Introduction
Non-small cell lung cancer (NSCLC) is a major type
of lung cancer, accounting for ~85% of all lung cancer cases
(1,2). To date, NSCLC remains the second most
common malignancy and the leading cause of cancer-associated
mortality worldwide (3,4). For NSCLC management, surgery is the
cornerstone treatment option for patients with early- and
intermediate-stage NSCLC; nonetheless, for patients with locally
advanced NSCLC, resection is not always feasible (5,6).
Consequently, to increase the feasibility of surgery and improve
survival, neoadjuvant therapy has been adopted for patients with
locally advanced NSCLC (7,8).
Immune checkpoint inhibitors (ICIs) are a class of
immune-oncology drugs that inhibit immune escape of tumor cells and
enhance T cell antitumor responses (9,10). The
Pacific study demonstrated that durvalumab (vs. placebo) after
chemoradiotherapy leads to prolonged estimated 5-year
progression-free survival (33.1 vs. 19.0%) and overall survival
(OS; 42.9 vs. 33.4%) in patients with stage III NSCLC (11). Recently, ICI in combination with
chemotherapy as a neoadjuvant treatment has been used for patients
with locally advanced NSCLC (12,13).
For example, a previous study demonstrated that complete
pathological response (CPR) and major pathological response (MPR)
are 41.67 and 33.33% in patients with locally advanced NSCLC
treated with neoadjuvant ICI + chemotherapy (12). Another study reported that following
treatment with neoadjuvant nivolumab + paclitaxel and carboplatin,
43 (95.6%) patients with locally advanced NSCLC achieve R0
resection; moreover, the 1-year disease-free survival (DFS) and OS
rates are 45.8 and 79.9%, respectively (13). Nevertheless, the application of
neoadjuvant ICI treatment + chemotherapy for patients with locally
advanced NSCLC requires more clinical evidence.
Therefore, the present retrospective cohort study
retrieved data of clinical and pathological response, survival and
adverse events (AEs) of 50 patients with locally advanced NSCLC who
received neoadjuvant ICI + chemotherapy or neoadjuvant chemotherapy
alone, aiming to evaluate the efficacy and safety of neoadjuvant
ICI treatment (camrelizumab or sintilimab) + chemotherapy for
patients with locally advanced NSCLC, which would provide evidence
for novel treatment of clinical NSCLC management.
Patients and methods
Patients
The present study was a retrospective cohort study
that analyzed 50 patients with locally advanced NSCLC [15 (30.0%)
females and 35 (70.0%) males] with a mean age of 59.2±7.6 years,
who received neoadjuvant therapy in The Third People's Hospital of
Chengdu (Chengdu, China) from April 2019 to December 2021. Of these
patients, 23 received ICI + chemotherapy (ICI + chemo), while 27
patients received chemotherapy alone (chemo group). The criteria
for inclusion were as follows: i) Diagnosis of NSCLC; ii) age
>18 years; iii) patients with locally advanced NSCLC with
tumor-node-metastasis (TNM) stage of IIIA-IIIB (T1-T4N2M0,
T3-T4N1M0 and T4N0M0) (14); iv)
Eastern Cooperative Oncology Group Performance Status (ECOG PS)
scores ranging from 0 to 1; v) patients who underwent ICI +
chemotherapy or chemotherapy alone as neoadjuvant therapy. The
exclusion criteria were as follows: i) Diagnosis of metastatic
NSCLC and ii) lack of clinical, pathological or follow-up
information. Moreover, at the initiation of the study, all the
surviving patients provided written informed consent; for the
deceased patients, informed consent forms were signed by their
family members. The protocol for the present study was approved by
the Ethics Committee of The Third People's Hospital of Chengdu
[Chengdu, China; ethics approval no. (2021) S-199].
Treatment
Patients who received camrelizumab or sintilimab +
chemotherapy (carboplatin combined with nab-paclitaxel or
paclitaxel) were defined as the ICI + chemo group; patients who
received carboplatin combined with nab-paclitaxel or paclitaxel
were defined as the chemo group. Camrelizumab or sintilimab were
administered at 200 mg for a 3-week cycle; paclitaxel was
administered at 200 mg/m2 for a 3-week cycle;
nab-paclitaxel was administered at 100 mg/m2 on the 1st,
8th and 15th days of a 3-week cycle. Carboplatin was administered
with an area under the concentration-time curve of 5 mg/ml on the
first day of a 3-week cycle. Following neoadjuvant therapy,
resectability was evaluated (two senior clinicians independently
evaluated the possibility of complete resection according to the
preoperative computed tomography CT results; in case of
disagreement, the two doctors discussed and decided on the best
results) (15).
Assessment
The clinical response rate [complete response (CR),
partial response (PR), stable disease (SD) and progressive disease
(PD)] was evaluated in all patients; moreover, Response Evaluation
Criteria In Solid Tumours (RECIST, version 1.1) was used as a
reference (16). MPR and CPR were
also evaluated in patients who underwent surgical resection.
Definition of MPR was as follows: Viable carcinoma cells did not
comprise >10% on the tumor cut side; the definition of CPR was:
0% tumor tissue observed on the surgical margin, as described in
previous studies (17,18). The follow-up data were collected on
the last follow-up time point in June 2022. DFS was evaluated in
patients who underwent surgery and was determined as the duration
from the surgical resection to disease relapse, mortality for any
reason or the last follow-up; OS was assessed in all patients and
calculated from the date of neoadjuvant therapy or mortality or the
last follow-up. In addition, AEs were documented and graded using
Common Toxicity Criteria for AEs, version 4.0 (19). Additionally, programmed death-ligand
1 (PD-L1) expression data, assessed by immunohistochemistry (IHC)
using Anti-PD-L1 antibody (Abcam) and evaluated based on the
percentage of the stained positive cells, was obtained.
Statistical analysis
SPSS 26.0 software (IBM Corp.) was utilized for
statistical analysis. GraphPad Prism 9.0 software (GraphPad
Software Inc.) was used to draw graphs. Mean ± standard deviation
(SD) was used to present continuous variables, while number
(percentage) was used to present categorical variables. Comparisons
between the ICI + chemo group and the chemo group were performed
using an un-paired Student's t, χ2, Mann-Whitney U and
Fisher's exact test. Kaplan-Meier curves and log-rank test were
used to compare the cumulative DFS and OS of patients between the
two groups. Logistic and Cox regression analyses were used to
analyze the superiority of ICI + chemotherapy over chemotherapy
alone, as well as other independent prognostic factors for patients
with locally advanced NSCLC (all factors analyzed in the univariate
analysis were subsequently input in multivariate analysis with the
entering mode). P<0.05 was considered to indicate a
statistically significant difference.
Results
Study flow and basic characteristics
of patients in the ICI + chemo group and chemo group
The study process is displayed in Fig. S1. The mean age of the patients in
the ICI + chemo group (n=23) was 57.8±6.3 years; the group
comprised 7 (30.4%) females and 16 (69.6%) males; the mean age of
the patients in the chemo group (n=27) was 60.4±8.6 years; the
chemo group comprised 8 (29.6%) females and 19 (70.4%) males
(Table I). No significant
differences were found in the basic characteristics the ICI + chemo
and chemo group, including age, sex, smoking status, ECOG PS score,
histological type, cT, cN and cTNM stage (all P>0.05). Moreover,
10 (43.5%) patients in the ICI + chemo group were assessed as
having a high PD-L1 expression (≥50%), while the other 13 (56.5%)
patients were evaluated as having a low PD-L1 expression (<50%).
The detailed basic characteristics of all subjects are presented in
Table I.
 | Table I.Basic characteristics of patients in
the ICI + chemo group and chemo group. |
Table I.
Basic characteristics of patients in
the ICI + chemo group and chemo group.
| Characteristic | ICI + chemo
(n=23) | Chemo (n=27) | Statistical value
(t/χ2/Z) | P-value |
|---|
| Mean age ± SD,
years | 57.783±6.310 | 60.370±8.607 | 1.194 | 0.239a |
| Sex, n (%) |
|
| 0.004 | 0.951b |
|
Female | 7 (30.400) | 8 (29.600) |
|
|
| Male | 16 (69.600) | 19 (70.400) |
|
|
| Smoking status, n
(%) |
|
| 2.820 | 0.244c |
|
Never | 5 (21.739) | 3 (11.111) |
|
|
|
Former | 12 (52.174) | 11 (40.741) |
|
|
|
Current | 6 (26.087) | 13 (48.148) |
|
|
| ECOG PS score, n
(%) |
|
| 0.119 | 0.730b |
| 0 | 18 (78.300) | 20 (74.100) |
|
|
| 1 | 5 (21.700) | 7 (25.900) |
|
|
| Histological type,
n (%) |
|
| 0.557 | 0.757c |
|
ADC | 8 (34.783) | 7 (25.926) |
|
|
|
SCC | 13 (56.522) | 18 (66.667) |
|
|
|
Others | 2 (8.696) | 2 (7.407) |
|
|
| cT stage, n
(%) |
|
| −0.305 | 0.761d |
|
cT2 | 11 (47.826) | 10 (37.037) |
|
|
|
cT3 | 9 (39.130) | 16 (59.259) |
|
|
|
cT4 | 3 (13.043) | 1 (3.704) |
|
|
| cN stage, n
(%) |
|
| 2.339 | 0.126b |
|
cN1 | 7 (30.435) | 14 (51.852) |
|
|
|
cN2 | 16 (69.565) | 13 (48.148) |
|
|
| cTNM stage, n
(%) |
|
| −0.259 | 0.796d |
|
cT2N2M0 | 11 (47.826) | 10 (37.037) |
|
|
|
cT3N1M0 | 4 (17.391) | 13 (48.148) |
|
|
|
cT3N2M0 | 5 (21.739) | 3 (11.111) |
|
|
|
cT4N1M0 | 3 (13.043) | 1 (3.704) |
|
|
| PD-L1 expression, n
(%) |
|
| - | - |
|
<50% | 10 (43.478) | 0 (0.000) |
|
|
|
≥50% | 13 (56.522) | 0 (0.000) |
|
|
| Not assessed | 0 (0.000) | 27 (100.000) |
|
|
Treatment information and pathological
(p)N2 stage at time of surgery
In the ICI + chemo group, 10 (43.5%), 4 (17.4%), 7
(30.4%) and 2 (8.7%) patients received sintilimab + paclitaxel +
carboplatin, sintilimab + nab-paclitaxel + carboplatin,
camrelizumab + paclitaxel + carboplatin and camrelizumab +
nab-paclitaxel + carboplatin, respectively. In the chemo group, 17
(63.0%) patients were treated with paclitaxel + carboplatin, while
the other 10 (37.0%) patients received nab-paclitaxel + carboplatin
(Table II). A total of 4 (17.4%)
patients in the ICI + chemo group and 9 (33.3%) patients in the
chemo group were evaluated as pN2 stage at the time of surgery
(P=0.200).
 | Table II.Treatment and pN2 at the time of
surgery. |
Table II.
Treatment and pN2 at the time of
surgery.
| Characteristic | ICI + chemo, n=23
(%) | Chemo n=27 (%) |
χ2 value | P-value |
|---|
| Treatment |
|
| - | - |
|
Sintilimab + paclitaxel +
carboplatin | 10 (43.478) | - |
|
|
|
Sintilimab + nab-paclitaxel +
carboplatin | 4 (17.391) | - |
|
|
|
Camrelizumab + paclitaxel +
carboplatin | 7 (30.435) | - |
|
|
|
Camrelizumab + nab-paclitaxel
+ carboplatin | 2 (8.700) | - |
|
|
|
Paclitaxel + carboplatin | - | 17 (63.000) |
|
|
|
Nab-paclitaxel +
carboplatin | - | 10 (37.000) |
|
|
| pN2 at time of
surgery | 4 (17.391) | 9 (33.333) | 1.641 | 0.200a |
ICI + chemo realized better treatment
response compared with chemo alone
The treatment response was superior in the ICI +
chemo group than in the chemo group (P=0.021, Fig. 1A). Specifically, the CR, PR, SD and
PD of patients treated with ICI + chemotherapy were 0.0, 73.9, 26.1
and 0.0%, respectively; while these were 0.0, 44.4, 40.7 and 14.8%
in the patients who received chemotherapy alone. The objective
response rate (ORR) was elevated in the ICI + chemo group compared
with the chemo group (73.9 vs. 44.4%, P=0.035, Fig. 1B). Nevertheless, surgical resection
rate (100.0 vs. 88.9%, P=0.240, Fig.
1C) and MPR (60.9 vs. 41.7%, P=0.188, Fig. 1D) did not differ significantly
between the ICI + chemo and chemo group. CPR was higher but not
significantly so in the ICI + chemo group compared with the chemo
group (30.4% vs. 8.3%, P=0.072, Fig.
1E).
 | Figure 1.Treatment response and ORR are
elevated in the ICI + chemo group (vs. chemo group). Comparison of
(A) treatment response, (B) ORR, (C) surgical resection rate, (D)
MPR and (E) CPR of patients with locally advanced non-small cell
lung cancer between the ICI + chemo and the chemo group. ORR,
objective response rate; ICI, immune checkpoint inhibitor; CR,
complete response; PR, partial response; SD, stable disease; PD,
progressive disease; MPR, major pathological response; CPR,
complete pathological response. |
To eliminate the potential confounding factors
influencing the comparison of the pathological response between the
ICI + chemo and chemo alone groups, multivariate logistic
regression analysis was applied. ICI + chemo (vs. chemo) was
independently associated with an elevated CPR in patients with
NSCLC [odds ratio (OR), 19.920; 95% confidence interval (CI),
1.363-291.038, P=0.029; Table
III].
 | Table III.Factors associated with CPR by
multivariate logistic regression analysis. |
Table III.
Factors associated with CPR by
multivariate logistic regression analysis.
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Variable | P-value | OR | Lower | Upper |
|---|
| Therapy (ICI +
chemo vs. Chemo) | 0.029 | 19.920 | 1.363 | 291.038 |
| Age (≥60 vs. <60
years) | 0.617 | 1.630 | 0.240 | 11.077 |
| Sex (male vs.
female) | 0.421 | 2.657 | 0.246 | 28.749 |
| Smoking status
(current vs. former + never) | 0.181 | 6.172 | 0.428 | 89.014 |
| ECOG PS score (1
vs. 0) | 0.475 | 0.392 | 0.030 | 5.123 |
| Histological type
(SCC vs. ADC + other) | 0.060 | 14.741 | 0.890 | 244.222 |
| Higher cT
stage | 0.894 | 0.857 | 0.089 | 8.231 |
| Higher cN
stage | 0.602 | 2.145 | 0.122 | 37.651 |
ICI + chemo led to an improving
survival profile compared with chemo alone
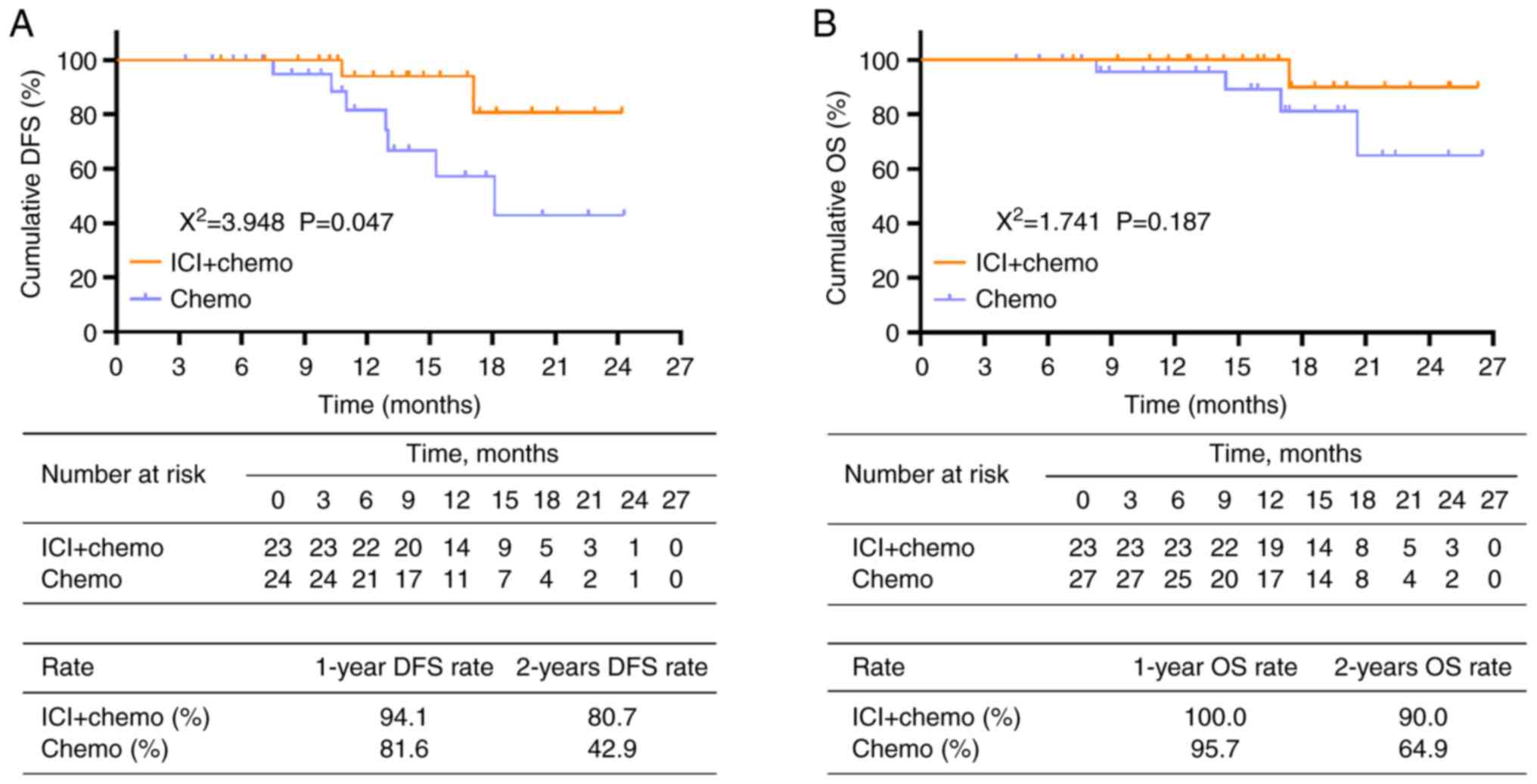
DFS was prolonged in patients who received ICI +
chemotherapy compared with those who received chemotherapy alone
(P=0.047, Fig. 2A). The 1-year and
2-year DFS rates were 94.1 and 80.7 in the ICI + chemo group but
only 81.6 and 42.9% in the chemo group, respectively. However, OS
did not differ significantly between the ICI + chemo group and
chemo group (P=0.187, Fig. 2B).
Specifically, the 1-year and 2-year OS rates were 100.0 and 90.0 in
the ICI + chemo and 95.7 and 64.9% in the chemo group,
respectively.
Furthermore, multivariate Cox proportional hazards
regression analysis revealed that the ICI + chemo group had a
non-inferior DFS [hazard ratio (HR), 1.893; 95% CI, 0.100-35.704;
P=0.670) and OS (HR, 0.189; 95% CI, 0.012-3.05; P=0.241] compared
with the chemo group (Table
IV).
 | Table IV.Factors associated with DFS and OS by
multivariate Cox's proportional hazards regression analysis. |
Table IV.
Factors associated with DFS and OS by
multivariate Cox's proportional hazards regression analysis.
| A, DFS |
|---|
|
|---|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|---|
| Variable | P-value | HR | Lower | Upper |
|---|
| Therapy (ICI +
chemo vs. Chemo) | 0.670 | 1.893 | 0.100 | 35.704 |
| Age (≥60 vs. <60
years) | 0.164 | 4.942 | 0.521 | 46.913 |
| Sex (male vs.
female) | 0.051 | 0.031 | 0.001 | 1.016 |
| Smoking status
(current vs. former + never) | 0.039 | 51.637 | 1.219 | 2,187.218 |
| ECOG PS score (1
vs. 0) | 0.055 | 17.194 | 0.944 | 313.078 |
| Histological type
(SCC vs. ADC + other) | 0.661 | 1.760 | 0.141 | 21.959 |
| Higher cT
stage | 0.092 | 0.018 | 0.000 | 1.928 |
| Higher cN
stage | 0.145 | 0.029 | 0.000 | 3.361 |
|
| B, OS |
|
|
|
|
| 95% CI |
|
|
|
|
|
|
Variable | P-value | HR | Lower | Upper |
|
| Therapy (ICI +
chemo vs. Chemo) | 0.241 | 0.189 | 0.012 | 3.055 |
| Age (≥60 vs. <60
years) | 0.331 | 5.623 | 0.172 | 183.294 |
| Sex (male vs.
female) | 0.534 | 0.407 | 0.024 | 6.902 |
| Smoking status
(current vs. former + never) | 0.519 | 2.508 | 0.153 | 41.146 |
| ECOG PS score (1
vs. 0) | 0.485 | 0.312 | 0.012 | 8.200 |
| Histological type
(SCC vs. ADC + other) | 0.197 | 8.906 | 0.320 | 247.496 |
| Higher cT
stage | 0.525 | 2.583 | 0.139 | 48.099 |
| Higher cN
stage | 0.281 | 8.748 | 0.170 | 450.532 |
Non-significant differences in
tolerance between ICI + chemo and chemo alone
The incidence of AEs did not differ significantly
between the ICI + chemo and the chemo group (all P>0.05), apart
from the increased leukopenia incidence in the ICI + chemo group
(47.8 vs. 18.5%; P=0.036). The incidences of all grade 3–4 AEs were
not different between the two groups, including leukopenia, anemia,
neutropenia, thrombocytopenia, fatigue, nausea and vomiting and
constipation (all P>0.050). Furthermore, the majority of AEs in
the ICI + chemo group were moderate and controllable. The most
frequent hematological AEs in the ICI + chemo group were leukopenia
(47.8%), anemia (39.1%) and neutropenia (34.8%), while the most
common non-hematological AEs were alopecia (43.5%), fatigue (43.5%)
and nausea and vomiting (39.1%). Grade 3–4 AEs in the ICI + chemo
group included three cases (13.0%) of neutropenia and one case
(4.3%) each of leukopenia, anemia and thrombocytopenia (Table V).
 | Table V.AEs. |
Table V.
AEs.
|
| ICI + chemo, n=23
(%) | Chemo, n=27
(%) |
|
|
|---|
|
|
|
|
|
|
|---|
| AE | Total | Grade 1–2 | Grade 3–4 | Total | Grade 1–2 | Grade 3–4 |
P-valuea |
P-valueb |
|---|
| Hematological |
|
|
|
|
|
|
|
|
|
Leukopenia | 11 (47.8) | 10 (43.5) | 1 (4.3) | 5 (18.5) | 5 (18.5) | 0 (0.0) | 0.036 | 0.460 |
|
Anemia | 9 (39.1) | 8 (34.8) | 1 (4.3) | 9 (33.3) | 8 (29.6) | 1 (3.7) | 0.771 | 1.000 |
|
Neutropenia | 8 (34.8) | 5 (21.7) | 3 (13.0) | 9 (33.3) | 8 (29.6) | 1 (3.7) | 0.914 | 0.322 |
|
Thrombocytopenia | 5 (21.7) | 4 (17.4) | 1 (4.3) | 9 (33.3) | 9 (33.3) | 0 (0.0) | 0.529 | 0.460 |
|
Non-hematological |
|
|
|
|
|
|
|
|
|
Alopecia | 10 (43.5) | 10 (43.5) | 0 (0.0) | 13 (48.1) | 13 (48.1) | 0 (0.0) | 0.782 | - |
|
Fatigue | 10 (43.5) | 10 (43.5) | 0 (0.0) | 7 (25.9) | 6 (22.2) | 1 (3.7) | 0.239 | 1.000 |
| Nausea
and vomiting | 9 (39.1) | 9 (39.1) | 0 (0.0) | 7 (25.9) | 6 (22.2) | 1 (3.7) | 0.373 | 1.000 |
|
Constipation | 6 (26.1) | 6 (26.1) | 0 (0.0) | 6 (22.2) | 5 (18.5) | 1 (3.7) | 1.000 | 1.000 |
|
Elevated transaminase | 6 (26.1) | 6 (26.1) | 0 (0.0) | 5 (18.5) | 5 (18.5) | 0 (0.0) | 0.733 | - |
|
Anorexia | 6 (26.1) | 6 (26.1) | 0 (0.0) | 5 (18.5) | 5 (18.5) | 0 (0.0) | 0.733 | - |
|
Rash | 5 (21.7) | 5 (21.7) | 0 (0.0) | 7 (25.9) | 7 (25.9) | 0 (0.0) | 1.000 | - |
|
Diarrhea | 3 (13.0) | 3 (13.0) | 0 (0.0) | 4 (14.8) | 4 (14.8) | 0 (0.0) | 1.000 | - |
|
Elevated bilirubin | 3 (13.0) | 3 (13.0) | 0 (0.0) | 4 (14.8) | 4 (14.8) | 0 (0.0) | 1.000 | - |
|
Hypothyroidism | 2 (8.7) | 2 (8.7) | 0 (0.0) | 2 (7.4) | 2 (7.4) | 0 (0.0) | 1.000 | - |
|
Peripheral neuropathy | 2 (8.7) | 2 (8.7) | 0 (0.0) | 1 (3.7) | 1 (3.7) | 0 (0.0) | 0.588 | - |
Discussion
The efficacy of ICI-based neoadjuvant treatment for
early-to-intermediate stage NSCLC has already been demonstrated in
previous studies (17,20,21).
Nevertheless, a limited number of studies have applied the
combination of ICI + chemotherapy as neoadjuvant therapy for
treatment of patients with locally advanced NSCLC (12,22).
For example, a previous study observed that the CPR (25.8 vs. 8.3%)
and MPR (61.3 vs. 37.5%) are higher in patients treated with
neoadjuvant camrelizumab + chemotherapy compared with patients who
received chemotherapy alone (22).
In line with the findings of previous studies (12,22),
the present study also demonstrated that neoadjuvant ICI treatment
+ chemotherapy led to a superior treatment response and ORR (73.9
vs. 44.4%) compared with chemotherapy alone in patients with
locally advanced NSCLC. Furthermore, ICI + chemotherapy (vs.
chemotherapy alone) was independently associated with elevated CPR
in patients with NSCLC. The reasons for this may be that ICI
directly enhanced the antitumor immune response in the tumor
microenvironment by targeting PD-1 or PD-L1 (17,23) or
there was a potential synergy between ICI and chemotherapy, which
enhanced the treatment efficacy (24,25).
ICI + chemotherapy achieved better treatment response compared with
chemotherapy alone.
Neoadjuvant ICI treatment + chemotherapy also leads
to a satisfactory survival profile in patients with locally
advanced NSCLC, according to previous studies (13,26).
For example, in the neoadjuvant nivolumab plus chemotherapy in
operable stage IIIA NSCLC trial, the 2-year DFS and OS rates were
77.1 and 89.9% in patients with locally advanced NSCLC who received
neoadjuvant nivolumab + paclitaxel-carboplatin therapy,
respectively (26). Another study
demonstrated 2-year DFS and OS rates of 45.8 and 79.9% in patients
treated with the neoadjuvant ICI + chemotherapy regimen (13). However, the majority of previous
studies are single-arm studies (13,26).
In the present study, neoadjuvant ICI + chemotherapy prolonged the
DFS of patients with NSCLC compared with chemotherapy alone;
however, no significant difference was observed in OS. The 2-year
DFS rate was 80.7% in the ICI + chemo group and 42.9% in the chemo
group. These findings revealed that ICI + chemotherapy led to an
improved survival profile compared with chemotherapy alone;
additionally, the survival outcome of the patients in the ICI +
chemo group in the present study was similar to that of previous
studies (13,26). A possible explanation for this may
be that combination of ICI + chemotherapy improved the pathological
response, which further suppressed disease progression and
recurrence of NSCLC (27).
Therefore, DFS was prolonged in patients who received ICI +
chemotherapy compared with those who received chemotherapy alone.
Multivariate Cox proportional hazards regression analysis suggested
that the therapy (ICI + chemo vs. chemo) was not an independent
factor of DFS or OS. This may be because mutual interference
between the therapy and the cT stage weakened the effects on DFS
and were not independent factors of DFS or the small number of
deaths weakened the statistical power and no factor was
independently associated with OS.
Moreover, the Pacific study found ORR and 1-year DFS
rate of 28.4 and 55.9%, respectively, in patients with stage III
NSCLC who received durvalumab after chemoradiotherapy, which
indicated the potency of ICI + chemoradiotherapy treatment pattern
in patients with NSCLC (28). To
the best of our knowledge, application of ICI + chemoradiotherapy
as neoadjuvant treatment in NSCLC patients is rarely reported
(29,30). Therefore, the comparison of
treatment efficacy between neoadjuvant ICI + chemotherapy and
neoadjuvant ICI + chemoradiotherapy in patients with NSCLC requires
further investigation.
Previous studies have reported the non-inferior
tolerance between neoadjuvant ICI + chemotherapy and neoadjuvant
chemotherapy alone in patients with locally advanced NSCLC; here,
the most common AEs in the ICI + chemo group included alopecia,
nausea and vomiting, anemia and fatigue (22,31,32).
Consistent with the aforementioned studies, the present study
revealed that the incidence of most AEs did not differ
significantly between the ICI + chemo and the chemo group, apart
from the increased leukopenia incidence in the ICI + chemo group.
Notably, the majority of AEs in the ICI + chemo group were moderate
and controllable, with a low incidence of grade 3–4 AEs, which
implied the reliable safety profile of neoadjuvant ICI +
chemotherapy for patients with locally advanced NSCLC.
There were limitations to the present study.
Firstly, the present study was a retrospective study and the
selection bias was difficult to avoid. Secondly, due to the small
number of patients in our department, it was hard to enroll more
eligible patients within the study period; thus, the sample size
(n=50) was small and further large-scale studies were warranted to
enhance the statistical power. Thirdly, the follow-up duration was
relatively short; hence, further studies with a long-term follow up
are required. Fourthly, pathological N staging before neoadjuvant
therapy was a reference for patients whose surgical feasibility was
hard to decide. However, due to the fact that this was a
retrospective study and most patients were originally assessed as
operable patients, pathological staging before neoadjuvant therapy
and surgery was not conducted in most patients. Consequently, most
patients only had the clinical TNM stage before neoadjuvant
treatment and CT images for surgical-feasibility reassessment
before surgery.
In conclusion, the present study demonstrated that
neoadjuvant ICI + chemotherapy provided an encouraging pathological
response, survival benefits and acceptable safety profiles compared
with chemotherapy alone in patients with locally advanced
NSCLC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZL contributed to the conception and the design of
the study. YY and ZL were responsible for the acquisition, analysis
and interpretation of the data. ZL and YY confirm the authenticity
of all the raw data. Both have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was permitted by the ethical
committee of The Third People's Hospital of Chengdu [ethics
approval no. (2021) S-199]. All surviving patients provided written
informed consent; for the deceased patients, informed consent was
signed by their family members.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Duma N, Santana-Davila R and Molina JR:
Non-small cell lung cancer: Epidemiology, screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mithoowani H and Febbraro M:
Non-small-cell lung cancer in 2022: A review for general
practitioners in oncology. Curr Oncol. 29:1828–1839. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ricotti A, Sciannameo V, Balzi W,
Roncadori A, Canavese P, Avitabile A, Massa I and Berchialla P:
Incidence and prevalence analysis of non-small-cell and small-cell
lung cancer using administrative data. Int J Environ Res Public
Health. 18:90762021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Alexander M, Kim SY and Cheng H: Update
2020: Management of non-small cell lung cancer. Lung. 198:897–907.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ettinger DS, Wood DE, Aisner DL, Akerley
W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, D'Amico
TA, et al: NCCN guidelines insights: Non-small cell lung cancer,
version 2.2021. J Natl Compr Canc Netw. 19:254–266. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Remon J, Soria JC and Peters S; ESMO
Guidelines Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Early and locally advanced non-small-cell lung cancer: An update of
the ESMO clinical practice guidelines focusing on diagnosis,
staging, systemic and local therapy. Ann Oncol. 32:1637–1642. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Puri S, Saltos A, Perez B, Le X and Gray
JE: Locally advanced, unresectable non-small cell lung cancer. Curr
Oncol Rep. 22:312020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ulas EB, Dickhoff C, Schneiders FL, Senan
S and Bahce I: Neoadjuvant immune checkpoint inhibitors in
resectable non-small-cell lung cancer: A systematic review. ESMO
Open. 6:1002442021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sławiński G, Wrona A, Dabrowska-Kugacka A,
Raczak G and Lewicka E: Immune checkpoint inhibitors and cardiac
toxicity in patients treated for non-small lung cancer: A review.
Int J Mol Sci. 21:71952020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Spigel DR, Faivre-Finn C, Gray JE, Vicente
D, Planchard D, Paz-Ares L, Vansteenkiste JF, Garassino MC, Hui R,
Quantin X, et al: Five-year survival outcomes from the PACIFIC
trial: Durvalumab after chemoradiotherapy in stage III
non-small-cell lung cancer. J Clin Oncol. 40:1301–1311. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen T, Ning J, Campisi A, Dell'Amore A,
Ciarrocchi AP, Li Z, Song L, Huang J, Yang Y, Stella F and Luo Q:
Neoadjuvant PD-1 inhibitors and chemotherapy for locally advanced
NSCLC: A retrospective study. Ann Thorac Surg. 113:993–999. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhai H, Li W, Jiang K, Zhi Y and Yang Z:
Neoadjuvant nivolumab and chemotherapy in patients with locally
advanced non-small cell lung cancer: A retrospective study. Cancer
Manag Res. 14:515–524. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu YL, Planchard D, Lu S, Sun H, Yamamoto
N, Kim DW, Tan DSW, Yang JC, Azrif M, Mitsudomi T, et al: Pan-Asian
adapted clinical practice guidelines for the management of patients
with metastatic non-small-cell lung cancer: A CSCO-ESMO initiative
endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann Oncol. 30:171–210.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shamji FM and Beauchamp G: Assessment of
operability and resectability in lung cancer. Thorac Surg Clin.
31:379–391. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cascone T, William WN Jr, Weissferdt A,
Leung CH, Lin HY, Pataer A, Godoy MCB, Carter BW, Federico L,
Reuben A, et al: Neoadjuvant nivolumab or nivolumab plus ipilimumab
in operable non-small cell lung cancer: The phase 2 randomized
NEOSTAR trial. Nat Med. 27:504–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Casarrubios M, Cruz-Bermúdez A, Nadal E,
Insa A, García Campelo MDR, Lázaro M, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, et al: Pretreatment tissue TCR
repertoire evenness is associated with complete pathologic response
in patients with NSCLC receiving neoadjuvant chemoimmunotherapy.
Clin Cancer Res. 27:5878–5890. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
National Institutes of Health and National
Cancer Institute, . Common terminology criteria for adverse events
(CTCAE) version 4.0. Published:. May 28–2009.https://evs.nci.nih.gov/ftp1/CTCAE/About.html
|
|
20
|
Duan H, Wang T, Luo Z, Tong L, Dong X,
Zhang Y, Afzal MZ, Correale P, Liu H, Jiang T and Yan X:
Neoadjuvant programmed cell death protein 1 inhibitors combined
with chemotherapy in resectable non-small cell lung cancer: An
open-label, multicenter, single-arm study. Transl Lung Cancer Res.
10:1020–1028. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Zhao Z and Long H: Application of
neoadjuvant immuno-chemotherapy in NSCLC. Zhongguo Fei Ai Za Zhi.
24:284–292. 2021.(In Chinese). PubMed/NCBI
|
|
22
|
Hou X, Shi X and Luo J: Efficacy and
safety of camrelizumab (a PD-1 inhibitor) combined with
chemotherapy as a neoadjuvant regimen in patients with locally
advanced non-small cell lung cancer. Oncol Lett. 24:2152022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang Y and Zhang Z: The history and
advances in cancer immunotherapy: Understanding the characteristics
of tumor-infiltrating immune cells and their therapeutic
implications. Cell Mol Immunol. 17:807–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Emens LA and Middleton G: The interplay of
immunotherapy and chemotherapy: Harnessing potential synergies.
Cancer Immunol Res. 3:436–443. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Salas-Benito D, Pérez-Gracia JL,
Ponz-Sarvisé M, Rodriguez-Ruiz ME, Martínez-Forero I, Castañón E,
López-Picazo JM, Sanmamed MF and Melero I: Paradigms on
immunotherapy combinations with chemotherapy. Cancer Discov.
11:1353–1367. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Provencio M, Nadal E, Insa A,
García-Campelo MR, Casal-Rubio J, Dómine M, Majem M,
Rodríguez-Abreu D, Martínez-Martí A, De Castro Carpeño J, et al:
Neoadjuvant chemotherapy and nivolumab in resectable non-small-cell
lung cancer (NADIM): An open-label, multicentre, single-arm, phase
2 trial. Lancet Oncol. 21:1413–1422. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mielgo-Rubio X, Uribelarrea EA, Cortés LQ
and Moyano MS: Immunotherapy in non-small cell lung cancer: Update
and new insights. J Clin Transl Res. 7:1–21. 2021.PubMed/NCBI
|
|
28
|
Antonia SJ, Villegas A, Daniel D, Vicente
D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, et
al: Durvalumab after chemoradiotherapy in stage III non-small-cell
lung cancer. N Engl J Med. 377:1919–1929. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hamada A, Soh J, Hata A, Nakamatsu K,
Shimokawa M, Yatabe Y, Oizumi H, Tsuboi M, Horinouchi H, Yoshino I,
et al: Phase II study of neoadjuvant concurrent
chemo-immuno-radiation therapy followed by surgery and adjuvant
immunotherapy for resectable stage IIIA-B (Discrete N2)
non-small-cell lung cancer: SQUAT trial (WJOG 12119L). Clin Lung
Cancer. 22:596–600. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dickhoff C, Senan S, Schneiders FL,
Veltman J, Hashemi S, Daniels JMA, Fransen M, Heineman DJ, Radonic
T, van de Ven PM, et al: Ipilimumab plus nivolumab and
chemoradiotherapy followed by surgery in patients with resectable
and borderline resectable T3-4N0-1 non-small cell lung cancer: The
INCREASE trial. BMC Cancer. 20:7642020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao ZR, Yang CP, Chen S, Yu H, Lin YB,
Lin YB, Qi H, Jin JT, Lian SS, Wang YZ, et al: Phase 2 trial of
neoadjuvant toripalimab with chemotherapy for resectable stage III
non-small-cell lung cancer. Oncoimmunology. 10:19960002021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aguado C, Chara L, Antoñanzas M, Matilla
Gonzalez JM, Jiménez U, Hernanz R, Mielgo-Rubio X, Trujillo-Reyes
JC and Couñago F: Neoadjuvant treatment in non-small cell lung
cancer: New perspectives with the incorporation of immunotherapy.
World J Clin Oncol. 13:314–322. 2022. View Article : Google Scholar : PubMed/NCBI
|