterior segment of the
left upper lobe, with maximum dimensions of 3.6×2.6 cm [maximum
standardized uptake value (SUVmax), 10.9]. Based on this evidence,
a malignant tumor was suspected. Systemic enlarged lymph nodes
(~1.2 cm in diameter; SUVmax, 3.0), splenomegaly (SUVmax, 3.6) and
bone metastases were also observed (Fig. 2). The patient was referred to the
Department of Thoracic Surgery of The Second Affiliated Hospital of
Zhejiang University School of Medicine (Hangzhou, China). A
CT-guided biopsy of the left lobe mass was performed and
histological analysis revealed diffuse sheets of small, round,
fusiform cells, with scant cytoplasm, and inconspicuous or absent
nucleoli with finely granular nuclear chromatin. The patient was
therefore diagnosed with SCLC (Fig.
3A). The immunohistochemical stains were positive for thyroid
transcription factor-1 (Fig. 3B),
synaptophysin (Fig. 3C),
glycoprotein hormones α-polypeptide (CgA) (Fig. 3D) and cluster of differentiation
(CD)56 (Fig. 3E), the
proliferation fraction (Ki-67) (Fig.
3F) was ~50%, and the staining was also positive for
neuron-specific enolase (Fig. 3G),
whereas staining was negative for cytokeratin 7 (Fig. 3H). The patient did not receive
chemotherapy due to thrombocytopenia. In August 2021, the patient
visited the College of Medicine, Lishui Hospital, Zhejiang
University (Lishui, China) again due to thrombocytopenia and was
admitted to the Department of Hematology.
The patient underwent laboratory tests, including a
complete blood count, an assessment of tumor markers and an
evaluation of lactate dehydrogenase activity (Table I). Monoclonal immunoglobulin gene
rearrangements were detected. The bone marrow smear indicated an
increased proportion of mature small lymphocytes, accounting for
28% of the total lymphocyte count, with occasional lymphoid cells.
Bone marrow immunophenotyping indicated that the small B
lymphocytes accounted for 19.83% of nuclear cells (60.91% of
lymphocytes), which mainly expressed CD19, human leukocyte antigen
DR isotype, immunoglobulin (Ig)M, CD79b and CD20, and weakly
expressed CD5 (Fig. 4). The
nuclear cells did not express CD10, CD23, FMC7 or CD200, and
CD19/CD20 double-positive cells were negative for the κ-light chain
and positive for the λ-light chain (Fig. 4). Ig variable heavy chain somatic
hypermutation testing was not performed. The detection of exons
2–11 of the TP53 gene was negative.
 | Table I.Laboratory tests, including reference
ranges. |
Table I.
Laboratory tests, including reference
ranges.
| Factor
assessed | Test results
(reference range) |
|---|
| White blood cell
count, ×109/l | 5.6 (3.5-9.5) |
| Hemoglobin level,
g/l | 118 (115–150) |
| Platelet count,
×109/l | 51 (125–350) |
| Serum tumor
markers |
|
| CEA,
ng/ml | 23.5 (<5.0) |
| CA19-9,
U/ml | 357 (<43) |
| CA72-4,
U/ml | 32.6 (<6.9) |
| NSE,
ng/ml | 157.9
(<16.3) |
| ProGRP,
pg/ml | >5,000
(<63) |
| Lactate
dehydrogenase level, U/l | 222 (109–245) |
| Cytogenetic
abnormalities | None |
Histopathological examination of the right axillary
lymph node revealed the presence of mature small lymphocytes
(Fig. 5A), which were positive for
CD20 (Fig. 5B), cyclin D1
(Fig. 5C), SRY-box transcription
factor 11 (Fig. 5D), Bcl-2
(Fig. 5E), paired box 5 (Fig. 5F) and Oct2 (Fig. 5G), the Ki-67 index was low (10–30%)
(Fig. 5H), and negative for CD3
(Fig. 5I), CD5 (Fig. 5J), Bcl6 (Fig. 5K), CD10 (Fig. 5L), CD21 (Fig. 5M), CD23 (Fig. 5N), CD30 (Fig. 5O), CD15 (Fig. 5P), CD43 (Fig. 5Q), multiple myeloma oncogene 1
(Fig. 5R), CD163 (Fig. 5S), epithelial membrane antigen
(Fig. 5T), cytokeratin AE1/AE3
(Fig. 5U) and Epstein-Barr virus
non-coding RNA (Fig. 5V), as
determined by immunohistochemical analysis. A bone marrow biopsy
was performed and the data indicated the presence of MCL
characterized by the infiltration of intermediate sized B-cells
into the mantle zones (Fig. 6A).
Immunohistochemical staining was positive for CD20 (Fig. 6B), PAX5 and cyclin D1 (Fig. 6C), whereas it was negative for CD3,
CD5 and CD23. The patient was diagnosed with stage IV MCL (symptom
status A) according to the Lugano 2014 Classification (5). A brain magnetic resonance imaging
scan demonstrated the absence of abnormal lesions. The patient was
finally diagnosed with MCLBMI complicated with ES-SCLC. A total of
six cycles (3 weeks per cycle) of chemotherapy were administered,
consisting of rituximab (600 mg on day 0), cisplatin (30 mg on days
1–3), etoposide (100 mg on days 1–3) and dexamethasone (10 mg on
days 1–5) (R-DEP) for both primary tumors at the College of
Medicine, Lishui Hospital, Zhejiang University (Lishui, China). In
August 2021, chest CT indicated a left lung mass with maximum
dimensions of 5.5×2.3 cm. Following the six aforementioned
treatment cycles, CT indicated that the left pulmonary masses were
reduced to maximum dimensions of 1.2×1.1 cm (Fig. 7A), and routine blood tests
indicated that the blood platelet count was gradually increasing
(Fig. 7B). Whole body PET-CT scans
in December 2021 indicated that, following therapy,
fluorodeoxyglucose (FDG) uptake was slightly increased in the
slightly larger lymph node noted under the right armpit. This
suggested that the lesion was still active, although the remaining
systemic lymph nodes and spleen lesions were reduced in size and
inactivated. FDG uptake was slightly increased locally in the left
upper lobe mass, and multiple bone metastases were noted throughout
the body (Fig. 7C). The patient
experienced a stable disease status in the 9-month follow-up
period. Follow-up was conducted once per month. The patient will be
followed up every 3 months for 2 years. This case achieved a
partial response according to the International Working Group
response criteria (Cheson classification) (6). The patient has a poor prognosis due
to ES-SCLC with synchronous MCLBMI.
Imaging examinations
PET/CT imaging (Biograph mCT; Siemens AG) was
performed 60 min after intravenous injection of 18F-FDG imaging
agent (Shanghai Atomic Kexing Pharmaceutical Co., Ltd.). Before the
examination, the patient fasted for >4 h, and the fasting blood
glucose level was controlled to within 10.0 mmol/l (reference
range, 3.90-6.10 mmol/l). 18F-FDG was injected through the
superficial forearm vein. The scanning range was from the base of
the skull to the middle of the femur. The current was 120 mA, the
voltage was 120 kV and the scanning time was 21–30 sec. The
thickness of the scanning slice was 5 mm. Body PET collection was
performed afterward, generally using 6 to 7 beds, with 2.0 to 3.0
min/bed. The head scanning method was performed with the same
values as the whole-body PET/CT, and 1 bed was collected. CT data
was automatically used by the CT machine to perform attenuation
correction on the PET images for image reconstruction and
fusion.
Flow cytometry (FCM)
immunophenotyping
The FCM immunophenotyping analysis was performed in
the Hangzhou Adicon Clinical laboratory (Hangzhou, China). Bone
marrow aspiration specimens were collected. Next, the sample was
incubated at 4°C for 5 min in the dark. Samples (1.5 ml) were added
into a conical centrifuge tube with 10 ml erythrocyte-lysing
solution (cat. no. 348202; BD Biosciences). The cells were
preserved after lysis procession. The cells were counted after
filtration in PBS with 0.1% BSA (cat. no. PM22316; Perfemiker) with
a 200-mesh nylon membrane filter (cat. no. QN3029; Beijing Biolab
Technology Co., Ltd.). Cell suspension (100 µl; adjusted to a
concentration of 5×106/ml) was added to each tube, and
then the antibodies were added for incubation at 4°C for 30 min.
Finally, the cells were preserved with incubation in the dark at
4°C to acquire data after cell washing. The antibodies used were as
follows: Anti-CD5 (PE; 1:2; cat. no. 347307; BD Biosciences),
anti-CD19 (PC5;1:2; cat. no. A07771; Beckman Coulter, Inc.),
anti-HLA-DR (FITC; 1:4; cat. no. 347363; BD Biosciences), anti-IgM
(APC; 1:2; cat. no. 750365; BD Biosciences), anti-CD79b (PE; 1:4;
cat. no. 557931; BD Biosciences), anti-CD20 (PC5; 1:2; cat. no.
IM2644U; Beckman Coulter, Inc.), anti-CD10 (FITC; 1:4; cat. no.
347503; BD Biosciences), anti-CD23 (PE; 1:4; cat. no. 341007; BD
Biosciences), anti-CD200 (PE-CY7; 1:2; 655735; BD Biosciences),
anti-FMC7 (FITC;1:4; cat. no. 340919; BD Biosciences), anti-κ
(FITC; 1:4; cat. no. C15623; Beckman Coulter, Inc.) and anti-λ (PE;
1:4; cat. no. C15189; Beckman Coulter, Inc.). All of the
fluorochromes and antibodies were acquired from BD Biosciences or
Beckman Coulter, Inc. FCM was performed using a NovoCyte D3000
(ACEA Bioscience, Inc.) and data were analyzed with NovoExpress™
software (V1.2.5; ACEA Bioscience, Inc.).
Biochemical examinations
The complete blood count was analyzed by a Coulter
LH750 Automatic Blood Cell Analyzer (Beckman Coulter, Inc.). The
biochemical tests, including those for lactate dehydrogenase and
tumor markers, were performed on a HITA CHI 7600 Automatic
Biochemical Analyzer (Hitachi, Ltd.) and a Tellgen Super Multiplex
Immunoassay System TESMI-F4000 (Tellgen Corporation), respectively.
All of the examinations were conducted and analyzed by the Clinical
Laboratory of Lishui Hospital, Zhejiang University.
Histopathological staining
The tissue was fixed with 4% neutral formalin at
room temperature for 12 h and embedded in paraffin after
dehydration, before 4-µm thick serial sections were prepared for
hematoxylin and eosin staining at room temperature for 90 min. The
pathological tissue slice was observed under an optical microscope
(Olympus BX45; Olympus Corporation).
Immunohistochemistry (IHC)
The formalin-fixed (4%) tissue specimens were used
for further pathological and immunohistochemical examinations by
the Department of Pathology of Lishui Hospital, Zhejiang
University. Immunohistochemical staining was performed with
antibodies from EnVision Systems (Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd. and Hangzhou HealthSky Biotechnology Co.,
Ltd.). The paraffin sections were deparaffinized in three changes
of xylene, 5 min each at 60°C, rehydrated with 100% ethanol (two
changes; 5 min each) and 95% ethanol (three changes; 2 min each),
and then immersed in distilled water. Afterwards, the paraffin
sections were rinsed (3 time for 5 min each) in PBS-T (0.01 M PBS
pH 7.4; 0.02% KH2PO4, 0.29%
N2HPO4, 0.02% KCl, 0.8% NaCl, 0.05% BSA,
0.05% Tween-20 and 0.0015% TritonX-100), and then blocked with 3%
peroxide-methanol at room temperature for 10 min for endogenous
peroxidase ablation. The duration and temperature of incubation
with primary antibodies were overnight at 4°C and those of
secondary antibodies were 30 min at 37°C. The paraffin sections
were coloured with 3,3-diaminobenzidin, and kept at room
temperature without light for 10 min. Finally the sections were
stained with hematoxylin at room temperature for 90 min,
dehydrated, cleared and mounted with neutral gum, and then images
were captured under a light microscope (Olympus BX45; Olympus
Corporation). The negative control group was assessed using the
same steps as the positive control, where the negative control
sample used PBS instead of primary antibody. The antibodies were as
follows: CD20 (1:100; cat. no. ZM-0039), cyclin D1 (1:200; cat. no.
ZA-0101), SRY-Box transcription factor 11 (1:200; cat. no.
ZM-0366), Bcl-2 (1:100; cat. no. ZA-0536), paired box 5 (1:100;
cat. no. ZA-0566), Oct2 (1:100; cat. no. ZA-0560), CD3 (1:100; cat.
no. ZA-0503), CD5 (1:100; cat. no. ZA-0510), Bcl-6 (1;100; cat. no.
ZA-0691), CD10 (1:100; cat. no. ZM-0283), CD21 (1;100; cat. no.
ZM-0525), CD23 (1:100; cat. no. ZA-0516), CD30 (1:100; cat. no.
ZA-0591), CD15 (1:100; cat. no. ZM0037), CD43 (1:100; cat. no.
ZM-0048), multiple myeloma oncogene 1 (1;100; cat. no. ZA-0583),
CD163 (1:100; cat. no. ZM-0428), epithelial membrane antigen
(1:100; cat. no. ZM-0095), cytokeratin AE1/AE3 (1:100; cat. no.
ZM-0069) and Epstein-Barr virus non-coding RNA (1:100; cat. no.
ZM-0105) (all Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd.), and thyroid transcription factor-1 (1:100; cat. no.
BFM-0379), CD56 (1:200; cat. no. BFM-0232), CgA (1:100; cat. no.
BFM-0102), Syn (1:100; cat. no. BFM-0147), neuron-specific enolase
(1:100; cat. no. BFM-0120), CK7 (1:100; cat. no. BFM-0373) and
Ki-67 (1:100; cat. no. BFM-0310) (all Hangzhou HealthSky
Biotechnology Co., Ltd.). The secondary antibody was from the
Histostain-SP Kit (1:200; cat. no. SPN-9001; Beijing Zhongshan
Golden Bridge Biotechnology Co., Ltd.).
Literature review
Search strategy
A comprehensive literature search was performed
using the PubMed (https://pubmed.ncbi.nlm.nih.gov), Web of Science
(http://webofscience.com) and Cochrane Library
(https://www.cochranelibrary.com/)
databases between January 2000 and May 2022, with the following
terms: ‘Mantle cell lymphoma and lung cancer’ OR ‘MCL and lung
carcinoma’ OR ‘synchronous and lymphoma’ OR ‘lymphoma and small
cell lung cancer’ OR ‘multiple primary malignant neoplasms and
lymphoma’.
Study identification
Studies were included if they met the following
criteria: i) Referring to lymphoma and lung cancer; ii) concerning
patients with a diagnosis of histologically confirmed lymphoma and
lung cancer; iii) containing relevant information on clinical
course, treatments and outcomes; iv) written in English; and v)
containing one or multiple clinical case reports or letters. Other
recognized solid malignant tumors and haematological malignancies
were excluded. Reviewers resolved all discrepancies by discussion
during the study identification process.
Discussion
MPMNs are defined as two or more primary malignant
tumors in the same patient at the same time or during different
time periods, which can occur in the same organ or other organs.
Multiple primary cancers occur at the same time and are
synchronized tumors (diagnosed within a 6-month period), while
those occurring at different time periods are called metachronous
(diagnosed at >6-month intervals) (7,8). MCL
is a rare and distinct type of B-cell NHL, which presents at an
advanced stage and frequently involves multiple extranodal sites,
such as the bone marrow, spleen and peripheral blood (9). SCLC is a high-grade malignancy with
the worst prognosis of all the pulmonary epithelial tumors. A large
quantity of epidemiological data has reported the risk factors of
the occurrence of two primary malignant tumors, including cancer
treatment strategies, common pathogenic factors of two types of
cancer (such as smoking and viral infection), and genetic
susceptibility (single nucleotide polymorphisms) (10,11).
A variety of second primary cancers may develop in patients with
lymphoma. Chien et al (12)
demonstrated that the probability of developing a second malignant
tumor in patients with NHL was 1.5-fold higher than that noted in
the general population; the second primary malignant tumor included
leukemia, multiple myeloma, bone or soft tissue tumors, and lung
cancer. The literature of MPMNs with regard to lymphoma and lung
cancer includes mainly case reports (13–15).
Previous studies have shown that lung cancer is the most common
second primary malignant tumor in patients with lymphoma. Lorenzo
Bermejo et al (16)
retrospectively analyzed the medical records of 60,901 patients
with NHL and demonstrated that 6,815 developed a second primary
malignant tumor, of which lung cancer was one of the most common
tumor types. An additional meta-analysis of 74,831 patients with
Hodgkin lymphoma indicated that 793 patients developed a second
primary lung cancer; the relative risk of lung cancer was higher
with time. The peak incidence of lung cancer was noted in 10–14
years of follow-up (17).
With regard to the differential diagnosis, lymphoma
and other primary malignant tumors can occur at the same site or at
different sites, which makes the diagnosis of the disease more
complicated (2,18). It is difficult to distinguish
synchronous primary malignant tumors from metastatic malignant
tumors (2). MCLBMI can be
misdiagnosed as SCLC involvement, since BM involvement has been
frequently reported in SCLC (19–21).
BM immunophenotyping and histopathological analysis can be used for
the differential diagnosis. Synchronous primary cancers can be
diagnosed if pathological examinations of the tumors in different
body regions favor the characteristics of independent primary
cancers (7). In the present case,
the diagnosis was straightforward, since the histological tumor
types between the MCL and SCLC were distinct. However, the reports
on MCL complicated with primary SCLC are very rare. Kampalath et
al (18) reported a rare case
of SCLC with synchronous MCL in a 58-year-old female. The patient
was treated with rituximab, cisplatin and etoposide, complete
remission was maintained for ~2.5 years, while partial remission
was achieved in the present case treated with rituximab, cisplatin,
etoposide and dexamethasone. In case a single tumor is not fully
characterized by its clinical manifestations, attention should be
paid to the possibility of multiple tumors occurring at the same
time. In the present case report, the patient exhibited symptoms of
chest pain and had a long history of heavy smoking. The CT scan
indicated a soft-tissue mass in the chest, which was localized in
the upper lobe of the left lung, with a maximum diameter of 2 cm.
In order to identify whether it was primary lung cancer, the
patient underwent a lung biopsy and immunohistochemical analysis to
confirm the diagnosis. The histological diagnosis of the
soft-tissue lesion was SCLC.
Although the association between solid tumors and
NHL is currently inconclusive, a correlation may exist between the
two tumor types. In the 2005 multicenter clinical study (13
centers, with 109,000 cases of NHL) by Brennan et al
(22), the incidence of NHL
secondary to other primary malignancies was considerably higher
than that of the normal population. However, the association
between the NHL subtypes and the pathological types of primary lung
malignancies is not clear when reviewing the existing literature
reports; diffuse large B-cell lymphoma combined with lung squamous
cell carcinoma is reported more frequently, followed by SCLC,
adenocarcinoma and large cell lung cancer (2,23–25).
Little is known regarding the risk factors of the
simultaneous occurrence of lymphoma combined with other primary
malignant tumors. The current clinical observational studies have
shown that it may be associated with smoking, age, the chemotherapy
used for the lymphoma and autologous stem cell transplantation
(22,26,27).
The current patient was >45 years old, a smoker and had a high
risk for developing lung cancer. The occurrence of primary lung
cancer may be related to smoking; however, it is not clear whether
other risk factors are involved. The occurrence of multiple primary
tumors may be associated with the following factors: Ethnicity,
environmental exposure (nuclear radiation), medical factors
(chemotherapy and radiotherapy) and genetic mutations (28,29).
The currently known single gene mutations in patients with multiple
primary tumors are as follows: PTEN, BRCA1/BRCA2, TP53,
retinoblastoma 1 and nth-like DNA glycosylase 1 (29,30).
In patients with multiple primary tumors with lung adenocarcinoma,
the EGFR mutation rate, especially that of exon 19 deletions, was
higher than that in patients without multiple primary tumors
(31).
The evidence found in the literature regarding
concurrent SCLC and MCL is scarce. Therefore, no standard guideline
is available for the treatment and prognosis of this disease. Upon
routine examination, the current patient presented with
thrombocytopenia (platelet count, 52×109/l) and systemic
lymphadenopathy in September 2020; however, the patient did not
initially realize the severity of the condition. The patient was
finally diagnosed with synchronous MCLBMI and ES-SCLC. In the
current case report, the treatment required consideration of the
respective clinical features and a treatment plan that would cover
both malignancies. Therefore, a multidisciplinary team meeting was
organized to discuss the optimal strategy. The combination of
cisplatin and etoposide was used as a standard therapy for the
treatment of SCLC. It is also the standard second-line chemotherapy
for NHL. Rituximab, a humanized anti-CD20 monoclonal antibody has
shown considerable activity in MCL. Therefore, the patient
underwent treatment with R-DEP. However, a lower dose of
chemotherapy than the standard dose was administered due to the
thrombocytopenia. The therapy was effective and the patient
achieved a partial response.
In the literature, a total of 6 cases with
synchronous MCL and lung carcinoma were reported, and all of them
received chemotherapy (18,32–36)
(Table II). Specifically,
Kampalath et al (18)
reported that a rare case of synchronous MCLBMI and ES-SCLC was
treated with cisplatin, etoposide and rituximab, and remained in
complete remission for ~2.5 years following the initial diagnosis.
No thrombocytopenia was reported in this case, unlike in the
current case.
 | Table II.Key characteristics of patients with
synchronous MCL and lung carcinoma. |
Table II.
Key characteristics of patients with
synchronous MCL and lung carcinoma.
| First author,
year | Age, years | Sex | Symptoms | Smoking status | Carcinoma
site/type | Lymphoma
site/type | Treatment
regimen | Follow-up time,
months, and status | (Refs.) |
|---|
| Kampalath et
al, 2004 | 58 | F | Cervical
lymphadenopathy | Moderate to
heavy | LUL/SCC | Right neck lymph
node/MCL | 6 cycles of
cisplatin and etoposide, and weekly cycles of rituximab for 8
weeks | 36, relapsed | (18) |
| Aqeel et al,
2018 | 55 | F | Severe lower flank
pain radiating to the lower abdomen and chest | 30 pack/year |
RUL/lepidic-predominant AC | RUL/MCL | 6 cycles of
chemotherapy (meprednisone, gemcitabine and cisplatin) | 12, survived | (32) |
| Hatzibougias et
al, 2008 | 73 | M | Dyspnea and
fever | Heavy | RUL/papillary
AC | RUL pleura/MCL | 6 cycles of
chemotherapy (Endoxan, Farmorubicin and vincristine) | 14, survived | (33) |
| Kai et al,
2018 | 71 | F | Abdominal
distension | Not available | PE/AC | PE and BM/MCL | DVCP and then
rituximab and bendamustine | 3, died | (34) |
| Samuel et
al, 2018 | 61 | M | Not available | Former smoker | RUL and
LRPLN/AC | LRPLN/MCL | NA | NA | (35) |
| Braham et
al, 2017 | 45 | M | Inguinal mass | Heavy | RUL/AC | Inguinal/MCL | 6 cycles of
alternating RCHOP and RDHAP regimens followed by ASCT | 36, died | (36) |
MCL is characterized by CD5 and cyclin D1
expression. However, weak CD5 expression was observed in the bone
marrow but no CD5 expression was observed in all biopsies in the
present case. Both FCM and IHC can be used to determine differences
in CD5 protein expression individually. FCM analysis could be used
for the quantitative detection of CD5-positive cells. It would be
quite hard to quantify the CD5 protein expression levels with IHC
staining. As for the reasons for different CD5 expression in bone
marrow species and all biopsies, on the one hand, FCM is more
sensitive than IHC in detecting CD5 expression, while on the other
hand, the antibody for the immunohistochemical detection was
different from that of FCM. Immunohistochemical examination is the
standard method of diagnosing lymphoma. It is important to
recognize the limitations of FCM and IHC for the detection of CD5
expression. If FCM could not have been performed in the present
case, it may have led to a different diagnosis of CD5-negative
MCLBMI. Finally, histopathological staining and IHC examination are
the standard methods to subclassify lymphoma, while FCM is probably
the most effective method that can be used to determine the
clonality and antigen expression of lymphoid cell populations
(37). Therefore, there are two
major limitations in the present study that could be addressed in
future research. First, FCM was applied to only bone marrow
immunophenotyping detection, but not to right axillary lymph node
immunophenotyping detection. Second, this study was based on a
single sample.
In conclusion, the present study described a case of
synchronous MCLBMI complicated with ES-SCLC. The patient underwent
treatment with R-DEP and achieved a partial response. Although the
patient did not achieve complete remission, the experience of this
case provides a new option for the treatment plan of synchronous
MCLBMI complicated with ES-SCLC. However, the treatment experience
in this case also shows that R-DEP therapy seems to be inadequate.
Therefore, more effective combination therapy options are required
to improve the effect of the treatment. Synchronous development of
these two malignant tumors is one of the most challenging problems
in cancer diagnosis and treatment. The treatment plan requires
comprehensive consideration and multidisciplinary cooperation
regarding both tumors. The incidence of synchronous MCLBMI and
ES-SCLC is extremely low; therefore, further studies are required
with a larger sample size. The present case report highlights the
importance of the detailed analysis of lymphoma lung involvement
and primary lung cancer. In similar cases, in which suspicion is
raised over the presence of MCLBMI and malignant tumors, the
application of immunohistochemical analysis is crucial to provide
an accurate diagnosis. Therefore, we propose a diagnostic workflow
to more accurately diagnose MCLBMI and ES-SCLC in the future as
follows: If the clinical manifestations of the patient included
unexplained lymphadenopathy, a lung mass and cytopenias, such as
thrombocytopenia, neutropenia, and anemia, a lymph node biopsy,
bone marrow biopsy and CT-guided biopsy of the lung mass should
first be performed. If the results of further IHC detection
indicate mantle cell lymphoma in combination with small cell lung
cancer, then an accurate diagnosis can be made.
Acknowledgements
The authors would like to thank Mr. Hong Ming Sun
(Department of Pathology of Lishui Hospital, Zhejiang University,
Lishui, China) for providing pathological images and Ms. Yu Ting
Zhang (Hangzhou Adicon Clinical Laboratory, Hangzhou China) for
providing flow cytometry immunophenotyping data.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
NX contributed to the analysis and interpretation of
the data and wrote the paper. LL conceived and designed the paper.
ZF and WL obtained medical images (e.g., PET-CT and CT scans) and
analyzed the data. CZ and JZ performed the bone marrow examination,
advised on patient treatment and collected the data. NX, LL, ZF,
WL, CZ and JZ performed the original draft preparation. LL and NX
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
All procedures were approved by the Ethics Committee
of College of Medicine, Lishui Hospital, Zhejiang University
(Lishui, China; approval no. 2022100).
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Karthikeyan VS, Sistla SC, Srinivasan R,
Basu D, Panicker LC, Ali SM and Rajkumar N: Metachronous multiple
primary malignant neoplasms of the stomach and the breast: Report
of two cases with review of literature. Int Surg. 99:52–55. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fujii M, Shirai T, Asada K, Saito Y,
Hirose M and Suda T: Synchronous diffuse large B-cell lymphoma and
squamous cell lung carcinoma. Respirol Case Rep. 2:33–35. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vose JM: Mantle cell lymphoma: 2015 update
on diagnosis, risk-stratification, and clinical management. Am J
Hematol. 90:739–745. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bernhardt EB and Jalal SI: Small cell lung
cancer. Cancer Treat Res. 170:301–322. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheson BD, Fisher RI, Barrington SF,
Cavalli F, Schwartz LH, Zucca E, Lister TA; Alliance, Australasian
Leukaemia and Lymphoma Group and Eastern Cooperative Oncology
Group, ; et al: Recommendations for initial evaluation, staging,
and response assessment of Hodgkin and non-Hodgkin lymphoma: The
Lugano classification. J Clin Oncol. 32:3059–3068. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheson BD: Staging and response assessment
in lymphomas: The new Lugano classification. Chin Clin Oncol.
4:52015.PubMed/NCBI
|
|
7
|
Lee TK, Myers RT, Scharyj M and Marshall
RB: Multiple primary malignant tumors (MPMT): Study of 68 autopsy
cases (1963–1980). J Am Geriatr Soc. 30:744–753. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Henne T and Schmähl D: Occurrence of
second primary malignancies in man-a second look. Cancer Treat Rev.
12:77–94. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Roy SD, Stafford JA, Scally J and
Selvachandran SN: A rare case of breast carcinoma co-existing with
axillary mantle cell lymphoma. World J Surg Oncol. 1:272003.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Travis LB, Rabkin CS, Brown LM, Allan JM,
Alter BP, Ambrosone CB, Begg CB, Caporaso N, Chanock S, DeMichele
A, et al: Cancer survivorship-genetic susceptibility and second
primary cancers: Research strategies and recommendations. J Natl
Cancer Inst. 98:15–25. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Park SL, Caberto CP, Lin Y, Goodloe RJ,
Dumitrescu L, Love SA, Matise TC, Hindorff LA, Fowke JH, Schumacher
FR, et al: Association of cancer susceptibility variants with risk
of multiple primary cancers: the population architecture using
genomics and epidemiology study. Cancer Epidemiol Biomarkers Prev.
23:2568–2578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chien SH, Liu CJ, Hong YC, Teng CJ, Hu YW,
Ku FC, Yeh CM, Chiou TJ, Gau JP and Tzeng CH: Development of second
primary malignancy in patients with non- Hodgkin lymphoma: A
nationwide population-based study. J Cancer Res Clin Oncol.
141:1995–2004. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim YJ, Shin HJ, Won CH, Chang SE, Lee MW,
Choi JH and Lee WJ: The incidence of other primary cancers in
patients with cutaneous lymphoma. Ann Dermatol. 30:335–341. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Alakeel F, Lee E, Baird-Howell M and
Easley S: Synchronous ductal carcinoma in situ and intravascular
large B-cell lymphoma of the breast. Appl Immunohistochem Mol
Morphol. 27:e91–e92. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li J, Zhou C, Liu W, Sun X and Meng X:
Synchronous diffuse large B cell lymphoma of the stomach and small
cell lung carcinoma: A case report. Medicine (Baltimore).
96:e88732017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bermejo JL, Pukkala E, Johannesen TB,
Sundquist J and Hemminki K: Age time risk patterns of solid cancers
in 60 901 non-Hodgkin lymphoma survivors from Finland, Norway and
Sweden. Br J Haematol. 164:675–683. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ibrahim EM, Kazkaz GA, Abouelkhair KM,
Al-Mansour MM, Al-Fayea TM, Al-Foheidi M, Bayer AM and Elmasri OA:
Increased risk of second lung cancer in Hodgkin's lymphoma
survivors: A meta-analysis. Lung. 191:117–134. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kampalath B, Abed N, Chitambar CR,
Vantuinen P, Chakrabarty G, Hanson G, Rao RN, Shidham VB and Chang
CC: Mantle cell lymphoma in lymph nodes with metastatic small cell
carcinoma of lung: A diagnostic and treatment dilemma. Leuk
Lymphoma. 45:409–414. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Argiris A and Murren JR: Staging and
clinical prognostic factors for small-cell lung cancer. Cancer J.
7:437–447. 2001.PubMed/NCBI
|
|
20
|
Loscocco GG, Piccini M, Bencini S and
Vergoni F: Bone marrow involvement in small cell lung cancer.
Hematol Transfus Cell Ther. 43:543–544. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pasini F, Cetto GL and Pelosi G: Does bone
marrow involvement affect prognosis in small-cell lung cancer? Ann
Oncol. 9:247–250. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brennan P, Scélo G, Hemminki K,
Mellemkjaer L, Tracey E, Andersen A, Brewster DH, Pukkala E,
McBride ML, Kliewer EV, et al: Second primary cancers among 109 000
cases of non-Hodgkin's lymphoma. Br J Cancer. 93:159–166. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fonseca D, Musthyala B, Ahmed F, Murthy SS
and Raju KVVN: A tale of synchronous lung carcinoma and diffuse
large B-cell lymphoma of ileum:a rare combination. Lung India.
32:398–401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tokuchi Y, Kamachi M, Harada M, Hasegawa
M, Mishina T, Yamashiro K, Suzuki H and Isobe H: Synchronous triple
lung cancers after treatment for non-Hodgkin's
lymphoma:metachronous quadruple cancers. Intern Med. 42:1031–1034.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
André M, Mounier N, Leleu X, Sonet A,
Brice P, Henry-Amar M, Tilly H, Coiffier B, Bosly A, Morel P, et
al: Second cancers and late toxicities after treatment of
aggressive non-Hodgkin lymphoma with the ACVBP regimen: A GELA
cohort study on 2837 patients. Blood. 103:1222–1228. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Moser EC, Noordijk EM, van Leeuwen FE,
Baars JW, Thomas J, Carde P, Meerwaldt JH, van Glabbeke M and
Kluin-Nelemans HC: Risk of second cancer after treatment of
aggressive non-Hodgkin's lymphoma;an EORTC cohort study.
Haematologica. 91:1481–1488. 2006.PubMed/NCBI
|
|
27
|
Smeland KB, Kiserud CE, Lauritzsen GF,
Blystad AK, Fagerli UM, Falk RS, Fluge O, Fosså A, Kolstad A, Loge
JH, et al: A national study on conditional survival, excess
mortality and second cancer after high dose therapy with autologous
stem cell transplantation for non-Hodgkin lymphoma. Br J Haematol.
173:432–443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mersheimer WL, Ringel A and Eisenberg H:
Some characteristics of multiple primary cancers. Ann NY Acad Sci.
114:896–921. 1964. View Article : Google Scholar
|
|
29
|
Cybulski C, Nazarali S and Narod SA:
Multiple primary cancers as a guide to heritability. Int J Cancer.
135:1756–1763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rivera B, Castellsagué E, Bah I, van
Kempen LC and Foulkes WD: Biallelic NTHL1 mutations in a woman with
multiple primary tumors. N Engl J Med. 373:1985–1986. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Luo YH, Ho HL, Tsai CM, Shih JF, Chiu CH,
Lai SL, Lee YC, Perng RP, Whang-Peng J, Chou TY and Chen YM: The
association between tumor epidermal growth factor receptor (EGFR)
mutation and multiple primary malignancies in patients with
adenocarcinoma of the lungs. Am J Clin Oncol. 38:147–151. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aqeel M, Uysal-Biggs N, Fenske TS and Rao
N: one pulmonary lesion, 2 synchronous malignancies. J Investig Med
High Impact Case Rep. 6:23247096187859342018.PubMed/NCBI
|
|
33
|
Hatzibougias D, Bobos M, Karayannopoulou
G, Karkavelas G, Karapanagiotidis GT, Foroulis CN and Kostopoulos
I: A rare tumoral combination, synchronous lung adenocarcinoma and
mantle cell lymphoma of the pleura. World J Surg Oncol. 6:1372008.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kai K, Ryu Y, Kamochi K, Nishioka A,
Kubota Y, Nakamura M, Kimura S, Sueoka E and Aishima S: Synchronous
mantle cell lymphoma and lung adenocarcinoma presenting in a
pleural effusion: A rare tumor combination and a potential pitfall
of cytodiagnosis. Cytopathology. 29:400–402. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Samuel G, Simoff M, Chaabaan S and
Diaz-Mendoza J: Synchronic diagnosis of non-hodgkin lymphoma and
lung adenocarcinoma via EBUS-guided TBNA. J Bronchology Interv
Pulmonol. 25:e41–e42. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Braham E, Zarrouk M, Mlika M, Kilani T and
El Mezni F: Synchronous mantle cell lymph node lymphoma and
pulmonary adenocarcinoma: A case report with literature review.
Clin Respir J. 11:430–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Craig FE and Foon KA: Flow cytometric
immunophenotyping for hematologic neoplasms. Blood. 111:3941–3967.
2008. View Article : Google Scholar : PubMed/NCBI
|