Introduction
Cancer of unknown primary site (CUP) is a rare
heterogeneous clinical syndrome of metastatic cancer for which the
primary site is difficult to determine. The pathogenesis of CUP
remains unclear (1). CUP accounts
for 2–5% of all cancer diagnoses (2–4). Most
CUPs are associated with clinical signs and symptoms of metastatic
tumors, such as weakness, loss of appetite, chest tightness and
abdominal distension (4). The most
commonly affected areas are the liver, lungs, bone and lymph nodes,
followed by pleura and the brain (5). The diagnosis of CUP requires
histopathological characterization, which is typically performed
with immunohistochemistry (IHC) and, more recently, molecular
analysis, which can detect the expression of specific genes in
tumor cells of patients, and the tumor classification and subtype
can be analyzed by comparing with the determined tumor
classification database (4,6). As the primary features of CUP are
unknown, most patients receive topical treatment or empiric
systemic chemotherapy (7). Despite
multiple combinations of chemotherapy, most patients have a poor
prognosis, with a survival time of 3–6 months (8). In the present case report, a patient
with a CUP of the mandible was successfully treated. The patient
was admitted to our hospital for treatment in November 2021 and
survived for nearly 1 year until October 2022, the patient is still
healthy now.
Case report
A 1×1-cm mass was found in the right mandible of a
71-year-old female patient in November 2006. The patient had no
obvious discomfort and was not treated. In November 2021, the
patient presented at Jinshazhou Hospital of Guangzhou University of
Chinese Medicine (Guangzhou, China) as the tumor had grown rapidly
to 5×4×5 cm within 2 weeks, accompanied by pain, bleeding,
salivation, a bad odour, and limited opening and closing of the
mouth (Fig. 1). Results of a
biopsy, achieved by scraping cells from the tumor surface, were
consistent with ulceration. A pathological biopsy was performed
subsequent to the initial superficial biopsy. MRI showed mandibular
bone destruction and a soft-tissue mass lesion, with invasion of
the bilateral sublingual glands, genioglossus muscle, right
masseter muscle and lower lip soft tissue. Slightly larger lymph
nodes were seen at the cervical Ia and bilateral Ib levels,
indicating the possibility of lymph node metastasis (Fig. 2A).
Ultrasound-guided puncture biopsy of the mandibular
tumor was performed and tumor bleeding was observed, which improved
after symptomatic hemostasis. The biopsy tissue was soaked in 10%
neutral formalin fixing solution for 8 h at 25°C (room
temperature), embedded in Leica paraffin wax and sliced into 4-µm
unstained sections. Antigen repair was conducted with three 250 ml
cylinders of xylene for 10 min each, two cylinders of 100 and 95%
ethanol for 5 min each, and 90, 85 and 75% ethanol for 3 min per
250 ml cylinder hydration, at 100°C constant temperature, followed
by TBS-T (0.05% Tween-20) washing for 3–8 min. After antigen
repair, the biopsy tissue was treated with 3% hydrogen peroxide
solution 25°C for 10 min for blocking. Pan-cytokeratin (CK) primary
antibody (1:400; cat. no. MAB-0671; Fuzhou Maixin Biotech Co.,
Ltd.) was then added, and the biopsy tissue was incubated at room
temperature for 50 min. The biopsy tissue was then incubated with
secondary antibody linked to horseradish peroxidase diluted by
TBS-T (1:2,500; cat. no. GK800711; Gene Tech Co., Ltd.) at room
temperature for 30 min. DAB staining was used for visualization,
which was conducted using an Olympus BX53 optical microscope. A
pathological report was generated indicating the presence of a
small round-cell malignant tumor and an immunohistochemical
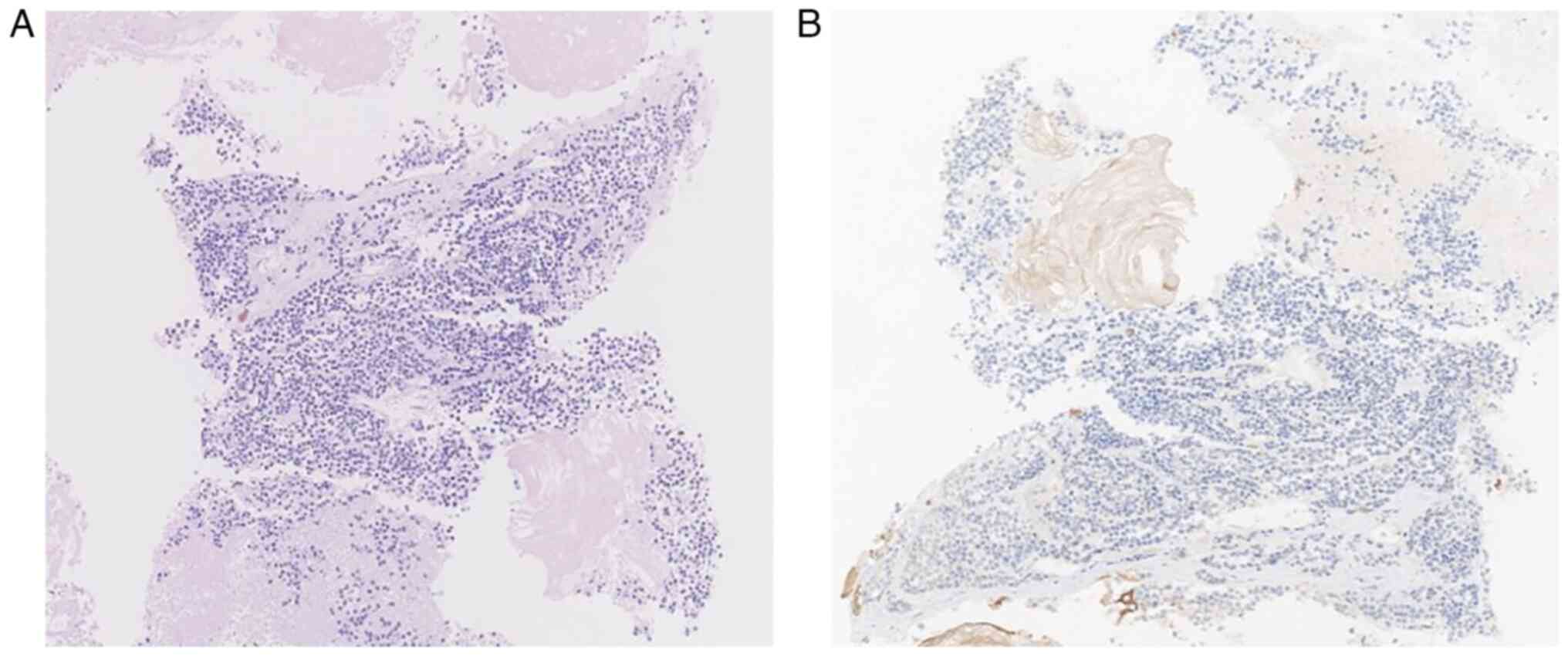
examination was recommended. The HE staining protocol of the 4-µm
unstained sections was as follows (all steps were carried out at
25°C, room temperature): i) 10% neutral formalin fixing solution
for 8 h; ii) xylene dewaxing I, II, III and IV (roman numerals
represent a different number of 250 ml cylinders) for 6 min each;
iii) rehydration in 100% (I and II), 95 and 75% ethanol for 1 min
each, followed by rinsing with tap water for 2 min; iv) staining
with hematoxylin for 5 min, followed by rinsing with tap water for
1 min; v) differentiation for 6 sec with 0.5% hydrochloric ethanol
solution and rinsing with tap water; vi) incubation with saturated
lithium carbonate solution for 5 sec, to prevent nuclei from being
too light; vii) staining with eosin for 1 min; viii) 75% ethanol,
two 250 ml cylinders of 95% ethanol, two 250 ml cylinders of 100%
ethanol 1 min each; ix) xylene I, II and III 1 min each; and x)
neutral gum sealing sheet to remove excess water and facilitate
microscopic observation (Fig. 3A).
The pathological diagnosis was of a malignant tumor.
Immunohistochemical analyses were not able to
identify small cell carcinoma, small cell osteosarcoma or a
primitive neuroectodermal tumor. The immunohistochemical results
were as follows: Cytokeratin (−), vimentin (−), synaptophysin (−),
chromogranin A (−), neural cell adhesion molecule 56 (−), Ki67 (5%
+), special AT-rich 2 (−), thyroid transcription 1 (−), CD99 (−),
desmin (−) and myoD1 (−). The tumor contained mostly necrotic
material with little living tissue, making determination of the
cancer type difficult and requiring further investigation. The IHC
protocol was as follows: The undyed slide was placed in the oven at
60°C for 120 min, and then xylene I, II, III (roman numerals
represent a different number of 250 ml cylinders)was added for
dewaxing, 10 min for each cylinder. The slides were washed in 100%
ethanol I, 100% ethanol II, 95% ethanol I, 95% ethanol II, 5 min
per cylinder; 90, 85, 75% ethanol, 3 min per cylinder, and then
rinsed with distilled water to complete the hydration. The slides
were placed in a 100°C constant temperature machine for 3–8 min for
antigen repair, washed with TBS-T (0.05% Tween-20), treated with 3%
hydrogen peroxide solution for 10 min and then rinsed with TBS-T
again. Primary CK antibody (1:400; cat. no. MAB-0671; Fuzhou Maixin
Biotech Co., Ltd.) was added and the slides were incubated at room
temperature for 50 min, before washing with TBS-T. Secondary
antibody linked to horseradish peroxidase diluted with TBS-T
(1:2,500; cat. no. GK800711; Shanghai GeneTech Co. Ltd.) was added
and the slides were incubated at room temperature for 30 min. The
slides were washed with TBS-T, DAB color developing solution was
added and slides were incubated at 25°C for 5 min, and then washed
with distilled water. The tissues were dyed with hematoxylin for 5
min, before washing with tap water for 1 min. Tissues were
differentiated for 6 sec with 0.5% hydrochloric ethanol solution
and washed with tap water. Saturated lithium carbonate solution was
added for 5 sec to prevent nuclear staining being too light.
Subsequently, the slides were washed with 75% ethanol, two 250 ml
cylinders of 95% ethanol, two 250 ml cylinders of 100% ethanol 1
min each, two 250 ml cylinders of 95% ethanol, two 250 ml cylinders
of 100% ethanol 1 min each to remove excess water and facilitate
microscopic observation. The slides were then placed in 75%
ethanol, two 250 ml cylinders of 95% ethanol and two 250 ml
cylinders of 100% ethanol for 1 min each, and then in two 250 ml
cylinders of 95% ethanol and two 250 ml cylinders of 100% ethanol
for 1 min each. The above steps were conducted to remove excess
water and facilitate microscopic observation. Finally, xylene
solution I, II and III was added for 1 min each. A neutral gum
sealing sheet was added (Fig. 3B).
The tumor site was prone to bleeding and, given the extensive
necrosis within the punctured tissue, the needle biopsy was not
re-performed. Therefore, the pathological type of the tumor
remained unknown, as did its site of origin, which may have been
the mandible, tongue or gums.
Arterial infusion chemotherapy with 60 mg docetaxel
and 40 mg lobaplatin was subsequently performed and the right
facial artery was embolized. The tumor had decreased in size 3 days
later, bleeding and salivation had decreased, and the opening and
closing of the mouth were more flexible than previously. A second
arterial infusion chemotherapy with the same drugs was performed 3
weeks later and the right facial artery was re-embolized. Visual
examination (Fig. 4) and MRI
(Fig. 2B) performed ~4 weeks after
the second arterial infusion chemotherapy showed that the
mandibular tumor had shrunk significantly and that the metastatic
lymph nodes in the neck had decreased in number and size. A total
of 4 cycles of chemotherapy according to the original scheme were
administered and the tumor size remained stable.
After 12 rounds of radiotherapy (planning target
volume 36 Gy/12 fractions) to the mandible, the tumor size had
decreased and the tumor had almost disappeared. There was a little
residual tumor on the CT scan, but the tumor was not active and was
considered clinically cured. The patient was checked every 3 months
and there has been no sign of recurrence since March 2022.
Discussion
Among CUP cases, 47% of patients suffer from highly
to moderately differentiated adenocarcinoma, 44% from poorly
differentiated or undifferentiated adenocarcinoma, 7% from squamous
carcinoma and 2% from undifferentiated malignant tumors (9). Two-thirds of patients with cervical
lymph node metastasis have squamous cell carcinoma (10). Melanoma of unknown primary site
(MUP) comprises 3–4% of all melanomas, is typically present in the
lymph nodes and more frequently involves the axillary lymph nodes,
followed by the cervical, inguinal and parotid lymph nodes; the
involvement of cervical metastatic lymph nodes is a negative
prognostic factor for MUP (11).
There are two hypotheses to explain the origins of CUP: i) A small,
dormant or degenerative undetectable primary tumor means that a
distinct primary lesion is the source; and ii) no primary tumor
exists and the CUP is independent of a primary tumor mass and
biologically different from other metastatic tumors (12). In support of the second hypothesis,
a previous study has shown that head and neck CUP is associated
with human papillomavirus (13).
At the time of CUP diagnosis, sufficient tissue for
immunohistochemical examination is desirable (14), but sometimes unavailable, as with
the present case study. Molecular tumor profiling (MTP) complements
standard pathological assessment to allow determination of CUP
tissue origin and is especially valuable when IHC produces
uncertain results (1,14,15).
These methods enable <87% of patients to receive a
tissue-of-origin diagnosis, compared with 30% using conventional
diagnostic tools (16). However,
MTP does not always translate into survival benefits (17). Site-specific therapy based on
accurate prediction using either reverse transcriptase polymerase
chain reaction or gene microarray techniques (18–20) to
determine the tissue of origin in patients with CUP, appears to
improve the prognosis for some patients (21), but there is no curative effect in
some tumor types, for example, breast, salivary gland, and adnexal
skin cancers, as these neoplasms have overlapping gene expression,
which may cause incorrect diagnosis of the tissue of origin
(22,23). Moreover, the clinical benefit of MTP
to CUP is not strongly supported (12).
Gene expression profiles can aid in the
identification of primary tumor sites and targeted mutations
(24). Liquid biopsy is a novel
technique to aid CUP diagnosis via gene expression profiling and
overcomes some limitations of tumor biopsy (12,25).
The acquisition and interpretation of tumor biopsy have inherent
limitations, and as a single tumor biopsy is typically very small,
it is uncertain whether it can represent the whole tumor (26). Liquid biopsy can detect tumor cells
and circulating tumor DNA fragments in bodily fluid specimens
including blood, tissue fluid and cerebrospinal fluid, to aid in
the diagnosis of CUP. However, to the best of our knowledge,
large-scale clinical trials have not yet been conducted to confirm
the benefits of gene sequencing (27). Such a clinical trial could involve a
subgroup of patients with CUP undergoing site-specific therapy with
or without a diagnosis from molecular cancer classifier assays,
under the guidance of a classical immunohistochemical panel. The
results of these trials could then be compared with those published
in historical cases of CUP that received similar treatment at a
known primary site. Despite the lack of prospective randomization,
similar results will generate rapid progress in this field
(28). Positron emission
tomography/CT is also valuable for primary tumor diagnosis
(29).
The prognosis of CUP is worse than that for most
other tumors (9). There is
currently no standard chemotherapy regimen for treatment (4,12), but
empiric platinum or paclitaxel-based chemotherapy is often used
(30–32), even though the level of evidence
supporting this method recommendation is low (4,33,34).
Combined chemotherapy with carboplatin and paclitaxel has been
found to be effective in patients with peritoneal carcinoma with
lymph node/pleural metastasis from a CUP; however, in patients with
liver, bone or multiple organ involvement, this regimen has limited
benefits (35). Gemcitabine, alone
or in combination with other drugs, may also be used (33). Most patients with metastatic
squamous cell carcinoma of the neck are treated with radiotherapy
(36). Notably, no significant
difference in 5-year survival rates among patients administered
radiotherapy or chemoradiotherapy alone and surgical treatment has
been found (37). Whether immune
checkpoint inhibitors (ICIs) are an effective CUP treatment option
is also currently an open question (38). ICIs are actively being evaluated in
CUP given their theoretical ability to mount an effective antitumor
immune response. Chromosomal instability is infrequent in CUP but
is a known driver of early dissemination and aggressive behavior,
reducing the response to ICIs (39). A number of patients with chromosomal
instability present with individual gene alterations with
implications for immune-evasion and resistance to ICIs (39). A 60-case clinical trial involved
treatment with carboplatin plus paclitaxel, followed by erlotinib
targeted therapy plus chemotherapy, and the median survival time of
patients was 13 months (40).
Another trial of 47 patients treated with bevacizumab and erlotinib
as second-line treatment had a median survival time of 7 months
(41). Immunotherapy may also be a
treatment option (12,42).
In conclusion, the diagnosis and treatment of CUP
presents difficulties and results in a poor prognosis. The present
case study is of the successful treatment of a patient with CUP.
The relevant literature has been reviewed and a comprehensive
treatment method including chemotherapy, interventional
embolization and radiotherapy is described in order to inform
treatment decisions for patients with CUP.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, LXQ, YXC, JPG and JML were involved in the
patient's treatment management process, BC participated in the
pathological analysis, XJZ obtained medical images. All authors
read and approved the final manuscript. XL, LXQ, BC and JML confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by The Ethics Committee of
Jinshazhou Hospital of Guangzhou University of Chinese Medicine
(Guangzhou, China).
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the present case report and any
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rassy E, Assi T and Pavlidis N: Exploring
the biological hallmarks of cancer of unknown primary: Where do we
stand today? Br J Cancesr. 122:1124–1132. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sacks D, Baxter B, Campbell BCV, Carpenter
JS, Cognard C, Dippel D, Eesa M, Fischer U, Hausegger K, Hirsch JA,
et al: Multisociety consensus quality improvement revised consensus
statement for endovascular therapy of acute ischemic stroke. Int J
Stroke. 13:612–632. 2018.PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pavlidis N and Pentheroudakis G: Cancer of
unknown primary site. Lancet. 379:1428–1435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chorost MI, Lee MC, Yeoh CB, Molina M and
Ghosh BC: Unknown primary. J Surg Oncol. 87:191–203. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bahrami A, Truong LD and Ro JY:
Undifferentiated tumor: True identity by immunohistochemistry. Arch
Pathol Lab Med. 132:326–348. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Greco FA and Pavlidis N: Treatment for
patients with unknown primary carcinoma and unfavorable prognostic
factors. Semin Oncol. 36:65–74. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang BL and Lim YS: Regarding the
manuscript of Huang et al. published in Acta Histochemica
(doi:10.1016/j.acthis.2010.06.003). Acta Histochem. 113:677–678.
675–676. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van de Wouw AJ, Janssen-Heijnen ML,
Coebergh JW and Hillen HF: Epidemiology of unknown primary tumours;
Incidence and population-based survival of 1285 patients in
Southeast Netherlands, 1984–1992. Eur J Cancer. 38:409–413. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strojan P, Ferlito A, Medina JE, Woolgar
JA, Rinaldo A, Robbins KT, Fagan JJ, Mendenhall WM, Paleri V,
Silver CE, et al: Contemporary management of lymph node metastases
from an unknown primary to the neck: I. A review of diagnostic
approaches. Head Neck. 35:123–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Boussios S, Rassy E, Samartzis E,
Moschetta M, Sheriff M, Pérez-Fidalgo JA and Pavlidis N: Melanoma
of unknown primary: New perspectives for an old story. Crit Rev
Oncol Hematol. 158:1032082021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Conway AM, Mitchell C, Kilgour E, Brady G,
Dive C and Cook N: Molecular characterisation and liquid biomarkers
in carcinoma of unknown primary (CUP): Taking the ‘U’ out of ‘CUP’.
Br J Cancer. 120:141–153. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Keller LM, Galloway TJ, Holdbrook T, Ruth
K, Yang D, Dubyk C, Flieder D, Lango MN, Mehra R, Burtness B and
Ridge JA: p16 status, pathologic and clinical characteristics,
biomolecular signature, and long-term outcomes in head and neck
squamous cell carcinomas of unknown primary. Head Neck.
36:1677–1684. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Losa F, Soler G, Casado A, Estival A,
Fernández I, Giménez S, Longo F, Pazo-Cid R, Salgado J and Seguí
MÁ: SEOM clinical guideline on unknown primary cancer (2017). Clin
Transl Oncol. 20:89–96. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tomuleasa C, Zaharie F, Muresan MS, Pop L,
Fekete Z, Dima D, Frinc I, Trifa A, Berce C, Jurj A, et al: How to
diagnose and treat a cancer of unknown primary site. J
Gastrointestin Liver Dis. 26:69–79. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moran S, Martinez-Cardús A, Boussios S and
Esteller M: Precision medicine based on epigenomics: The paradigm
of carcinoma of unknown primary. Nat Rev Clin Oncol. 14:682–694.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rassy E and Pavlidis N: The diagnostic
challenges of patients with carcinoma of unknown primary. Expert
Rev Anticancer Ther. 20:775–783. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Erlander MG, Ma XJ, Kesty NC, Bao L,
Salunga R and Schnabel CA: Performance and clinical evaluation of
the 92-gene real-time PCR assay for tumor classification. J Mol
Diagn. 13:493–503. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pillai R, Deeter R, Rigl CT, Nystrom JS,
Miller MH, Buturovic L and Henner WD: Validation and
reproducibility of a microarray-based gene expression test for
tumor identification in formalin-fixed, paraffin-embedded
specimens. J Mol Diagn. 13:48–56. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Varadhachary GR, Spector Y, Abbruzzese JL,
Rosenwald S, Wang H, Aharonov R, Carlson HR, Cohen D, Karanth S,
Macinskas J, et al: Prospective gene signature study using microRNA
to identify the tissue of origin in patients with carcinoma of
unknown primary. Clin Cancer Res. 17:4063–4070. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hainsworth JD, Rubin MS, Spigel DR, Boccia
RV, Raby S, Quinn R and Greco FA: Molecular gene expression
profiling to predict the tissue of origin and direct site-specific
therapy in patients with carcinoma of unknown primary site: A
prospective trial of the Sarah Cannon research institute. J Clin
Oncol. 31:217–223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Boorjian S: Commentary on ‘Predicted
plasma 25-hydroxyvitamin D and risk of renal cell cancer.’ Joh HK,
Giovannucci EL, Bertrand KA, Lim S, Cho E, Department of medicine,
seoul national university college of medicine, Seoul, South Korea.
J Natl Cancer Inst. 2013.105((10)): 726–32, [Epub 2013 Apr 8]. doi:
10.1093/jnci/djt082. Urol Oncol. 32:933–934. 2014.
|
|
23
|
Greco FA: Cancer of unknown primary or
unrecognized adnexal skin primary carcinoma? Limitations of gene
expression profiling diagnosis. J Clin Oncol. 31:14792013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Daud AI: Removing the unknown from the
carcinoma of unknown primary. J Clin Oncol. 31:174–175. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
El Rassy E, Khaled H and Pavlidis N:
Liquid biopsy: A new diagnostic, predictive and prognostic window
in cancers of unknown primary. Eur J Cancer. 105:28–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thiele JA, Bethel K, Králíčková M and Kuhn
P: Circulating tumor cells: Fluid surrogates of solid tumors. Annu
Rev Pathol. 12:419–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Economopoulou P, Mountzios G, Pavlidis N
and Pentheroudakis G: Cancer of unknown primary origin in the
genomic era: Elucidating the dark box of cancer. Cancer Treat Rev.
41:598–604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rassy E, Labaki C, Chebel R, Boussios S,
Smith-Gagen J, Greco FA and Pavlidis N: Systematic review of the
CUP trials characteristics and perspectives for next-generation
studies. Cancer Treat Rev. 107:1024072022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maghami E, Ismaila N, Alvarez A, Chernock
R, Duvvuri U, Geiger J, Gross N, Haughey B, Paul D, Rodriguez C, et
al: Diagnosis and management of squamous cell carcinoma of unknown
primary in the head and neck: ASCO guideline. J Clin Oncol.
38:2570–2596. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lynch HT, Slostad B and Silberstein P:
Familial carcinoma of unknown primary. JAMA Oncol. 2:346–347. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hainsworth JD and Fizazi K: Treatment for
patients with unknown primary cancer and favorable prognostic
factors. Semin Oncol. 36:44–51. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rassy E, Parent P, Lefort F, Boussios S,
Baciarello G and Pavlidis N: New rising entities in cancer of
unknown primary: Is there a real therapeutic benefit? Crit Rev
Oncol Hematol. 147:1028822020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bochtler T, Löffler H and Krämer A:
Diagnosis and management of metastatic neoplasms with unknown
primary. Semin Diagn Pathol. 35:199–206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Oldenburg J, Aparicio J, Beyer J,
Cohn-Cedermark G, Cullen M, Gilligan T, De Giorgi U, De Santis M,
de Wit R, Fosså SD, et al: Personalizing, not patronizing: The case
for patient autonomy by unbiased presentation of management options
in stage I testicular cancer. Ann Oncol. 26:833–838. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Briasoulis E, Kalofonos H, Bafaloukos D,
Samantas E, Fountzilas G, Xiros N, Skarlos D, Christodoulou C,
Kosmidis P and Pavlidis N: Carboplatin plus paclitaxel in unknown
primary carcinoma: A phase II hellenic cooperative oncology group
study. J Clin Oncol. 18:3101–3107. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Galloway TJ and Ridge JA: Management of
squamous cancer metastatic to cervical nodes with an unknown
primary site. J Clin Oncol. 33:3328–3337. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balaker AE, Abemayor E, Elashoff D and
John MA: Cancer of unknown primary: Does treatment modality make a
difference? Laryngoscope. 122:1279–1282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Olivier T, Fernandez E, Labidi-Galy I,
Dietrich PY, Rodriguez-Bravo V, Baciarello G, Fizazi K and
Patrikidou A: Redefining cancer of unknown primary: Is precision
medicine really shifting the paradigm? Cancer Treat Rev.
97:1022042021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chebly A, Yammine T, Boussios S, Pavlidis
N and Rassy E: Chromosomal instability in cancers of unknown
primary. Eur J Cancer. 172:323–325. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hainsworth JD, Spigel DR, Thompson DS,
Murphy PB, Lane CM, Waterhouse DM, Naot Y and Greco FA:
Paclitaxel/carboplatin plus bevacizumab/erlotinib in the first-line
treatment of patients with carcinoma of unknown primary site.
Oncologist. 14:1189–1197. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hainsworth JD, Spigel DR, Farley C,
Thompson DS, Shipley DL and Greco FA; Minnie Pearl Cancer Research
Network, : Phase II trial of bevacizumab and erlotinib in
carcinomas of unknown primary site: The minnie pearl cancer
research network. J Clin Oncol. 25:1747–1752. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kourie HR, Awada G and Awada AH: Unknown
primary tumors: Is there a future therapeutic role for immune
checkpoint inhibitors? Future Oncol. 12:429–431. 2016. View Article : Google Scholar : PubMed/NCBI
|