Introduction
Colorectal cancer is the fourth most common cancer,
with 1.8 million cases worldwide as of 2017. It is also the second
most common cause of cancer-related mortality, accounting for
896,000 deaths globally in 2017 (1). The depth of tumor invasion, which is
classified into T status, is one of the poor prognostic factors,
and T4 disease accounts for approximately 15% of all primary colon
cancer (2,3). In the 7th edition of AJCC TNM
classification in 2010, pathological T4 (pT4) was divided as tumor
invasion through the visceral peritoneum (T4a) and to the adjacent
organs or structures (T4b) (4)
because a large study using National Cancer Data Base showed that
pathological T4a (pT4a) had better 5-year survival rate than
pathological T4b (pT4b) (5,6).
This classification is still applied today; however,
there is no difference in the prognosis between pT4a and pT4b
(7,8). A noteworthy point may be differences
in the recurrence pattern between pT4a and pT4b colon cancer. The
ratios of peritoneal recurrence were higher in pT4 colon cancer
than in the other pT status colon cancer, ranging from 18.3 to
42.1% in previous studies (7–10).
pT4a colon cancer had more frequent peritoneal recurrence than pT4b
colon cancer (8). Conversely, R1
resection leads to some recurrence (especially locoregional
recurrence) in pT4b colon cancer requiring multivisceral en block
resection (MVR), and surgical technique also plays a role in
prognosis (11). R1 resection is a
poor prognostic factor for locally advanced colon cancer requiring
MVR (12).
Poor prognostic factors for pT4 colon cancer remain
incompletely elucidated. Understanding the oncological
characteristics of pT4 colon cancer, including the tumor-specific
differences between pT4a and pT4b colon cancer, can assist surgeons
in providing the best possible treatment. Therefore, we
investigated the prognostic risk factors for pT4 colon cancer in
patients who underwent R0 resection. Toward this goal, we compared
oncological outcomes, including overall survival (OS), relapse-free
survival (RFS), and recurrence pattern, between patients with pT4
vs. pT4b colon cancer. Additionally, the independent risk factors
for pT4 colon cancer were examined.
Materials and methods
Patient selection criteria
Overall, 1,066 patients underwent surgery for
primary colorectal cancer between 2014 and 2021 at Tokyo Women's
Medical Hospital. Among these cases, 83 patients who underwent
curative resection excluding emergency surgery and were diagnosed
with pT4 colon cancer were enrolled. The patients with stage II–III
cancer with lesions located between the cecum and rectosigmoid
colon were included, but patients with stage IV colon cancer or R1
resection were excluded. En bloc MVR with complete mesocolic
excision to achieve R0 resection was performed for patients with
locally advanced colon cancer if tumors were invading or adhering
to multiple organs or structures. The surgical approach included
open, laparoscopic, and robotic surgery, and the most appropriate
method was selected based on patient and tumor factors. A robotic
approach was first used at our institution in 2017. The anastomosis
in the minimally invasive surgery was not performed using the
intra-corporeal functional end-to-end anastomosis technique.
Conversion from the minimally invasive approach to an open approach
was assigned to the open group. We collected medical data using the
electronic medical records of these patients and analyzed them
retrospectively. This study was approved by the Ethics Committee of
Tokyo Women's Medical Hospital (Institutional Review Board number
5266).
Patient evaluation and perioperative
treatment
Patient characteristics (age, sex, and body mass
index [BMI]) were collected from the medical database. All patients
were diagnosed with adenocarcinoma via biopsy and underwent
colonoscopy and multidetector-row computed tomography (CT) or
positron emission tomography (PET-CT) pre-operatively. Tumor status
(tumor size, histology, pathological T stage and pathological N
stage) and details concerning the surgical approach were obtained
from the medical database. Pathological stage was determined
according to the 8th edition of the TNM classification of malignant
tumors (13). Regarding tumor
location, the right colon was defined as the cecum and transverse
colon, and the left colon was defined as the descending colon and
the rectosigmoid colon.
Postoperative follow-up
Postoperative adjuvant chemotherapy was recommended
for all patients based on their individual health status. Routine
follow-up was performed every 3 months by consultation and included
blood tests for tumor markers. Either CT or PET-CT was performed
every 6 months. Additional examinations were performed in cases of
suspected recurrence.
Statistical analysis
The baseline demographic information and long-term
oncological outcomes (follow-up duration and recurrence site) were
described and compared between the pT4a and T4b groups. Categorical
data are presented as numbers and proportions and were compared
between the two patient groups using Fisher's exact test.
Continuous numerical variables are presented as median and
interquartile range (IQR). The Mann-Whitney U test was used
to compare the distribution of continuous numerical variables
between the pT4a and pT4b groups. Patient characteristics (age,
sex, BMI), tumor (tumor size, histology, tumor location, pT status,
and pN status), and treatment-related variables (surgical approach,
adjuvant chemotherapy) were used as potential risk factors for
postoperative peritoneal recurrence, as described in previous
studies (7,8). Factors of interest identified at the
univariate level (P<0.10) were entered into a multivariate Cox
regression model. The effects of these putative factors on
recurrence risks are described as the hazard ratio (HR) and
corresponding 95% confidence intervals (CIs). Statistical
significance was set at a two-sided P-value of <0.05.
Kaplan-Meier estimates were used to examine OS and RFS curves. The
log-rank test was performed to compare long-term outcomes between
the pT4a and pT4b groups. Among the patients with recurrence, OS
with and without peritoneal recurrence, and with and without
distant metastasis alone were also analyzed similarly. All
statistical analyses were performed using SPSS version 24 (SPSS
Inc., Chicago, IL, USA).
Results
Baseline demographics of patients
The baseline patient demographics, tumor
characteristics, and treatment-related factors of the sample are
summarized in Table I. There were
no significant differences in patient characteristics, including
age, sex, and BMI, between the pT4a group (n=62) and the pT4b group
(n=21). Tumor size was, however, significantly larger in the pT4b
group than in the pT4a group (P=0.033). The difference in the tumor
location between the two groups was 37 cases (59.5%) (cecum: 6,
ascending colon: 16, transverse colon: 15) vs. 8 cases (38.1%)
(cecum: 2, ascending colon: 6) for the right-sided colon and 25
cases (40.3%) (descending colon: 4, sigmoid colon: 14, rectosigmoid
colon: 7) vs. 13 cases (61.9%) (sigmoid colon: 11, rectosigmoid
colon: 2) for the left-sided colon (P=0.128). The proportions of
MVR were 4.8 and 100% in the pT4a and pT4b groups, respectively.
The adjacent organs or structures resected for en bloc complete
tumor resection were the peritoneum or abdominal wall (n=13),
bladder (n=5), small intestine (n=4), other parts of the colon
(n=3), duodenum (n=2), liver (n=1), ovary (n=1), gonadal vessels
(n=1), omentum (n=1), and pararenal fat (n=1). Of these, it is in
the pT4a group that there were the peritoneum or abdominal wall
(n=3), omentum (n=1), and pararenal fat (n=1). Conversion from
laparoscopy to open surgery was observed in one case. There were no
significant differences in the surgical approach or use of
postoperative adjuvant chemotherapy between the pT4a and pT4b
groups.
 | Table I.Baseline patient demographics compared
between the pT4a and pT4b groups. |
Table I.
Baseline patient demographics compared
between the pT4a and pT4b groups.
|
|
| pT status |
|
|---|
|
|
|
|
|
|---|
| Variables | Total (n=83) | pT4a (n=62) | pT4b (n=21) | P-value |
|---|
| Age, years | 73.0 (62.0–77.0) | 72.5 (61.0–77.3) | 73.0 (66.0–78.0) | 0.971 |
| Sex, male/female n
(%) | 49 (52.4%)/34
(41.5%) | 34 (54.8%)/28
(45.2%) | 15 (71.4%)/6
(28.6%) | 0.209 |
| BMI,
kg/m2 | 21.6 (18.9–23.9) | 21.8 (19.1–23.5) | 21.3 (18.4–25.1) | 0.773 |
| Tumor size, cm | 5.5 (4.5–7.0) | 5.0 (4.1–7.0) | 6.5 (5.5–8.3) | 0.033 |
| Site of tumor |
|
|
| 0.128 |
|
Right-sided colon | 45 (54.9%) | 37 (59.7%) | 8 (38.1%) |
|
|
Left-sided colon | 38 (46.3%) | 25 (40.3%) | 13 (61.9%) |
|
| Histology |
|
|
| 0.200 |
|
Well/moderately | 66 (79.5%) | 47 (75.8%) | 19 (90.5%) |
|
|
Mucinous | 14 (16.9%) | 13 (21.0%) | 1 (4.8%) |
|
|
Poorly | 3 (3.6%) | 2 (3.2%) | 1 (4.8%) |
|
| pN
statusa, n (%) |
|
|
| 0.619 |
| N0 | 45 (54.2%) | 32 (51.6%) | 13 (61.9%) |
|
| N1 | 23 (27.7%) | 19 (30.6%) | 4 (19.0%) |
|
| N2 | 15 (18.1%) | 11 (17.7%) | 4 (19.0%) |
|
| Stent insertion
before surgery, n (%) | 15 (18.1%) | 13 (20.1%) | 2 (9.5%) | 0.334 |
| Approach, n (%) |
|
|
| 0.234 |
| Open | 18 (21.7%) | 11 (17.7%) | 7 (33.3%) |
|
|
Laparoscopic | 62 (74.7%) | 49 (79.0%) | 13 (61.9%) |
|
|
Robotic | 3 (3.6%) | 2 (3.2%) | 1 (4.8%) |
|
| MVR, n (%) | 24 (28.9%) | 3 (4.8%) | 21 (100%) |
|
| Adjuvant
chemotherapy, n (%) | 46 (55.4%) | 33 (53.2%) | 13 (61.9%) | 0.613 |
Oncological outcomes including
recurrence pattern
The oncological outcomes are summarized in Table II. The median (IQR) follow-up
duration was comparable between the two groups (38.5 [15.8-55.0]
months vs. 34 [14.5-58.5] months, P=0.917). Disease recurrence was
observed in 17 (27.4%) and 8 (38.1%) patients in the pT4a and pT4b
groups, respectively (P=0.413). Furthermore, peritoneal recurrence
tended to occur more frequently in the pT4a group than in the pT4b
group (19.4 vs. 9.5%, P=0.501), while distant metastasis ratio
tended to be higher in the pT4b group than the pT4a group (11.3 vs.
28.6%, P=0.082). Five (71.4%) of the seven patients in the pT4b
group who experienced recurrence were distant metastasis alone. The
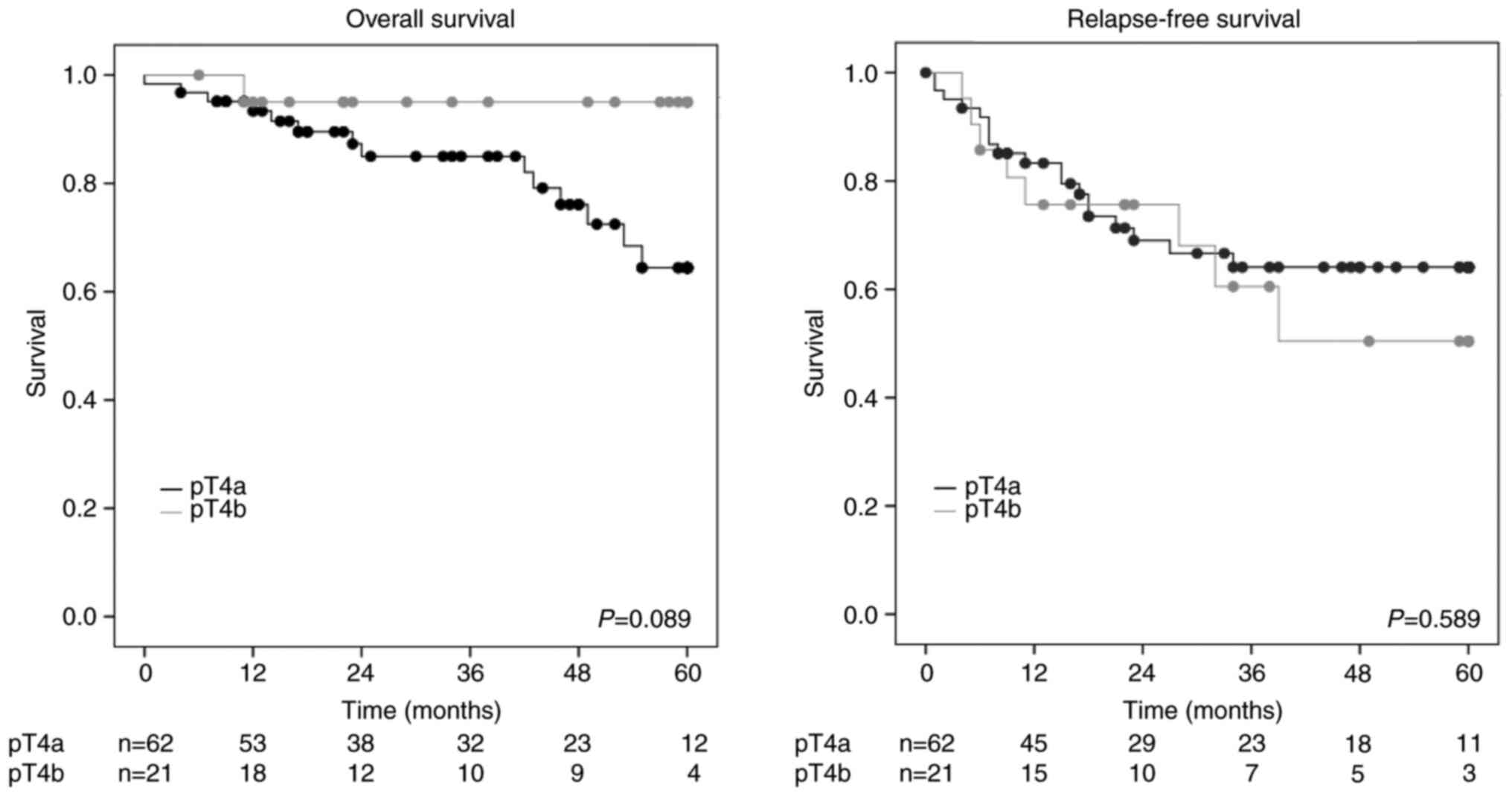
3-year OS rates were 85.1% in the pT4a group and 95.0% in the pT4b
group, respectively (P=0.089) (Fig.
1). The 3-year RFS rates were 64.1% in the pT4a group and 60.5%
in the pT4b group, respectively (P=0.589) (Fig. 1). The 3-year peritoneal
recurrence-free survival rate tended to be lower in the pT4a group
than in the pT4b group (71.0 vs. 90.2%, P=0.085) (Fig. 2).
 | Table II.Oncological outcomes compared between
the pT4a and pT4b groups. |
Table II.
Oncological outcomes compared between
the pT4a and pT4b groups.
|
|
| pT4 status |
|
|---|
|
|
|
|
|
|---|
| Variables | Total (n=83) | pT4a (n=62) | pT4b (n=21) | P-value |
|---|
| Follow-up duration,
months | 38.0
(16.0–57.0) | 38.5
(15.8–55.0) | 34.0
(14.5–58.5) | 0.917 |
| Recurrent
disease |
|
|
|
|
|
Overall, n (%)a | 25 (30.1%) | 17 (27.4%) | 8 (38.1%) | 0.413 |
|
Locoregional, n (%) | 8 (9.6%) | 6 (9.7%) | 2 (9.5%) | 1.000 |
|
Distant, n (%) | 13 (15.7%) | 7 (11.3%) | 6 (28.6%) | 0.082 |
|
Peritoneum, n (%) | 14 (16.9%) | 12 (19.4%) | 2 (9.5%) | 0.501 |
| 3-year overall
survival | 87.5% | 85.1% | 95.0% | 0.089 |
| 3-year relapse-free
survival | 63.3% | 64.1% | 60.5% | 0.589 |
Risk factors for overall and
recurrence-free survival
Table III
presents the independent risk factors associated with OS and RFS.
In univariate and multivariable analyses, OS was associated with
mucinous or poorly differentiated adenocarcinoma (HR, 6.828; 95%
CI, 2.173-21.456; P=0.001), right-sided colon cancer (HR, 4.290;
95% CI, 1.119-16.446; P=0.034), and pN status positive (HR, 5.060;
95% CI, 1.387-18.462; P=0.014). Conversely, poor prognostic factors
were histologically confirmed mucinous or poorly differentiated
adenocarcinoma (HR, 3.114; 95% CI, 1.375-7.052; P=0.006) and pN
status positive (HR, 7.845; 95% CI, 2.706-22.745; P<0.001).
 | Table III.Risk factors for overall survival and
relapse-free survival in patients with pT4 colon cancer. |
Table III.
Risk factors for overall survival and
relapse-free survival in patients with pT4 colon cancer.
| A, Overall
survival |
|---|
|
|---|
|
| Univariate | Multivariable |
|
|---|
|
|
|
|
|
|---|
| Variables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Age (≥75 years
old/<75 years old) | 1.239
(0.449–3.417) | 0.679 |
|
|
| Sex
(male/female) | 0.794
(0.288–2.191) | 0.656 |
|
|
| BMI (≥22
kg/m2/<22 kg/m2) | 0.625
(0.222–1.762) | 0.374 |
|
|
| Tumor size (≥5
cm/<5 cm) | 1.048
(0.357–3.077) | 0.932 |
|
|
| Mucinous/Poorly
(yes/no) | 6.012
(2.134–16.937) | 0.001 | 6.828
(2.173–21.456) | 0.001 |
| Location
(right-sided/left-sided) | 3.296
(0.929–11.694) | 0.065 | 4.290
(1.119–16.446) | 0.034 |
| pT
statusa (T4a/4b) | 4.893
(0.643–37.233) | 0.125 |
|
|
| pN
statusa
(N1-2/N0) | 4.778
(1.347–16.941) | 0.015 | 5.060
(1.387–18.462) | 0.014 |
| Stent insertion
(yes/no) | 1.721
(0.547–5.414) | 0.354 |
|
|
| Approach
(Laparoscopic or robotic/open) | 0.767
(0.243–2.421) | 0.651 |
|
|
| Adjuvant
chemotherapy (yes/no) | 0.909
(0.330–2.508) | 0.909 |
|
|
|
| B, Relapse-free
survival |
|
|
|
Univariate |
Multivariable |
|
|
|
|
|
|
|
Variables | Hazard ratio
(95% CI) | P-value | Hazard ratio
(95% CI) | P-value |
|
| Age (≥75 years
old/<75 years old) | 0.537
(0.235–1.228) | 0.141 |
|
|
| Sex
(male/female) | 1.128
(0.516–2.465) | 0.763 |
|
|
| BMI (≥22
kg/m2/<22 kg/m2) | 0.962
(0.452–2.048) | 0.920 |
|
|
| Tumor size (≥5
cm/<5 cm) | 0.910
(0.408–2.029) | 0.818 |
|
|
| Mucinous/Poorly
(yes/no) | 3.328
(1.481–7.481) | 0.004 | 3.114
(1.375–7.052) | 0.006 |
| Location
(right-sided/left-sided) | 1.525
(0.698–3.331) | 0.290 |
|
|
| pT
statusa (T4a/4b) | 0.797
(0.349–1.822) | 0.591 |
|
|
| pN
statusa
(N1-2/N0) | 8.075
(2.789–23.374) | <0.001 | 7.845
(2.706–22.745) | <0.001 |
| Stent insertion
(yes/no) | 1.074
(0.403–2.862) | 0.887 |
|
|
| Approach
(Laparoscopic or robotic/open) | 1.118
(0.423–2.953) | 0.822 |
|
|
| Adjuvant
chemotherapy (yes/no) | 1.156
(0.536–2.493) | 0.712 |
|
|
Discussion
This study investigated prognostic risk factors for
pT4 colon cancer when radical resection was performed, including a
comparison between the pT4a and pT4b groups. There were no
statistical differences in OS and RFS between the pT4a and pT4b
groups; however, there was a trend toward more disseminated
recurrence in the pT4a group. Histology (mucinous or poorly
differentiated adenocarcinoma) and pN status (positive) were
associated with OS and RFS, and tumor location (right-sided) was an
independent risk factor for OS in patients with pT4 colon
cancer.
Before 2010, several studies had shown that tumor
invasion through the visceral peritoneum is associated with a worse
prognosis than invasion to the adjacent organs or structures
(14–16). Subsequently, in the 7th edition of
AJCC TNM classification (2010), pT4 was divided into pT4a and T4b4,
since a large study using National Cancer Data Base showed pT4a had
a better 5-year survival rate than pT4b (5). However, recent studies showed no
difference between pT4a and pT4b, which is similar to our results
(7,8). In our study, pT groups were not
significantly associated with survival. Tumor location, histology,
and pN status were associated with OS, and histology and pN status
were risk factors for RFS. These findings are consistent with those
of previous studies, which suggested that tumor location
(right-sided), histology (mucinous or poor differentiated
adenocarcinoma) and pN status were associated with a poorer
prognosis (5,12,17,18),
especially right-sided colon cancers characterized by mucinous
histology, high microsatellite instability, and BRAF
mutation carrier status (17,18).
Although pT4a colon cancer is expected to be more
likely to cause peritoneal recurrence than pT4b colon cancer,
studies have often failed to prove this with statistical
significance. Our study also demonstrated a tendency for the pT4a
group to be more prone to recurrence of peritoneal recurrence than
the pT4b group (19.4 vs. 4.8%, P=0.168). At least one prior study
showed this effect could be due to a type II statistical error, and
demonstrated that the pT4a group had more peritoneal recurrence
than the pT4b group in the absence of this error (8). Interestingly, in our study, the pT4b
group showed a trend toward more distant as opposed to peritoneal
recurrence (11.3 vs. 28.6%, P=0.082). Peritoneal recurrence was
associated with a poorer prognosis than was no peritoneal
recurrence (P=0.009). However. the prognosis was poor in the
presence of peritoneal recurrence, while distant metastasis alone
was associated with a better prognosis than other recurrence
patterns (P=0.039) (Fig. 2).
Therefore, strategies that consider the recurrence pattern of pT4a
vs. pT4b colon cancer are required for oncologists. A previous
review of three large phase III randomized trials suggested that
developing peritoneal recurrence led to poorer OS than other forms
of recurrence (19).
A randomized controlled trial comparing open and
laparoscopic surgery for colon cancer (JCOG0404) also found no
significant differences in the rate of peritoneal recurrence
between the two approaches (20).
Conversely, a conflicting study showed an association between the
laparoscopic approach and increased risk for peritoneal recurrence
(21). We believe that it is
important to avoid direct contact with the tumor as much as
possible during laparoscopic surgery for pT4a, since the cancer
cells are exposed on the surface of the serosa and may be
disseminated by the surgical procedure. Conversely, it must be
recommended that R0 resection with MVR is always required for
patients in the pT4b group. The surgical approach should be
selected according to the surgical-skill level of the institution
and that of the surgeon to prevent R1 resection by the surgical
procedure, as proposed in our previous study (12).
This study has some limitations. First, this was a
retrospective study with a small sample size that included surgical
data from a single institution. Second, this study had a selection
bias for patients in terms of the surgical approach. However, this
decision was based on the capacities of the research institution,
which is in keeping with real-world clinical practice. Third, only
a few patients received postoperative adjuvant chemotherapy. In
contrast, the major strength of this study is its focus on patients
with colon cancer who underwent R0 resection without primary
involvement of the rectum. This is because locally advanced colon
cancer is more prone to peritoneal recurrence than rectal cancer,
and the treatment including surgical difficulty, is standardized.
Finally, large-scale studies with multicenter designs would be
required to generalize our findings.
In conclusion, our findings suggest that patients
with pT4b colon cancer and R0 resection may not have a poorer
prognosis compared to those with pT4a colon cancer. However, the
possibility that patients with pT4a colon cancer had more
peritoneal recurrence than those with pT4b colon cancer may have
affected the survival rates. Histology (mucinous or poorly
differentiated adenocarcinoma) and pN status (positive) were
significant prognostic factors for OS and RFS, and tumor location
(right-sided) was also associated with OS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
The study concept and design were developed by TK
and SY. Data collection was performed by TK, RN, YN, FM, KT, HK,
KK, YK, YB, SO, YI and MI. The analysis was performed by TK. The
manuscript was prepared by TK. The manuscript was supervised by SY.
All authors reviewed the manuscript. TK and RN confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Tokyo Women's Medical Hospital (Institutional Review Board number
5266). The committee waived the requirement for informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr Tsutomu Kumamoto, ORCID: 0000-0002-7177-9328.
Glossary
Abbreviations
Abbreviations:
|
pT4
|
pathological T4
|
|
OS
|
overall survival
|
|
RFS
|
relapse-free survival
|
|
MVR
|
multivisceral en bloc resection
|
|
BMI
|
body mass index
|
|
CT
|
computed tomography
|
|
PET
|
positron emission tomography
|
|
IQR
|
interquartile range
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Global Burden of Disease Cancer
Collaboration, . Fitzmaurice C, Abate D, Abbasi N, Abbastabar H,
Abd-Allah F, Abdel-Rahman O, Abdelalim A, Abdoli A, Abdollahpour I,
et al: Global, regional, and national cancer incidence, mortality,
years of life lost, years lived with disability, and
disability-adjusted life-years for 29 cancer groups, 1990 to 2017:
A systematic analysis for the global burden of disease study. JAMA
Oncol. 5:1749–1768. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Segelman J, Akre O, Gustafsson UO, Bottai
M and Martling A: External validation of models predicting the
individual risk of metachronous peritoneal carcinomatosis from
colon and rectal cancer. Colorectal Dis. 18:378–385. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Neree Tot Babberich MPM, Detering R,
Dekker JWT, Elferink MA, Tollenaar RAEM, Wouters MWJM and Tanis PJ;
Dutch ColoRectal Audit Group, : Achievements in colorectal cancer
care during 8 years of auditing in the Netherlands. Eur J Oncol.
44:1361–1370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A III: Colon and rectum. AJCC Cancer Staging
Manual. 7th edition. Springer-Verlag; New York, NY: pp. 143–164.
2010
|
|
5
|
Gunderson LL, Jessup JM, Sargent DJ,
Greene FL and Stewart AK: Revised TN categorization for colon
cancer based on national survival outcomes data. J Clin Oncol.
28:264–271. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Frankel WL and Jin M: Serosal surfaces,
mucin pools, and deposits, oh my: Challenges in staging colorectal
carcinoma. Mod Pathol. 28 (Suppl 1):S95–S108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Eom T, Lee Y, Kim J, Park I, Gwak G, Cho
H, Yang K, Kim K and Bae BN: Prognostic factors affecting
disease-free survival and overall survival in T4 colon cancer. Ann
Coloproctol. 37:259–265. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bastiaenen VP, Aalbers AGJ, Arjona-Sánchez
A, Bellato V, van der Bilt JDW, D'Hoore AD, Espinosa-Redondo E,
Klaver CEL, Nagtegaal ID, van Ramshorst B, et al: Risk of
metachronous peritoneal metastases in patients with pT4a versus
pT4b colon cancer: An international multicentre cohort study. Eur J
Surg Oncol. 47:2405–2413. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hompes D, Tiek J, Wolthuis A, Fieuws S,
Penninckx F, Van Cutsem E and D'Hoore A: HIPEC in T4a colon cancer:
A defendable treatment to improve oncologic outcome? Ann Oncol.
23:3123–3129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Santvoort HC, Braam HJ, Spekreijse KR,
Koning NR, de Bruin PC, de Vries Reilingh TS, Boerma D, Smits AB,
Wiezer MJ and van Ramshorst B: Peritoneal carcinomatosis in T4
colorectal cancer: Occurrence and risk factors. Ann Surg Oncol.
21:1686–1691. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eveno C, Lefevre JH, Svrcek M, Bennis M,
Chafai N, Tiret E and Parc Y: Oncologic results after multivisceral
resection of clinical T4 tumors. Surgery. 156:669–675. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kumamoto T, Toda S, Matoba S, Moriyama J,
Hanaoka Y, Tomizawa K, Sawada T and Kuroyanagi H: Short- and
long-term outcomes of laparoscopic multivisceral resection for
clinically suspected T4 colon cancer. World J Surg. 41:2153–2159.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
UICC, . TNM Classification of Malignant
Tumours. 8th edition. John Wiley & Sons Ltd.; New York, NY:
2017
|
|
14
|
Shepherd NA, Baxter KJ and Love SB: The
prognostic importance of peritoneal involvement in colonic cancer:
A prospective evaluation. Gastroenterology. 112:1096–1102. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Compton C, Fenoglio-Preiser CM, Pettigrew
N and Fielding LP: American Joint Committee on cancer prognostic
factors consensus conference: Colorectal working group. Cancer.
8:1739–1757. 2000. View Article : Google Scholar
|
|
16
|
Keshava A, Chapuis PH, Chan C, Lin BP,
Bokey EL and Dent OF: The significance of involvement of a free
serosal surface for recurrence and survival following resection of
clinicopathological stage B and C rectal cancer. Colorectal Dis.
9:609–618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Petrelli F, Tomasello G, Borgonovo K,
Ghidini M, Turati L, Dallera P, Passalacqua R, Sgroi G and Barni S:
Prognostic survival associated with left-sided vs right-sided colon
cancer: A systematic review and meta-analysis. JAMA Oncol.
3:211–219. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park JS, Huh JW, Park YA, Cho YB, Yun SH,
Kim HC, Lee WY and Chun HK: Prognostic comparison between mucinous
and nonmucinous adenocarcinoma in colorectal cancer. Medicine
(Baltimore). 94:e6582015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mayanagi S, Kashiwabara K, Honda M, Oba K,
Aoyama T, Kanda M, Maeda H, Hamada C, Sadahiro S, Sakamoto J, et
al: Risk factors for peritoneal recurrence in stage II to III colon
cancer. Dis Colon Rectum. 61:803–808. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saito S, Akagi T, Katayama H, Wakabayashi
M, Inomata M, Yamamoto S, Ito M, Kinugasa Y, Egi H, Munakata Y, et
al: Identification of patient subgroups with unfavorable long-term
outcomes associated with laparoscopic surgery in a randomized
controlled trial comparing open and laparoscopic surgery for colon
cancer (Japan Clinical Oncology Group Study JCOG0404). Ann
Gastroenterol Surg. 5:804–812. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huynh C, Minkova S, Kim D, Stuart H and
Hamilton TD: Laparoscopic versus open resection in patients with
locally advanced colon cancer. Surgery. 170:1610–1615. 2021.
View Article : Google Scholar : PubMed/NCBI
|