Introduction
A pituitary adenoma is a benign neuroendocrine tumor
that originates from the adenohypophysis and accounts for 10–20% of
all primary intracranial tumors (1,2).
According to the diameter of the tumor, pituitary adenomas are
divided into micro-adenoma, macro-adenoma and giant adenoma
(3). Among them, giant pituitary
adenoma refers to a pituitary adenoma with a diameter >4 cm,
accounting for 6–10% of all pituitary adenomas (4). Due to its large size, the giant
pituitary adenoma can confer a series of clinical symptoms on
patients, such as headaches, dizziness, amaurosis, vision loss and
endocrine abnormalities, among others (5). In addition, giant pituitary adenomas
can be divided into functional adenomas and non-functional adenomas
(6,7). For some functional giant pituitary
adenomas, such as prolactinomas and growth hormone adenomas,
symptomatic treatment to reduce prolactin and growth hormone levels
can be adopted (8). However, once
drug treatment is not effective, surgical treatment should be
considered. For other types of functional giant pituitary adenomas
and non-functional giant pituitary adenomas, surgical treatment is
considered as the first-line treatment (9,10).
Giant pituitary adenomas tend to extend to multiple anatomical
compartments, often enclose neurovascular structures and show high
invasiveness (11). Due to the
characteristics of a huge volume and extracellular extension,
surgical resection of giant pituitary adenomas is still a challenge
(12). Generally, the surgical
strategy can be divided into single surgery and staged surgery. It
has been reported that radical resection of giant pituitary
adenomas through a single surgery is achieved in less than
one-third of cases (13). Staged
surgery can greatly improve the tumor resection rate, even up to
100% (14). Furthermore, irregular
giant pituitary adenomas have unique shapes and locations, which
further increases the difficulty of surgery and the risk of
postoperative complications. Therefore, for irregular giant
pituitary adenomas, it is necessary to customize personalized
staged surgery. The present study reports two cases of giant
pituitary adenomas with an irregular growth shape and position,
respectively. To achieve the radical resection of the tumor, the
staged surgery was customized according to the location and shape
of the tumor, specifically, resection of most of the tumor by
first-stage surgery using the microscopic transcranial approach,
followed by the total resection of the residual tumor by
second-stage surgery using the endoscopic transsphenoidal
approach.
Case report
Case 1
A 51-year-old man was admitted to the Department of
Neurosurgery of Chongqing General Hospital (Chongqing, China) in
August 2015 due to memory loss for 2 months. No obvious
abnormalities were found in terms of other clinical symptoms or on
physical examination. Brian MRI revealed a large space-occupying
lesion in the sellar region, and the tumor grew from the sellar
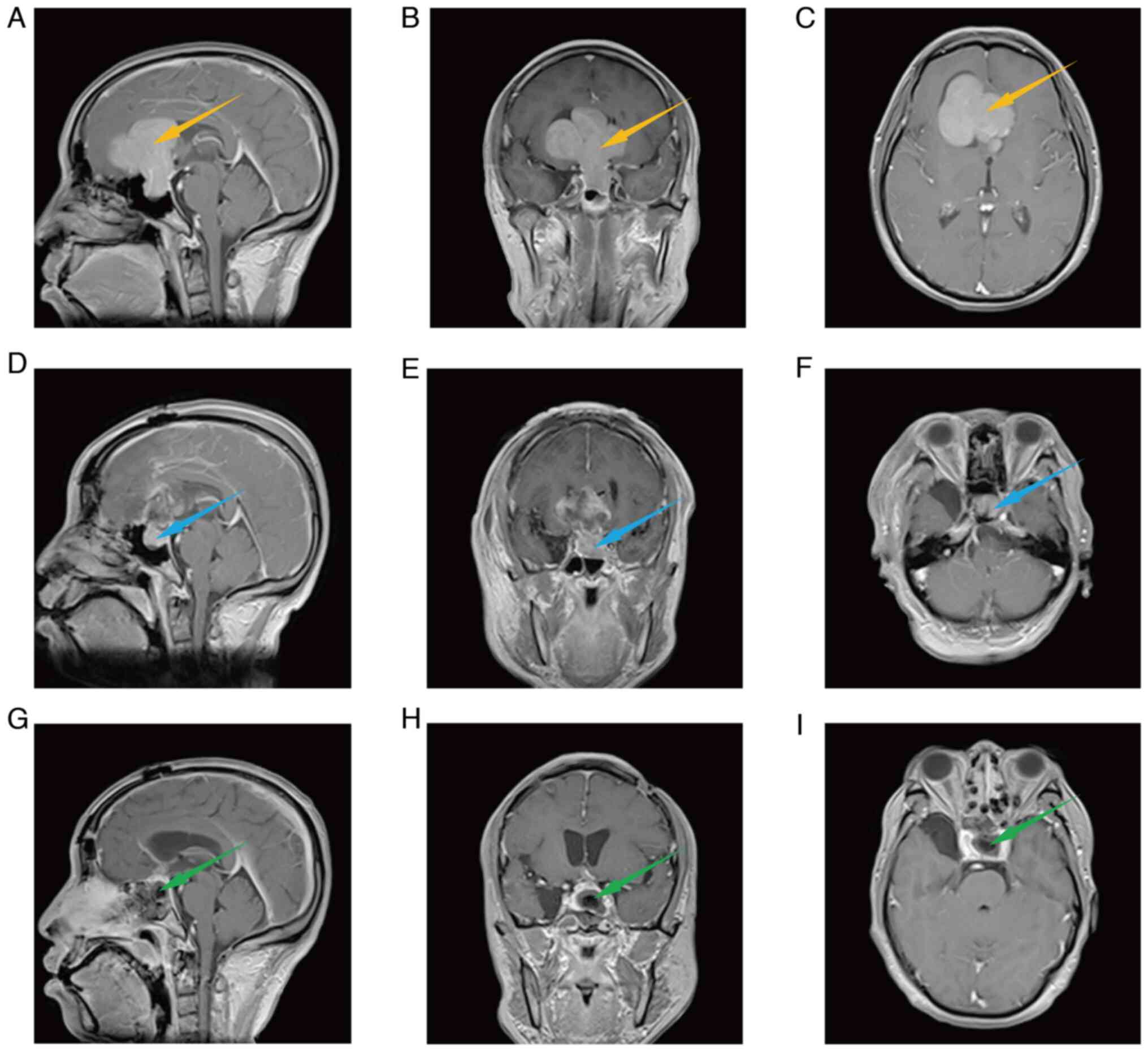
region to the right lateral ventricle. Fig. 1A-C shows the axial, coronal and
sagittal MRI, indicating that the size of the tumor was
6.15×6.11×5.69 cm and the shape of the tumor was paginated. The
tumor had the characteristics of a huge volume, a high probability
of adhesion with surrounding tissues (such as arteries, optic
nerves and optic chiasma) and an irregular shape, leading to large
blind areas of the visual field, all of which would make it
difficult to achieve a total tumor resection through a single
operation and greatly increase the incidence of postoperative
complications. Therefore, two-stage surgery was planned to totally
remove the irregular giant pituitary adenoma. In the first-stage
operation, most of the tumor was removed by the microscopic
transcranial approach, while in the second-stage operation, the
residual tumor was removed by the endoscopic transsphenoidal
approach.
In the first stage of the operation, the right
frontal dura was radially cut, the right frontal lobe was
decompressed and the tumor was exposed. It was observed that the
tumor adhered to the right optic nerve and the tumor base was
located within the sellar septum. After the tumor parenchyma had
been exposed to the surgical vision and was removed, part of the
tumor capsule remained and this was repeatedly burned by
electrocoagulation. A tumor cavity measuring 5.00×4.00×5.00 cm was
formed. After the bleeding had completely stopped, the tumor cavity
was filled with absorbable hemostatic yarn, the dura was repaired
using artificial dura mater, the titanium mesh was cut and used to
repair bone holes, the bone flap was reset and fixed, and the
muscles, cap aponeurosis and scalp were repaired. Fig. 1D-F shows the sagittal, coronal and
transverse cranial MRI after the first stage of surgery, from which
it can be seen that most of the tumors have been removed. However,
some residual tumor was left in the sellar region, as forcibly
removing it would cause postoperative complications, such as
cerebrospinal fluid rhinorrhea. The patient developed transient
diabetes insipidus and hyponatremia on the 6th day after the
operation. After symptomatic treatment, urine output and
electrolyte levels returned to normal. After the first stage of the
operation, the patient's memory improved slightly. There were no
other symptoms or discomfort between the first-stage operation and
the second-stage operation.
After 1 year, the patient came to the hospital again
for the second-stage operation. The residual tumor was completely
removed through the endoscopic transsphenoidal approach. After the
dura mater at the sellar base was cut, the tumor was exposed, and
the residual tumor tissue was separated and removed along the
normal pituitary interface. During the operation, the tumor was
found to be slightly firm with some chylous necrosis. Fig. 1G-I shows the brain MRI after the
second-stage operation, indicating that the residual tumor was
totally removed. Postoperative complications, such as diabetes
insipidus, electrolyte imbalance and pituitary dysfunction, did not
occur. During the follow-up of 4 years, there was no recurrence. In
addition, a telephone follow-up was conducted 6 years after the
second-stage operation, and the patient stated that their memory
had improved further.
Case 2
A 60-year-old man who had suffered from intermittent
dizziness for 10 years and paroxysmal amaurosis for 1 year was
admitted to the Department of Neurosurgery of Chongqing General
Hospital in February 2019. The intermittent dizziness occurred
mainly in the afternoon without obvious triggers, while the
bilateral paroxysmal amaurosis occurred 1–2 times per month and
lasted for only seconds. The patient received a brain MRI
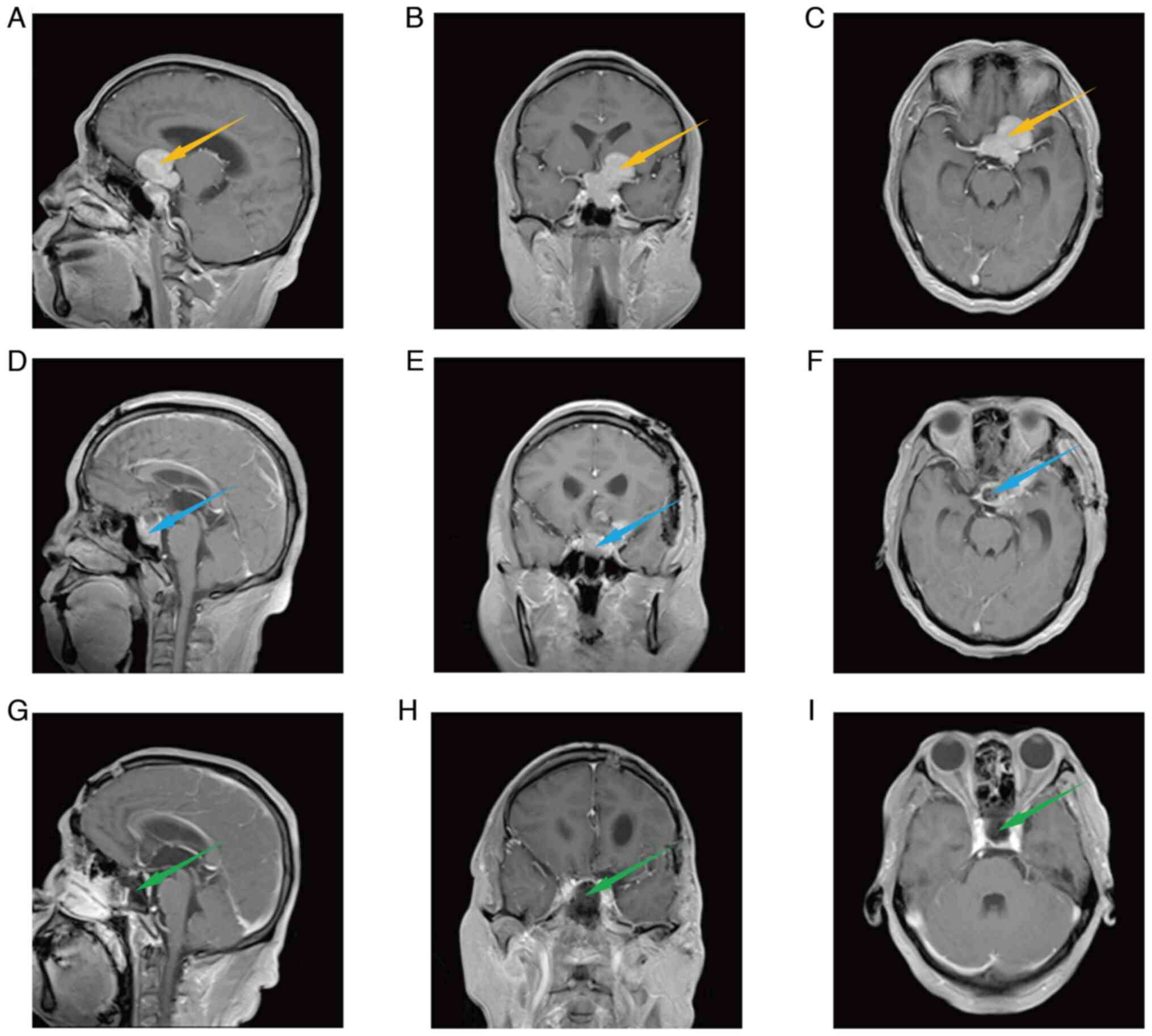
examination, which revealed a 4.35×3.96×3.07-cm lesion in the
sellar region, as shown in Fig.
2A-C. The lesion was deep and broke through the sellar septum.
Furthermore, the tumor showed lateral eccentric growth. Due to the
special location and huge size of the tumor, there was a blind area
in the surgical visual field, which would make it difficult to
totally remove the tumor in a single operation. Therefore, for this
giant pituitary adenoma with lateral eccentric growth, staged
surgery was planned to achieve a complete resection of the tumor.
Specifically, the first-stage operation was microscopic, to achieve
a total resection of the suprasellar tumor, and the second-stage
operation was endoscopic transsphenoidal surgery to achieve a total
resection of the residual tumor.
In the first stage of surgery, the dura mater was
radially opened and suspended to release the cerebrospinal fluid.
After the intracranial pressure decreased to a certain extent, the
frontal lobes were raised to expose the tumor. The tumor's surface
was gray and slightly adhered to the surrounding tissues. As the
tumor was lifted, it was observed that the tumor grew from the
sellar region to the intracranial region through between the optic
nerve and the internal carotid artery on the left. The capsule was
cut, and the tumor parenchyma had a tough texture. During the
operation, it was observed that the tumor compressed the left optic
nerve and internal carotid artery, and adhered to the branches of
the basilar artery, failing to totally separate the tumor from the
surrounding normal tissues. The intracranial tumor was nearly
totally removed, with only a little residual tumor capsule. It is
worth noting that to reduce brain tissue traction, the residual
intrasellar tumor was left for the second stage of surgery. The
brain MRI after the first stage of surgery showed that the
intracranial tumor had been nearly totally removed, with residual
intrasellar tumor tissue, as shown in Fig. 2D-F. On the 4th day after the
operation, the patient developed transient diabetes insipidus and
recovered after symptomatic treatment. After the first stage of the
operation, the patient reported that the paroxysmal amaurosis had
been relieved but that slight intermittent dizziness still
existed.
After 6 months, the patient was admitted to the
hospital for the second stage of surgery. In the second stage of
the operation, the endoscope was placed into the right nasal
cavity, the opening of the sphenoid sinus was found under the
endoscope, the sphenoid sinus and sellar bone were ground open, the
dura was exposed and the dura was cut with a sharp knife. After
opening the dura, the residual tumor was observed to be dark
yellow-white and hard, as shown in Fig.
3A. The intrasellar tumor was separated along the normal
structure of the left cavernous sinus. After scraping off part of
the tumor, scar hyperplasia was observed at the sellar septum, as
shown in Fig. 3B. Then, the tumor
on the scar was removed to achieve gross total resection (GTR) of
the tumor. Fig. 3C and D shows the
tumor at the stump scar and tumor cavity. Video S1, Video S2, Video S3 and Video S4 are representative surgical
videos, which correspond to the surgical procedures shown in
Fig. 3A, B, C and D, respectively.
The brain MRI after the second stage of surgery indicated that the
intrasellar tumor had also been totally removed, as shown in
Fig. 2G-I. The patient developed
transient diabetes insipidus on the second day after the operation
and recovered after symptomatic treatment. Overall, through the
staged surgery, the giant irregular pituitary adenoma, including
the intracranial and intrasellar regions, had successfully
undergone GTR without serious postoperative complications. During
the follow-up period of 10 months, there was no recurrence.
Moreover, a telephone follow-up that was conducted 3 years after
the second-stage operation revealed that the patient's dizziness
and amaurosis had disappeared.
Both patients had a good prognosis during long-term
follow-up.
Discussion
Due to its large size and irregular growth, a giant
pituitary adenoma can cause obvious clinical symptoms such as
visual field defects, headaches, dizziness and abnormal hormone
levels, with the most common clinical symptoms caused by
non-secretory giant pituitary adenomas being visual impairment and
visual field defects, followed by headaches (15). Although very rare, giant pituitary
adenomas may also sometimes compress the third ventricle, which may
lead to the accumulation of cerebrospinal fluid in the lateral
ventricle, hydrocephalus and increased intracranial pressure,
resulting in nausea, vomiting and other symptoms (16,17).
Therefore, it is necessary to treat giant pituitary adenomas to
improve the quality of life of the patients. At present, for giant
prolactinomas and giant growth hormone adenomas, drug therapy can
be used. For other giant pituitary adenomas, surgery is the first
choice.
At present, giant pituitary adenomas are mainly
removed by a single surgery via a single surgical approach or a
combined surgical approach. The common surgical approaches are the
transsphenoidal and transcranial approaches, both of which have
advantages and limitations (18).
Generally, the transsphenoidal approach is considered to be the
best method for resection of intrasellar pituitary adenomas, but
due to the limitations of a deep and narrow working space, the
resection of giant pituitary adenomas is still controversial
(11). The transcranial approach is
usually used to remove the lateral or complex extension of the
tumor, which provides more opportunities for a radical resection of
the tumor, but also increases the risk of complications and causing
higher damage to the surrounding tissue structure (19). Previous studies have shown that
single surgery with a single surgical approach (transsphenoidal or
transcranial) for giant pituitary adenomas not only has a low GTR
rate (44%), but also has very high postoperative complication rate
(11%) (20–24). The low GTR rate of single surgery
with a single surgical approach may be mainly due to the limited
visual field of the microscope and endoscope. To expand the visual
field and improve the GTR rate, the combined surgical approaches
(transsphenoidal + transcranial or transcranial + transcranial) can
be adopted in a single operation (25,26).
The main advantage of this is that the tumor can be operated on
simultaneously from two approaches, so that the hidden part of the
tumor that cannot be seen or touched in the single approach can
still be removed (27–29). Compared with that of a single
transcranial approach or single transsphenoidal approach, the GTR
rate of the combined surgical approaches can be improved to a
certain extent (30). However, the
cost of improving the GTR rate using a single surgery with combined
surgical approaches is an increase in the risk of cerebrospinal
fluid leakage. Moreover, this type of surgery requires two surgical
teams, two sets of operation equipment and a large operation room,
which means that the medical cost and operation difficulty are
increased. Therefore, overall, for giant pituitary adenomas, the
GTR rates of a single operation via transcranial approach,
transsphenoidal approach and combined surgical approaches are still
relatively low, but the medical cost and surgical difficulty are
increased. Moreover, the GTR of giant tumors may also introduce
additional trauma to the patients, including damage to the
surrounding normal tissues, massive intraoperative bleeding, an
increased possibility of postoperative complications and a longer
healing time. To avoid these risk factors, it is not recommended to
totally remove the tumor in a single operation, and the staged
operation is advocated.
Staged surgery refers to performing a first-stage
operation and then a later second-stage operation, in which the
specific surgical approach depends on the location of the tumor and
the habits of the surgeon. If the transsphenoidal approach is
selected for the primary operation, the degree of tumor resection
will be small, resulting in an increased probability of
complications such as hydrocephalus and cerebrospinal fluid
rhinorrhea. Therefore, transcranial surgery is recommended for the
first stage of surgery to achieve large-scale resection of the
tumor and to reduce the possibility of hydrocephalus, while
endoscopic transsphenoidal surgery is recommended for the second
stage of surgery to remove the residual tumor. In the present
study, 2 patients with giant pituitary adenomas underwent staged
surgery, and GTR of the tumor was achieved. The resection of most
of the tumor was achieved through the first stage of microscopic
transcranial surgery, while GTR was achieved through the second
stage of endoscopic transsphenoidal surgery. After the operation,
there were no obvious complications except temporary diabetes
insipidus. Case 1 was followed up for 4 years without any
complications or tumor recurrence. Case 2 was followed up for 10
months without any complications or tumor recurrence. Therefore,
for irregular giant pituitary adenomas, staged surgery (first-stage
transcranial surgery and second-stage transsphenoidal surgery) can
not only improve the GTR rate but also reduce postoperative
complications and ensure the quality of life of patients.
In conclusion, the present study retrospectively
analyzed two patients with irregular giant pituitary adenoma who
underwent staged surgery to achieve total tumor resection without
postoperative complications. Furthermore, staged surgery is not
limited to the removal of irregular giant pituitary adenomas, but
can also be performed for other irregular giant brain tumors.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CT, JWW, PW, DWZ and NW participated in the
conception, design and data acquisition of the paper. CT
participated in drafting and revising the manuscript. JWW
critically revised the paper. NW ensured that questions related to
the integrity of any part of the work were appropriately
investigated and resolved. CT, JWW, PW, DWZ and NW confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
Chongqing General Hospital (Chongqing, China).
Patient consent for publication
Written informed consent was obtained from the
patients for the publication of anonymized data and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen Y, Wang CD, Su ZP, Chen YX, Cai L,
Zhuge QC and Wu ZB: Natural history of postoperative nonfunctioning
pituitary adenomas: A systematic review and meta-analysis.
Neuroendocrinology. 96:333–342. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ostrom QT, Gittleman H, Truitt G, Boscia
A, Kruchko C and Barnholtz-Sloan JS: CBTRUS statistical report:
Primary brain and other central nervous system tumors diagnosed in
the United States in 2011–2015. Neuro Oncol. 20 (suppl_4):iv1–iv86.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Raghib MF, Salim A, Angez M, Ghazi SM,
Hashmi S, Tariq MB, Hashmi F, Anis SB, Shamim MS, Tanwir A and Enam
SA: Prognostic implication of size on outcomes of pituitary
macroadenoma: A comparative analysis of giant adenoma with
non-giant macroadenoma. J Neurooncol. 160:491–496. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Iglesias P, Rodriguez Berrocal V and Diez
JJ: Giant pituitary adenoma: Histological types, clinical features
and therapeutic approaches. Endocrine. 61:407–421. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koutourousiou M, Gardner PA,
Fernandez-Miranda JC, Paluzzi A, Wang EW and Snyderman CH:
Endoscopic endonasal surgery for giant pituitary adenomas:
Advantages and limitations. J Neurosurg. 118:621–631. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Asa SL, Mete O, Perry A and Osamura RY:
Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr
Pathol. 33:6–26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Iglesias P, Arcano K, Trivino V,
Guerrero-Pérez F, Rodríguez Berrocal V, Vior C, Cordido F,
Villabona C and Díez JJ: Giant non-functioning pituitary adenoma:
Clinical characteristics and therapeutic outcomes. Exp Clin
Endocrinol Diabetes. 129:309–313. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Koylu B, Firlatan B, Sendur SN, Oguz SH,
Dagdelen S and Erbas T: Giant growth hormone-secreting pituitary
adenomas from the endocrinologist's perspective. Endocrine. Nov
1–2022.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tang OY, Hsueh WD, Eloy JA and Liu JK:
Giant pituitary adenoma-special considerations. Otolaryngol Clin
North Am. 55:351–379. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Micko A, Agam MS, Brunswick A, Strickland
BA, Rutkowski MJ, Carmichael JD, Shiroishi MS, Zada G, Knosp E and
Wolfsberger S: Treatment strategies for giant pituitary adenomas in
the era of endoscopic transsphenoidal surgery: A multicenter
series. J Neurosurg. 136:776–785. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mortini P, Barzaghi R, Losa M, Boari N and
Giovanelli M: Surgical treatment of giant pituitary adenomas:
Strategies and results in a series of 95 consecutive patients.
Neurosurgery. 60:993–1002; discussion 1003–1004. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oruçkaptan HH, Senmevsim Ö, Özcan OE and
Özgen T: Pituitary adenomas: Results of 684 surgically treated
patients and review of the literature. Surg Neurol. 53:211–219.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nishioka H, Hara T, Nagata Y, Fukuhara N,
Yamaguchi-Okada M and Yamada S: Inherent tumor characteristics that
limit effective and safe resection of giant nonfunctioning
pituitary adenomas. World Neurosurg. 106:645–652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
D'Ambrosio AL, Syed ON, Grobelny BT, Freda
PU, Wardlaw S and Bruce JN: Simultaneous above and below approach
to giant pituitary adenomas: Surgical strategies and long-term
follow-up. Pituitary. 12:217–225. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsuyama J, Kawase T, Yoshida K, Hasegawa
M, Hirose Y, Nagahisa S, Watanabe S and Sano H: Management of large
and giant pituitary adenomas with suprasellar extensions. Asian J
Neurosurg. 5:48–53. 2010.PubMed/NCBI
|
|
16
|
Joshi SM, Chopra IS and Powell M:
Hydrocephalus caused by giant pituitary tumors: Case series and
guidelines for management. Br J Neurosurg. 23:30–32. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koktekir E, Karabagli H and Ozturk K:
Simultaneous transsphenoidal and transventricular endoscopic
approaches for giant pituitary adenoma with hydrocephalus. J
Craniofac Surg. 26:e39–e42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Buchfelder M, Schlaffer SM and Zhao Y: The
optimal surgical techniques for pituitary tumors. Best Pract Res
Clin Endocrinol Metab. 33:1012992019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Buchfelder M and Kreutzer J: Transcranial
surgery for pituitary adenomas. Pituitary. 11:375–384. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goel A, Nadkarni T, Muzumdar D, Desai K,
Phalke U and Sharma P: Giant pituitary tumors: A study based on
surgical treatment of 118 cases. Surg Neurol. 61:436–445;
discussion 445–436. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
van Lindert EJ, Grotenhuis JA and Meijer
E: Results of follow-up after removal of non-functioning pituitary
adenomas by transcranial surgery. Br J Neurosurg. 5:129–133. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Takakura K and Teramoto A: Management of
huge pituitary adenomas. Acta Neurochir Suppl. 65:13–15.
1996.PubMed/NCBI
|
|
23
|
Elshazly K, Kshettry VR, Farrell CJ,
Nyquist G, Rosen M and Evans JJ: Clinical outcomes after endoscopic
endonasal resection of giant pituitary adenomas. World Neurosurg.
114:e447–e456. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Komotar RJ, Starke RM, Raper DM, Anand VK
and Schwartz TH: Endoscopic skull base surgery: A comprehensive
comparison with open transcranial approaches. Br J Neurosurg.
26:637–648. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Greenfield JP, Leng LZ, Chaudhry U, Brown
S, Anand VK, Souweidane MM and Schwartz TH: Combined simultaneous
endoscopic transsphenoidal and endoscopic transventricular
resection of a giant pituitary macroadenoma. Minim Invasive
Neurosurg. 51:306–309. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nishioka H, Hara T, Usui M, Fukuhara N and
Yamada S: Simultaneous combined supra-infrasellar approach for
giant/large multilobulated pituitary adenomas. World Neurosurg.
77:533–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Alleyne CH Jr, Barrow DL and Oyesiku NM:
Combined transsphenoidal and pterional craniotomy approach to giant
pituitary tumors. Surg Neurol. 57:380–390; discussion 390. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ojha BK, Husain M, Rastogi M, Chandra A,
Chugh A and Husain N: Combined trans-sphenoidal and simultaneous
trans-ventricular-endoscopic decompression of a giant pituitary
adenoma: Case report. Acta Neurochir (Wien). 151:843–847;
discussion 847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maesawa S, Fujii M, Nakahara N, Watanabe
T, Wakabayashi T and Yoshida J: Intraoperative tractography and
motor evoked potential (MEP) monitoring in surgery for gliomas
around the corticospinal tract. World Neurosurg. 74:153–161. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kuga D, Toda M, Ozawa H, Ogawa K and
Yoshida K: Endoscopic endonasal approach combined with a
simultaneous transcranial approach for giant pituitary tumors.
World Neurosurg. 121:173–179. 2019. View Article : Google Scholar : PubMed/NCBI
|