Introduction
Cervical cancer has the fourth highest incidence and
mortality among gynecological neoplasms worldwide. In 2018, the
annual estimates for cervical cancer were ~570,000 new cases and
311,000 mortalities (1). However,
this neoplasm affects each country according to its degree of
economic development, social factors and lifestyle, and is an
imminent and serious crisis for developing countries. In Mexico
from 2011 to 2015, the mortality rate for cervical cancer was 6.45
per 100,000 women (2), indicating
that it a highly prevalent health issue. Furthermore, the use of
screening programs based on cytology, known as pap smear testing,
has not been successful in developing countries due to the poor
quality of the tests resulting in high rates of false negatives
(3,4). Additionally, patients frequently first
present at health centers with advanced lesions and are diagnosed
with locally advanced stages IB2 to IVA according to the FIGO
classification (5). The suggested
treatment comprises cisplatin (CDDP)-based chemotherapy concomitant
with radiation therapy plus brachytherapy, which represents the
standard of care in patients with locally advanced disease
(6–9). The average prognosis for 5-year
survival is 56% (5,10).
Although patients with a poor response to standard
treatment are treated with secondary systemic therapies (7,8), there
is no standard treatment for patients with progressive or
metastatic cervical cancer due to its heterogeneous manifestations
(11). Notably, chemotherapeutic
treatments for cervical cancer have shown limited success due to
the lack of specificity associated with systemic administration. In
addition, higher doses are required to achieve a therapeutic
effect, which increases the adverse cytotoxic effects that
exacerbate those of the first treatment and may reduce the physical
integrity of the patient; therefore, survival is limited. The
resistance of cancer cells to physical and chemical methods, low
efficiency of drug delivery and highly heterogeneous tumor
microenvironments represent significant impediments in clinical
oncology. Furthermore, even when drug administration is optimized,
the efficiency of chemotherapy has several challenges, one of which
is the typical hypovascularization of cervical cancer tissues
(12), which reduces the efficiency
of systemic drug distribution. The cellular origin of cervical
cancer also contributes to the development and diversity of the
tumor microenvironment, which creates different obstacles to drug
transport, even in tumors of the same size and stage. Additionally,
it has been shown that the density of the tumor cells and formation
of intercellular junctions serve key roles in the pharmacokinetics
of chemotherapeutic agents in solid tumors (13).
Nano-oncology is a subdivision of nanomedicine in
which nanotechnology is used in the treatment of cancer (14,15).
Specific delivery strategies for anticancer agents have been
developed, generally in the nanoscale range, using materials such
as organic nanoparticles made from lipids, polymers, liposomes,
polymeric micelles, dendrimers and engineered peptides and nucleic
acids, and inorganic nanoparticles such as carbon, metal and metal
oxide nanoparticles (16,17). Nanomaterials have distinctive
physical, chemical and optical properties and may be modified with
biological molecules to direct them toward specific targets. In
this regard, membrane receptors and their ligands have great
relevance as biomarkers and therapeutic targets in the treatment of
different neoplasms. The insulin-like growth factor (IGF) system
has been reported in epithelial and glandular tumors, including
prostate cancer, breast cancer and colon cancer, and is an
excellent target for nano-oncology (18–20).
The present review provides a brief overview of the
IGF system, its relevance in cervical cancer and the development of
new nanotechnology-based therapies targeting IGF complex molecules
for the treatment of cervical cancer.
IGF axis
The IGF system is a complex network comprising
growth factors IGF-1 and −2, cell surface receptors IGF-1R and −2R,
the IGF binding protein (IGFBP) family of high-affinity specific
binding proteins (IGFBP-1 to −6) and IGFBP proteases, as well as
molecules that interact with IGFBP to regulate and disseminate the
actions of IGF in tissues (21).
IGFs are peptide hormones from a family that also includes insulin.
While the main role of IGF-2 is as a regulator of embryonic and
fetal development, IGF-1 is maintained throughout life as a
broad-spectrum growth factor (22).
These factors bind with a specific receptor on the cell surface and
stimulate different signaling pathways.
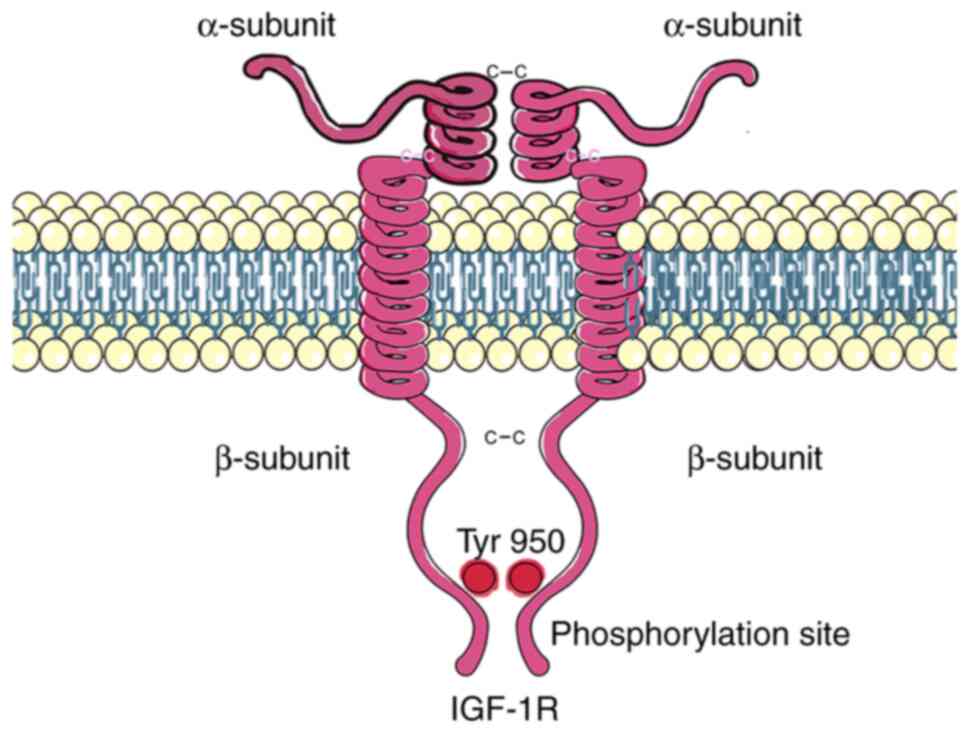
The sequence of IGF-1R has 60% homology with that of
the insulin receptor. It is generated as a polypeptide precursor
that is post-translationally modified by glycosylation, proteolytic
cleavage and dimerization to form a heterotetramer comprising two α
subunits and two β subunits bonded together via α-α and β-β
disulfide linkages. The α subunits are located outside the cell and
contain the ligand binding site, while the β subunits have
extracellular and transmembrane domains and an intracellular
portion that contains the tyrosine kinase catalytic domain
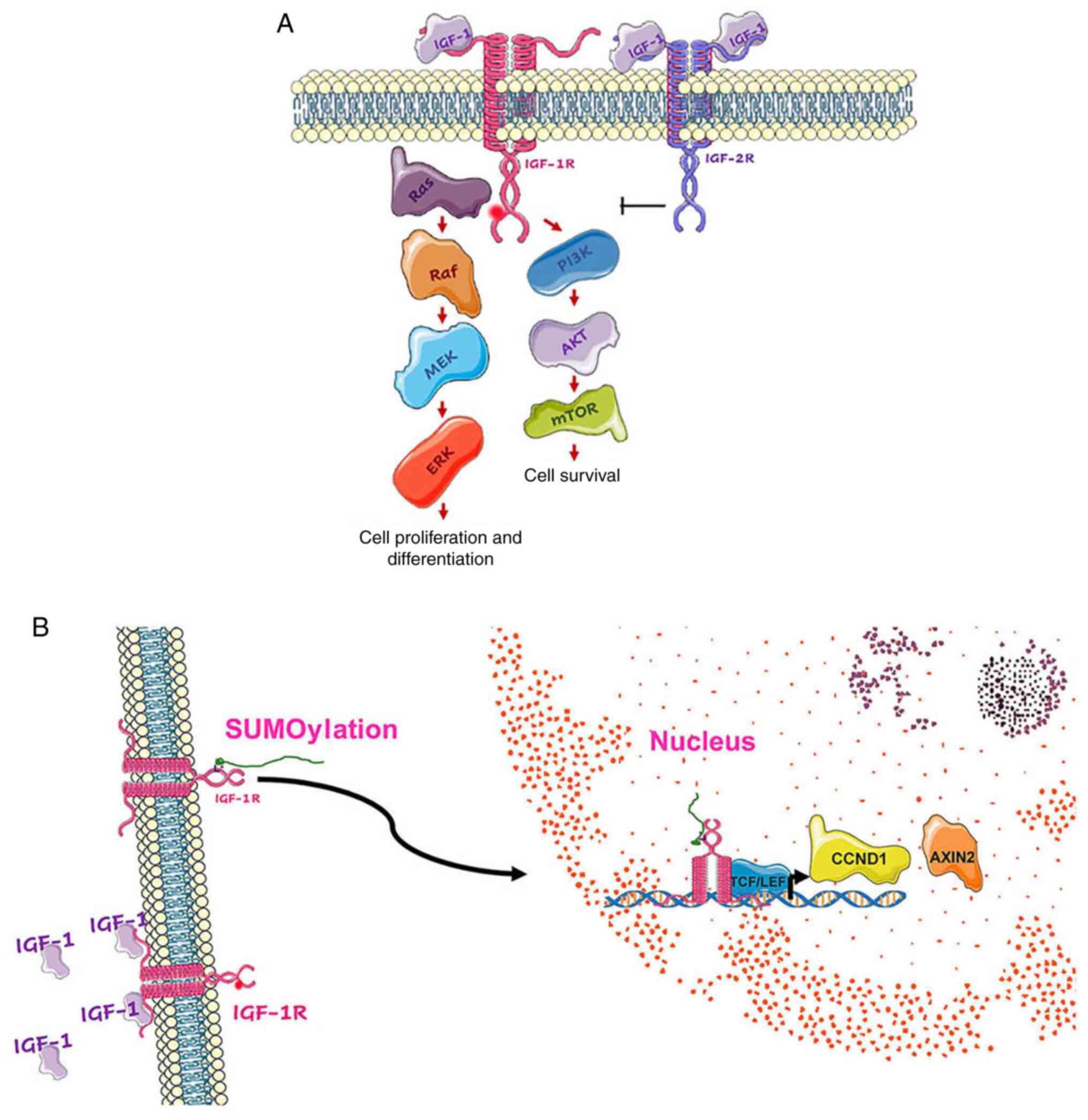
(Fig. 1) (23). The binding of an IGF ligand to its
receptor activates the tyrosine kinase domain, which induces a
conformational change allowing autophosphorylation at the
Tyr950 site. This phosphorylation site is a docking
point for substrates such as the insulin receptor substrate 1–4
proteins, where activation of the PI3K/Akt/mTOR and Ras/Raf/MEK/ERK
signaling pathways occurs. The former regulates cell survival and
protein synthesis, while the latter regulates gene expression, cell
proliferation and differentiation (Fig.
2A) (24). IGF-1R has a
tyrosine-kinase domain, whereas IGF-2R does not. Due to this fact
and its high affinity for the IGF-2 ligand, it is proposed that the
function of IGF-2R is to limit the interaction of IGF-2 with
IGF-1R, thereby acting as a tumor suppressor (25,26).
The covalent attachment of a small ubiquitin-like modifier (SUMO)
family protein to three lysine residues in the b-subunit of IGF-1R
via SUMOylation induces its translocation to the nucleus in a
ligand-independent manner after (27). In the nucleus, IGF-1R and T cell
factor/lymphoid enhancer factor act as transcriptional coactivators
to increase the promoter activity and expression of downstream
target genes, including cyclin D1 and Axin2 (Fig. 2B), which promote cell cycle
progression (19,27).
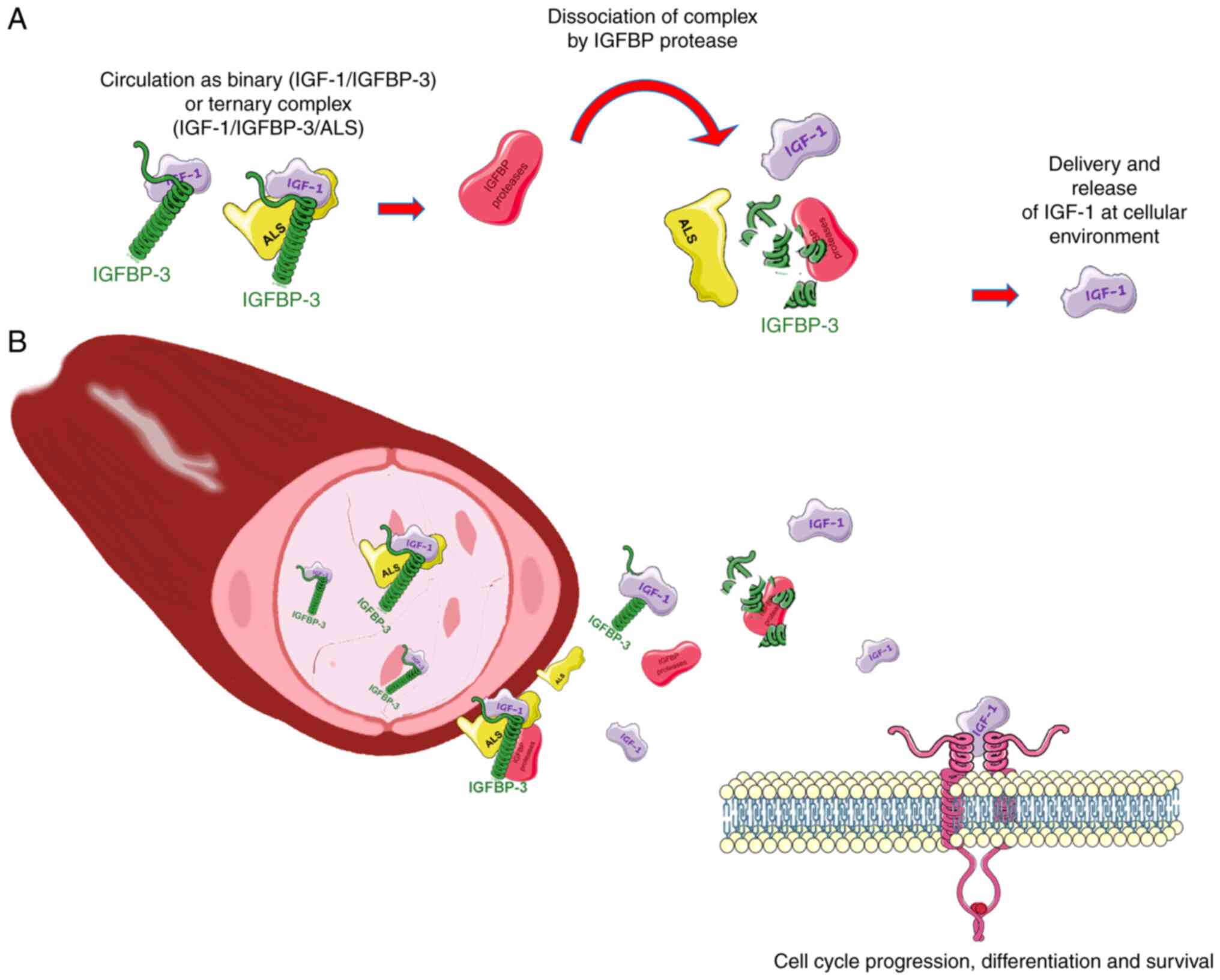
The actions of IGFs are regulated by interaction
with soluble IGFBPs and IGFBP proteases. The IGFBP family comprises
six IGFBPs that bind to IGF with high affinity and specificity, and
IGFBP-related proteins that are structurally similar to IGFBPs but
have lower IGF-binding affinity (22). The six IGFBP proteins are
structurally similar to each other but not to cell surface
receptors. Each of these binding proteins is the product of
different genes and has different functional properties; however,
they are all mostly present as high-molecular-weight complexes with
IGF-1 and IGF-2 in the circulation and extracellular space, for
example, as ~150-kDa complexes with IGFBP-3 and the acid-labile
subunit (28,29). These complexes inhibit extravascular
transit and help to retain the IGF-1 ligand in the circulation. The
IGFBP proteases are critical for modulating the availability of
IGF-1 at the cellular level and regulating its half-life via the
degradation of IGF-1-containing complexes (Fig. 3A). The dynamic balance of IGF-1,
IGFBP and IGFBP proteases constitutes the IGF-1 axis that
ultimately determines the extent of the cellular effects dependent
on this hormone (30–32). Following dissociation of the ternary
complex, the IGFBP/IGF binary complexes are cleared from the
circulation via the endothelium, from where they are delivered to
tissues and interact with cell surface receptors (Fig. 3B). Since the binding affinity of
IGFs for IGFBPs is higher than that for their receptors, IGFBPs in
tissues inhibit the interaction of IGF with its receptors and
thereby regulate the action of IGF, promoting a microenvironment
that functions as a reservoir for the slow release of ligands. This
prolongs the half-life of IGFs in the circulation and prevents them
from crossing the capillary barrier (28,33).
IGFBP-3 is the most abundant binding protein in human serum; it is
present in several glycosylated forms weighing between 40 to 44 kDa
and has been shown to regulate the apoptosis induced by p53
(34).
IGF axis in cervical cancer
Human papillomavirus (HPV) infection is the primary
etiological factor of cervical carcinogenesis (35). The HPV E6 and E7 viral proteins
serve well-established oncogenic functions: E6 binds to p53 in a
trimeric complex with E6-associated protein, a ubiquitin-ligase,
which induces the degradation of p53 in proteasomes (36), while E7 binds to hypophosphorylated
retinoblastoma-associated protein (pRB), which is rapidly degraded
by proteasomes and constitutively releases the transcription factor
E2F (37,38). The HPV-induced loss of function of
these two tumor suppressors is a fundamental cause of cervical
cancer carcinogenesis. However, additional elements are involved in
the inactivation of p53 and pRB (39).
Studies have demonstrated the relationships between
viral proteins and members of the IGF system during the neoplastic
process. In a study conducted by Kuramoto et al (39), it was shown that the expression of
IGF-1R is gradually upregulated in cervical intraepithelial
neoplasia (CIN) 3 and invasive cancer lesions while its expression
is moderate in CIN 1 and 2. The study also suggested that the viral
oncoprotein E6 represses p53 and causes transcriptional
dysregulation by activating the upregulation of the expression of
this receptor. Furthermore, it confirmed that the phosphorylation
of IGF-1R increases as the disease progresses. The phosphorylation
of IGF-1R activates the MAPK (Ras/Raf/MEK/ERK) and PI3K survival
signaling pathways, which contribute to cell survival and drug
resistance and thereby serve an important role in progression of
the neoplasia (39). It has also
been observed in other human neoplasms, including clear cell kidney
cancer, colorectal carcinoma and pediatric glioma, that the nuclear
translocation of IGF-1R is associated with advanced disease and
poor prognosis (19,40). In the study of Codony-Servat et
al (19), it was observed that
the treatment of patients with metastatic colorectal cancer using
IGF-1R blocking antibodies induced an increase in nuclear
translocation, suggesting that receptor nuclear sequestration may
contribute to resistance. In another study, in which the
upregulation of IGF-1R was shown to be associated with resistance
to radiotherapy in patients with HPV-16-positive cervical cancer,
IGF-1R was proposed as a predictive biomarker of the response to
radiation (41). Similarly, in a
recent study IGF-2R was proposed as a poor prognostic biomarker for
patients with cervical cancer since it may be involved in the
recurrence of the disease. In that study, Takeda et al
(42) describe an oncogenic
mechanism of IGF-2R, in which it participates in the regulation of
lysosomal transport via Golgi bodies, together with cathepsins B
and L loaded with mannose-6-phosphate, resulting in increased
lysosomal homeostasis and decreased apoptosis. Thus, IGF-2R appears
to have a dual oncogenic role in cervical cancer.
The upregulation of the IGF receptors in tumors
resistant to radiation therapy indicates that they are potential
targets for alternative therapies. Furthermore, the expression of
ligands of the IGF system has been reported in different events
that contribute to the pathogenesis and progression of various
neoplasms (43,44). In non-small cell lung cancer, a
study reported that the expression of IGF-1 and IGF-1R was
upregulated and associated with progression and poor prognosis, and
suggested that the autocrine/paracrine activity of IGF-1 may play
an important role in the development of lung cancer (45). In cervical cancer, a review of the
IGF axis indicated that the presence of IGF-1 may contribute to
each stage of tumor progression, from malignant transformation,
tumor growth, local invasion, distal metastasis and resistance to
treatment (46). Elevated levels of
IGF-1 and IGF-2 promote signaling via the stimulation of IGF-1R in
cervical cancer from the CIN phase (47,48),
with a dose-dependent effect on the growth and invasiveness of
tumor cells, mainly mediated by IGF-1. Furthermore, an unexpected
role of IGF-1 as a stimulator of the invasion and proliferation of
cervical cells through interaction with IGF-1R with the cooperation
of integrin αvβ3 has been reported (49). It is important to note that
relatively low IGF-2 mRNA levels have been reported in primary
tumor samples and cervical tumor cell models (29,50,51).
Therefore, it appears that the production of IGF-2 by cervical
epithelial cells is insufficient to transduce a strong mitogenic
signal. Nevertheless, Steller et al (50) proposed that the autocrine function
of IGF-2 in cervical cancer cells involves the mitogenic signaling
of epidermal growth factor (EGF).
Studies on IGF-binding proteins in cervical
neoplasia have mainly reported on IGFBP-2 and −3. The role of
IGFBP-2 in tumorigenesis is complex and multifaceted, as it can
both promote and suppress tumors. The prolonged expression of HPV16
E6 and E7 suppresses IGFBP-2 expression; IGFBP-2 generally inhibits
the actions of IGF and thereby inhibits mitogenesis,
differentiation, survival and other cellular processes, which may
be due to the ability of IGFBP-2 to compete with IGF-1R or −2R for
the binding of IGF-1 or −2 ligands (47,52).
However, IGFBP-2 has also been demonstrated to interact with
integrins to exert oncogenic effects that promote cell
proliferation and invasion and suppress apoptosis. Specifically,
studies have shown that IGFBP-2 is associated with metastasis and
uses integrin-dependent mechanisms to reduce cell adhesion and
promote invasion, suggesting that IGFBP-2 has IGF-independent
oncogenic effects (52–54). By contrast, IGFBP-3 is known to
protect against cancer via the p53-mediated activation of
apoptosis. However, IGFBP-3 upregulation is a late event after
E6/E7 expression in infected cells, after which E6 inhibits p53
activity and consequently blocks apoptosis (55). Additionally, E7 impedes the ability
of IGFBP-3 to induce apoptosis. This appears to be mediated via the
binding of E7 to the nuclear localization sequence of IGFBP-3 in
the nucleus, which reduces the half-life of nuclear IGFBP3 and
subsequently induces the polyubiquitination and proteolysis of
IGFBP-3 in cervical cancer cells (28,56).
However, the functions of IGFBP-3 in the nucleus are not clearly
understood, although it may regulate transcription and modify
cellular functions through intranuclear pathways (57). Notably, a study of 226 patients
found that a high nuclear concentration of IGFBP-3 was a powerful
predictor of recurrence in prostate cancer (57,58).
IGF axis members as therapeutic targets in
cervical cancer
As explained above, the components of the IGF system
are activated in an aberrant way during carcinogenesis and,
importantly, the expression of certain components confers
resistance to the treatments used for this neoplasia, making them a
key target for new therapeutic strategies. Several approaches have
been used to target components of the IGF system, in particular
IGF-1R, due to its involvement in cancer cell growth. These include
interference RNAs, antisense oligonucleotides and RNAs, triple
helix-forming oligonucleotides, specific kinase inhibitors, single
chain antibodies and humanized anti-IGF-1R monoclonal antibodies.
Tyrosine kinase inhibitors and monoclonal antibodies are among the
most useful; they include ganitumab (AMG-479), dalotuzumab
(MK-0646), cixutumumab (IMC-A12), teprotumumab (R1507) and
figitumumab (CP-751,871), which are fully human recombinant
monoclonal antibodies commonly used to target IGF-1R. They prevent
IGF-1 from binding to IGF-1R and inhibit downstream signaling via
the PI3K/Akt pathway (18,59–63).
The PI3K/Akt pathway is known to promote cell growth and survival
in response to extracellular signals. However, a study
investigating advances in the treatment of solid tumors with these
IGF-1R inhibitory antibodies, alone or in combination with other
therapies, revealed they had non-significant effects on overall
survival and progression-free survival, and furthermore, adverse
effects were observed for dalotuzumab in the breast, colorectal and
prostate cancer subgroups (63).
Although monoclonal antibodies are highly selective, their
development as therapeutic agents is challenging due to their poor
tumor penetration and high production costs (28).
Extracellular domain of IGF-1R used as a
trap nanoparticle
The action of cell surface receptors can be
effectively blocked via the use of soluble decoys that specifically
bind to a ligand with high affinity, thereby limiting the
bioavailability of the ligand and the signaling it would otherwise
mediate at the membrane receptor (64,65).
Furthermore, other studies have demonstrated that the efficiency of
these decoys is significantly improved by the addition of the Fc
domain of human IgG1 to form a more stable chimeric
protein known as a ‘Trap’. Specific Traps have been used to treat
various diseases, including rheumatoid arthritis (66), cryopyrin-associated periodic
syndromes (67), wet macular
degeneration and metastatic colorectal cancer (65). In addition, an EGFR-Fc fusion decoy
comprising the truncated extracellular domains of EGFR/ErbB-1 and
ErbB-4 was shown to have high affinity for EGF-like growth factor
and inhibit the proliferation, invasion and metastasis of breast
cancer cells (64,68).
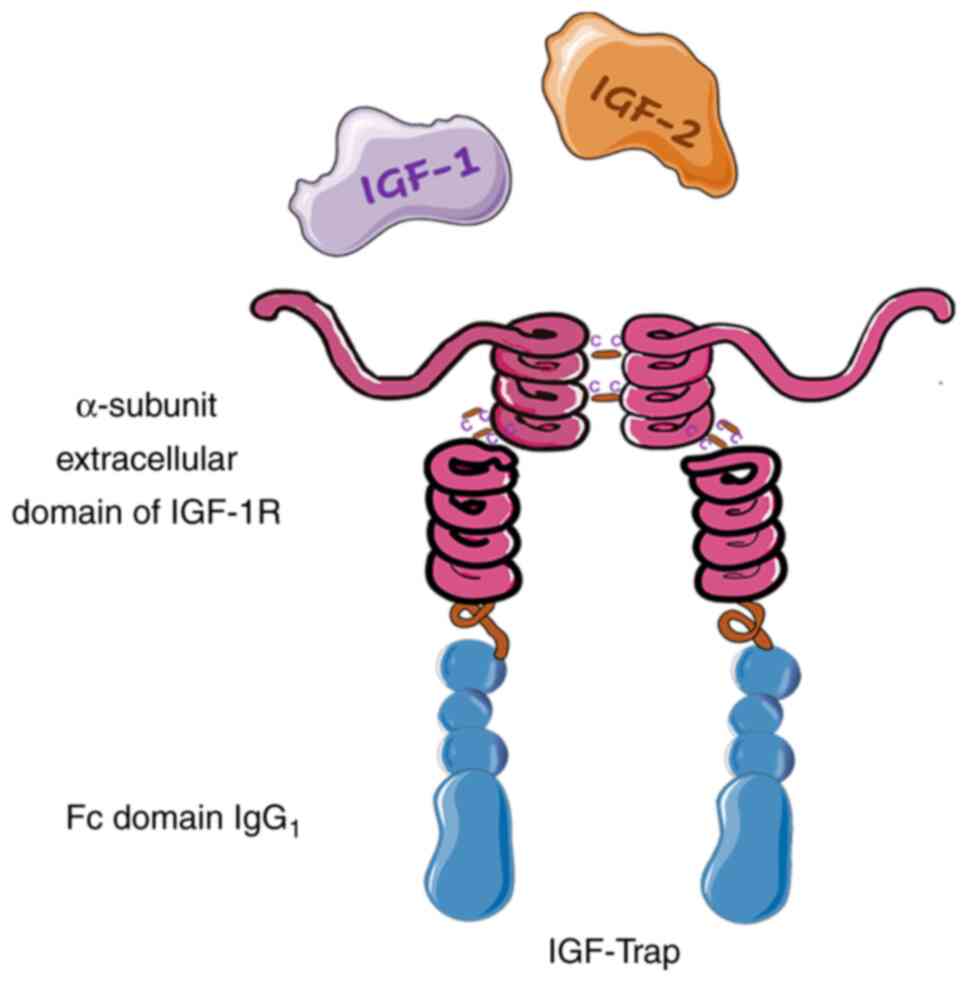
The identification of elements of the IGF system as
therapeutic targets in different tumors has stimulated the
development of decoys based on the IGF receptor system. A study
conducted by Samani et al (69) initially designed a truncated protein
of IGF-1R comprising the first 933 amino acids of the native
receptor and encompassing its extracellular domain. This protein
was expressed in H-59 highly metastatic murine lung carcinoma cells
and detected as a secreted heterotetramer
(βm-α-α-βm) that exogenously neutralized the
IGF-1 ligand and inhibited the proliferation, invasion and
resistance to apoptosis of the cells via the regulation of IGF-1R
signaling. Similarly, the expression of this protein markedly
reduced the metastatic potential of the H-59 cells following their
intrasplenic/portal inoculation in mice, reducing the formation of
liver metastases by 90% and significantly extending the
disease-free survival time. In a second study, a gutless adenovirus
expressing soluble IGF-1R (sIGFIR) was intravenously injected into
mice, which led to the production of measurable plasma levels of
sIGFIR for up to 21 days and significantly inhibited liver
metastasis (70). Subsequently, to
optimize this soluble decoy for translation to the clinic, its
pharmacokinetic properties and therapeutic potential were improved
via fusion with the Fc portion of human IgG1 to form
sIGFIR/hFc-IgG1. The addition of the Fc fragment did not
alter the binding kinetics of the recombinant protein. Furthermore,
this IGF-Trap decoy had high binding affinity for hIGF-1,
moderately lower affinity for mouse IGF-2 and IGF-1, and a
three-log lower affinity for insulin (20). IGF-Trap displayed similar effects to
sIGFIR, with the ability to inhibit IGF-1, IGF-2 and
IGF-1R-regulated cell signaling and functions in various types of
carcinoma cells in vitro, including breast, lung and colon
carcinoma cells. However, the pharmacokinetic profile of IGF-TRAP
was more favorable than that of sIGFIR in vivo, as
demonstrated by half-lives of 47.5 and 21.9 h, respectively, which
confirmed that the two Fc domains improved the stability of the
protein in vivo (20,64).
A frequent limitation of fusion proteins is that
they may form high-molecular-weight complexes via the formation of
disulfide bonds between Fc fragments. This is an issue for the
IGF-Trap decoy, a tetramer that comprises two subunits each fused
to an IgG1 Fc domain, in which the proximity of adjacent
Fc domains facilitates the formation of disulfide bonds and large
molecular complexes. For this reason, the IGF-Trap decoy was
redesigned by the replacement of cysteine with serine in the hinge
region of the Fc fragment of human IgG1, and the
introduction of a longer, more flexible linker between the IGF-1R
ectodomain and the Fc domain (Fig.
4). This modification decreased the formation of high molecular
weight complexes by this Trap and increased its stability, thereby
improving its pharmacodynamic properties (64,71).
Using the kinase receptor activation (KIRA) assay, it was shown
that the serum bioavailability of IGF-1 is closely associated with
the pharmacokinetic/pharmacodynamic profile of the IGF-Trap. In
this assay, the bioavailability of the ligand was measured via
quantification of the phosphorylated IGF-1 receptor. Unlike
traditional endpoint bioassays that measure the downstream effects
of IGF-1R activation, the KIRA assay directly measures receptor
activation, thereby eliminating the confounding effects of other
factors that may also activate downstream signaling pathways. In
addition, since the bioavailability and bioactivity of IGF-1 are
affected by IGF-BP and naturally occurring proteases in the
circulation, the KIRA assay provides a more accurate measure of
bioactive ligands (72). The
aforementioned studies indicate that IGF-Trap has high specificity
for IGF-1 and IGF-2 and low affinity for insulin, and therefore
should minimally influence the physiological functions of insulin.
In addition, the penetration and diffusion of IGF-Trap into solid
tumors may exert beneficial effects via the neutralization of
locally produced IGFs. Furthermore, reducing the bioavailability of
IGFs using IGF-Trap may affect various components of the tumor
microenvironment and thereby provide an enhanced growth inhibiting
effect. These data also suggest that IGF-Trap could provide a
surrogate marker for response assessment and a potential tool for
the classification of patients with resistant cervical tumors.
Nanoparticles targeting IGF-1R with
theragnostic advantages
Magnetic nanoparticles (MNPs) have shown promising
results in the personalized therapy and clinical management of
patients with resistant tumors. Due to the unique physicochemical
properties of MNPs, they may be used for multiple applications
simultaneously, particularly for theragnostic purposes, such as
imaging combined with the administration of therapeutic drugs.
Magnetic iron oxide nanoparticles (IONPs) are biocompatible and
biodegradable with low toxicity. Therefore, various types of IONPs
have been used clinically and have been shown to be safe.
Furthermore, IONPs have unique paramagnetic properties that provide
T2- and T2*-weighted images with a strong contrast, and a T1 effect
at very low concentrations (73,74).
Biodegradable IONPs have been generated and directed
against different target receptors, including the urokinase
plasminogen activator (uPA) receptor (uPAR). In one study,
amphiphilic polymer-coated IONPs were conjugated to the
amino-terminal fragment of uPA, the natural high-affinity ligand
for uPAR (75). In addition, the
polymer coating was modified to allow the encapsulation of
hydrophobic chemotherapeutic drugs to form nanoparticulate drug
delivery vehicles that are also sensitive to magnetic resonance
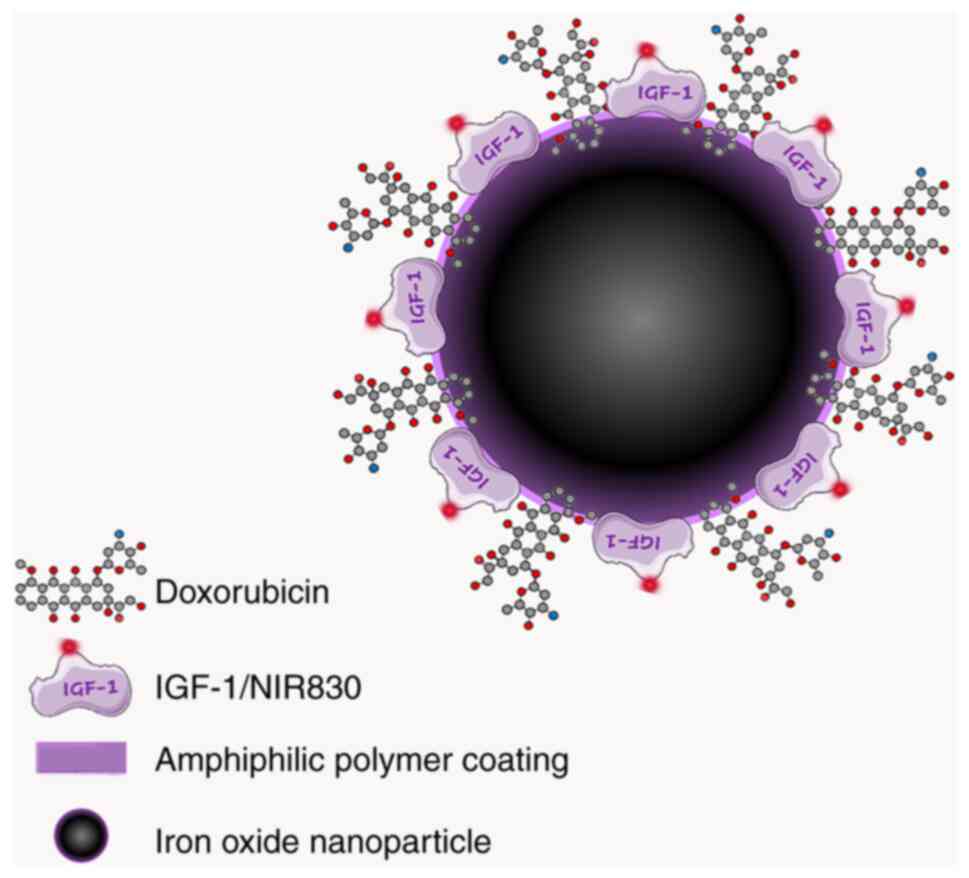
imaging (MRI). The fluorescent hydrophobic drug doxorubicin (Dox)
was efficiently encapsulated into the IONPs to form compact
Dox-loaded nanoparticles that were stable at pH 7.4 but released
Dox at an acidic pH of 4.0-5.0 within 2 h. These Dox-encapsulating
IONPs were observed to retain their T2 MRI contrast effect
following their internalization in tumor cells (75). Notably, this IONP system can be
conjugated with different ligands and thus be directed to different
target receptors to perform theranostic functions. IGF-1R appears
to be an ideal target receptor due to its upregulation in tumor
cells resistant to treatments. In another study, Zhou et al
(76) aimed to exploit the
theranostic capacities of IONPs directed at this receptor by
loading IONPs with Dox and conjugating them with recombinant human
IGF-1 for targeting purposes (Fig.
5). The efficacy of these theranostic IONPs, referred to as
IBF-1-IONP-Dox, was evaluated using human patient-derived xenograft
(PDX) models in which pancreatic cancer tissue was implanted into
severe combined immunodeficient mice. The repeated systemic
administration of the IGF-1-targeted theragnostic IONPs was
monitored by optical imaging and near infrared magnetic resonance,
and the results revealed that IGF-1-IONP-Dox induced a
significantly greater reduction in PDX growth than was achieved
using free Dox or undirected IONP-Dox in both subcutaneous and
orthotopic locations. In summary, theragnostic nanoparticles that
can easily be modified using a variety of targeting molecules and
therapeutic agents, such as antibodies, peptides, small molecules
and aptamers, via several conjugation strategies have been directed
to specific targets including IGF-1R.
These IONPs constitute a novel model for the imaging
and targeted administration of drugs for the treatment of tumors
(77). Human pancreatic PDX models,
which are highly similar to tumors in patients in terms of their
intratumoral heterogeneity, histological features and tumor
microenvironments, were used to assess the effect of IONPs. The
strategy of using IGF-1 for the targeted therapy of pancreatic
cancer is promising. Although this system has not been tested in
cervical tumors, it appears to be a promising innovation for the
management of resistant tumors.
Protein nanotubes
The self-assembly of peptides to form nanostructured
materials is a research area in which the non-covalent interactions
within or between peptide building blocks have been investigated
for their contribution to the self-assembly process (78). Based on the role of IGFBPs in the
initiation, development, progression and survival of cancer and
their function as natural antagonists of IGFs, IGFBP mimetics have
been created as potential alternative therapies for cancer
treatment using IGFBP-2 as a template. It was observed that by
fragmenting the IGFBP-2 protein at the single tryptophan residue
within the conserved CWCV motif, the carboxyl terminal fragment was
stable and able to inhibit the binding of IGF-1 to IGF 1R (79). Therefore, this fragment was
subjected to further investigation.
The native sequence of the
hIGFBP-2249-289 fragment includes two cysteine residues
in its primary sequence, and cysteine-rich regions have been
observed to increase the specificity of the ligand (79). Previously, in a study by Binkert
et al (80), the amino acid
sequences of the mature forms of human IGFBP-1, IGFBP-2 and the rat
BRL-BP proteins were aligned, and they observed that the three
IGFBPs share a cysteine-rich region homologous at its amino
terminus, plus an RGD motif embedded in a conserved pentapeptide.
However, there are differences between the three proteins of this
family. IGFBP-2 has the highest number of cysteines at its carboxyl
end and carries an Arg-Gly-Asp (RGD) motif embedded in a conserved
pentapeptide, which implies a structural or functional relevance
(80). Therefore, following the
addition of an extra cysteine at residue 281, an
hIGFBP-2249-289 (R281C) polypeptide with an odd number
of cysteines was obtained (79,81,82),
which spontaneously self-assembled to form soluble nanotubular
structures via the formation of intermolecular disulfide bonds. The
formation and disassembly of the nanotubes can be controlled by the
choice of appropriate redox conditions. Furthermore, the
polypeptide fragment contains an RGD motif in its sequence
(81,82), and an RGD array is present on the
surface of the nanotubes, which serves as a site for the active
targeting of cancer cells via integrin binding. RGD is an adhesive
peptide widely studied in the field of biomaterials. It has been
established that RGD is very effective in promoting the attachment
of numerous types of cells to various materials. It constitutes the
main binding domain of integrins present in the extracellular
matrix, including fibronectin, vitronectin, fibrinogen, osteopontin
and bone sialoprotein (83,84).
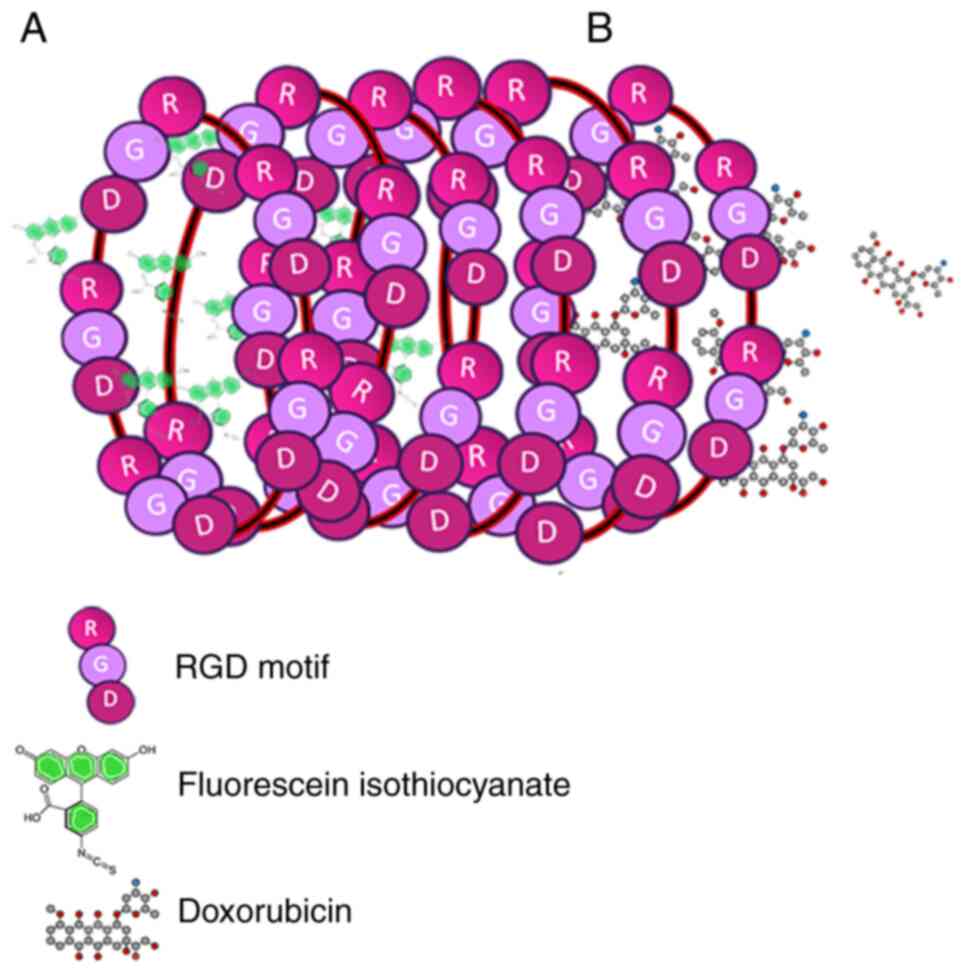
An interesting application of this protein nanotube
system was reported in the study by Asampille et al
(81). The interior of the
nanotubes was loaded with Dox as a representative hydrophobic
cytotoxic drug (Fig. 6A) or with
the dye fluorescein isothiocyanate as a representative imaging
agent (Fig. 6B). In order to
determine the ability of the multi-RGD moieties to specifically
deliver the nanotubes to cancer cells, integrins were overexpressed
on HeLa and MDA-MB-231 cell lines in vitro. Confocal
microscopy showed that the nanotubes remained attached to the
membrane of these cells, while flow cytometry revealed an increase
in apoptosis caused by the action of Dox at the cell periphery
(Fig. 7b-2). These results
demonstrate the theragnostic potential of these nanotubes (28,81) in
resistant tumors, including cervical cancer.
Conclusions
Cervical cancer is a public health issue that
particularly affects developing countries. The lack of efficiency
in screening methods, the prevalence of locally advanced stages and
intrinsic resistance to common treatments are the main reasons for
the failure to control this neoplasm. The conventional treatment
recommended by The International Federation of Gynecology and
Obstetrics, which comprises 50-Gy radiotherapy concomitant with
CDDP-based chemotherapy and brachytherapy, is applied
indiscriminately to the majority of patients (8,9). A
prediction system has been proposed that indicates the response to
treatment and/or the risk of metastasis via the molecular analysis
of transcriptional gene signatures (7,85,86),
which are molecular tools that enable oncologists to select the
optimum therapeutic strategy for each patient. However, the poor
prognosis of cervical cancer to conventional treatment necessitates
the development of novel therapeutic alternatives that are more
efficient in eliminating resistant tumors. Nanotechnology has been
used to prepare dual or theragnostic systems that can be
manufactured using various materials, including nanogels, polymeric
micelles, liposomes and targeting agents such as cell-penetrating
peptides (81,87–92),
functionalized with drugs and combined with bioactive cellular
molecules that increase the specificity and effectiveness of
diagnosis and treatment. Furthermore, advances in the manufacture
of advanced biological materials such as protein nanomaterials have
highlighted their potential in bioengineering and biomedical
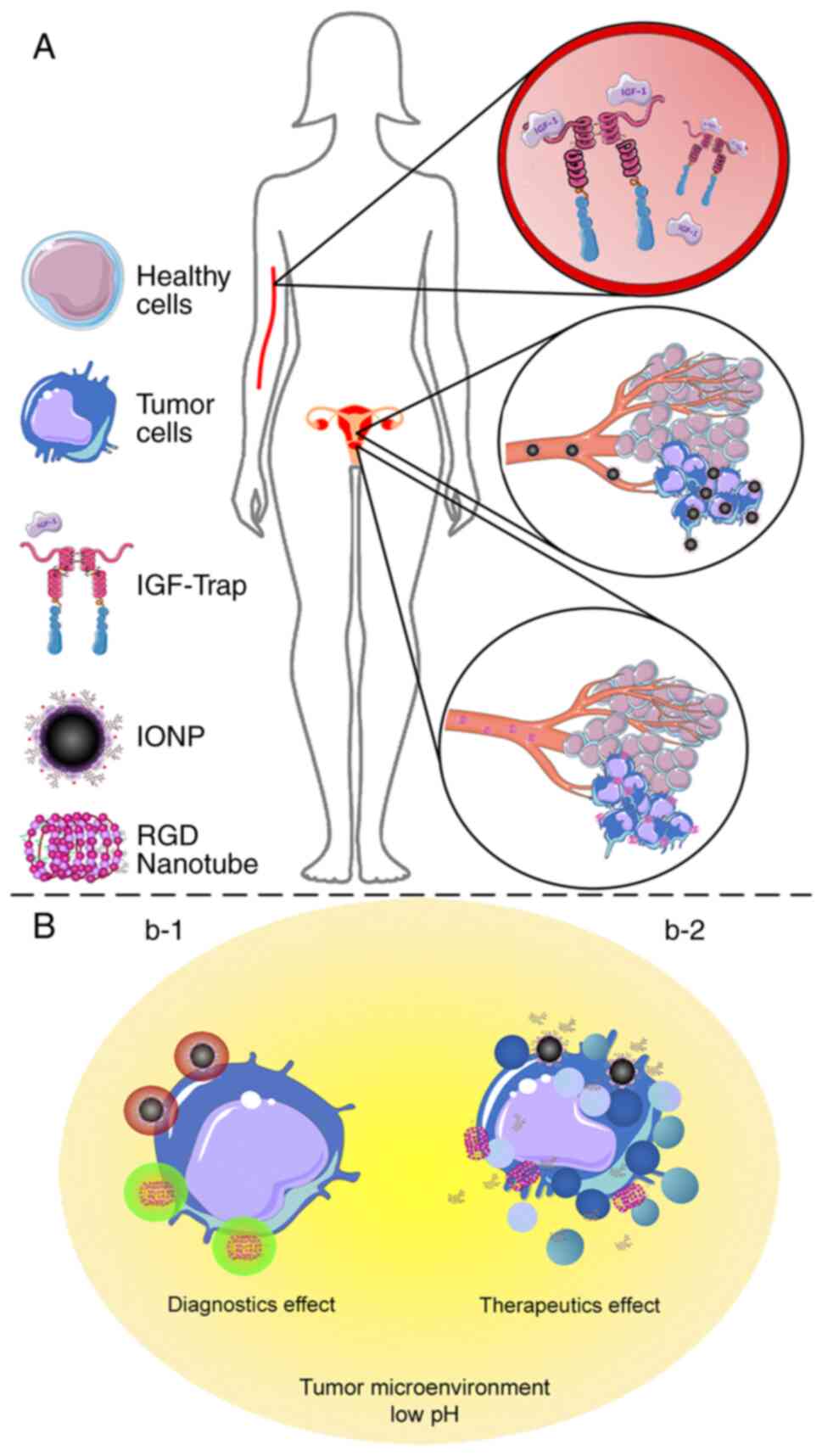
applications (93) (Fig. 7). The present review emphasizes the
participation of three elements of the IGF system (Fig. 7A), which actively participate in
tumor survival and resistance mechanisms, summarizing their use as
bioactive molecules and/or therapeutic targets of nanocarriers
(Table I). It may be concluded that
they represent a breakthrough in nano-oncology and have potential
in the treatment of resistant cervical tumors.
 | Table I.Nanotherapy therapeutics and
diagnostics based on elements of the IGF-axis. |
Table I.
Nanotherapy therapeutics and
diagnostics based on elements of the IGF-axis.
| Authors | Nanoparticle | IGF-axis
target | Theragnostic
capacity | Bioactive
molecules | Activity | Action
mechanism | (Refs.) |
|---|
| Chen et al,
2020; Samani et al, 2004; Vaniotis et al, 2018; | Trap decoys | IGF-1 and IGF-2
ligands | Therapeutic | Chimeric
protein | Systemic | Blocks the binding
of IGF-1 and −2 with cell receptors | (64,69,71) |
| Yang et al,
2008; Zhou et al, 2016 | Magnetic iron oxide
therapeutic | IGF-1R | Diagnostic and
nanoparticles | Magnetic
nanoparticles conjugated with IGF-1 | Targeted membrane
receptors | Nanoparticles
loaded with drug or fluorescent molecules, targeting IGF-1R in the
cell membrane | (75,76) |
| Kibbey et
al, 2006; Binkert et al, 1989; Asampille et al,
2018; Swain et al, 2010; | Protein
nanotubes | Tumor cell membrane
proteins, i.e., integrins | Diagnostic and
therapeutic | IGFBP-2 carboxyl
end (repeated RGD motif) conjugated to leader molecule
(ligands) | Targeted membrane
receptors | Nanotubes loaded
with drug or fluorescent molecules, targeting membrane
proteins | (79–82) |
Acknowledgements
The authors acknowledge Professor Alejandro Ariel
García-Arriaga (Division of Health, Biological and Environmental
Sciences, Open and Distance University of Mexico, Mexico City,
México) and Professor Fernando Ferrara-Suárez (Nanotechnology and
Biotechnology Engineering Division, Polytechnic University of the
Valley of Mexico. Tultitlán, Mexico State, México) for language
editing of the manuscript. Parts of the figures were drawn by using
pictures from Servier Medical Art. Servier Medical Art by Servier
is licensed under a Creative Commons Attribution 3.0 Unported
License (https://creativecommons.org/licenses/by/3.0/).
Funding
This work was supported by the Research Support of the
Polytechnic University of the Valley of Mexico.
Availability of data and materials
Not applicable.
Authors' contributions
JFR and MMR conceived the study, wrote the
introduction and conclusions sections, and reviewed and edited the
manuscript. LPG, HZM and CCCG wrote the IGF axis, IGF axis in
cervical cancer and IGF axis members as therapeutic targets in
cervical cancer sections. JN and BMP wrote the extracellular domain
of IGF-1R used as a trap nanoparticle and nanoparticles targeting
IGF-1R with theragnostic advantages sections. JFR and RVMT wrote
the protein nanotubes section. MMR and JAJL participated in the
acquisition of data on the IGF axis, extracellular domain of IGF-1R
used as a trap nanoparticle, nanoparticles targeting IGF-1R with
theragnostic advantages, and protein nanotubes and worked on the
development of all figures. Data authentication is not applicable.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aldaco-Sarvide F, Pérez-Pérez P,
Cervantes-Sánchez G, Torrecillas-Torres L, Erazo-Valle-Solís AA,
Cabrera-Galeana P, Motola-Kuba D, Anaya P, Rivera-Rivera S and
Cárdenas-Cárdenas E: Mortalidad por cáncer en México: Actualización
2015. Gac Mex Oncol. 17:28–34. 2018.
|
|
3
|
Granados-García V, Flores YN, Pérez R,
Rudolph SE, Lazcano-Ponce E and Salmerón J: Cost of the cervical
cancer screening program at the Mexican social security institute.
Salud Publica Mex. 56:502–510. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murillo R, Almonte M, Pereira A, Ferrer E,
Gamboa OA, Jerónimo J and Lazcano-Ponce E: Cervical cancer
screening programs in Latin America and the Caribbean. Vaccine. 26
(Suppl 11):L37–L48. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McCormack M, Kadalayil L, Hackshaw A,
Hall-Craggs MA, Symonds RP, Warwick V, Simonds H, Fernando I,
Hammond M, James L, et al: A phase II study of weekly neoadjuvant
chemotherapy followed by radical chemoradiation for locally
advanced cervical cancer. Br J Cancer. 108:2464–2469. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gadducci A and Cosio S: Neoadjuvant
chemotherapy in locally advanced cervical cancer: Review of the
literature and perspectives of clinical research. Anticancer Res.
40:4819–4828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fernandez-Retana J, Lasa-Gonsebatt F,
Lopez-Urrutia E, Coronel-Martínez J, Cantu De Leon D,
Jacobo-Herrera N, Peralta-Zaragoza O, Perez-Montiel D,
Reynoso-Noveron N, Vazquez-Romo R and Perez-Plasencia C: Transcript
profiling distinguishes complete treatment responders with locally
advanced cervical cancer. Transl Oncol. 8:77–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Monk BJ, Enomoto T, Kast WM, McCormack M,
Tan DSP, Wu X and González-Martín A: Integration of immunotherapy
into treatment of cervical cancer: Recent data and ongoing trials.
Cancer Treatment Reviews. 106:1023852022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri: 2021 Update. Int J
Gynecol Obstet. 155 (Suppl 1):S28–S44. 2021. View Article : Google Scholar
|
|
10
|
Naga Ch P, Gurram L, Chopra S and
Mahantshetty U: The management of locally advanced cervical cancer.
Curr Opin Oncol. 30:323–329. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li H, Wu X and Cheng X: Advances in
diagnosis and treatment of metastatic cervical cancer. J Gynecol
Oncol. 27:e432016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Höckel S, Schlenger K, Vaupel P and Höckel
M: Association between host tissue vascularity and the
prognostically relevant tumor vascularity in human cervical cancer.
Int J Oncol. 19:827–832. 2001.PubMed/NCBI
|
|
13
|
Sims LB, Curry KC, Parupalli S, Horner G,
Frieboes HB and Steinbach-Rankins JM: Efficacy of surface-modified
PLGA nanoparticles as a function of cervical cancer Type. Pharm
Res. 36:662019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sau S, Alsaab HO, Bhise K, Alzhrani R,
Nabil G and Iyer AK: Multifunctional nanoparticles for cancer
immunotherapy: A groundbreaking approach for reprogramming
malfunctioned tumor environment. J Control Release. 274:24–34.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chaturvedi VK, Singh A, Singh VK and Singh
MP: Cancer nanotechnology: A new revolution for cancer diagnosis
and therapy. Curr Drug Metab. 20:416–429. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Buzea C, Pacheco II and Robbie K:
Nanomaterials and nanoparticles: Sources and toxicity.
Biointerphases. 2:MR17–MR71. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhu L, Zhou Z, Mao H and Yang L: Magnetic
nanoparticles for precision oncology: Theranostic magnetic iron
oxide nanoparticles for image-guided and targeted cancer therapy.
Nanomedicine (Lond). 12:73–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hewish M, Chau I and Cunningham D:
Insulin-like growth factor 1 receptor targeted therapeutics: Novel
compounds and novel treatment strategies for cancer medicine.
Recent Pat Anticancer Drug Discov. 4:54–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Codony-Servat J, Cuatrecasas M, Asensio E,
Montironi C, Martínez-Cardús A, Marín-Aguilera M, Horndler C,
Martínez-Balibrea E, Rubini M, Jares P, et al: Nuclear IGF-1R
predicts chemotherapy and targeted therapy resistance in metastatic
colorectal cancer. Br J Cancer. 117:1777–1786. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang N, Rayes RF, Elahi SM, Lu Y, Hancock
MA, Massie B, Rowe GE, Aomari H, Hossain S, Durocher Y, et al: The
IGF-Trap: Novel inhibitor of carcinoma growth and metastasis. Mol
Cancer Ther. 14:982–993. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lelbach A, Muzes G and Feher J: The
insulin-like growth factor system: IGFs, IGF-binding proteins and
IGFBP-proteases. Acta Physiol Hung. 92:97–107. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Schaffer A, Koushik A, Trottier H,
Duarte-Franco E, Mansour N, Arseneau J, Provencher D, Gilbert L,
Gotlieb W, Ferenczy A, et al: Insulin-like growth factor-I and risk
of high-grade cervical intraepithelial neoplasia. Cancer Epidemiol
Biomarkers Prev. 16:716–722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
De Meyts P and Whittaker J: Structural
biology of insulin and IGF1 receptors: Implications for drug
design. Nat Rev Drug Discov. 1:769–783. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hakuno F and Takahashi SI: IGF1 receptor
signaling pathways. J Mol Endocrinol. 61:T69–T86. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liefers-Visser JAL, Meijering RAM, Reyners
AKL, van der Zee AGJ and de Jong S: IGF system targeted therapy:
Therapeutic opportunities for ovarian cancer. Cancer Treat Rev.
60:90–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Huang Z, Wen Y, Shandilya R, Marks JR,
Berchuck A and Murphy SK: High throughput detection of M6P/IGF2R
intronic hypermethylation and LOH in ovarian cancer. Nucleic Acids
Res. 34:555–563. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sehat B, Tofigh A, Lin Y, Trocmé E,
Liljedahl U, Lagergren J and Larsson O: SUMOylation mediates the
nuclear translocation and signaling of the IGF-1 receptor. Sci
Signal. 3:ra102010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Brahmkhatri VP, Prasanna C and Atreya HS:
Insulin-like growth factor system in cancer: Novel targeted
therapies. Biomed Res Int. 2015:5380192015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mathur SP, Mathur RS and Young RC:
Cervical epidermal growth factor-receptor (EGF-R) and serum
insulin-like growth factor II (IGF-II) levels are potential markers
for cervical cancer. Am J Reprod Immunol. 44:222–230. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bayes-Genis A, Conover CA and Schwartz RS:
The insulin-like growth factor axis: A review of atherosclerosis
and restenosis. Circ Res. 86:125–130. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Blat C, Villaudy J and Binoux M: In vivo
proteolysis of serum insulin-like growth factor (IGF) binding
protein-3 results in increased availability of IGF to target cells.
J Clin Invest. 93:2286–2290. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rajah R, Katz L, Nunn S, Solberg P, Beers
T and Cohen P: Insulin-like growth factor binding protein (IGFBP)
proteases: Functional regulators of cell growth. Prog Growth Factor
Res. 6:273–284. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Butt AJ and Williams AC: IGFBP-3 and
apoptosis-a licence to kill? Apoptosis. 6:199–205. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grimberg A, Liu B, Bannerman P, El-Deiry
WS and Cohen P: IGFBP-3 mediates p53-induced apoptosis during serum
starvation. Int J Oncol. 21:327–335. 2002.PubMed/NCBI
|
|
35
|
zur Hausen H: Papillomaviruses and cancer:
From basic studies to clinical application. Nat Rev Cancer.
2:342–350. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Scheffner M, Werness BA, Huibregtse JM,
Levine AJ and Howley PM: The E6 oncoprotein encoded by human
papillomavirus types 16 and 18 promotes the degradation of p53.
Cell. 63:1129–1136. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Boyer SN, Wazer DE and Band V: E7 protein
of human papilloma virus-16 induces degradation of retinoblastoma
protein through the ubiquitin-proteasome pathway. Cancer Res.
56:4620–4624. 1996.PubMed/NCBI
|
|
38
|
Jones DL, Thompson DA and Münger K:
Destabilization of the RB tumor suppressor protein and
stabilization of p53 contribute to HPV type 16 E7-induced
apoptosis. Virology. 239:97–107. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kuramoto H, Hongo A, Liu YX, Ojima Y,
Nakamura K, Seki N, Kodama J and Hiramatsu Y: Immunohistochemical
evaluation of insulin-like growth factor I receptor status in
cervical cancer specimens. Acta Med Okayama. 62:251–259.
2008.PubMed/NCBI
|
|
40
|
Aleksic T, Chitnis MM, Perestenko OV, Gao
S, Thomas PH, Turner GD, Protheroe AS, Howarth M and Macaulay VM:
Type 1 insulin-like growth factor receptor translocates to the
nucleus of human tumor cells. Cancer Res. 70:6412–6419. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Moreno-Acosta P, Vallard A, Carrillo S,
Gamboa O, Romero-Rojas A, Molano M, Acosta J, Mayorga D, Rancoule
C, Garcia MA, et al: Biomarkers of resistance to radiation therapy:
A prospective study in cervical carcinoma. Radiat Oncol.
12:1202017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Takeda T, Komatsu M, Chiwaki F,
Komatsuzaki R, Nakamura K, Tsuji K, Kobayashi Y, Tominaga E, Ono M,
Banno K, et al: Upregulation of IGF2R evades lysosomal
dysfunction-induced apoptosis of cervical cancer cells via
transport of cathepsins. Cell Death Dis. 10:8762019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Scagliotti GV and Novello S: The role of
the insulin-like growth factor signaling pathway in non-small cell
lung cancer and other solid tumors. Cancer Treat Rev. 38:292–302.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
You L, Liu C, Tang H, Liao Y and Fu S:
Advances in targeting insulin-like growth factor signaling pathway
in cancer treatment. Curr Pharm Des. 20:2899–2911. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fu S, Tang H, Liao Y, Xu Q, Liu C, Deng Y,
Wang J, Wang J and Fu X: Expression and clinical significance of
insulin-like growth factor 1 in lung cancer tissues and
perioperative circulation from patients with non-small-cell lung
cancer. Curr Oncol. 23:12–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Durzyńska J: IGF axis and other factors in
HPV-related and HPV-unrelated carcinogenesis (review). Oncol Rep.
32:2295–2306. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pickard A, Durzynska J, McCance DJ and
Barton ER: The IGF axis in HPV associated cancers. Mutat Res Rev
Mutat Res. 772:67–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wu X, Tortolero-Luna G, Zhao H, Phatak D,
Spitz MR and Follen M: Serum levels of insulin-like growth factor I
and risk of squamous intraepithelial lesions of the cervix. Clin
Cancer Res. 9:3356–3361. 2003.PubMed/NCBI
|
|
49
|
Shen MR, Hsu YM, Hsu KF, Chen YF, Tang MJ
and Chou CY: Insulin-like growth factor 1 is a potent stimulator of
cervical cancer cell invasiveness and proliferation that is
modulated by alphavbeta3 integrin signaling. Carcinogenesis.
27:962–971. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Steller MA, Delgado CH, Bartels CJ,
Woodworth CD and Zou Z: Overexpression of the insulin-like growth
factor-1 receptor and autocrine stimulation in human cervical
cancer cells. Cancer Res. 56:1761–1765. 1996.PubMed/NCBI
|
|
51
|
van der Veeken J, Oliveira S, Schiffelers
RM, Storm G, van Bergen En Henegouwen PM and Roovers RC: Crosstalk
between epidermal growth factor receptor- and insulin-like growth
factor-1 receptor signaling: Implications for cancer therapy. Curr
Cancer Drug Targets. 9:748–760. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pickard A, McDade SS, McFarland M,
McCluggage WG, Wheeler CM and McCance DJ: HPV16 down-regulates the
insulin-like growth factor binding protein 2 to promote epithelial
invasion in organotypic cultures. PLoS Pathog. 11:e10049882015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kaur G, Balasubramaniam SD and Lee YJ:
IGFBP-2 in cervical cancer development. Exp Mol Pathol.
113:1043622020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schütt BS, Langkamp M, Rauschnabel U,
Ranke MB and Elmlinger MW: Integrin-mediated action of insulin-like
growth factor binding protein-2 in tumor cells. J Mol Endocrinol.
32:859–868. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Berger AJ, Baege A, Guillemette T, Deeds
J, Meyer R, Disbrow G and Schlegel R and Schlegel R: Insulin-like
growth factor-binding protein 3 expression increases during
immortalization of cervical keratinocytes by human papillomavirus
type 16 E6 and E7 proteins. Am J Pathol. 161:603–610. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mannhardt B, Weinzimer SA, Wagner M,
Fiedler M, Cohen P, Jansen-Dürr P and Zwerschke W: Human
papillomavirus type 16 E7 oncoprotein binds and inactivates
growth-inhibitory insulin-like growth factor binding protein 3. Mol
Cell Biol. 20:6483–6495. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Baxter RC: Nuclear actions of insulin-like
growth factor binding protein-3. Gene. 569:7–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Seligson DB, Yu H, Tze S, Said J, Pantuck
AJ, Cohen P and Lee KW: IGFBP-3 nuclear localization predicts human
prostate cancer recurrence. Horm Cancer. 4:12–23. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Arcaro A: Targeting the insulin-like
growth factor-1 receptor in human cancer. Front Pharmacol.
4:302013. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
King ER and Wong KK: Insulin-like growth
factor: Current concepts and new developments in cancer therapy.
Recent Pat Anticancer Drug Discov. 7:14–30. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Navarro M and Baserga R: Limited
redundancy of survival signals from the type 1 insulin-like growth
factor receptor. Endocrinology. 142:1073–1081. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Park S, Chapuis N, Tamburini J, Bardet V,
Cornillet-Lefebvre P, Willems L, Green A, Mayeux P, Lacombe C and
Bouscary D: Role of the PI3K/AKT and mTOR signaling pathways in
acute myeloid leukemia. Haematologica. 95:819–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Qu X, Wu Z, Dong W, Zhang T, Wang L, Pang
Z, Ma W and Du J: Update of IGF-1 receptor inhibitor (ganitumab,
dalotuzumab, cixutumumab, teprotumumab and figitumumab) effects on
cancer therapy. Oncotarget. 8:29501–29518. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Chen YM, Qi S, Perrino S, Hashimoto M and
Brodt P: Targeting the IGF-axis for cancer therapy: Development and
validation of an IGF-Trap as a potential drug. Cells. 9:10982020.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Holash J, Davis S, Papadopoulos N, Croll
SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, et
al: VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc
Natl Acad Sci USA. 99:11393–11398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Messori A, Santarlasci B and Vaiani M: New
drugs for rheumatoid arthritis. N Engl J Med. 351:937–938. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Hoffman HM, Throne ML, Amar NJ, Sebai M,
Kivitz AJ, Kavanaugh A, Weinstein SP, Belomestnov P, Yancopoulos
GD, Stahl N and Mellis SJ: Efficacy and safety of rilonacept
(interleukin-1 Trap) in patients with cryopyrin-associated periodic
syndromes: Results from two sequential placebo-controlled studies.
Arthritis Rheum. 58:2443–2452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Lindzen M, Carvalho S, Starr A,
Ben-Chetrit N, Pradeep CR, Köstler WJ, Rabinkov A, Lavi S, Bacus SS
and Yarden Y: A recombinant decoy comprising EGFR and ErbB-4
inhibits tumor growth and metastasis. Oncogene. 31:3505–3515. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Samani AA, Chevet E, Fallavollita L,
Galipeau J and Brodt P: Loss of tumorigenicity and metastatic
potential in carcinoma cells expressing the extracellular domain of
the type 1 insulin-like growth factor receptor. Cancer Res.
64:3380–3385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang N, Lu Y, Pinard M, Pilotte A, Gilbert
R, Massie B and Brodt P: Sustained production of a soluble IGF-I
receptor by gutless adenovirus-transduced host cells protects from
tumor growth in the liver. Cancer Gene Ther. 20:229–236. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Vaniotis G, Moffett S, Sulea T, Wang N,
Elahi SM, Lessard E, Baardsnes J, Perrino S, Durocher Y, Frystyk J,
et al: Enhanced anti-metastatic bioactivity of an IGF-TRAP
re-engineered to improve physicochemical properties. Sci Rep.
8:173612018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sadick MD, Intintoli A, Quarmby V, McCoy
A, Canova-Davis E and Ling V: Kinase receptor activation (KIRA): A
rapid and accurate alternative to end-point bioassays. J Pharm
Biomed Anal. 19:883–891. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Bulte JWM and Kraitchman DL: Iron oxide MR
contrast agents for molecular and cellular imaging. NMR Biomed.
17:484–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Miller-Kleinhenz JM, Bozeman EN and Yang
L: Targeted nanoparticles for image-guided treatment of
triple-negative breast cancer: Clinical significance and
technological advances. Wiley Interdiscip Rev Nanomed
Nanobiotechnol. 7:797–816. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Yang L, Cao Z, Sajja HK, Mao H, Wang L,
Geng H, Xu H, Jiang T, Wood WC, Nie S and Wang YA: Development of
receptor targeted magnetic iron oxide nanoparticles for efficient
drug delivery and tumor imaging. J Biomed Nanotechnol. 4:439–449.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Zhou H, Qian W, Uckun FM, Zhou Z, Wang L,
Wang A, Mao H and Yang L: IGF-1 receptor targeted nanoparticles for
image-guided therapy of stroma-rich and drug resistant human
cancer. Proc SPIE Int Soc Opt Eng. Apr 17–2016.(Epub ahead of
print).
|
|
77
|
Yu MK, Park J and Jon S: Targeting
strategies for multifunctional nanoparticles in cancer imaging and
therapy. Theranostics. 2:3–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Gao X and Matsui H: Peptide-based
nanotubes and their applications in bionanotechnology. Adv Mater.
17:2037–2050. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kibbey MM, Jameson MJ, Eaton EM and
Rosenzweig SA: Insulin-like growth factor binding protein-2:
Contributions of the C-terminal domain to insulin-like growth
factor-1 binding. Mol Pharmacol. 69:833–845. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Binkert C, Landwehr J, Mary JL, Schwander
J and Heinrich G: Cloning, sequence analysis and expression of a
cDNA encoding a novel insulin-like growth factor binding protein
(IGFBP-2). EMBO J. 8:2497–2502. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Asampille G, Verma BK, Swain M, Shettar A,
Rosenzweig SA, Kondaiah P and Atreya HS: An ultra-stable
redox-controlled self-assembling polypeptide nanotube for targeted
imaging and therapy in cancer. J Nanobiotechnology. 16:1012018.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Swain M, Thirupathi R, Krishnarjuna B,
Eaton EM, Kibbey MM, Rosenzweig SA and Atreya HS: Spontaneous and
reversible self-assembly of a polypeptide fragment of insulin-like
growth factor binding protein-2 into fluorescent nanotubular
structures. Chem Commun (Camb). 46:216–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Arnaout MA, Mahalingam B and Xiong JP:
Integrin structure, allostery, and bidirectional signaling. Annu
Rev Cell Dev Biol. 21:381–410. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Bellis SL: Advantages of RGD peptides for
directing cell association with biomaterials. Biomaterials.
32:4205–4210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Pedroza-Torres A, López-Urrutia E,
García-Castillo V, Jacobo-Herrera N, Herrera LA, Peralta-Zaragoza
O, López-Camarillo C, De Leon DC, Fernández-Retana J, Cerna-Cortés
JF and Pérez-Plasencia C: MicroRNAs in cervical cancer: Evidences
for a miRNA profile deregulated by HPV and its impact on
radio-resistance. Molecules. 19:6263–6281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fernandez-Retana J, Zamudio-Meza H,
Rodriguez-Morales M, Pedroza-Torres A, Isla-Ortiz D, Herrera L,
Jacobo-Herrera N, Peralta-Zaragoza O, López-Camarillo C,
Morales-Gonzalez F, et al: Gene signature based on
degradome-related genes can predict distal metastasis in cervical
cancer patients. Tumour Biol. Jun 22–2017.(Epub ahead of print).
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Cuggino JC, Molina M, Wedepohl S,
Igarzabal CIA, Calderón M and Gugliotta LM: Responsive nanogels for
application as smart carriers in endocytic pH-triggered drug
delivery systems. Eur Polym J. 78:14–24. 2016. View Article : Google Scholar
|
|
88
|
Patel SG, Sayers EJ, He L, Narayan R,
Williams TL, Mills EM, Allemann RK, Luk LYP, Jones AT and Tsai YH:
Cell-penetrating peptide sequence and modification dependent uptake
and subcellular distribution of green florescent protein in
different cell lines. Sci Rep. 9:62982019. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Poshteh Shirani M, Rezaei B, Khayamian T,
Dinari M, Karami K, Mehri-Lighvan Z, Hosseini Shamili F, Ramazani M
and Alibolandi M: Folate receptor-targeted multimodal fluorescence
mesosilica nanoparticles for imaging, delivery palladium complex
and in vitro G-quadruplex DNA interaction. J Biomol Struct Dyn.
36:4156–4169. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Tomitaka A, Arami H, Huang Z, Raymond A,
Rodriguez E, Cai Y, Febo M, Takemura Y and Nair M: Hybrid
magneto-plasmonic liposomes for multimodal image-guided and
brain-targeted HIV treatment. Nanoscale. 10:184–194. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Trujillo-Nolasco M, Cruz-Nova P,
Ferro-Flores G, Gibbens-Bandala B, Morales-Avila E, Aranda-Lara L,
Vargas M and Ocampo-García B: Development of
177Lu-DN(C19)-CXCR4 ligand nanosystem for combinatorial
therapy in pancreatic cancer. J Biomed Nanotechnol. 17:263–278.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wei T, Chen C, Liu J, Liu C, Posocco P,
Liu X, Cheng Q, Huo S, Liang Z, Fermeglia M, et al: Anticancer drug
nanomicelles formed by self-assembling amphiphilic dendrimer to
combat cancer drug resistance. Proc Natl Acad Sci USA.
112:2978–2983. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sun H, Li Y, Yu S and Liu J: Hierarchical
self-assembly of proteins through rationally designed
supramolecular interfaces. Front Bioeng Biotechnol. 8:2952020.
View Article : Google Scholar : PubMed/NCBI
|