Introduction
Immune thrombocytopenia purpura (ITP) is an
autoimmune disease that destroys platelets. In certain patients,
ITP can be resolved after appropriate treatment in the early stages
of the disease. However, some patients with ITP suffer relapse or
have continuous progression even after treatment. ITP is a
recognized complication in patients with non-Hodgkin's lymphoma
(NHL) (1,2). The incidence of NHL after ITP is low,
and in one previous study, only 76 out of 8,067 patients with ITP
had NHL, so the increased risk of NHL could not be considered as
very large (3). The incidence of
immune platelet destruction in patients with NHL is ~5% (4–6);
however, most cases occur in patients with chronic lymphoblastic
leukemia. To the best of our knowledge, there have been no reported
cases of secondary lymphoproliferative diseases in patients with
primary ITP. Diffuse large B-cell lymphoma (DLBCL) is the most
common type of NHL and accounts for 30–40% of NHL (7). DLBCL is characterized by a broad
heterogeneity in its clinical manifestations and prognoses
(8).
Monoclonal gammopathy (MG) is a disease in which the
body produces a higher concentration of immunoglobulins due to the
abnormal proliferation of monoclonal B cells, tending to result in
tumor formation. The association between MG and well-differentiated
B-cell NHL has been reported (7).
MG has been reported in certain patients with DLBCL; however, the
number of MG cases in patients with DLBCL is still insufficient for
further statistical research (9).
Little is known about the pathogenesis and prognosis of patients
with DLBCL coinciding with MG (10). To the best of our knowledge, there
have been no previous case reports describing DLBCL secondary to
ITP. The current study presents the case of a patient diagnosed
with DLBCL and MG secondary to ITP.
Case report
A 67-year-old man presented to the Department of
Hematology, Gansu Provincial People's Hospital (Lanzhou, China) in
August 2009 complaining of oral hematoma and gingival bleeding. A
routine blood examination demonstrated a white blood cell count of
9.3×109/l (reference range, 4.0-10.0×109/l),
a platelet count of 6.0×109/l (reference range,
100.0-300.0×109/l) and a hemoglobin level of 153 g/l
(reference range, 120–165 g/l). Primary ITP was diagnosed after
bone marrow cytomorphological examination of the anterior superior
iliac spine, and ultrasound examination (used for finding any
masses on the body surface) excluded other primary diseases. A
treatment regime consisting of glucocorticoid, γ immune globulin
and danazol was initiated, but the platelet count remained at
10.0×109/l. Upon failure of this ITP treatment, splenic
embolization was performed on the patient. The patient's platelet
count increased to 120×109/l after 1 month. Due to
marked bleeding in both lower limbs, the patient was admitted to
the 940th Hospital of the Joint Logistics Support Force of the
Chinese People's Liberation Army (Lanzhou, China) in November 2009,
100 days after the initial presentation. A routine blood
examination demonstrated a white blood cell count of
7.9×109/l, a platelet count of 1.0×109/l and
a hemoglobin level of 95 g/l. B-scan ultrasonography of the abdomen
demonstrated normal splenic color blood flow, which indicated a
failure of the splenic embolization. Maturation arrest of
megakaryocytes was found upon morphological examination of the bone
marrow cells, which indicated ITP (Fig.
1A and B).
The patient underwent a splenectomy in January 2010,
152 days after the initial presentation, and the platelet count
increased to 140×109/l. In May 2016, subsequent B-scan
ultrasonography of the pelvis revealed multiple enlarged lymph
nodes (3.0×2.5 cm) in the left inguinal region. The patient
reported pain in the anterior tibial region due to the compression
of the enlarged lymph nodes of the left leg, which limited its use.
A biopsy of the lymph nodes demonstrated DLBCL in the left inguinal
lymph node originating from the germinal center, based on
assessment using the Hans algorithm (11). Immunohistochemical analysis of the
tissue from the left inguinal lymph node demonstrated the presence
of CD20+, Bcl-6+, interferon regulatory
factor-1−, CD3− and CD10+ B cells.
IgM was highly expressed in the cytoplasm of certain neoplastic
cells, but the protein expression levels of IgG, CD38 and CD138
were below detection limits.
Ki-67+ cells accounted for ~75% of the
tumor cells. The cells were negative for BCL-6 rearrangement as
assessed using fluorescence in situ hybridization (FISH).
Dysregulation of BCL-2 and MYC was also negative.
Whole-body bone scintigraphy revealed lesions that
indicated bone destruction. Abnormally high radioactivity was
demonstrated in the bilateral scapula, bilateral humeri and femurs,
bilateral anterior ribs, vertebral bodies, the left anterior iliac
spine, the upper tibia, and the left knee and ankle joints
(Fig. 2A and B). These lesions were
considered metastatic foci. The patient received eight courses of
an rituximab, cyclophosphamide, doxorubicin, vincristine and
prednisone (R-CHOP) regimen as follows: 375 mg/m2
rituximab, intravenous (IV), on day 0; 750 mg/m2
cyclophosphamide, 50 mg/m2 doxorubicin and 1.4
mg/m2 vincristine, IV, all on day 1; and 100 mg
prednisone, per os, on days 1–5. After R-CHOP treatment, a
positron emission tomography-computed tomography (PET-CT) scan
found no further bone destruction.
The patient returned for a follow-up examination in
May 2019, 3,555 days after the initial presentation. An
investigation of bone marrow cell morphology demonstrated that a
proportion of lymphocytes (19%) and plasmacyte (1.2%) were normal,
and bone marrow hyperplasia was active (Fig. 1C and D). A total of ~8% of nucleated
cells were lymphocytes, of which mature B lymphocytes accounted for
14.5%. No abnormal immune phenotype was observed in the
proliferative B lymphocytes. Enlarged lymph nodes (1.5×0.5 cm) were
found in the left neck upon superficial B-scan ultrasonography.
Immunofixation electrophoresis, using a 1% agarose gel,
demonstrated the presence of λ light chains and IgGs. Serum protein
electrophoresis demonstrated a γ band (40.7%), a β band (6.1%), an
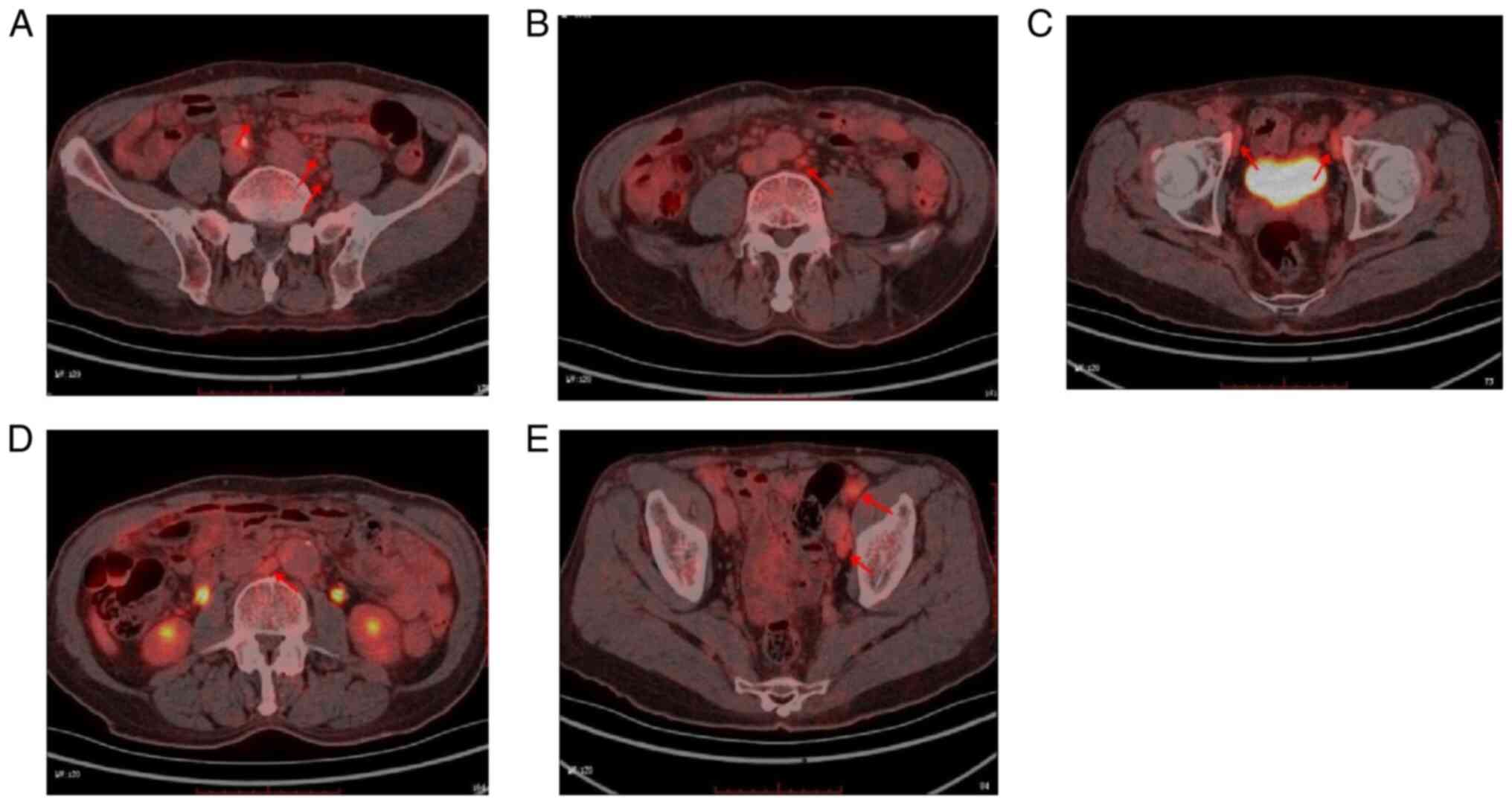
α2 band (6.5%) and an albumin band (44.6%). PET-CT demonstrated an
abnormal increase of F-18 fluorodeoxyglucose lymph node metabolism
in numerous body regions (Fig.
3A-E), which was consistent with metabolic changes in the
progressive disease stage after lymphoma treatment.
The malignant lymphoma showed a progressive increase
in globulin and IgG levels (Fig. 4A and
B), and the patient was diagnosed with DLBCL and MG secondary
to ITP. The patient was administered one course of the R-CHOP
regimen after the DLBCL diagnosis. The patient's levels of
globulins (42 g/l; reference range, 20–40 g/l) and IgG (2,520
mg/dl; reference range, 700–1,660 mg/dl) decreased but remained
higher than normal. Immunofixation electrophoresis testing
demonstrated the presence of λ light chains. The patient remains
under follow-up treatment using the R-CHOP regimen at the time of
writing.
Methods
Immunohistochemical analysis
Tissue from the left inguinal lymph node were fixed
using 4% paraformaldehyde at room temperature for 24 h, paraffin
embedded and sliced into 5 µm sections. Sections were dewaxed by
heating to 65°C for 5 min, washed with xylene three times for 10
min and rehydrated using a descending ethanol series. Antigen
repair was performed by incubation with EDTA for 3 min. Sections
were incubated with 5% bovine serum albumin (Shengshi Technology
Co., Ltd.) for 10 min at 37°C to block endogenous peroxidase
activity and then washed three times with PBS for 3 min. Sections
were incubated with primary antibodies against CD20 (L26) (1:100;
cat. no. M0755; Dako; Agilent Technologies, Inc.) for 60 min at
25°C. Sections were incubated with Goat Anti-Mouse IgG HRP
Conjugate secondary antibodies (1:500; cat. no sc-2005; Santa Cruz
Biotechnology, Inc.) for 30 min at 25°C. Sections were imaged using
a BX53 biological light microscope (magnification ×20; Olympus
Corporation).
FISH
Vysis LSI IGH/BCL2 Dual Color, Dual Fusion
Translocation Probe Kit (Abbott Molecular Inc.) was used to perform
FISH. Tissues were fresh frozen at −20°C and cut into 5 µm
sections. Sections were fixed using a methanol and glacial acetic
acid mixture (30 and 10 ml, respectively) at 25°C For 10 min.
Colcemid (0.1%; MedChemExpress) was used to arrest the cells in
metaphase. Cells were centrifuged at 447.2 × g for 10 min at room
temperature, the supernatant was discarded and 5 ml fixative (3:1
mixture of methanol to glacial acetic acid) was added for 10 min at
room temperature. The aforementioned process was repeated for
secondary fixation. KCl (0.075 M) was used as the hypotonic
solution with incubation for 25 min at 37°C. The suspension was
dropped on to dry glass slides.
The probe was thawed at room temperature, mixed
manually, and centrifuged at 600 × g for 10 min at 25°C. A total of
5 µl (10 ng/µl) probe working solution (50 ng DNA template, 0.3 µM
primer and 1 unit Taq DNA polymerase were required, and the total
reaction volume was 25 µl) with saline sodium citrate buffer (2X
SSC; 0.3 M NaCl, 0.03 M sodium citrate dihydrate, distilled water,
adjusted to pH 7.0 with hydrochloric acid or sodium hydroxide) was
used per sample and covered with a coverslip, which spread out the
probe. Liquid adhesive was used to seal the edges of the coverslip.
Slides were heated to 75°C for 2 min to denature the probe. Slides
were incubated at 37°C overnight for hybridization.
The coverslips were removed and slides were placed
in 72°C 0.4X SSC [60 mM NaCl, 20 mM Tris-HCl (pH 7.5), 0.5% SDS and
1 mM EDTA] solution for 2 min. Redundant probes were removed using
buffer (0.05% Tween-20/2× SSC solution: 75 nM NaCl, 37.5 mM sodium
citrate, 0.05% Tween-20, pH 7.0). Blocking was performed using 5%
bovine serum albumin (Sangon Biotech Co., Ltd.) at room temperature
for 1 h.
Slides were counterstained using 10 µl DAPI and
immediately covered with a coverslip and placed in the dark at 4°C
for 5–10 min. The slides were assessed using a fluorescence
microscope. First, the cell area was confirmed under a
low-magnification objective lens, and then a 40X objective lens was
used to select areas with better nuclear distribution (such as
areas in which a single nucleus could be distinguished). In order
to accurately observe and interpret the results of FISH, a
representative area for observation that accurately reflected the
distribution of the signals was selected. After selecting the
observation area, the FISH results of the nucleus were accurately
counted.
Immunofixation electrophoresis
The serum of the patient was centrifuged at 5,180 ×
g for 10 min at room temperature and the supernatant was collected.
Total protein was extracted using 20% ammonium sulfate and
separated by 2% agarose gel electrophoresis. The separated protein
was fixed and immunoprecipitated using an Immunoprecipitation Kit,
Protein A/G Plus Agarose (cat. no. GS4780; Beijing Biolab
Technology Co., Ltd.). The unprecipitated protein was removed using
absorbent paper and washed with distilled water. The protein was
stained using Coomassie brilliant blue at 37°C for 10 min and the
locations of these immunoprecipitation bands were compared with the
abnormal protein bands observed after electrophoresis.
Discussion
ITP is an autoimmune hemorrhagic disease
characterized by increased platelet destruction and inhibition of
platelet production (12,13). Patients display purpura in regions
of the body. B-cell production is increased in the spleen of
patients with ITP (14). Chen et
al (15) reported increased
antibody levels, and increased B-cell regulator and B-cell
activating factor levels in the plasma of patients with ITP.
Another study reported that the function of CD19+,
CD41hi and CD38hi regulatory B cells (Bregs),
which promoted peripheral immune tolerance in patients with ITP,
were impaired (16). In a previous
study, the levels of CD19+, CD24+ and
FOXP3+ Breg subsets in the spleen of patients with ITP
were increased (17). Impairment of
Bregs and B cells in patients with ITP was reported to lead to the
production of pathogenic antibodies, which triggered platelet
destruction and megakaryocyte-formation defects in the spleen and
liver (18).
One previous study reported a significant
correlation between platelet count and the number of megakaryocytes
in the bone marrow (13). The
decrease in megakaryocyte levels was reported to be the main reason
for the reduction in platelet count in patients with ITP (19). Anti-platelet antibodies have been
reported in patients with chronic lymphoblastic leukemia, hairy
cell leukemia, lymphoma, Hodgkin's disease and Waldenstrom
macroglobulinemia, and indicated that an immune regulation
mechanism is involved in the development of thrombocytopenia in
lymphocyte proliferative diseases (20–24).
A previous study reported that platelet antibody IgG
(PA-IgG) levels were elevated in 46% of patients with
lymphoproliferative disorders (25). There was a certain correlation
between thrombocytopenia and the increase of PA-IgG levels in
patients with ITP after resection (26). The high incidence of elevated
anti-platelet antibodies in patients undergoing splenectomy
indicated that other organs could produce autoantibodies (27).
DLBCL is the most common malignant tumor of the
lymphatic system in adults (7); it
is one in a group of malignant tumors that demonstrate
heterogeneity in terms of clinical manifestations and prognosis.
Studies showed that the response and survival rates of patients
with DLBCL were greatly improved after treatment with CD20
monoclonal antibodies; however, up to 40% of patients relapsed and
10% of the entire cohort of treated patients progressed to
refractory diseases (28,29).
An increased level of free light chains in serum is
a factor for a poor prognosis in DLBCL (30). MG is common in well-differentiated
indolent lymphoma and is usually characterized by increased
immunoglobulin levels (31).
However, the frequency and prognostic significance of MG in
patients with DLBCL remains unclear.
A previous study reported that of all patients with
lymphocytic lymphoma, ~44% had elevated serum immunoglobulin levels
and ~20% had MG, usually of the IgM type (32). However, another study reported that
MG was present in 14.6% of patients with DLBCL (33). IgM was the most commonly reported
immunoglobulin in numerous MG cases (32). However, Kim et al (34) and Li et al (35) reported that patients with DLBCL were
susceptible to IgG-type MG. Furthermore, Zhang et al
(36) reported that the presence of
MG was unrelated to the adverse effects on overall survival and
progression-free survival. However, Li et al (35) and Cox et al (37) reported that non-IgM MG was a
negative prognostic factor for both overall and progression-free
survival.
In the present case study, DLBCL concurrent with MG
secondary to ITP was reported. Different clinical manifestations of
MG and lymphoma have previously confused diagnoses and presented
dilemmas in treatment. In the present case report, IgG was observed
in the patient's serum and it was hypothesized that the excessive
production of immunoglobulin could have promoted MG.
Immunohistochemical analysis was used for differential diagnosis
and classification; however, the diagnosis of DLBCL was confirmed
using biopsy. To the best of our knowledge, no previous study has
reported that ITP could cause DLBCL. Although the probability of
ITP patients with DLBCL is minimal, the diagnosis and treatment of
the three diseases in this patient is still worth discussing.
Different clinical manifestations of MG and lymphoma have caused
confusion in diagnoses and dilemmas in treatment. The present study
highlights the unusual source of immunoglobulin, and suggests that
the excessive production of immunoglobulin may promote MG. Although
the prognosis of patients with subsequent monoclonal immunoglobulin
disease due to lack of renal biopsy remains unclear, it reminds us
that a comprehensive and detailed assessment of the patient's
condition is necessary.
The present case demonstrated the importance of a
complete clinical assessment and examination of patients with
lymphoproliferative diseases. This information assists in making a
comprehensive assessment and helps direct effective treatment
protocols.
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the Gansu
Province Innovation Base and Talent Plan (Gansu Province Leukemia
Clinical Research Center; grant no. 21JR7RA015).
Availability of data and materials
The datasets used or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
LR analyzed the data and wrote the manuscript. WL,
FX and DM collected and provided data on the treatment of the case
presented. LY, WL, FX and DM acquired the data and participated in
critical revision of the manuscript. TW and HB analyzed the data,
compiled diagnostic data and contributed to the writing of the
manuscript. All authors read and approved the final manuscript. LY,
TW and HB confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient to publish this case report and any accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fattizzo B and Barcellini W: Autoimmune
cytopenias in chronic lymphocytic leukemia: Focus on molecular
aspects. Front Oncol. 9:14352020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barcellini W, Capalbo S, Agostinelli RM,
Mauro FR, Ambrosetti A, Calori R, Cortelezzi A, Laurenti L,
Pogliani EM, Pedotti P, et al: Relationship between autoimmune
phenomena and disease stage and therapy in B-cell chronic
lymphocytic leukemia. Haematologica. 91:1689–1692. 2006.PubMed/NCBI
|
|
3
|
Fallah M, Liu X, Ji J, Försti A, Sundquist
K and Hemminki K: Autoimmune diseases associated with non-Hodgkin
lymphoma: A nationwide cohort study. Ann Oncol. 25:2025–2030. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Visco C, Ruggeri M, Laura Evangelista M,
Stasi R, Zanotti R, Giaretta I, Ambrosetti A, Madeo D, Pizzolo G
and Rodeghiero F: Impact of immune thrombocytopenia on the clinical
course of chronic lymphocytic leukemia. Blood. 111:1110–1116. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mittal S, Blaylock MG, Culligan DJ, Barker
RN and Vickers MA: A high rate of CLL phenotype lymphocytes in
autoimmune hemolytic anemia and immune thrombocytopenic purpura.
Haematologica. 93:151–152. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kyle RA and Lust JA: Monoclonal
gammopathies of undetermined significance. Semin Heamatol.
26:176–200. 1989.PubMed/NCBI
|
|
7
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y and Barta SK: Diffuse large B-cell
lymphoma: 2019 Update on diagnosis, risk stratification, and
treatment. Am J Hematol. 94:604–616. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Papageorgiou SG, Thomopoulos TP, Spathis
A, Bouchla A, Glezou I, Stavroulaki G, Gkontopoulos K, Bazani E,
Foukas PG and Pappa V: Prognostic significance of monoclonal
gammopathy in diffuse large B-cell lymphoma. Hematol Oncol.
37:634–637. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harris NL, Jaffe ES, Diebold J, Flandrin
G, Muller-Hermelink HK, Vardiman J, Lister TA and Bloomfield CD:
World Health Organization classification of neoplastic diseases of
the hematopoietic and lymphoid tissues: Report of the clinical
advisory committee meeting. Airlie House, Virginia, November 1997.
J Clin Oncol. 17:3835–3849. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hans CP, Weisenburger DD, Greiner TC,
Gascoyne RD, Delabie J, Ott G, Müller-Hermelink HK, Campo E,
Braziel RM, Jaffe ES, et al: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cooper N: State of the art-how I manage
immune thrombocytopenia. Br J Haematol. 177:39–54. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zufferey A, Kapur R and Semple JW:
Pathogenesis and therapeutic mechanisms in immune thrombocytopenia
(ITP). J Clin Med. 6:162017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Olsson B, Ridell B, Jernås M and Wadenvik
H: Increased number of B-cells in the red pulp of the spleen in
ITP. Ann Hematol. 91:271–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen JF, Yang LH, Chang LX, Feng JJ and
Liu JQ: The clinical significance of circulating B cells secreting
anti-glycoprotein IIb/IIIa antibody and platelet glycoprotein
IIb/IIIa in patients with primary immune thrombocytopenia.
Hematology. 17:283–290. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li X, Zhong H, Bao W, Boulad N,
Evangelista J, Haider MA, Bussel J and Yazdanbakhsh K: Defective
regulatory B-cell compartment in patients with immune
thrombocytopenia. Blood. 120:3318–3325. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Aslam R, Segel GB, Burack R, Spence SA,
Speck ER, Guo L and Semple JW: Splenic lymphocyte subtypes in
immune thrombocytopenia: Increased presence of a subtype of
B-regulatory cells. Br J Haematol. 173:159–160. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Min YN, Wang CY, Li XX, Hou Y, Qiu JH, Ma
J, Shao LL, Zhang X, Wang YW, Peng J, et al: Participation of
B-cell-activating factor receptors in the pathogenesis of immune
thrombocytopenia. J Thromb Haemost. 14:559–571. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miltiadous O, Hou M and Bussel JB:
Identifying and treating refractory ITP: Difficulty in diagnosis
and role of combination treatment. Blood. 135:472–490. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
De Rossi G, Granati L, Girelli G, Gandolfo
G, Arista MC, Martelli M, Conti L, Marini R, La Tagliata R, Leone
R, et al: Incidence and prognostic significance of autoantibodies
against erythrocytes and platelets in chronic lymphocytic leukemia
(CLL). Nouv Rev Fr Hematol (1978). 30:403–406. 1988.PubMed/NCBI
|
|
21
|
De Rossi G, Granati L, Girelli G, Gandolo
G, Perrone P, Martelli M, Conti L, Marini R, Pastorelli D, Coluzzi
S, et al: Prognostic value of autoantibodies against erythrocytes
and platelets in chronic lymphocytic leukemia (CLL). Tumori.
77:100–104. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Garvey MB and Freedman J: Indirect
platelet radioactive antiglobulin test in patients with
lymphoproliferative disease. J Lab Clin Med. 107:123–128.
1986.PubMed/NCBI
|
|
23
|
Liu EB, Zhang PH, Li ZQ, Sun Q, Yang QY,
Fang LH, Sun FJ and Qiu LG: Clinicopathologic features of
lymphoplasmacytic lymphoma. Zhonghua Bing Li Xue Za Zhi.
39:308–312. 2010.(In Chinese). PubMed/NCBI
|
|
24
|
Lechman HA, Lehman LO, Rutagi PK, Rustgi
RN, Plunkett RW, Farolino DL, Conway J and Logue GL:
Complement-mediated autoimmune thrombocytopenia. Monoclonal IgM
antiplatelet antibody associated with lymphoreticular malignant
disease. N Engl J Med. 316:194–198. 1987.
|
|
25
|
Kuznetsov AI, Ivanov AL, Idelson LI and
Mazurov AV: Mechanisms of thrombocytopenia in patients with
lymphoproliferative diseases. Eur J Haematol. 49:113–118. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Myers TJ, Kim BK, Steiner M and Baldini
MG: Platelet-associated complement C3 in immune thrombocytopenic
purpura. Blood. 59:1023–1028. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaiho T, Miyazaki M, Iinuma K, Ito H,
Koyama T, Nakagawa K and Nakajima N: Long-term prognosis of
idiopathic thrombocytopenic purpura treated by partial splenic
embolization. Nihon Geka Gakkai Zasshi. 94:383–393. 1993.(In
Japanese). PubMed/NCBI
|
|
28
|
Vardhana SA, Sauter CS, Matasar MJ,
Zelenetz AD, Galasso N, Woo KM, Zhang Z and Moskowitz CH: Outcomes
of primary refractory diffuse large B-cell lymphoma (DLBCL) treated
with salvage chemotherapy and intention to transplant in the
rituximab era. Br J Haematol. 176:591–599. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Coiffier B, Thieblemont C, Van Den Neste
E, Lepeu G, Plantier I, Castaigne S, Lefort S, Marit G, Macro M,
Sebban C, et al: Long-term outcome of patients in the LNH-98.5
trial, the first randomized study comparing rituximab-CHOP to
standard CHOP chemotherapy in DLBCL patients: A study by the Groupe
d'Etudes des Lymphomes de l'Adulte. Blood. 116:2040–2045. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Witzig TE, Maurer MJ, Stenson MJ, Allmer
C, Macon W, Link B, Katzmann JA and Gupta M: Elevated serum
monoclonal and polyclonal free light chains and interferon
inducible protein-10 predicts inferior prognosis in untreated
diffuse large B-cell lymphoma. Am J Hematol. 89:417–422. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaseb H, Annamaraju P and Babiker HM:
Monoclonal gammopathy of undetermined significance. StatPearls
[Internet] Treasure Island (FL): StatPearls Publishing; 2023
|
|
32
|
Wright DH: Malignant lymphomas other than
Hodgkin's disease: Histology, cytology, ultrastructure, immunology.
J Clin Pathol. 32:4141979. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Economopoulos T, Papageorgiou S, Pappa V,
Papageorgiou E, Valsami S, Kalantzis D, Xiros N, Dervenoulas J and
Raptis S: Monoclonal gammopathies in B-cell non-Hodgkin's
lymphomas. Leuk Res. 27:505–508. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim YR, Kim SJ, Cheong JW, Kim Y, Jang JE,
Lee JY, Min YH, Song JW, Yang WI and Kim JS: Monoclonal and
polyclonal gammopathy measured by serum free light chain and
immunofixation subdivide the clinical outcomes of diffuse large
B-cell lymphoma according to molecular classification. Ann Hematol.
93:1867–1877. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li Y, Wang L, Zhu HY, Liang JH, Wu W, Wu
JZ, Xia Y, Fan L, Li JY and Xu W: Prognostic significance of serum
immunoglobulin paraprotein in patients with diffuse large B cell
lymphoma. Br J Haematol. 182:131–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Y, Wei Z, Li J, Gao R and Liu P:
Monoclonal gammopathies regardless of subtypes are associated with
poor prognosis of diffuse large B-cell lymphoma: A STROBE-compliant
article. Medicine (Baltimore). 97:e117192018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cox MC, Di Napoli A, Fabbri A, Cencini E
and Ruco L: The significance of serum immunoglobulin paraprotein in
diffuse large B-cell lymphoma. Br J Haematol. 182:741–742. 2018.
View Article : Google Scholar : PubMed/NCBI
|