Introduction
Immunotherapy has profoundly changed the history of
several types of cancer such as the improvement of survival,
duration of response, and quality of life compared with
chemotherapy. Immune checkpoint inhibitors (ICIs) are comprehensive
of different monoclonal antibodies targeting cytotoxic T
lymphocyte-associated antigen 4 (CTLA-4), programmed death protein
1 (PD-1) or programmed death-ligand 1 (PD-L1) (1).
Interaction between PD-1 (on T cells) and PD-L1 (on
tumor cells) induces immune system tolerance and promotes tumor
growth. Consequently, the inhibition of the immune system
down-regulation permits the reactivation of T-cells (2). However, the immunity activation can
disrupt peripheral immune tolerance and cause immune-related
adverse events (irAEs) (3).
Pembrolizumab is a humanized monoclonal antibody
IgG4 PD-1 inhibitor. It blocks the interaction with PDL1 and PDL2
expressed by antigen-presenting cells (APC), tumor and other cells
in the tumor microenvironment (4).
It has shown efficacy in different malignancies, specifically in
Non-Small cell lung cancer (NSCLC). The first-line approval came
with the Keynote024 trial (5) that
involved patients with metastatic NSCLC non-oncogene addicted,
PD-L1 ≥50%. In this study, patients were randomized to receive
pembrolizumab 200 mg q3w vs. platinum-based chemotherapy.
Clinically significant results in overall survival (OS) and
progression-free survival (PFS) were obtained in this population
expressing high levels of PD-L1.
First-line treatment standard for patients with
PD-L1 <50% is the combination of chemo and immunotherapy. In the
KEYNOTE-189 trial (6), patients
were randomized to receive chemotherapy + pembrolizumab +
pemetrexed or chemotherapy + placebo, followed by maintenance with
pembrolizumab + pemetrexed or placebo + pemetrexed. A median
follow-up of 4 years confirms trial results: experimental arm OS is
significantly better than the placebo arm (HR 0,60). Subgroup
analysis shows that the combination is efficient even in patients
with brain and liver metastasis. Positive effects are independent
of PD-L1 expression.
Evidence shows that more than 60% of patients
treated with immunotherapy develop irAEs. Dermatological irAEs
concern between 30 and 50% of patients on ICIs and are the most
frequent and the earliest (7). More
than 30% reported mild skin toxicity, alone or associated with
other symptoms (8). Unfortunately
some severe irAEs such as Stevens-Johnson syndrome (SJS) and toxic
epidermal necrolysis (TEN) and Drug Rash with Eosinophilia and
Systemic Symptoms (DRESS) are life-threating are described and
require the cessation of ICIs (8).
The average incidence of skin irAEs reported in meta-analysis
includes 16.7% for Pembrolizumab (9). For patients treated with the
chemoimmunotherapy combination, the skin reactions mostly reported
(frequency ≥1/10) were: alopecia, skin eruption, and pruritus.
Common adverse events (≥1/100, <1/10) were severe cutaneous
reactions, erythema, dermatitis, and dry skin. Uncommon adverse
events (≥1/1.000, <1/100) were psoriasis, eczema, lichenoid
keratosis, acneiform dermatitis, and vitiligo. Rare adverse events
(≥1/10.000, <1/1.000) were nodosum erythema, papules, and change
in color of skin or hair (4).
Psoriatic plaques are the most frequent presentation of psoriasis
but the incidence has been difficult to estimate (9). The mechanisms of pembrolizumab-induced
psoriasis remains unclear. A reason could be related to the
activation of Th17 lymphocytes, which typically are inactivated by
PD-1 (10).
In this paper, we describe a case of psoriasis in a
68-years-old man with advanced NSCLC who developed this specific
dermatology adverse event during immunotherapy.
Case report
A 68-year-old Caucasian male patient with Eastern
Cooperative Oncology Group (ECOG) Performance Status 0, came to our
observation, at Multispeciality Department of Oncology, ASL Latina,
‘Sapienza’ University of Rome, Aprilia, Italy in February 2020 with
a histologically confirmed diagnosis of right upper lobe lung
adenocarcinoma. Immunohistochemistry (IHC) showed ALK and ROS1
negative, PD-L1 <1%, and EGFR wild type in quantitative PCR.
A CT scan and brain MRI demonstrated a lung lesion
of 34×25×29 mm and a single right hemispheric lesion of 7 mm
asymptomatic. In April 2020 the patient started stereotactic
radiation therapy, completed in 3 sessions (33,75 Gy total dose).
Following, a front-line chemo-immunotherapy was set up with
carboplatin AUC 5 i.v. day 1, pemetrexed 500 mg/sqm i.v. day 1 and
pembrolizumab 200 mg day 1, every 21 days. Initially the patient
developed diarrhea G1 as a single adverse event (AE). Then, after 2
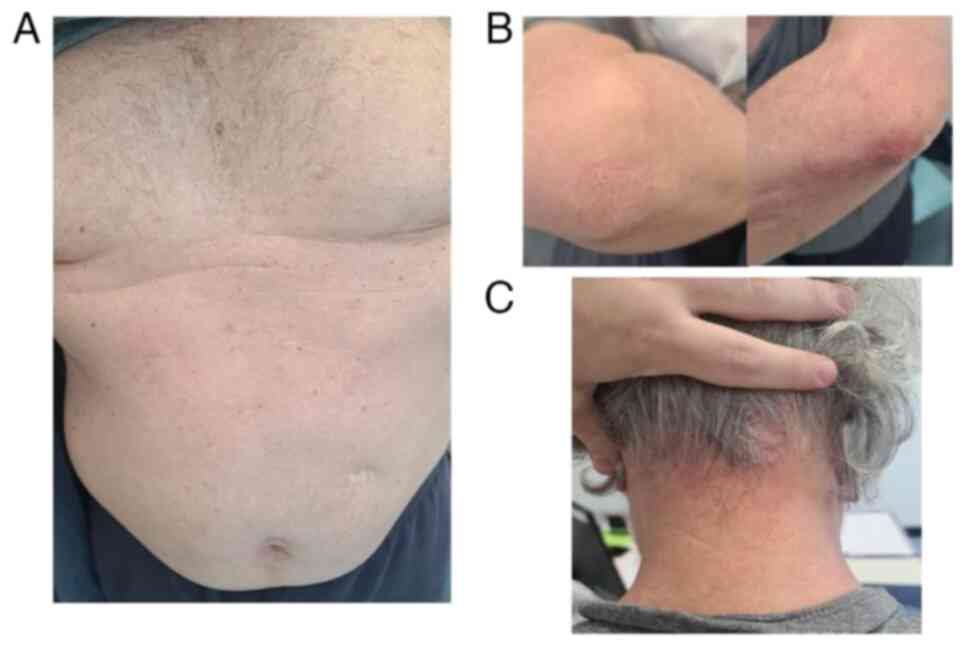
cycles of chemo-immunotherapy, a cutaneous rash G3 (Fig. 1) appeared on the back of the chest,
upper and lower limbs appeared associated with pruritus G1.
In June 2020 the patient stopped chemo-immunotherapy
treatment and daily oral prednisolone 50 mg/daily for three days +
ebastine 10 mg was administered. Steroids have been weaned in 10
days, with complete regression of the rash. After 3 months a CT
scan showed stability of disease of the primary lesion and complete
response of the hemispheric lesion post-radiotherapy.
In August 2020 the patient restarted the
chemoimmunotherapy combination after seven weeks of interruption.
In September 2020, after four cycles of therapy, a CT scan showed a
partial response of the primary lesion. The patient started
maintenance therapy with pemetrexed 500 mg/sqm and pembrolizumab
200 mg day 1, every 21 days. After 3 maintenance cycles, he
manifested a re-occurrence of the rash with scaly papules on the
head, abdomen, back and gluteus (Fig.
2). This was treated with prednisolone 50 mg/daily for three
days, weaned over 10 days. After the interruption of prednisolone
appeared an exacerbation of the cutaneous rash and pruritus G1.
In December 2020 a consultant dermatologist
diagnosed psoriasiform reaction on the elbows, gluteus and scalp; a
cutaneous biopsy confirmed diagnosis of psoriasis (Fig. 3). The patient denied any personal or
family history of psoriasis.
Topical treatments: clobetasol foam for scalp twice
daily for 15/20 days, calcipotriol + betamethasone foam once a day
for 4 weeks, dexamethasone + clotrimazole on skin folds twice daily
and then soothing cream.
After the complete regression of the lesions, the
patient continued the maintenance treatment without pembrolizumab.
The patient has completed 28 maintenance cycles with only
pemetrexed with stable disease at CT revaluations. The skin
manifestations of psoriasis are controlled without any local or
systemic treatment (Fig. 4).
Discussion
Immunotherapy has brought the development of new
strategies against NSCLC. Previously negative PD-L1 patients were
considered less responsive to these new treatments, now we know
that PD-L1 is not an affordable biomarker of immunotherapy
sensitivity, indeed also patients with lower expression of PD-L1
could benefit from this treatment, above all combined protocols as
established by KEYNOTE-189 (6). The
scenery of non-oncogene addicted NSCLS is thus changed because
immunotherapy constitutes the standard of care (combined with
chemotherapy or not). This has certainly increased the number of
patients treated with immunotherapy, as well as the experiences of
both responses and adverse events.
Skin AEs are one of the most frequent AEs in
patients treated with anti-PD-1 (nivolumab and pembrolizumab).
Onset is quite early, within the first weeks after the infusion.
Despite these serious manifestations being rare (<3%), and
usually do not require the interruption of treatment (11).
Psoriasis is a chronic autoimmune disease that
affects about 0,27-11,4% of the population, hits the skin, and it
could impact lives of patients both physically and psychologically
(12). It's often associated with
multiple comorbidities such as obesity (13). This disease causes red, itchy scaly
patches, most commonly on the knees, elbows, trunk and scalp
(14). The histopathological
features are similar to typical psoriasis vulgaris, with the
presence of hyperkeratosis, hypogranulosis, acanthosis with
elongated rete ridges, and a perivascular lymphocytic infiltration
(7).
A health-related quality of life (HRQoL) impairment
is associated with psoriasis. Itching and skin pain have been
reported in 62–97 and 42–59% of patients with psoriasis,
respectively (15). Furthermore,
although a correlation between anxiety and psoriasis has not been
demonstrated, the levels of anxiety recorded in patients with
psoriasis were higher than average (16).
The mechanism of disease is a higher expression of
Th1 and Th17 lymphocytes and, consequentially, cytokines such as
IL-1, IL-6, and IL-17 (17). It is
known that immunotherapy might exacerbate psoriatic lesions in
those with positive familial or personal history of psoriasis. The
most frequent presentation is plaque-type psoriasis, and it is
associated with a family history (17). The mechanism under this adverse
event is explained by the inhibition of PD-1/PD-L1 axis that, as
reported by Dulos et al (18) and Okiyama and Tanaka (19), activates Th1, Th17 and Th22
lymphocytes with the production of IL-6 (20). In turn, IL-6 stimulates IL-17, a
cytokine that downregulates T-reg cells. The balance between these
types of cells is the key to autoimmune regulation and cancer
(21). Another mechanism described
in literature is overweight or obesity. Western Diet fed mice
became obese and they have higher expression of Th-17 cytokines and
PD-1 on γδ-low T cells. Imiquimod treatment elevate psoriasis
inflammation and PD-1 as inhibitory regulation. Anti-PD1 treatment,
as Pembrolizumab, induced a prominent PD-1 blockage effect and
severe psoriasiform dermatitis (8).
Our patient is overweight with a BMI of 28.7.
As in Table I, not
many cases of pembrolizumab-induced psoriasis are described in the
literature. We have reported patients without a personal or family
history of psoriasis. The median onset time was 6 weeks, 2 cycles.
Only 3 cases of NSCLC were registered.
 | Table I.Case reports of pembrolizumab-induced
psoriasis. |
Table I.
Case reports of pembrolizumab-induced
psoriasis.
| First author,
year | Sex | Age, years | Cancer type | Onset time of
psoriasis after the start of immunotherapy, weeks | Personal and familial
history | Psoriasis
management | (Refs.) |
|---|
| Huang and Chu,
2020 | M | 71 | Squamous NSCLC | 2 | No | Topical steroids | (17) |
| Suzuki et al,
2020 | F | 78 | NSCLC | 9 | No | Topical and oral
steroids | (22) |
| Johnson et al,
2019 | M | 80 | Melanoma | 12 | No | Topical steroids and
moisturizers, then retinoids and oral steroids, then
secukinumab | (3) |
| Siciliano et
al, 2020 | M | 75 | Melanoma | 30 | No | Apremilast | (23) |
| Takama et
al, 2020 | M | 74 | Bladder | 2 | No | Oral steroids +
vitamin D, then apremilast | (25) |
| Totonchy et
al, 2016 | F | 80 | Melanoma | 4.5 | NA | Topical
steroids | (26) |
| Bonigen et
al, 2017 | M | 59 | Melanoma | NA | No | Topical
steroids | (27) |
| Bonigen et
al, 2017 | M | 77 | Melanoma | NA | No | Topical
steroids | (27) |
| Voudouri et
al, 2017 | M | 69 | Squamous cell
carcinoma of the tonsil | 6 | No | UVB-NB and topical
steroids | (12) |
| Present study | M | 68 | NSCLC | 6 | No | Oral and topical
steroids, and antihistaminic and antifungal cream | - |
For G1 toxicity (skin rash with or without symptoms,
>10% body surface area (BSA) ESMO Guidelines recommend avoiding
skin irritants, sun exposure, topical emollients, topical steroids
(mild strength) cream and/or, topical or oral antihistamines for
itch. For G2 (rash covers 10–30% of BSA) is recommended supportive
management as above, a topical steroid (moderate strength) cream
and or potent cream bd +/- topical or oral antihistamines for itch.
The treatment can be continued. For G3 (rash covers >30% BSA or
grade 2 with substantial symptoms) withhold ICIs, topical
treatments as above (potent) and steroids. If mild to moderate
0.5/1 mg/kg prednisolone once daily for three days then wean over
1–2 weeks; or if severe, i.v. (methyl)prednisolone 0.5–1 mg/kg and
convert to oral steroids on response, wean over 2–4 weeks. For G4
(skin sloughing >30% BSA with associated symptoms) i.v.
(methyl)prednisolone 1–2 mg/kg with urgent dermatology review and
discontinuation of ICIs (11).
Our patient, as described above, needed both topical
and systemic steroids, soothing and antifungal cream to solve
maculopapular rash. With the aim of preserving the recurrence and
severity of psoriasis, the immunotherapy treatment was
discontinued.
The majority of cases reported in Table I were treated with topical steroids.
One case was treated with anti-IL-17 (secukinumab) (3), and two cases with anti-PDE4
(apremilast) (22,23). Despite the discontinuation of the
treatment, the benefit of our patient, in terms of progression free
survival, is consistent if compared with the median PFS of the
Keynote 189 trial, which is 9.0 months (24). A meta-analysis conducted on 52
studies including 9,156 patients affected by different malignancies
treated with anti-PD-1/PD-L1 agents (22), demonstrated how irAEs were
statistically associated with better PFS and OS. Observing
subgroups analysis, these results have been described in NSCLC,
endocrine and skin toxicities but not for gastrointestinal and lung
AE. The major grade of adverse events was not associated with
favorable PFS or OS.
In conclusion, pembrolizumab, as an immunotherapy
drug, has changed the prognosis and the quality of life of
patients. Compared to chemotherapy, ICIs have a different spectrum
of adverse events, mostly well tolerated. However, as in our case,
they could be so severe to discontinue the treatment, even without
a positive history of autoimmune disease. As mentioned above, our
patient had a long response from immunotherapy despite its
interruption. Although the mechanism behind irAEs remains not
clear, it might be explained by a reactivation of T-cells, so that
patients with a more responsive immune system are the ones who
benefit more by ICIs. For these reasons, it might be important to
conduct other studies to identify predictive factors of
immune-related toxicities and objective response.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FG, LR, IP and AC made substantial contributions to
conception and design of the manuscript. SC, AA, MB, CP and GC
contributed to acquisition and interpretation of data, and wrote
the abstract. DS, VP and VS performed the histological examination
of the psoriasis and were major contributors in writing the
manuscript, confirm the authenticity of all the raw data and gave
final approval of the version to be published. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AE
|
adverse event
|
|
APC
|
antigen-presenting cells
|
|
BSA
|
body surface area
|
|
CTLA-4
|
cytotoxic T lymphocyte-associated
antigen 4
|
|
DRESS
|
Drug Rash with Eosinophilia and
Systemic Symptoms
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
HRQoL
|
health related quality of life
|
|
ICIs
|
immune checkpoint inhibitors
|
|
IHC
|
immunohistochemistry
|
|
irAEs
|
immune-related adverse events
|
|
NA
|
not applicable
|
|
NSCLC
|
non-small cell lung cancer
|
|
OS
|
overall survival
|
|
PD-1
|
programmed death protein 1
|
|
PD-L1
|
programmed death ligand 1
|
|
PFS
|
progression-free survival
|
|
SJS
|
Stevens-Johnson syndrome
|
|
TEN
|
toxic epidermal necrolysis
|
References
|
1
|
Nikolaou V, Sibaud V, Fattore D, Sollena
P, Ortiz-Brugués A, Giacchero D, Romano MC, Riganti J, Lallas K,
Peris K, et al: Immune checkpoint-mediated psoriasis: A multicenter
European study of 115 patients from the European network for
cutaneous adverse event to oncologic drugs (ENCADO) group. J Am
Acad Dermatol. 84:1310–1320. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Monsour EP, Pothen J and Balaraman R: A
novel approach to the treatment of pembrolizumab-induced psoriasis
exacerbation: A case report. Cureus. 11:e58242019.PubMed/NCBI
|
|
3
|
Johnson D, Patel AB, Uemura MI, Trinh VA,
Jackson N, Zobniw CM, Tetzlaff MT, Hwu P, Curry JL and Diab A:
IL17A blockade successfully treated psoriasiform dermatologic
toxicity from immunotherapy. Cancer Immunol Res. 7:860–865. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
European Medicines Agency, . https://www.ema.europa.eu/en/documents/product-information/keytruda-epar-product-information_it.pdf
|
|
5
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gandhi L, Rodríguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen CH, Yu HS and Yu S: Cutaneous adverse
events associated with immune checkpoint inhibitors: A review
article. Curr Oncol. 29:2871–2886. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cutroneo P, Ingrasciotta Y, Isgrò V, Rullo
EV, Berretta M, Fiorica F, Trifirò G and Guarneri C: Psoriasis and
psoriasiform reactions secondary to immune checkpoint inhibitors.
Dermatol Ther. 34:e148302021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Belum VR, Benhuri B, Postow MA, Hellmann
MD, Lesokhin AM, Segal NH, Motzer RJ, Wu S, Busam KJ, Wolchok JD
and Lacouture ME: Characterisation and management of dermatologic
adverse events to agents targeting the PD-1 receptor. Eur J Cancer.
60:12–25. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Scarfì F, Lacava R, Patrizi A, Tartari F,
Ravaioli GM, Veronesi G, Lambertini M and Dika E: Follicular
psoriasis induced by pembrolizumab in a patient with advanced
non-small-cell lung cancer. Int J Dermatol. 58:e151–e152. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Haanen JBAG, Carbonnel F, Robert C, Kerr
KM, Peters S, Larkin J and Jordan K; ESMO Guidelines Committee, :
Management of toxicities from immunotherapy: ESMO clinical practice
guidelines for diagnosis, treatment and follow-up. Ann Oncol. 28
(Suppl 4):iv119–iv142. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Voudouri D, Nikolaou V, Laschos K,
Charpidou A, Soupos N, Triantafyllopoulou I, Panoutsopoulou I,
Aravantinos G, Syrigos K and Stratigos A: Anti-PD1/PDL1 induced
psoriasis. Curr Probl Cancer. 41:407–412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu S, Wu X, Shi Z, Huynh M, Jena PK, Sheng
L, Zhou Y, Han D, Wan YY and Hwang ST: Diet-induced obesity
exacerbates imiquimod-mediated psoriasiform dermatitis in anti-PD-1
antibody-treated mice: Implications for patients being treated with
checkpoint inhibitors for cancer. J Dermatol Sci. 97:194–200. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
https://www.mayoclinic.org/diseases-conditions/psoriasis/symptoms-causes/syc-20355840
|
|
15
|
Honma M, Kanai Y, Murotani K, Nomura T,
Ito K and Imafuku S: Itching and skin pain in real-life patients
with plaque psoriasis: Baseline analysis of the ProLOGUE study. J
Dermatol Sci. 105:189–191. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu S, Tu HP, Huang YC and Lan CCE: The
incidence of anxiety may not be correlated with severity of
psoriasis: A prospective pilot study. Med Hypotheses.
130:1092542019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang PW and Chu CY: Pembrolizumab-induced
linear psoriasis. Lung Cancer. 146:378–379. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dulos J, Carven GJ, van Boxtel SJ, Evers
S, Driessen-Engels LJ, Hobo W, Gorecka MA, de Haan AF, Mulders P,
Punt CJ, et al: PD-1 blockade augments Th1 and Th17 and suppresses
Th2 responses in peripheral blood from patients with prostate and
advanced melanoma cancer. J Immunother. 35:169–178. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Okiyama N and Tanaka R: Varied
immuno-related adverse events induced by immune-check point
inhibitors-nivolumab-associated psoriasiform dermatitis related
with increased serum level of interleukin-6. Nihon Rinsho Meneki
Gakkai Kaishi. 40:95–101. 2017.(In Japanese). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hara T, Mikita N, Ikeda T, Inaba Y,
Kunimoto K, Kaminaka C, Kanazawa N, Yamamoto Y and Jinnin M:
Psoriatic arthritis induced by anti-programmed death 1 antibody
pembrolizumab. J Dermatol. 46:e466–e467. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gadgeel S, Rodríguez-Abreu D, Speranza G,
Esteban E, Felip E, Dómine M, Hui R, Hochmair MJ, Clingan P, Powell
SF, et al: Updated analysis from KEYNOTE-189: Pembrolizumab or
placebo plus pemetrexed and platinum for previously untreated
metastatic nonsquamous non-small-cell lung cancer. J Clin Oncol.
38:1505–1517. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzuki M, Matsumoto S, Takeda Y and
Sugiyama H: Systemic psoriasiform dermatitis appeared after the
administration of pembrolizumab. Intern Med. 59:871–872. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Siciliano MA, Dastoli S, d'Apolito M,
Staropoli N, Tassone P, Tagliaferri P and Barbieri V:
Pembrolizumab-induced psoriasis in metastatic melanoma: Activity
and safety of apremilast, a case report. Front Oncol.
10:5794452020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fan Y, Xie W, Huang H, Wang Y, Li G, Geng
Y, Hao Y and Zhang Z: Association of immune related adverse events
with efficacy of immune checkpoint inhibitors and overall survival
in cancers: A systemic review and meta-analysis. Front Oncol.
11:6330322021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takama H, Shibata T, Ando Y, Yanagishita
T, Ohshima Y, Akiyama M and Watanabe D: Pembrolizumab-induced
psoriasis vulgaris successfully treated with apremilast. Eur J
Dermatol. 30:188–190. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Totonchy MB, Ezaldein HH, Ko CJ and Choi
JN: Inverse psoriasiform eruption during pembrolizumab therapy for
metastatic melanoma. JAMA Dermatol. 152:590–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bonigen J, Raynaud-Donzel C, Hureaux J,
Kramkimel N, Blom A, Jeudy G, Breton AL, Hubiche T, Bedane C,
Legoupil D, et al: Anti-PD1-induced psoriasis: A study of 21
patients. J Eur Acad Dermatol Venereol. 31:e254–e257. 2017.
View Article : Google Scholar : PubMed/NCBI
|