Introduction
The breakdown of fibrin formed during blood clotting
is called fibrinolysis (1,2). Fibrinolysis is an important
physiological function of the human body and regulates the process
of bleeding and hemostasis together with the coagulation system
(3,4). Abnormal enhancement of fibrinolytic
activity is called hyperfibrinolysis. Hyperfibrinolysis is further
divided into the primary and secondary types. Primary
hyperfibrinolysis refers to a hemorrhagic syndrome caused by
increased plasminogen activator (PA) [such as tissue (t)-PA or
urokinase (u)-PA] or decreased fibrinolytic system inhibitor [such
as plasminogen activator inhibitor (PAI-1), thrombin-activated
plasminogen inhibitor (TAFI) and α2-antiplasmin] levels during the
pathophysiological process of the primary disease, causing
hyperfibrinolysis (5). Primary
hyperfibrinolysis is rare in clinical practice, but can be observed
in patients with chronic liver disease, acute leukemia, severe
trauma and postpartum hemorrhage (6,7).
Secondary hyperfibrinolysis refers to the massive production of
fibrin due to the activation of coagulation function [such as
thrombosis and disseminated intravascular coagulation (DIC)] in the
early stage of the disease, which subsequently causes
hyperfibrinolysis (8,9). Malignancies may affect the
fibrinolytic process leading to hyperfibrinolysis, and may induce
bleeding (1).
The case of a patient with metastatic breast cancer
with bleeding caused by hyperfibrinolysis as the first symptom is
discussed in the present study. The detailed case history,
diagnosis, treatment process and follow-up of this case are
reported to provide a reference for the clinical detection and
diagnosis of tumor-related coagulation dysfunction. The case is
presented in accordance with the CARE reporting checklist. The
literature is also reviewed with regard to hyperfibrinolysis in
patients with breast cancer or other solid malignant neoplasms.
Information was compiled by searching for the role of fibrinolytic
function in tumorigenesis and metastasis.
Case report
A 52-year-old woman was admitted to the First
Hospital of Jilin University (Changchun, China) in February 2018
due to bleeding as a result of hyperfibrinolysis. There was no
history of neoplastic disease. Laboratory results were significant
for hyperfibrinolysis: Thrombin time was 15.2 sec (normal range,
11.0-17.8 sec), prothrombin time (PT) was 14.9 sec (normal range,
9.0-13.0 sec) and activated partial thromboplastin time (APTT) was
36.6 sec (normal range, 20.0-40.0 sec), while the fibrinogen level
was very low at 0.5 g/l (normal range, 1.8-4.0 g/l), the D-dimer
assay result was 7,070 µg/l (normal range, 0–232 µg/l) and the
fibrin (fibrinogen) degradation products (FDP) level was 129.4
µg/ml (normal range, 0–5 µg/ml). Meanwhile, the blood routine
suggested anemia and thrombocytopenia [red blood cell count,
2.41×1012/l (normal range, 3.8-5.1×1012/l);
hemoglobin level, 77 g/l (normal range, 115–150 g/l); platelet
count, 62×109/l (normal range,
125–350×109/l); and white blood cell count,
4.22×109/l (normal range, 3.5-9.5×109/l)]. A
bone marrow biopsy revealed abnormal cell clusters, and the
pathological report revealed primary tumors of the breast, with
estrogen receptor (ER)-positive (+80%), progesterone receptor
(PR)-negative and Ki-67 (+20%) results by immunohistochemistry.
Human epidermal growth factor receptor 2 (HER2) testing was not
performed due to limited specimen availability. A mammogram and
breast MRI revealed a mass in the upper outer quadrant of the left
breast, ~1.8×1.4 cm in size, of Breast Imaging-Reporting and Data
System category 4C (10) (Fig. 1A). The systemic evaluation suggested
metastatic cancer in liver segments S5 and S8 (liver function tests
showed no significant abnormalities) and multiple bone metastases
throughout the body. The diagnosis was infiltrating ductal
carcinoma with immunohistochemical expression of ER (+90%), PR
(+45%), Ki-67 (+35%), but not HER-2, as indicated by breast mass
puncture pathology. The TNM stage was stage IV (cT1N0M1) according
to the 8th edition of the American Joint Committee on Cancer
staging manual (11). Genetic
testing suggested no treatment-related genetic variations.
Fibrinogen and blood transfusions were administered during the
course of the disease to improve the patient's coagulation function
based on the patient's condition.
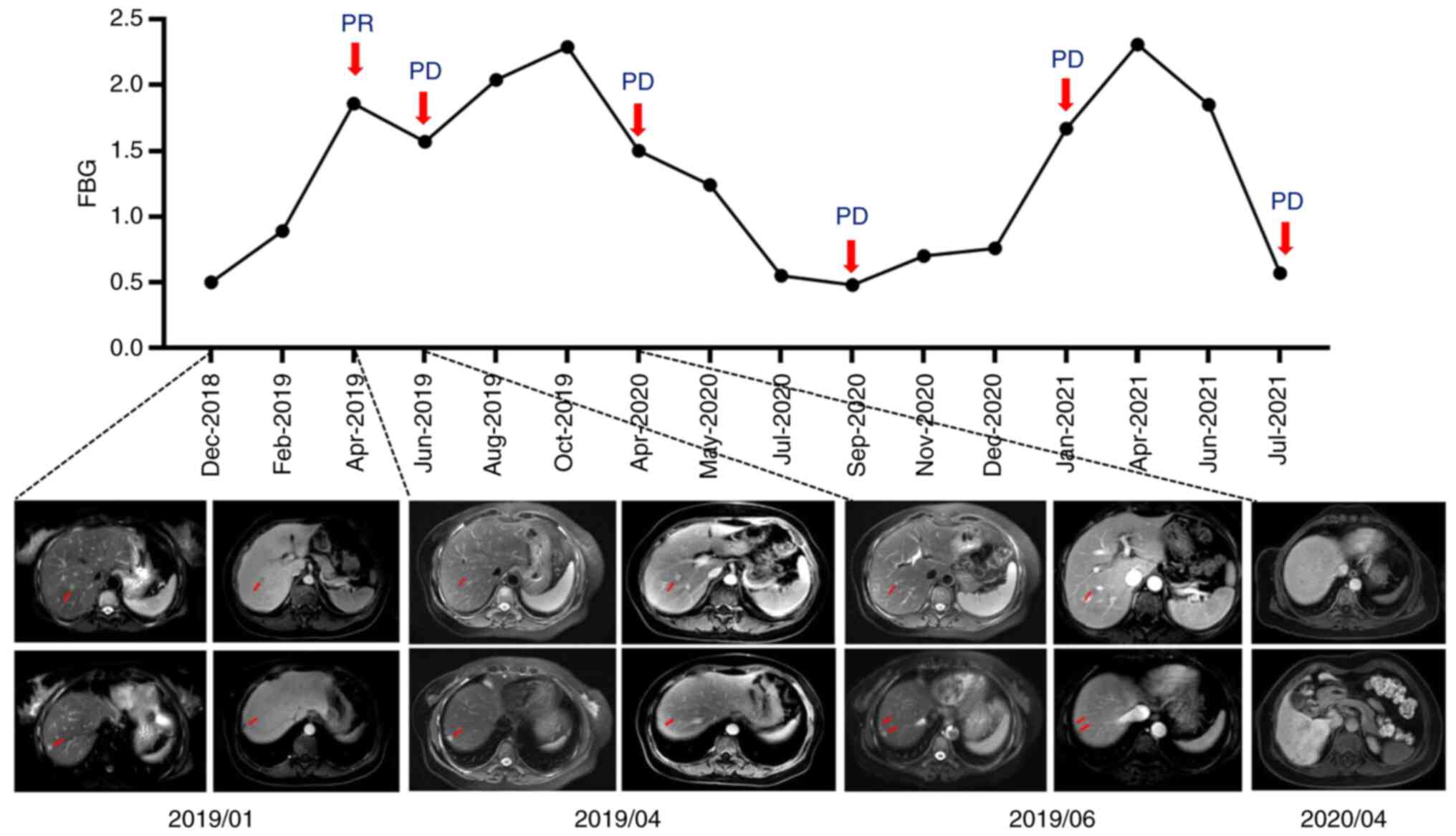
In January 2019, the patient was administered 200 mg
nab-paclitaxel weekly as first-line treatment (Fig. 2). After 4 cycles (3 months) of
chemotherapy, the liver metastases had shrunk (from 1.1 to 0.5 cm;
Fig. 3) and the breast lesions had
shrunk (from 1.8×1.4 to 0.3×0.4 cm) compared with previously; the
evaluation of the response was of a partial response. Meanwhile,
the blood routine suggested that the bone marrow suppression due to
bone marrow metastasis had improved (red blood cell count,
3×1012/l; hemoglobin level, 94 g/l; platelet count,
139×109/l; and white blood cell count,
4.93×109/l) and liver function tests showed no
significant abnormalities. After 7 cycles of nab-paclitaxel
treatment (5 months) in total, the breast lesions were still
enlarged (0.6×0.3 cm; Fig. 1B), and
new S8 liver lesions appeared (Fig.
3), suggesting disease progression. Blood routine analysis
indicated a decrease in white blood cells and platelets compared
with previously (red blood cell count, 2.94×1012/l;
hemoglobin level, 90 g/l; white blood cell count,
3.33×109/l; and platelet count, 121×109/l).
The progression-free survival (PFS) time was 5 months. A decrease
in neutrophil count was recorded as a grade 2 adverse event in this
period, and no grade 3–4 adverse events occurred, based on National
Cancer Institute Common Terminology Criteria for Adverse Events
version 5.0 (12). During
treatment, the hemogram values were higher than before, and
hyperfibrinolysis was relieved.
Second-line treatment for the patient was
palbociclib and letrozole. Oral letrozole (2.5 mg) was administered
once daily for 28 days and palbociclib (125 mg) once daily for 21
days. Each cycle was a 28-day cycle. After 2 months of treatment,
the evaluation of response was of stable disease (SD). At this
time, the patient's ancillary tests suggested a decrease in the
number of red blood cells, white blood cells and platelets (red
blood cell count, 2.79×1012/l; hemoglobin level, 90 g/l;
white blood cell count, 1.74×109/l; platelet count,
34×109/l), and liver function tests showed no
significant abnormalities. The patient's coagulation function and
imaging assessment were not abnormal, so the changes in blood
routine were considered to be treated-related adverse effects. The
patient received palliative surgery for the breast cancer. The
pathological report revealed that only a few invasive ductal
carcinomas remained in the breast tissue, with a maximum diameter
of 0.2 cm, no cancer invasion of the vessels and nerves, and no
cancer metastasis in the axillary lymphoid tissue (0/19). The
patient continued to receive palbociclib and letrozole treatment at
the same dose for 6 months after surgery. In April 2020, imaging
suggested an increased number of liver metastases compared with
before (the liver function tests showed no significant
abnormalities). The PFS time was 10 months. During this period, due
to the antitumor drugs, the patient experienced a decrease in
neutrophil count and platelet count, which were recorded as grade 3
adverse events. This improved with granulocyte colony-stimulating
factor administration and platelet transfusion after
discontinuation, based on the patient's condition.
Third-line treatment for the patient was
capecitabine chemotherapy. Oral capecitabine (1,500 mg) was
administered twice daily for 14 days. Each cycle was a 21-day
cycle. After 4 cycles of treatment, the liver metastases showed no
significant change compared with before, while the evaluation of
response was of SD. At this time, the patient's blood routine
suggested a decrease in the number of red blood cells and platelets
(red blood cell count, 2.3×1012/l; hemoglobin, 87 g/l;
and platelet count, 57×109/l), and liver function tests
showed no significant abnormalities. The patient exhibited a
progressively decreased level of fibrinogen, and physical
examination showed skin ecchymosis at the right chest wall port,
lower limbs and buttocks, and atypical cell clusters on bone marrow
puncture. Laboratory tests showed a fibrinogen level of 0.38 g/l,
which was considered progression of the bone marrow metastasis and
aggravation of the hyperfibrinolysis. The third-line treatment
produced a PFS time of 5 months. Aminocaproic acid antifibrinolytic
therapy, a fibrinogen intravenous drip, thrombopoietin and other
symptomatic treatments were administered to the patient based on
the patient's condition.
Fourth-line treatment was eribulin chemotherapy (2
mg on day 1 and 8, every 3 weeks). After 4 cycles of treatment, the
liver metastases were not significantly changed compared with
previously, while the evaluation of response was of SD. The patient
had poor disease control due to the progressive decline in
fibrinogen level and the hyperfibrinolysis aggravation, with a PFS
time of 5 months. Meanwhile, the patient's blood routine suggested
a decrease in the number of red blood cells, white blood cells and
platelets (red blood cell count, 2.62×1012/l; hemoglobin
level, 95 g/l; white blood cell count, 1.31×109/l; and
platelet count, 47×109/l), and liver function tests
showed liver injury caused by liver metastases and antitumor
therapy (aspartate aminotransferase, 127.9 U/l; alanine
transaminase, 54.6 U/l; γ-glutamyl transferase, 335.3 U/l; and
blood bilirubin, 43.1 µmol/l).
In February 2021, the patient started to receive
fulvestrant as endocrine therapy (500 mg administered
intramuscularly every 4 weeks after an initial 2-week induction).
After 4 cycles of treatment, the liver metastases showed no
significant change from previously. Routine blood and liver
function tests indicated that the degree of bone marrow suppression
and liver damage was improved. In July 2021, the patient
experienced subcutaneous bleeding in the lower abdomen and left
lower extremity, with a low platelet count and fibrinogen level,
which was considered as aggravated hyperfibrinolysis (fibrinogen,
0.84 g/l; platelet count, 29×109/l). The patient died
due to a cerebral hemorrhage in September 2021. The overall
survival time of the patient was 30 months.
Discussion
Paraneoplastic syndrome (PNS) refers to a series of
diseases caused by malignant tumors that are not associated with
direct tumor invasion; it is caused by immune cross-reactivity
between tumor-produced bioactive substances (e.g., hormones,
peptides and cytokines) and normal tissues (13). PNS may affect multiple organ systems
throughout the body, especially the endocrine, nervous, rheumatic
and hematological systems. Some patients with malignant tumors such
as small cell lung cancer often present with PNS as the first
manifestation before the diagnosis of the tumor is confirmed
(14). Correct identification of
PNS can allow the timely diagnosis of the primary disease and avoid
a missed diagnosis. The present study reports a case of metastatic
breast cancer with hemorrhage as the first manifestation, and
acquired hyperfibrinolysis, which is a rare paraneoplastic
phenomenon in breast cancer.
Hyperfibrinolysis is uncommon in clinical practice,
and in most cases is secondary to severe diseases such as DIC,
liver disease and trauma. Hyperfibrinolysis is divided into the
primary and secondary types. Primary hyperfibrinolysis refers to
the release of plasminogen activators (t-PA and u-PA) into the
blood under the condition of basically normal coagulation function,
which promotes the activation of plasminogen to become plasmin or
decreases PAI-1 and TAFI levels, increases the activity of plasmin
and finally leads to hyperfibrinolysis (1,6,15).
Secondary hyperfibrinolysis, on the other hand, refers to extensive
microthrombosis in the setting of abnormal coagulation, such as
DIC, leading to the generation of large amounts of coagulant active
substances and excessive consumption of hemostatic components,
followed by activation of the fibrinolytic system (16). Hyperfibrinolysis is mainly
manifested as bleeding, such as skin petechiae, ecchymosis, wounds,
wound bleeding, and in severe cases, hematemesis, hematochezia,
intracranial hemorrhage and other manifestations.
In the present case, hyperfibrinolysis was the first
manifestation, and fibrinogen levels were examined. Combined with
the results of a bone marrow aspiration examination, the findings
revealed that the possible cause of thrombocytopenia was bone
marrow hematopoietic suppression caused by bone marrow metastasis
of breast cancer. The patient showed no abnormality in liver
function and had no history of hepatitis or liver cirrhosis.
Decreased fibrinogen synthesis caused by liver disease could
therefore be excluded. PT and APTT were approximately normal, and
D-dimer and FDP levels were significantly increased. At the same
time, active antitumor therapy and antifibrinolytic therapy
significantly improved the fibrinogen levels, which also provided
strong evidence for the diagnosis of hyperfibrinolysis. Low-grade
DIC occurs in patients with extensive systemic metastases and may
be one of the causes of hyperfibrinolysis (17). Although there was ample evidence
that the cause of bleeding symptoms in the present patient was
tumor-associated hyperfibrinolysis, hyperfibrinolysis is uncommon
in association with solid tumors (18).
The mechanism of tumor-induced primary
hyperfibrinolysis is not fully understood. Possible causes include:
i) Tumor cells themselves can produce proteins during fibrinolysis,
such as u-PA and PAI-1 (19,20).
Urinary tract and genital tract tumors are rich in u-PA, which
releases large amounts of u-PA into the blood during surgical or
traumatic injuries and triggers primary hyperfibrinolysis (21). Winther-Larsen et al (18) systematically evaluated
hyperfibrinolysis in 21 patients with malignant solid tumors, with
prostate cancer (76%) being the most common type, while there have
been few reports of hyperfibrinolysis in patients with breast
cancer (17,21). Breast cancer cells contain abundant
plasminogen activators (t-PA and u-PA), and with the progression of
tumors, plasminogen activator in tumor cells is released into the
blood in large amounts, causing enhanced fibrinolytic system
function (22–24). ii) The tumor cell membrane also
carries a specific u-PA-receptor (u-PAR), which contributes to the
assembly of fibrinolytic components and promotes the activation of
the fibrinolytic cascade (24,25).
In addition, the fibrinolytic system plays an
important role in the process of tumor invasion and metastasis
(19,26). Studies found that u-PA and u-PAR
levels were significantly higher in patients with breast cancer
with regional lymph node metastasis and other organ metastasis
(27,28). The invasion of breast cancer cells
is closely associated with u-PA and u-PAR activity, and is an
independent risk factor. Levels of u-PA in tumor specimens can also
be used to assess prognosis in breast cancer (27). Distant metastases have already
developed in 85% of patients at the time of a hyperfibrinolysis
diagnosis (18). u-PA is involved
in multiple stages of tumor formation and development through the
regulation of invasion, metastasis and cell adhesion (29). u-PA effectively degrades the
extracellular matrix and basement membrane by binding to specific
receptors (u-PAR) on the surface of tumor cells and activating the
formation of plasmin (30). At the
same time, u-PA and u-PAR form complexes with vitronectin and
integrins, which can promote the adhesion of tumor cells to the
extracellular matrix and promote cell proliferation by binding to
G-protein-coupled receptors (19,31).
PAI-1 can inhibit apoptosis and improve the survival rate of tumor
cells (32). Therefore,
hyperfibrinolysis can reduce the adhesion and stability of tumor
cells, and promote tumor metastasis.
Bone marrow metastases from solid tumors are common
in breast, prostatic and gastric adenocarcinomas, and can lead to
hematological disorders such as anemia, leukopenia and
thrombocytopenia (33,34). For patients with hormone
receptor-positive and HER2-negative metastatic breast cancer with
visceral crisis such as bone marrow metastasis, chemotherapy is the
primary means of treatment (35),
and its purpose is to rapidly control the tumor burden and provide
opportunities for subsequent antitumor therapy. Combined endocrine
therapy with CDK4/6 inhibitors is a promising regimen after
effective control of tumor burden by chemotherapy. In the present
study, the administration of palbociclib combined with letrozole
after nab-paclitaxel monotherapy for the patient resulted in a PFS
time of 10 months, which was significantly longer than the PFS time
after the other treatments, such as chemotherapy or single drug
endocrine therapy The overall survival time was also longer than
the average of 19 months recorded previously in patients with bone
marrow metastases (33,34). This result may be associated with
the application of CDK4/6 inhibitors combined with endocrine
therapy. There are also studies on the clinical attempts using
CDK4/6 inhibitors combined with endocrine therapy as first-line
treatment for patients with metastatic breast cancer with hormone
receptor-positive, HER2-negative and visceral crisis (34).
In conclusion, patients with breast cancer who
exhibit bleeding as the first symptom are rare, and attention
should be focused on them in the process of diagnosis and treatment
to avoid misdiagnosis or a missed diagnosis. Early recognition and
appropriate treatment can improve the clinical symptoms and
prognosis, which improves the quality of life. For patients with
cancer and acquired hyperfibrinolysis, in addition to the
inhibition fibrinolytic therapy, the active treatment of the
primary disease and the effective control of the tumor burden while
experiencing hyperfibrinolysis can also be improved. Fibrinogen
levels can be used as biomarkers for efficacy prediction and
specifically reflect the tumor burden and disease changes in
patients. This process may be associated with u-PA secreted by
breast malignancies. Furthermore, for patients with
HR-positive/HER2-negative breast cancer with a visceral crisis such
as bone marrow metastasis, CDK4/6 inhibitors combined with
endocrine therapy can potentially improve the survival time and
quality of life, and provide evidence for subsequent treatment
strategies for patients with a visceral crisis.
Acknowledgements
Not applicable.
Funding
This case report was funded by the National Natural Science
Youth Foundation of China (grant no. 82001670).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
GL and RY were involved in the identification and
selection of patient cases and drafted the manuscript. LJ and ZL
reviewed and edited the manuscript. GL, LJ, RY, ZL and JC were
involved in the patient's clinical management. LJ, ZL and JC were
involved in the identification, selection and management of patient
cases, and reviewed and edited the manuscript. GL, ZL and JC
confirm the authenticity of all the raw data. All authors
contributed to the article and read and approved the final
version.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants followed the ethical standards of the institutional
and/or national research committee and the 1964 Helsinki
Declaration and its later amendments or comparable ethical
standards. This case report was approved by The Ethics Committee of
the First Hospital of Jilin University (Changchun, China).
Patient consent for publication
The patient's family provided oral consent for the
article and accompanying images to be published. The Ethics
Committee of the First Hospital of Jilin University approved that
oral consent was sufficient in this case report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kolev K and Longstaff C: Bleeding related
to disturbed fibrinolysis. Br J Haematol. 175:12–23. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Longstaff C and Kolev K: Basic mechanisms
and regulation of fibrinolysis. J Thromb Haemost. 13 (Suppl
1):S98–S105. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rijken DC and Lijnen HR: New insights into
the molecular mechanisms of the fibrinolytic system. J Thromb
Haemost. 7:4–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lin H, Xu L, Yu S, Hong W, Huang M and Xu
P: Therapeutics targeting the fibrinolytic system. Exp Mol Med.
52:367–379. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chapin JC and Hajjar KA: Fibrinolysis and
the control of blood coagulation. Blood Rev. 29:17–24. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Franchini M and Mannucci PM: Primary
hyperfibrinolysis: Facts and fancies. Thromb Res. 166:71–75. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rein-Smith CM and Church FC: Emerging
pathophysiological roles for fibrinolysis. Curr Opin Hematol.
21:438–444. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leebeek FW and Rijken DC: The fibrinolytic
status in liver diseases. Semin Thromb Hemost. 41:474–480. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rabizadeh E, Cherny I, Lederfein D,
Sherman S, Binkovsky N, Rosenblat Y and Inbal A: The cell-membrane
prothrombinase, fibrinogen-like protein 2, promotes angiogenesis
and tumor development. Thromb Res. 136:118–124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vanel D: The American college of radiology
(ACR) breast imaging and reporting data system (BI-RADS): A step
towards a universal radiological language? Eur J Radiol.
61:1832007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Plichta JK, Ren Y, Thomas SM, Greenup RA,
Fayanju OM, Rosenberger LH, Hyslop T and Hwang ES: Implications for
breast cancer restaging based on the 8th edition AJCC staging
manual. Ann Surg. 271:169–176. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
National Institutes of Health, National
Cancer Institute, U.S. Department of Health and Human Services, .
Common Terminology Criteria for Adverse Events (CTCAE) version 5.0.
https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5×11.pdfPublished
November 27, 2017. June 20–2020
|
|
13
|
Pelosof LC and Gerber DE: Paraneoplastic
syndromes: An approach to diagnosis and treatment. Mayo Clin Proc.
85:838–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soomro Z, Youssef M, Yust-Katz S, Jalali
A, Patel AJ and Mandel J: Paraneoplastic syndromes in small cell
lung cancer. J Thorac Dis. 12:6253–6263. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franchini M, Zaffanello M and Mannucci PM:
Bleeding disorders in primary fibrinolysis. Int J Mol Sci.
22:70272021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hunt BJ: Bleeding and coagulopathies in
critical care. N Engl J Med. 370:21532014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Naina HVK, Patnaik MM, Ali UA, Chen D and
Ashrani AA: Systemic fibrinolysis caused by tissue plasminogen
activator-producing metastatic breast cancer. J Clin Oncol.
28:e167–e168. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Winther-Larsen A, Sandfeld-Paulsen B and
Hvas AM: Hyperfibrinolysis in patients with solid malignant
neoplasms: A systematic review. Semin Thromb Hemost. 47:581–588.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kwaan HC and Lindholm PF: Fibrin and
fibrinolysis in cancer. Semin Thromb Hemost. 45:413–422. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cesarman-Maus G and Hajjar KA: Molecular
mechanisms of fibrinolysis. Br J Haematol. 129:307–321. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sacco E, Pinto F, Sasso F, Racioppi M,
Gulino G, Volpe A and Bassi P: Paraneoplastic syndromes in patients
with urological malignancies. Urol Int. 83:1–11. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Look MP, van Putten WL, Duffy MJ, Harbeck
N, Christensen IJ, Thomssen C, Kates R, Spyratos F, Fernö M,
Eppenberger-Castori S, et al: Pooled analysis of prognostic impact
of urokinase-type plasminogen activator and its inhibitor PAI-1 in
8377 breast cancer patients. J Natl Cancer Inst. 94:116–128. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ruszkowska-Ciastek B, Kwiatkowska K,
Bielawska S, Robakowska M, Bielawski K and Rhone P: Evaluation of
the prognostic value of fibrinolytic elements in invasive breast
carcinoma patients. Neoplasma. 67:1146–1156. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wrzeszcz K, Słomka A, Zarychta E, Rhone P
and Ruszkowska-Ciastek B: Tissue plasminogen activator as a
possible indicator of breast cancer relapse: A preliminary,
prospective study. J Clin Med. 11:23982022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kulić A, Cvetković Z and Libek V: Primary
hyperfibrinolysis as the presenting sign of prostate cancer: A case
report. Vojnosanit Pregl. 73:877–880. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Stephens RW, Brünner N, Jänicke F and
Schmitt M: The urokinase plasminogen activator system as a target
for prognostic studies in breast cancer. Breast Cancer Res Treat.
52:99–111. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Duffy MJ, McGowan PM, Harbeck N, Thomssen
C and Schmitt M: uPA and PAI-1 as biomarkers in breast cancer:
validated for clinical use in level-of-evidence-1 studies. Breast
Cancer Res. 16:4282014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malmström P, Bendahl PO, Boiesen P,
Brünner N, Idvall I and Fernö M; South Sweden Breast Cancer Group,
: S-phase fraction and urokinase plasminogen activator are better
markers for distant recurrences than nottingham prognostic index
and histologic grade in a prospective study of premenopausal lymph
node-negative breast cancer. J Clin Oncol. 19:2010–2019. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Duffy MJ: The urokinase plasminogen
activator system: Role in malignancy. Curr Pharm Des. 10:39–49.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kyyriäinen J, Bolkvadze T, Koivisto H,
Lipponen A, Pérez LO, Ekolle Ndode-Ekane X, Tanila H and Pitkänen
A: Deficiency of urokinase-type plasminogen activator and its
receptor affects social behavior and increases seizure
susceptibility. Epilepsy Res. 151:67–74. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Resnati M, Pallavicini I, Wang JM,
Oppenheim J, Serhan CN, Romano M and Blasi F: The fibrinolytic
receptor for urokinase activates the G protein-coupled chemotactic
receptor FPRL1/LXA4R. Proc Natl Acad Sci USA. 99:1359–1364. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kortlever RM, Higgins PJ and Bernards R:
Plasminogen activator inhibitor-1 is a critical downstream target
of p53 in the induction of replicative senescence. Nat Cell Biol.
8:877–884. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kopp HG, Krauss K, Fehm T, Staebler A,
Zahm J, Vogel W, Kanz L and Mayer F: Symptomatic bone marrow
involvement in breast cancer-clinical presentation, treatment, and
prognosis: A single institution review of 22 cases. Anticancer Res.
31:4025–4030. 2011.PubMed/NCBI
|
|
34
|
Garufi G, Carbognin L, Orlandi A, Palazzo
A, Tortora G and Bria E: The therapeutic challenge of disseminated
bone marrow metastasis from HR-positive HER2-negative breast
cancer: Case report and review of the literature. Front Oncol.
11:6517232021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gradishar WJ, Anderson BO, Abraham J, Aft
R, Agnese D, Allison KH, Blair SL, Burstein HJ, Dang C, Elias AD,
et al: Breast cancer, version 3.2020, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 18:452–478. 2020.
View Article : Google Scholar : PubMed/NCBI
|