Introduction
For endometrial cancer (EC), poor prognostic factors
driving tumor recurrence are directly related to mortality
(1). Tumor grade, histological
subtype, degree of myometrial invasion, cervical involvement, tumor
size, lymph-vascular space invasion (LVSI), and lymph node status
are the most important clinicopathological prognostic markers in
patients diagnosed with EC (2).
However, these markers have been proven to be of limited utility,
particularly in the case of recurrent or high-grade EC (2,3). It is
therefore crucial to identify prognostic biomarkers for metastatic
and/or recurrent EC to estimate disease risk and treatment options.
High-grade and metastatic/recurrent EC patients have few
therapeutic options due to the ineffectiveness of traditional
platinum and taxane chemotherapy regimens and the limited impact of
newer agents such as Lenvatinib, Pembrolizumab and Bevacizumab
(4,5). New therapeutic approaches are
necessary to address this critical unmet need. A deep understanding
of the molecular alterations associated with EC offers the
opportunity of adding targeted therapies to the current treatment
arsenal.
Excess lipids and cholesterol are converted to
triglycerides and cholesteryl esters (CE) in cancer cells (6). Intra-tumoral CE has been shown to
increase tumor cell proliferation, invasiveness, and survival
(7–10); thus, inhibiting CE synthesis may be
a useful anti-cancer therapeutic strategy (10). Because sterol O-acyltransferase
(SOAT1), also known as acyl-CoA cholesterol acyltransferase1
(ACAT1) is involved in maintaining appropriate levels of CE within
cells by converting excess cholesterol to CE, this enzyme became
the target of research in tumor cells. SOAT1 expression and CE
levels were reported to be abnormal in a variety of cancers,
including ovarian cancer, leukemia, glioma, prostate cancer,
pancreatic cancer, breast cancer, and colon cancer (11–16).
However, information on the role of SOAT1/CE accumulation in EC is
limited. We have recently reported increased SOAT1 and CE levels in
ovarian cancer cell lines, tumor tissue, and peritoneal fluid
compared to the non-malignant group, confirming SOAT1-mediated CE
accumulation as a cancer-specific event (15). The expression of these mediators
correlated with malignancy and tumor aggressiveness in ovarian
cancer (16,17). These findings prompted us to
investigate SOAT1/CE as potential prognostic or therapeutic targets
in advanced EC.
The Cancer Genome Atlas (TCGA) has reported SOAT1
expression (mRNA and protein) levels in normal and tumor tissue
from EC patients; however, to date, there are no data documenting
SOAT1 levels in peritoneal fluid or plasma of EC patients.
Consequently, we examined the levels of SOAT1 and CE in tumor
tissue, peritoneal fluid, and plasma from subjects diagnosed with
EC and subjects with normal endometrium (controls) to ascertain the
relationship between SOAT1/CE levels and a variety of factors,
including malignancy, tumor aggressiveness (ki67 expression), and
survival. Additionally, possible correlations of SOAT1/CE levels
between peritoneal fluid, plasma and tumor tissue were assessed to
evaluate their diagnostic potential. A strong, positive, linear
correlation between increasing BMI and disease incidence, as well
as a strong, negative, linear correlation between increasing BMI
and oncological outcomes have been previously reported (18). Moreover, comorbidities such as
obesity, hyperlipidemia, diabetes, hypertension and hyperthyroidism
are known to alter cholesterol metabolism; therefore, BMI and other
comorbidities were adjusted in logistic regressions to analyze
their potential influence on the association between SOAT1/CE
levels and malignancy.
Materials and methods
Study design
This was an observational, cross-sectional pilot
study involving patients scheduled for an oophorectomy, bilateral
salpingo-oophorectomy (BSO), hysterectomy, hysterectomy/BSO,
staging and/or debulking via laparotomy or laparoscopy for surgical
management of biopsy confirmed EC, between 2016 and 2021, at the
Division of Gynecological Oncology, Department of Obstetrics &
Gynecology, Southern Illinois University School of Medicine,
Springfield, IL. Patients scheduled to undergo the aforementioned
procedures for the management of other gynecologic diagnoses (e.g.,
pelvic prolapse), were enrolled within the divisions of General
Gynecology and Urogynecology. Exclusion criteria included a
previous malignancy, chemotherapy or radiation therapy prior to
surgery. All sample collections were performed on the day of
surgery. After surgery, subjects were grouped into three study
cohorts based on their diagnosis: i) Subjects with a confirmed
diagnosis of EC (‘EC’ group; N=32); and ii) subjects with normal
endometrium (‘control’ group; N=16). Relevant clinical information
was collected from SIU electronic health records, including: age,
menopausal status, cancer diagnosis, FIGO stage/grade (confirmed by
independent pathologists), and presence of comorbidities such as
obesity, dyslipidemia, diabetes, hypertension and
hypothyroidism.
For analysis, data from subjects diagnosed with
stage I and stage II were pooled together into the ‘early stage EC’
group (N=18) whereas data from subjects diagnosed with stage III
and stage IV patients were pooled into the ‘advanced stage EC’
group N=14). The clinical and pathological characteristics of the
study population are summarized in Table I.
 | Table I.Clinical and pathological
characteristics of samples. |
Table I.
Clinical and pathological
characteristics of samples.
| Parameter | Control | EC |
|---|
| Sample size,
na (%) | 16 (33) | 32 (67) |
| Age, median years
(min-max) | 61.5 (52–81) | 63 (49–83) |
| BMI, median
kg/m2 (min-max) | 26.7
(20.9-35.3) | 35.9
(19.8-52.0) |
| Premenopausal, n
(%) | 2 (13) | 1 (3) |
| Postmenopausal, n
(%) | 14 (88) | 31 (97) |
| Obesity, n (%) | 4 (25) | 17 (53) |
| Diabetes, n
(%) | 3 (19) | 7 (22) |
| Hypertension, n
(%) | 3 (19) | 17 (53) |
| Hypothyroidism, n
(%) | 3 (19) | 8 (25) |
| FIGO stage, n (% of
EC) |
|
|
| Stage
I |
| 16 (50) |
| Stage
II |
| 2 (6) |
| Stage
III |
| 12 (38) |
| Stage
IV |
| 2 (6) |
| FIGO grade, n (% of
EC) |
|
|
| Grade
1 |
| 15 (47) |
| Grade
2 |
| 7 (22) |
| Grade
3 |
| 4 (13) |
| No
information |
| 6 (19) |
| Histotype, n (% of
EC) |
|
|
|
Endometrioid |
| 27 (85) |
|
Serous |
| 3 (9) |
|
Other |
| 2 (6) |
Ethic statement, standard protocol
approvals, registrations and patient consents
This study was approved by the local Institutional
Review Board (Springfield Committee for Research Involving Human
Subjects) under protocol 16–493. Eligible patients (age ≥30 years)
were invited to participate during their preoperative evaluation
and diagnostic workup. If patients expressed interest in
participation, written informed consent was obtained at this
preoperative visit.
Peripheral blood, peritoneal fluid and
tumor tissue sample collection
Peripheral blood was collected into sodium heparin
tubes just prior to surgery. Peritoneal fluid was collected during
the surgical procedure as previously described (19). To summarize, collection involves
aspiration of ascites, infusion of saline and re-aspiration of the
fluid. Plasma and peritoneal fluid samples were centrifuged at
1,500 r/min for 10 min and stored at −80°C before being tested.
Fragments (≥1 cm3) of fresh tissue without necrotic
areas were collected from the endometrium of subjects immediately
after the hysterectomy was completed. A macro-dissection of the
tissue samples was performed to remove fatty tissue and exclusively
collect tumor or normal endometrial tissue. The tissue specimens
were flash frozen in liquid nitrogen and stored at −80°C until
analyzed.
SOAT1 protein quantification by
enzyme-linked immunosorbent assay (ELISA)
SOAT1 protein concentrations in plasma, peritoneal
fluid, and tissue lysates were determined using the SOAT1 ELISA Kit
(Human) from Mybiosource according to the manufacturer's protocol.
Quantification of SOAT1 in plasma and peritoneal fluid was done in
50 µl aliquots of sample. Tissue lysates for ELISA were prepared as
described previously (16,17). Protein concentrations were
quantified using a bicinchoninic acid (BCA) protein assay kit
(Bio-Rad). Absorbance was measured at 450 nm using a Synergy H1MFD
(Hybrid multimode) microplate reader (BioTek). Concentration of
SOAT1 (pg/ml) was calculated by interpolation from a standard
curve.
Quantitative analysis of CE, free
cholesterol (FC) and total cholesterol (TC) from plasma and
peritoneal fluid
We used the Total Cholesterol and Cholesteryl Ester
Colorimetric Assay Kit from Biovision to quantify TC, FC, and CE
from plasma and peritoneal fluid, as previously described (15). Following the manufacturer's
instructions, concentrations were determined in 50 µl aliquots of
sample. The absorbance at 570 nm was measured using a Synergy H1MFD
(Hybrid multimode) microplate reader (BioTek). Interpolation from a
standard curve was used to calculate TC concentrations (mg/dl).
Immunohistochemistry (IHC)
As described previously, immunohistochemical
analysis for SOAT1 was performed on paraffin-embedded endometrial
tissue sections generated by Springfield Memorial Hospital
Laboratory as surgical pathology standard of care samples. We also
purchased endometrial cancer disease spectrum tissue microarray
slides from US Biomax (T091a and T094) for SOAT1 and Ki67 staining.
IHC was performed as per standard protocol previously described
(16,17). Briefly, the slides were
deparaffinized, rehydrated and heated to unmask the antigenic
sites. The slides were further incubated with appropriate primary
antibodies (SOAT1 1:500 dilution, Ki67 1:500 dilution) and their
respective secondary antibodies (Abcam), followed by detection with
3.3′-diaminobenzidine (DAB) substrate (Abcam) and counterstain with
hematoxylin. Negative controls were performed without the addition
of any primary antibody to rule out any nonspecific staining of the
secondary antibodies. Images were captured using an inverted
microscope (Olympus H4-100, CCD camera) with a 20X objective. Five
images were recorded in each core, and 1 µm wide z-stacks were
obtained. ImageJ software (NIH) was used to analyze the images. For
pathological investigation, one slide per sample was stained with
hematoxylin and eosin. We have included appropriate IgG isotype
negative controls to establish the background staining during IHC
method optimization studies. We used recombinant Anti-Ki67
(ab92742) and anti-SOAT 1/SOAT1 (ab39327) primary antibodies from
Abcam.
RNA extraction and cDNA synthesis
Total RNA from EC tumors and normal endometrial
tissues were isolated using TRIzol Reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). After assessing the yield and quality by
spectrophotometry, the RNA was stored at −80°C until use. A total
of 1 µg RNA from each sample was reverse transcribed into cDNA
using the iScript cDNA synthesis kit (Bio-Rad).
Gene expression analyses by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
SOAT1 and Ki67 mRNA levels were determined by
real-time RT-qPCR using their respective specific primers purchased
from Integrated DNA Technologies, Inc. RPl4 and β-actin were used
as housekeeping genes. Transcript analysis was done as described
previously using PowerUP SYBR-Green Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.). Applied Biosystems 7500 Real Time
PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.) was
used for RT-qPCR analysis. The thermal expression levels were
measured in triplicate. The threshold cycle (Ct) values were
normalized to RPl4 and β-actin and relative mRNA expression was
determined using the ΔΔCt method (20).
Statistical and bioinformatics
analysis
The clinical/pathological variables and
comorbidities were described using descriptive statistics.
Categorical data are presented as frequencies (percentages) and
continuous data as medians (interquartile ranges). Continuous
variables were compared between the control group and EC group
using the Mann-Whitney non-parametric U test. When more than two
groups were compared (i.e., control, early stage EC and advanced
stage EC), statistical significance was determined using
Kruskal-Wallis test with Dunn's post hoc correction. P<0.05 was
considered significant. Analysis of correlation between variables
was assessed using Spearman's rank correlation coefficient test. To
assess the diagnostic potential of biomarkers, ROC curve analysis
was used to determine the area under the curve (AUC), sensitivity,
specificity and optimal cut-off values of individual biomarkers.
Logistic regressions were performed to understand the influence of
confounding variables (BMI and comorbidities) on the SOAT1/CE
content in malignancy (EC). Model 1 is an unadjusted model whereas
model 2 adjusts predictor variables with different cofounder
variables individually. Differences were considered statistically
significant when adjusted P<0.05. Bioinformatics analysis for
SOAT1 expression and its prognostic significance was retrieved from
UALCAN and cBioPortal platforms. Kaplan Meier curves were generated
to understand the relationship between SOAT1 gene and overall
survival. Log rank test was used to compare the survival curves of
samples with high gene expression and low gene expression. All
statistical analyses were performed using GraphPad Prism 7.04 and
SPSS statistical software (SPSS Inc.).
Results
SOAT1 mRNA and protein expression
increased in EC tumor tissues
EC tumor tissue contained significantly higher
levels of SOAT1 mRNA than control endometrial tissue (P=0.0002;
Mann-Whitney U test; data not shown). When dividing the EC group
into early EC (stage I–II) and advanced EC (stage III–IV), only the
advanced EC group had significantly higher SOAT1 mRNA levels than
the control (P=0.0010; Kruskal-Wallis test with Dunn's post hoc
correction; Fig. 1A). No
significant difference was observed between early and advanced EC
groups. Similar to mRNA, SOAT1 protein levels were also higher in
malignant EC tissue compared to normal endometrial tissue
(P=0.0006; Mann Whitney non-parametric U test; data not shown).
When dividing the EC group by disease stage, SOAT1 protein levels
were significantly higher only in the advanced stage EC group
compared to the control group (P=0.0013; Kruskal-Wallis test with
Dunn's post hoc correction; Fig.
1B). No significant difference was observed between early and
advanced EC groups. IHC analysis further confirmed increased SOAT1
expression in tumor tissue collected from EC subjects (evidenced by
increased DAB staining) compared to normal tissues (Fig. 1C). There was a positive correlation
between SOAT1 protein and mRNA levels in tissue samples (Table SI; Spearman correlation analysis,
r=0.721, P<0.0001).
 | Figure 1.SOAT1 mRNA and protein levels in
tissue samples. Samples were collected from control subjects and
endometrial cancer (EC) patients diagnosed with early (Stage I–II)
or advanced (Stage III–IV) disease. Box plots show medians
(interquartile ranges) and whiskers (the minimum and maximum
values). Asterisks indicate statistically significant difference
compared to control, **P<0.01. (A) SOAT1 mRNA transcript levels
assessed in endometrial tissue via RT-qPCR (Control, n=11; early
stage EC, n=9; advanced stage EC, n=8; triplicate experiments). (B)
SOAT1 protein expression levels in tissue assessed via ELISA
(control, n=15; early stage EC, n=9; advanced stage EC, n=8;
triplicate experiments). (C) SOAT1 expression shown by DAB staining
(brown) in human normal endometrium, low stage and high stage EC
tumor samples. Representative images were taken with an inverted
microscope (Olympus H4-100, CCD camera) and a 10X objective. n,
number of samples. Insets show images obtained with a 40X
objective. EC, endometrial cancer; SOAT1, sterol-o-acyl transferase
1. |
SOAT1 expression evaluation in UALCAN
and cBioportal cancer databases
We used the UALCAN platform (http://ualcan.path.uab.edu) to validate SOAT1
expression at both mRNA and protein levels in endometrial cancer
and matched normal tissues. Statistical analysis in the UALCAN
database revealed that SOAT1 expression was significantly higher in
the primary EC group compared with the normal group (P<0.0001)
at both mRNA and protein levels (Fig.
S1). According to the cBioPortal cancer genomics database
(https://www.cbioportal.org/), genetic
alteration of SOAT1 gene was reported in 6% of EC patients (83 of
1638 patients), most of which are copy number amplifications.
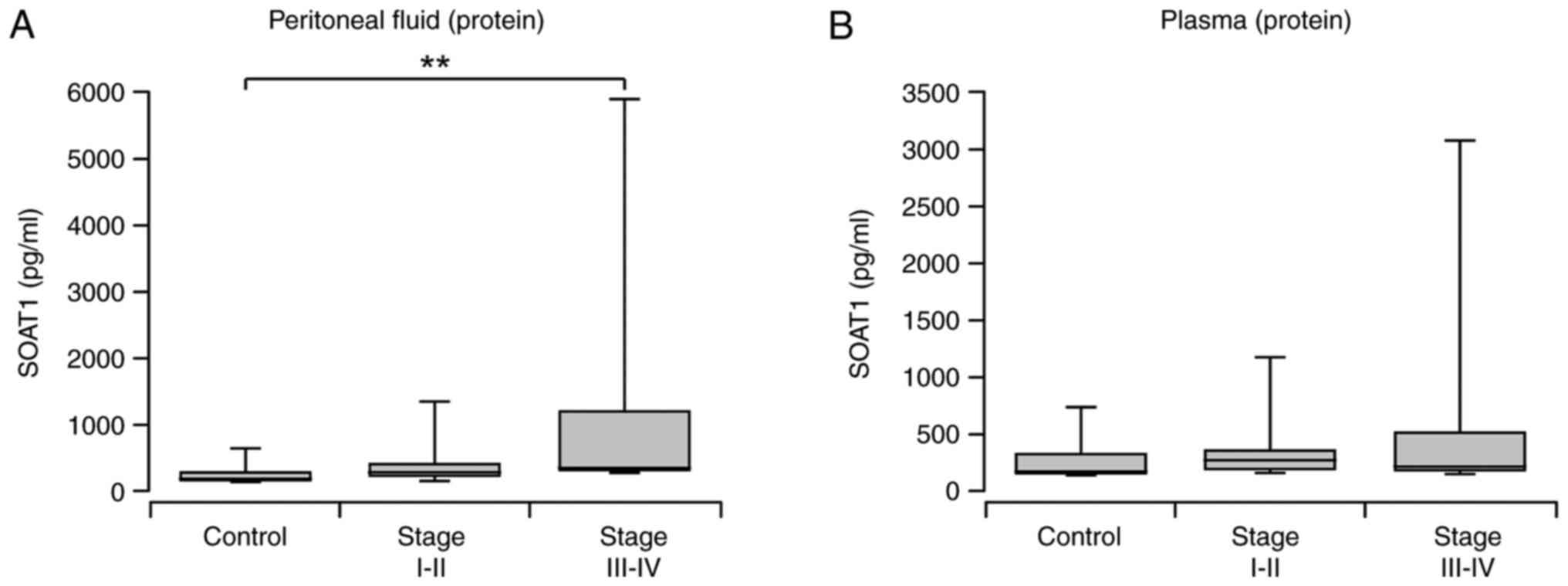
SOAT1 elevation in peritoneal fluid of
EC patients
Peritoneal fluid collected from subjects diagnosed
with EC had significantly higher levels of SOAT1 protein compared
to those in peritoneal fluid collected from control subjects
(P=0.0082; Mann-Whitney non-parametric U test; data not shown).
When dividing the EC group by stage, statistical significance was
only observed in the advanced stage group (P=0.0015; Kruskal-Wallis
test with Dunn's post hoc correction; Fig. 2A). No significant difference was
observed between early and advanced EC groups. As shown in Table SI, peritoneal fluid SOAT1 protein
levels positively correlated with tissue SOAT1 mRNA (Spearman
r=0.506, P=0.008) and tissue SOAT1 protein (Spearman r=0.388,
P=0.049).
Plasma SOAT1 concentration in EC and
control group
Plasma SOAT1 levels did not differ between the EC
and control groups (P>0.05; Mann-Whitney non-parametric U test;
data not shown). Early or advanced stages did not differ
significantly from the control group or between stages (P>0.05;
Kruskal-Wallis test with Dunn's post hoc correction; Fig. 2B). The plasma SOAT1 protein and
tissue SOAT1 mRNA transcript levels did not correlate (Table SI; Spearman r=0.342, P=0.081).
Correlation between SOAT1 protein
concentrations in tissue, peritoneal fluid, and plasma
To determine if peritoneal fluid and plasma SOAT1
levels can predict tumor SOAT1 status, we assessed the correlation
between peritoneal fluid, plasma and tissue SOAT1 protein levels.
As shown in Fig. S2A, peritoneal
fluid and tissue SOAT1 levels correlated positively (Spearman
r=0.388, P=0.049) but plasma SOAT1 did not correlate with tissue
(Fig. S2B, Spearman r=0.324,
P>0.05) or peritoneal fluid SOAT1 levels (Fig. S2C, Spearman r=0.231,
P>0.05).
CE, TC, and FC levels in peritoneal
fluid and plasma
TC, FC, and CE levels did not differ significantly
in the peritoneal fluid of women with EC compared to the control
group (P>0.05; Mann-Whitney non-parametric U test; data not
shown). However, comparing the control group to the early and
advanced stage EC groups separately, significantly higher levels of
TC and CE were observed in advanced stage EC group than control
group (P=0.0473 and P=0.0293, respectively; Kruskal-Wallis test
with Dunn's post hoc correction; Fig.
3A-C). Interestingly, significant difference in CE levels were
observed between early and advanced EC groups (P=0.0033; Fig. 3C). There was a strong positive
correlation between TC, FC, and CE levels in peritoneal fluid
(P<0.0001). FC levels did not differ significantly between
malignant and control groups (Fig.
3B). Plasma levels of TC, FC, and CE did not differ between the
EC and control groups (P>0.05; Mann-Whitney non-parametric U
test; data not shown). No significant differences were observed
when compared between control, early and advanced stage groups
(P>0.05; Kruskal-Wallis test with Dunn's post hoc correction;
Fig. 3D-F).
 | Figure 3.Total cholesterol (TC), free
cholesterol (FC) and cholesteryl ester (CE) levels in biological
samples. Samples were collected from control subjects (n=12) and
endometrial cancer (EC) patients diagnosed with early (Stage I–II;
n=14) or advanced (Stage III–IV; n=14) disease. Lipids from
peritoneal fluid and plasma were quantified using Total Cholesterol
and Cholesteryl Ester Colorimetric Assay Kit. Box plots show
medians (interquartile ranges) and whiskers (the minimum and
maximum values). Asterisks indicate statistically significant
difference between the indicated groups, *P<0.05; **P<0.01.
Experiments were performed in triplicate. (A) TC in peritoneal
fluid from control, early stage and advanced stage EC patients; (B)
FC in peritoneal fluid from control, early stage and advanced stage
EC patients; (C) CE in peritoneal fluid from control, early stage
and advanced stage EC patients; (D) TC in plasma from control,
early stage and advanced stage EC patients; (E) FC in plasma from
control, early stage and advanced stage EC patients; (F) CE in
plasma from control, early stage and advanced stage EC patients.
EC, endometrial cancer; SOAT1, sterol-o-acyl transferase 1; TC,
total cholesterol; FC, free cholesterol; CE, cholesterol ester. |
Correlation between SOAT1 and CE
levels and in endometrial tissue, plasma and peritoneal fluid
Table SI shows a
significant correlation between CE and SOAT1 protein levels in
peritoneal fluid (spearman r=0.453, P=0.005). Interestingly, a
strong correlation was also observed between peritoneal fluid CE
levels and tissue SOAT1 protein (Spearman r=0.467, P=0.016). This
may also imply that tissue SOAT1 regulates CE secretion into the
peritoneal fluid, and that CE levels in peritoneal fluid reflect
tissue SOAT1 levels and tumor aggressiveness. In contrast, plasma
CE did not show any correlation with plasma, tissue or peritoneal
fluid SOAT1 protein.
Correlation between Ki67 expression
and SOAT1 (protein, mRNA), CE, TC and FC levels
Tumor tissue collected from subjects diagnosed with
EC has higher levels of Ki67 mRNA transcripts than endometrial
tissue collected from control subjects (P=0.0012; Mann-Whitney
non-parametric U test; data not shown). When multiple comparisons
were done between the control, early EC (stage I–II) and advanced
EC groups (stage III–IV), Ki67 mRNA levels were significantly
higher only in advanced EC group (P=0.0001, Kruskal-Wallis test
with Dunn's post hoc correction, Fig.
4A). Interestingly, significant difference in Ki67 mRNA levels
were observed between early and advanced EC groups (P=0.0152;
Fig. 4A). Spearman correlation
analysis (Table II) indicated a
significant positive correlation between tissue Ki67 and SOAT1 mRNA
transcripts (r=0.581, P=0.0015). Additionally, Ki67 mRNA correlated
significantly with SOAT1 protein from tissue (r=0.506, P=0.007) and
peritoneal fluid (r=0.614, P=0.0011). A positive association was
also seen between Ki67mRNA and peritoneal fluid CE (r=0.628,
P=0.0008) and TC levels (r=0.562, P=0.0035). Moreover, high SOAT1
immunostaining was associated to high Ki67 expression in tissue
sections (Fig. 4B). EC tumors of
advanced stage and high grade had the highest levels of SOAT1
immunostaining. There was no significant correlation between Ki67
and any of the plasma analytes.
 | Table II.Spearman's rank coefficient analyses
of Ki67 correlation with SOAT1, TC, FC and CE levels. |
Table II.
Spearman's rank coefficient analyses
of Ki67 correlation with SOAT1, TC, FC and CE levels.
| Ki67 (mRNA) | Spearman r | P-value |
|---|
| vs. Grade | 0.660 | 0.0010a |
| vs. Tissue SOAT1
protein | 0.506 | 0.0070a |
| vs. Tissue SOAT1
mRNA | 0.581 | 0.0015a |
| vs. Peritoneal
Fluid SOAT1 protein | 0.614 | 0.0011a |
| vs. Plasma SOAT1
protein | 0.469 | 0.0157b |
| vs. Peritoneal
Fluid TC | 0.562 | 0.0035a |
| vs. Peritoneal
Fluid FC | 0.439 | 0.0280b |
| vs. Peritoneal
Fluid CE | 0.628 | 0.0008c |
| vs. Plasma TC | −0.383 | 0.0533d |
| vs. Plasma FC | −0.287 | 0.1554d |
| vs. Plasma CE | −0.314 | 0.1180d |
Assessment of SOAT1 and CE as
diagnostic markers for EC
The diagnostic potential of SOAT1 and CE in plasma,
peritoneal fluid, and tissue was evaluated using ROC curves to
determine optimal cut-off levels for SOAT1 and CE in EC diagnosis.
As shown in Table SII, assessing
SOAT1 levels in peritoneal fluid and EC tissue has better
diagnostic power as compared to plasma levels. Tissue SOAT1 mRNA
and protein has the highest area under the curve (AUC) of ROC
(0.834 and 0.893 respectively) followed peritoneal fluid SOAT1
(0.767). Other peritoneal fluid or plasma analytes have not shown
significant diagnostic potential and thus may not be ideal for
diagnostic assessments. Tissue SOAT1 protein had a sensitivity of
59% and a specificity of 100% at a cut off concentration of
>1.56 pg/mg protein. Tissue SOAT1 mRNA had a sensitivity of 82%
and a specificity of 100% at a cut off concentration of
>1.67-fold change. Peritoneal fluid SOAT1 level had a
sensitivity of 80% and a specificity of 67% at a cut off
concentration of >234 pg/ml.
Prognostic evaluation of SOAT1
expression in endometrial cancer (Bioinformatics analysis)
Survival data could not be analyzed in our study
population due to the short time span of the study and the small
sample size. However, we used cBioPortal database to evaluate the
significance of SOAT1 expression as a prognostic biomarker in EC.
According to cBioportal, comparison of groups based on their median
SOAT1 gene expression, revealed that subjects with low SOAT1
expression survived significantly longer than those with high SOAT1
expression (Log Rank test P<0.0001; Fig. S3). This suggests that SOAT1
expression can be a potential prognostic marker for EC. Further
bioinformatics analyses are needed to confirm the utility of SOAT1
expression and to establish prognostic algorithms.
Assessment of the potential influence
of comorbidities on the association of SOAT1/CE with EC
BMI differed significantly between the non-malignant
and EC groups (P=0.003, data not shown). This result is consistent
with the previously established correlation between BMI and
increased EC risk (14). Spearman
correlation analysis showed a significant correlation between BMI
and EC grade and stage (spearman r=0.362, P=0.025, and r=0.298,
P=0.049, respectively). Moreover. BMI positively correlated with
tissue SOAT1 levels (spearman r=0.387, P=0.042). Comorbidities such
as obesity, hyperlipidemia, diabetes, hypertension, and
hyperthyroidism are known to alter cholesterol metabolism,
therefore we analyzed the impact of BMI and other comorbidities on
SOAT1 and CE levels. When these co-variables were adjusted in
logistic regressions (Model 2), the association of SOAT1 or CE
levels with EC remained statistically significant (P<0.05;
Table SIII).
Discussion
Despite advances in the development of new
therapeutic strategies, the prognosis for advanced EC patients
remains dismal. Indeed, histologic subtype and FIGO staging alone
may not effectively predict prognosis due to the molecular
heterogeneity of EC (21). There is
an urgent need to identify sensitive and specific molecular markers
for prognosis to achieve personalized treatment and improve
clinical outcomes. In this study, the prognostic relevance of SOAT1
in EC was investigated. SOAT1 expression in tumor tissue,
peritoneal fluid and plasma were comprehensively analyzed in
patients diagnosed with EC and normal healthy subjects. SOAT1 and
CE levels, were significantly elevated in tumor tissue and
peritoneal fluid samples collected from the EC group. Significant
positive associations between CE and SOAT1, SOAT1/CE and Ki67, and
SOAT1/CE and poor overall survival in EC patients suggest that
SOAT1/CE may be associated with malignancy, aggressiveness, and
poor prognosis, thus may serve as potential biomarker(s) for
prognosis and target-specific treatment in EC.
Chemo-resistance and metastatic spread continue to
be key challenges for EC patients, and various chemotherapy
resistance mechanisms have been proposed. Many malignancies have
abnormal cholesterol metabolism, which contributes to tumor
aggressiveness and resistance to treatment (6–9,11–14).
As a result, cholesterol metabolism has been identified as a
potential cancer therapeutic target. Numerous studies have
established SOAT1/CE as a new prognostic marker in various cancers
(11–14), but few have examined its role in
endometrial cancer. Previously, we demonstrated the prognostic
significance of CE and SOAT1 in ovarian cancer (15,17).
Ovarian and endometrial malignancies have similar epidemiological
and genetic characteristics (22).
Ovulatory cycles are linked to both endometrial and ovarian cancer
risk due to accumulation of p53 or PTEN mutations associated with
reproductive tissue turnover (23).
Due to these common factors, it is intriguing to investigate
SOAT1/CE levels in endometrial cancer as well. In this study, we
systematically analyzed SOAT1 expression and CE levels in
peritoneal fluid, plasma and tumor tissue in EC patients and
compared them to normal controls. We also correlated the expression
of these mediators with a marker of tumor aggressiveness. Ki67.
SOAT1/CE levels in plasma were not significantly
different between normal and EC cohorts, implying that SOAT1 cannot
be used as a non-invasive biomarker for diagnosis or prognosis. In
tumor tissue, significant SOAT1/CE levels were observed in both
early and advanced stage EC group compared to normal group. Our
result is in accordance with TCGA data and other similar studies
which reported 3–7 fold higher SOAT1 levels in endometrial cancer
tissue as compared to normal secretory endometrium (24). SOAT1 and CE-rich tumors were
associated with higher aggressive potential and poor survival in
many cancers (16). cBioportal
reported poor overall survival with higher SOAT1 expression.
Consistent with these reports, we observed a significant positive
correlation between SOAT1 (protein and mRNA), CE levels and Ki67,
an established tumor proliferation marker known to predict disease
outcome in many human malignancies. Many studies demonstrated a
positive relation between high proliferation rates and poor
survival or increased recurrence (25).
SOAT1 expression in peritoneal fluid, on the other
hand, was low in the normal and early stage groups but
significantly higher in advanced stage EC. The peritoneal fluid
(tumor microenvironment) contains oncogenic cellular and acellular
mediators that promote tumor invasiveness and treatment resistance
(26,27). In ascites, cholesterol levels are
greatly elevated and may be utilized as a marker for malignant
ascites (28). Indeed, our research
revealed SOAT1 and CE as additional key components of the EC tumor
microenvironment that vary based on stage/grade and may contribute
to cancer aggressiveness and treatment resistance.
While cholesterol is required for cell
proliferation, excessive cellular cholesterol is toxic (29). SOAT1 mediated cholesterol
esterification has been hypothesized to keep signaling pathways
active and protect cells from FC toxicity, while also evading
feedback inhibition and maintaining the high metabolic activity
required for disease progression (7–10).
Corroboration for this idea was found in the form of a significant
positive connection between SOAT1 and CE levels (12–17).
As a result, inhibiting CE generation may suppress tumor
proliferation and disease progression. Indeed, suppression of SOAT1
by pharmacologic (avasimibe) or genetic (SOAT1 shRNA) agents
decreased cancer cell proliferation, migration, and invasion in
vitro and in vivo, as observed in colon, pancreas,
prostate, and EOC models (12,14,15,30).
The mechanism(s) underlying the relation between CE accumulation
and cancer aggressiveness has not been precisely established.
Inhibiting CE generation was linked to inactivation of SREBP1
leading to downregulation of SREBP1 regulated processes such as
caveolin-1/MAPK activation, reduced LDLr expression and reduced
LDLr mediated uptake of essential fatty acids, such as arachidonic
acid, a proliferation factor in many cancers (13,14,31).
According to Yue et al 2014, CE accumulation
is a consequence of the loss of the tumor suppressor PTEN
(phosphatase and tensin homolog), and of the subsequent activation
of the PI3K/AKT pathway (13).
Other possible signaling mechanisms include the downregulation of
Wnt/β-catenin, pAkt and ERK1/2 pathways and inhibition of TLR4
(Toll-like receptor 4), all of which play significant roles in
cancer cell proliferation, metastatic cancer recurrence, and
chemotherapy resistance (32,33).
For all of the gynecologic malignancies, Wnt signaling is being
evaluated as a possible therapeutic target (34–38),
therefore this pathway can be targeted via inhibition of CE and
SOAT1.
Chemoresistance and metastatic dissemination remain
major hurdles for EC patients. Abnormal accumulation of SOAT1/CE
may lead to resistance to drugs such as tamoxifen, gemcitabine,
imatinib and cisplatin as shown in various in vitro and
in vivo cancer models (15,30,39,40).
Therefore, inhibition of CE accumulation may enhance the
sensitivity of cancer cells to drugs. We previously shown that
SOAT1-inhibited SKOV-3 and IGROV-1 cell lines were more sensitive
to cisplatin than their respective controls (15). Although we do not have evidence
supporting a specific mechanism(s), others have reported that SOAT1
inhibition and depletion of CE lead to inhibition of PI3K/Akt,
caveolin and MAPK pathways contributing to increased sensitivity to
drugs (13,40). Further studies are needed to fully
elucidate the mechanisms linking cholesterol metabolism and cancer
drug resistance in EC.
We concluded that SOAT1/CE levels are associated
with malignancy and tumor aggressiveness, and thus can be
considered druggable targets that reflect molecular modifications
during endometrial cancer, especially in advanced stages. Tissue
and peritoneal fluid SOAT1/CE levels, in addition to predictable
clinical-pathological characteristics, could be utilized to
categorize patients into groups with varying risk of recurrence for
better treatment guidance. Additional research and a larger sample
cohort are needed to validate SOAT1/CE as therapeutic targets in
EC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was supported in part by the Laboratory Directed
Research and Development Program, of Oak Ridge National Laboratory
awarded to ABF and LB. This program is managed by UT-Battelle, LLC,
for the U.S. Department of Energy. Oak Ridge National Laboratory is
managed by UT-Battelle, LLC, for the U.S. Department of Energy
under contract DEAC05-00OR22725. Funding was also provided by the
Simmons Cancer Institute at Southern Illinois University School of
Medicine through the Team Science Grant (TSG) awarded to ABF and
LB.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LB and ABF provided resources and acquired funding
for the investigation. VNA and LB supervised the study, and
participated in its conceptualization and design. LB, PDS, EMS, KG
and TW collected clinical samples, administered sample allocation,
developed and submitted the clinical research protocol, and
reviewed and reporting any unanticipated problems and protocol
deviations to the Institutional Review Board. ABF participated in
the processing and storage of clinical samples. VNA, ML and ZP
conducted all the experiments. VNA, ML and PDS participated in the
formal analysis of data. VNA and ML confirm the authenticity of all
the raw data. VNA wrote the original draft. VNA and PDS designed
the figures and tables. All authors reviewed, edited, read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the local Institutional
Review Board (Springfield Committee for Research Involving Human
Subjects) under protocol 16–493. Written informed consent was
obtained from all participants for participation in the study and
use of their biological samples and clinical data.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
SOAT1
|
sterol-O-acyl transferase 1
|
|
ACAT-1
|
acyl-CoA cholesterol acyl
transferase
|
|
CE
|
cholesterol ester
|
|
FC
|
free cholesterol
|
|
TC
|
total cholesterol
|
References
|
1
|
Bendifallah S, Canlorbe G, Raimond E,
Hudry D, Coutant C, Graesslin O, Touboul C, Huguet F, Cortez A,
Daraï E and Ballester M: A clue towards improving the European
society of medical oncology risk group classification in apparent
early stage endometrial cancer? Impact of lymphovascular space
invasion. Br J Cancer. 110:2640–2646. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Colombo N, Creutzberg C, Amant F, Bosse T,
González-Martín A, Ledermann J, Marth C, Nout R, Querleu D, Mirza
MR, et al: ESMO-ESGO-ESTRO consensus conference on endometrial
cancer: Diagnosis, treatment and follow-up. Ann Oncol. 27:16–41.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gilks CB, Oliva E and Soslow RA: Poor
interobserver reproducibility in the diagnosis of high-grade
endometrial carcinoma. Am J Surg Pathol. 37:874–881. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aghajanian C, Filiaci V, Dizon DS, Carlson
JW, Powell MA, Secord AA, Tewari KS, Bender DP, O'Malley DM,
Stuckey A, et al: A phase II study of frontline
paclitaxel/carboplatin/bevacizumab,
paclitaxel/carboplatin/temsirolimus, or
ixabepilone/carboplatin/bevacizumab in advanced/recurrent
endometrial cancer. Gynecol Oncol. 150:274–281. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Makker V, Taylor MH, Aghajanian C, Oaknin
A, Mier J, Cohn AL, Romeo M, Bratos R, Brose MS, DiSimone C, et al:
Lenvatinib plus pembrolizumab in patients with advanced endometrial
cancer. J Clin Oncol. 38:2981–2992. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chang TY, Li BL, Chang CC and Urano Y:
Acyl-coenzyme A: Cholesterol acyltransferases. Am J Physiol
Endocrinol Metab. 297:E1–E9. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Danilo C, Gutierrez-Pajares JL, Mainieri
MA, Mercier I, Lisanti MP and Frank PG: Scavenger receptor class B
type I regulates cellular cholesterol metabolism and cell signaling
associated with breast cancer development. Breast Cancer Res.
15:R872013. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paillasse MR, de Medina P, Amouroux G,
Mhamdi L, Poirot M and Silvente-Poirot S: Signaling through
cholesterol esterification: A new pathway for the cholecystokinin 2
receptor involved in cell growth and invasion. J Lipid Res.
50:2203–2211. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tosi MR and Tugnoli V: Cholesteryl esters
in malignancy. Clin Chim Acta. 359:27–45. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mayengbam SS, Singh A, Pillai AD and Bhat
MK: Influence of cholesterol on cancer progression and therapy.
Transl Oncol. 14:1010432021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Antalis CJ, Arnold T, Rasool T, Lee B,
Buhman KK and Siddiqui RA: High ACAT1 expression in estrogen
receptor negative basal-like breast cancer cells is associated with
LDL-induced proliferation. Breast Cancer Res Treat. 122:661–670.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bemlih S, Poirier MD and El Andaloussi A:
Acyl-coenzyme A: Cholesterol acyltransferase inhibitor avasimibe
affect survival and proliferation of glioma tumor cell lines.
Cancer Biol Ther. 9:1025–1032. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yue S, Li J, Lee SY, Lee HJ, Shao T, Song
B, Cheng L, Masterson TA, Liu X, Ratliff TL and Cheng JX:
Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT
activation underlies human prostate cancer aggressiveness. Cell
Metab. 19:393–406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Gu D, Lee SS, Song B, Bandyopadhyay
S, Chen S, Konieczny SF, Ratliff TL, Liu X, Xie J and Cheng JX:
Abrogating cholesterol esterification suppresses growth and
metastasis of pancreatic cancer. Oncogene. 35:6378–6388. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ayyagari VN, Wang X, Diaz-Sylvester PL,
Groesch K and Brard L: Assessment of acyl-CoA cholesterol
acyltransferase (ACAT-1) role in ovarian cancer progression-an in
vitro study. PLoS One. 15:e02280242020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Gonzalo-Calvo D, López-Vilaró L,
Nasarre L, Perez-Olabarria M, Vázquez T, Escuin D, Badimon L,
Barnadas A, Lerma E and Llorente-Cortés V: Intratumor cholesteryl
ester accumulation is associated with human breast cancer
proliferation and aggressive potential: A molecular and
clinicopathological study. BMC Cancer. 15:4602015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ayyagari V, Li M, Pasman Z, Wang X, Louis
S, Diaz-Sylvester P, Groesch K, Wilson T and Brard L: Assessment of
the diagnostic and prognostic relevance of ACAT1 and CE levels in
plasma, peritoneal fluid and tumor tissue of epithelial ovarian
cancer patients-a pilot study. BMC Cancer. 22:3872022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Secord AA, Hasselblad V, Von Gruenigen VE,
Gehrig PA, Modesitt SC, Bae-Jump V and Havrilesky LJ: Body mass
index and mortality in endometrial cancer: A systematic review and
meta-analysis. Gynecol Oncol. 140:184–190. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miao R, Badger TC, Groesch K,
Diaz-Sylvester PL, Wilson T, Ghareeb A, Martin JA, Cregger M, Welge
M, Bushell C, et al: Assessment of peritoneal microbial features
and tumor marker levels as potential diagnostic tools for ovarian
cancer. PLoS One. 15:e02277072020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schmittgen TD and Livak KJ: Analyzing
real-time PCR data by the comparative C(T) method. Nat Protoc.
3:1101–1108. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Piulats JM, Guerra E, Gil-Martín M,
Roman-Canal B, Gatius S, Sanz-Pamplona R, Velasco A, Vidal A and
Matias-Guiu X: Molecular approaches for classifying endometrial
carcinoma. Gynecol Oncol. 145:200–207. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cramer DW: The epidemiology of endometrial
and ovarian cancer. Hematol Oncol Clin North Am. 26:1–12. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Merritt MA and Cramer DW: Molecular
pathogenesis of endometrial and ovarian cancer. Cancer Biomark.
9:287–305. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Omsjø IH and Norum KR: Cholesterol
esterification in human secretory endometrium and in endometrial
cancer tissue. Demonstration of microsomal acyl-CoA-cholesterol
acyl-transferase (ACAT) activity. Acta Obstet Gynecol Scand.
64:473–476. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Odagiri T, Watari H, Hosaka M, Mitamura T,
Konno Y, Kato T, Kobayashi N, Sudo S, Takeda M, Kaneuchi M and
Sakuragi N: Multivariate survival analysis of the patients with
recurrent endometrial cancer. J Gynecol Oncol. 22:3–8. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sahoo SS, Zhang XD, Hondermarck H and
Tanwar PS: The emerging role of the microenvironment in endometrial
cancer. Cancers (Basel). 10:4082018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hanahan D and Coussens LM: Accessories to
the crime: Functions of cells recruited to the tumor
microenvironment. Cancer cell. 21:309–322. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rana SV, Babu SG and Kocchar R: Usefulness
of ascitic fluid cholesterol as a marker for malignant ascites. Med
Sci Monit. 11:Cr136–Cr142. 2005.PubMed/NCBI
|
|
29
|
Tabas I: Consequences of cellular
cholesterol accumulation: Basic concepts and physiological
implications. J Clin Invest. 110:905–911. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li J, Qu X, Tian J, Zhang JT and Cheng JX:
Cholesterol esterification inhibition and gemcitabine
synergistically suppress pancreatic ductal adenocarcinoma
proliferation. PLoS One. 13:e01933182018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu L, Xu HX, Wang WQ, Wu CT, Chen T, Qin
Y, Liu C, Xu J, Long J, Zhang B, et al: Cavin-1 is essential for
the tumor-promoting effect of caveolin-1 and enhances its
prognostic potency in pancreatic cancer. Oncogene. 33:2728–2736.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee HJ, Li J, Vickman RE, Li J, Liu R,
Durkes AC, Elzey BD, Yue S, Liu X, Ratliff TL and Cheng JX:
Cholesterol esterification inhibition suppresses prostate cancer
metastasis by impairing the Wnt/β-catenin pathway. Mol Cancer Res.
16:974–985. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Scott CC, Vossio S, Vacca F, Snijder B,
Larios J, Schaad O, Guex N, Kuznetsov D, Martin O, Chambon M, et
al: Wnt directs the endosomal flux of LDL-derived cholesterol and
lipid droplet homeostasis. EMBO Rep. 16:741–752. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Goad J, Ko YA, Kumar M, Jamaluddin MFB and
Tanwar PS: Oestrogen fuels the growth of endometrial hyperplastic
lesions initiated by overactive Wnt/β-catenin signalling.
Carcinogenesis. 39:1105–1116. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Meng F, Xu Y, Yang S, Xiao M, Chen
X and Lou G: Overexpression of Wnt7a is associated with tumor
progression and unfavorable prognosis in endometrial cancer. Int J
Gynecol Cancer. 23:304–311. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Coopes A, Henry CE, Llamosas E and Ford
CE: An update of Wnt signalling in endometrial cancer and its
potential as a therapeutic target. Endocr Relat Cancer. Aug
9–2018.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang Y, Hanifi-Moghaddam P, Hanekamp EE,
Kloosterboer HJ, Franken P, Veldscholte J, van Doorn HC, Ewing PC,
Kim JJ, Grootegoed JA, et al: Progesterone inhibition of
Wnt/beta-catenin signaling in normal endometrium and endometrial
cancer. Clin Cancer Res. 15:5784–5793. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshioka S, King ML, Ran S, Okuda H,
MacLean JA II, McAsey ME, Sugino N, Brard L, Watabe K and Hayashi
K: WNT7A regulates tumor growth and progression in ovarian cancer
through the WNT/β-catenin pathway. Mol Cancer Res. 10:469–482.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hultsch S, Kankainen M, Paavolainen L,
Kovanen RM, Ikonen E, Kangaspeska S, Pietiäinen V and Kallioniemi
O: Association of tamoxifen resistance and lipid reprogramming in
breast cancer. BMC Cancer. 18:8502018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liscovitch M and Lavie Y: Multidrug
resistance: A role for cholesterol efflux pathways? Trends Biochem
Sci. 25:530–534. 2000. View Article : Google Scholar : PubMed/NCBI
|