Introduction
Esophageal squamous cell carcinoma (ESCC) remains a
significant global challenge, having the 6th highest mortality
worldwide and killing over 500,000 people in 2020 (1). Despite recent progress in
multidisciplinary treatments against ESCC, many patients die from
distant metastasis or recurrence after surgery (2). Circulating tumor cells (CTCs) are
defined as cancer cells that depart from the primary tumor to enter
the bloodstream (3) and are
considered predictors of distant metastasis and cancer recurrence
(4,5). In esophageal cancer, researchers
associate CTC detection with advanced disease stage, poor
therapeutic response, and prognosis (6,7).
Most CTC separation techniques are two-step:
firstly, cell enrichment of the sample and secondly, CTC detection.
Enrichment protocols for CTCs generally use cell surface markers or
morphological features enabling CTC isolation via immunological
assays, microfluidic devices, or density gradient centrifugation
(6–8). Although subsequent detection methods
include flow cytometry, biomechanical discrimination, and
polymerase chain reaction (7,9), with
marker-stained cell manual detection by microscope the most common
method. Increased attention is being paid to these approaches
thanks to recent reports exploring cancer cell heterogeneity in
terms of malignant potential and stem cell properties. Accordingly,
identifying heterogeneity and malignant subsets in CTCs is a
priority, with the usefulness of various surface markers reported
(4,10–13).
However, the use of multiple markers makes CTC detection more
complex and time-consuming. Therefore, an accurate, easy-to-use,
and rapid detection method is required for clinical
application.
Artificial intelligence (AI) is the simulation of
human intelligence processes demonstrated by a computer program. AI
can extract important information from large amounts of diverse
data, classifying and summarizing common patterns. Potentially
alleviating a significant quantity of human workload (14,15).
Recently, attention has focused on a method called deep learning,
which uses multiple layers of artificial neural networks and is
modeled after the human cerebral cortex (16). Object recognition is a major
application of deep learning, with convolutional neural networks
(CNN) applied facilitate image diagnosis (17).
The aim of this study was to establish an accurate
and rapid image processing algorithm based on CNN for CTC detection
in patients with ESCC. We first investigated the AI algorithm's
accuracy in distinguishing ESCC cell lines from peripheral blood
mononuclear cells (PBMCs), then used the AI algorithm to detect
CTCs in peripheral blood samples obtained from ESCC patients.
Materials and methods
Patients' eligibility and
sampling
This study was approved by the ethics review board
of the University of Toyama Hospital (R2021042) and written
informed consent was obtained from all ESCC participants.
Peripheral blood was collected from 10 newly diagnosed ESCC
patients and 5 healthy volunteers. Patient samples were collected
between January 2022 and October 2022. The eligibility criteria for
patients were i) a confirmed diagnosis of ESCC, ii) undergoing
treatment at the University of Toyama Hospital, and iii) no ESCC
treatment prior to enrollment. All cases were diagnosed according
to the 7th edition of the Union for International Cancer Control
system (18).
For the four surgical patients, peripheral blood
samples were extracted from each patient during general anesthesia
via the arterial pressure line prior to the operation. From the six
patients who underwent chemotherapy, blood samples were extracted
via a median cubital vein. Peripheral blood samples were obtained
from each healthy volunteer via a median cubital vein. Blood
samples were collected in 3 ml ethylenediaminetetraacetic acid
(EDTA) tubes. Samples were processed within 3 h of the collection
as described below.
Cell lines and cell culture
Human ESCC cell lines (KYSE30, KYSE140, KYSE520, and
KYSE1440) were purchased from the Japanese Collection of Research
Bioresources (JCRB, Tokyo, Japan). These cell lines are
authenticated using STR profiling in the JCRB. Cells were cultured
in Dulbecco's Modified Eagle (DMEM) medium (Nacalai tesque, Kyoto,
Japan), supplemented with 1% penicillin-streptomycin and 10%
heat-inactivated fetal calf serum (FCS). The culture was grown in
cell culture dishes in a humidified atmosphere containing 5%
CO2 at 37°C. Cells were washed with phosphate-buffered
saline without calcium and magnesium (PBS, FUJIFILM Wako Pure
Chemical Corporation, Osaka, Japan) and harvested with Trypsin-EDTA
(0.25%) (ThermoFisher, Massachusetts, USA). The harvested cells
were processed immediately for imaging as described below.
Sample collection and processing
We collected 2.5 ml of peripheral blood samples from
ESCC patients and healthy volunteers in EDTA tubes. Density
gradient centrifugation was performed using the RosetteSep™ Human
Circulating Epithelial Tumor Cell Enrichment Cocktail (StemCell™
Technologies, Vancouver, Canada) combined with Lymphoprep™
(StemCell™ Technologies, Vancouver, Canada). To the 2.5 ml blood
sample was added 250 µl (50 µl/ml) of the RosetteSep™ cocktail and
then incubated for 20 min at room temperature. Blood samples were
diluted with equal volumes of PBS and carefully layered onto
Lymphoprep™ then centrifuged at 3,600 rpm at room temperature for
20 min. After centrifugation, supernatants were transferred to
another 15 ml conical tube with cells pelleted by centrifugation at
1,800 rpm for 20 min at room temperature. The enriched cells were
collected, red blood cells were lysed by BD Pharm Lyse lysing
solution (Becton, Dickinson and Company, New Jersey, USA), and
washed in PBS.
Cell labeling
Cell fixation was performed using 4%
paraformaldehyde. For staining, human monoclonal
EpCAM-phycoerythrin (PE) (clone REA764; MACS Miltenyi Biotec,
Cologne, Germany) antibodies were used. Antibodies were diluted
1:50 in 50 µl PBS containing 5% FBS. After incubation for 60 min,
the cells were washed in PBS and pelleted by centrifuge at 1,200
rpm for 5 min at 4°C. SlowFade™ Diamond Antifade Mountant with DAPI
(ThermoFisher, Massachusetts, USA) was added and deposited on a
microscope slide to be prepared for imaging.
Imaging, processing, and computational
classification of cells using AI
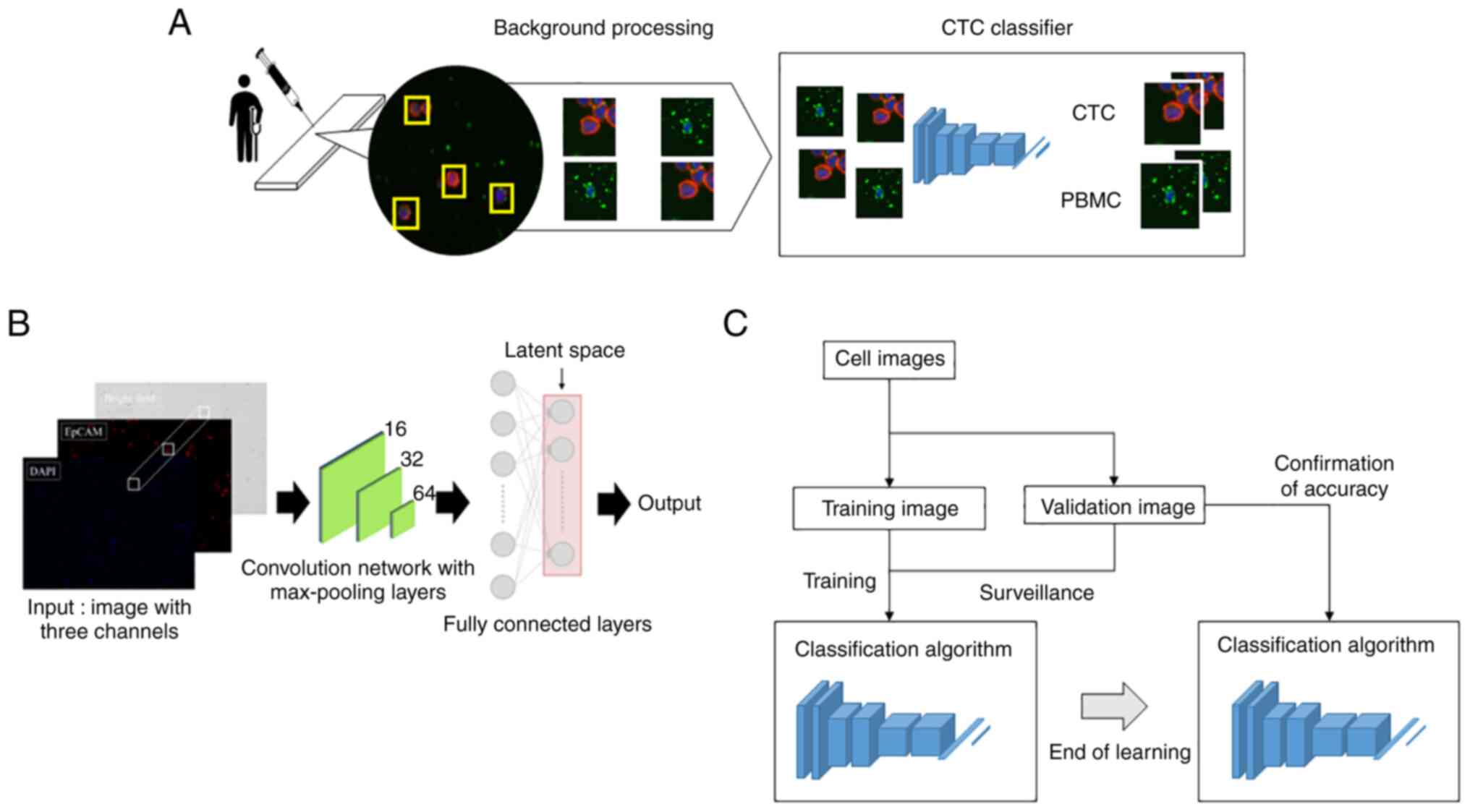
The cell classification process using the CNN-based
algorithm is shown in Fig. 1A.
Cellular regions were extracted from the microscopic image of KYSEs
and blood samples. Luminance characteristic analysis was performed
on these to control cell image background information. Then the
brightness value components of each fluorescence were combined into
a single image. Images were then fed into the CNN-based classifier
for cancer cell evaluation.
Specifically, KYSEs and blood samples were prepared
as above. Images were captured using the inverted microscope
BZ-X800 (KEYENCE, Osaka, Japan). Images were taken at a 20×
magnification through the objective lens. The acquired images were
processed using an algorithm constructed in cooperation with the
Department of Mechanical and Intellectual Systems Engineering,
Faculty of Engineering, University of Toyama. Cell image cropping
used the following morphological criteria: Extract the
DAPI-positive site using Otsu's method (19). Narrowing down by requiring an EpCAM
luminance of 20 or more. Furthermore, DAPI- and/or EpCAM-positive
cells with an area greater than 700 pixels were excluded.
Fig. 1B shows the
model with the classification network. The CNN consists of an input
layer, hidden layer, and output layer. We input images of 64 pixels
×64 pixels to the input layer. The hidden layer includes many
convolutional layers, pooling layers, and fully connected layers.
The convolutional layer extracts various local features of the
input layer through the convolution operation and normalizes the
features for each channel image. The CNN performs feature
extraction again at the pooling layer and semantically combines
similar features to make the features robust to noise and
deformation. The CNN samples these features, outputting them in a
reduced processing size, this operation is repeated and continues
with the fully connected layer. Each neuron in the fully connected
layer is fully connected to all neurons in the previous layers. The
fully connected layer integrates local information with class
discrimination from the previous layers by the rectified linear
unit (ReLU) function. Finally, the output value of the fully
connected layer is passed to the output layer.
A schematic diagram of the evaluation method for the
cell classifying AI algorithm accuracy is shown in Fig. 1C. The cell identification algorithm
is trained using training images of cancer cells and PBMCs. Cell
identification accuracy is then confirmed using validation images.
By repeating this process, the AI algorithm accuracy is evaluated
with regard to the training data variability.
The hardware environment used for computation was;
CPU:Intel(r) Core(TM) i9-10980XE CPU @ 3.00GHz, Memory: 96GB,
GPU:NVIDIA GeForce RTX 3090, V-RAM:24GB. The software environments
used for computation were Python Ver.3.6.13, CUDA Ver.10.1, opencv
Ver.4.5.3.56, cuDNN Ver.7.6.5, TensorFlow Ver.2.6.0, Keras
Ver.2.6.0, NumPy Ver.1.19.5, pandas Ver.1.1.5, openpyel Ver.3.0.9,
matplotlib Ver.3.3.4, scikit-learn Ver.0.24.2, seaborn Ver.0.11.2,
shap Ver.0.40.0.
Classification of ESCC cell lines
To validate AI image recognition accuracy in
distinguishing ESCC cells from PBMCs, we used images of ESCC cell
lines stained with DAPI and EpCAM (KYSE30: 640 images, KYSE140: 194
images, KYSE520: 1037 images, KYSE1440: 347 images) and PBMCs from
healthy volunteers (400 images). Specifically, we trained the AI
using images of a KYSE and PBMC, shown a pair at a time and in
order. Then the AI evaluated other image sets of KYSEs and PBMCs
(KYSE30 vs. PBMCs, KYSE140 vs. PBMCs, KYSE520 vs. PBMCs, KYSE1440
vs. PBMCs), withholding the answers, to identify cell image as KYSE
or PBMC.
Comparison of cell detection between
AI image processing and manual cell count
To compare cell-detecting speeds between AI and
humans, the AI and three researchers (TA, YN, TY) counted a total
cell number in three identical images of KYSE140 and PBMCs each.
Specifically, KYSE140 (1.0×105 cells) and PBMCs
(1.0×107 cells), stained with DAPI as above, with 2×2
view images taken and merged to create 3 images for each KYSE140
and PBMCs from healthy volunteers. The AI and three researchers
then counted the DAPI-positive areas recognized as cells, recording
the time required to count.
Comparing AI and human image
recognition accuracy
To compare image recognition accuracy between AI and
humans, four researchers and pre-trained AI were tested to
distinguish between KYSE140 and PBMCs. As described in the previous
section, the AI was pre-trained using segmented 194 and 400 single
cell images of KYSE140 and PBMCs, respectively, both stained with
DAPI and EpCAM. Three sets of 100 images (50 images each were
randomly selected from the 194 and 400 images of KYSE140 and PBMCs,
respectively) were presented to the AI and four researchers
separately (TA, YN, TY, NM), to identify the cell images as either
KYSE140 or PBMC. To each image was assigned a hidden answer as to
whether it was a KYSE or a PBMC. The researchers classified cells
as KYSE140 and PBMCs based on the detection of
EpCAM-positive/DAPI-stained cells and EpCAM-negative/DAPI-stained
cells, respectively. The analysis time required for the 100 images
was also noted.
Statistical analysis
All analyses were carried out with JMP 16.0 software
(SAS Institute Inc., Cary, NC, USA). A confusion matrix was used to
observe specificity, sensitivity, and accuracy. Difference between
the AI and manual accuracy using image sets of KYSE140 and PBMCs
was determined using the Wilcoxon rank-sum test P<0.05 was
considered to indicate a statistically significant difference.
Results
Validation of the image recognition
accuracy of AI
Firstly, the image recognition accuracy of the
trained AI in distinguishing ESCC cell lines from PBMCs was
evaluated. The AI was trained using identified paired images of
single cells from ESCC cell lines and PBMC. Then the AI was shown
paired images of a KYSE and PBMC, with the answer hidden, and
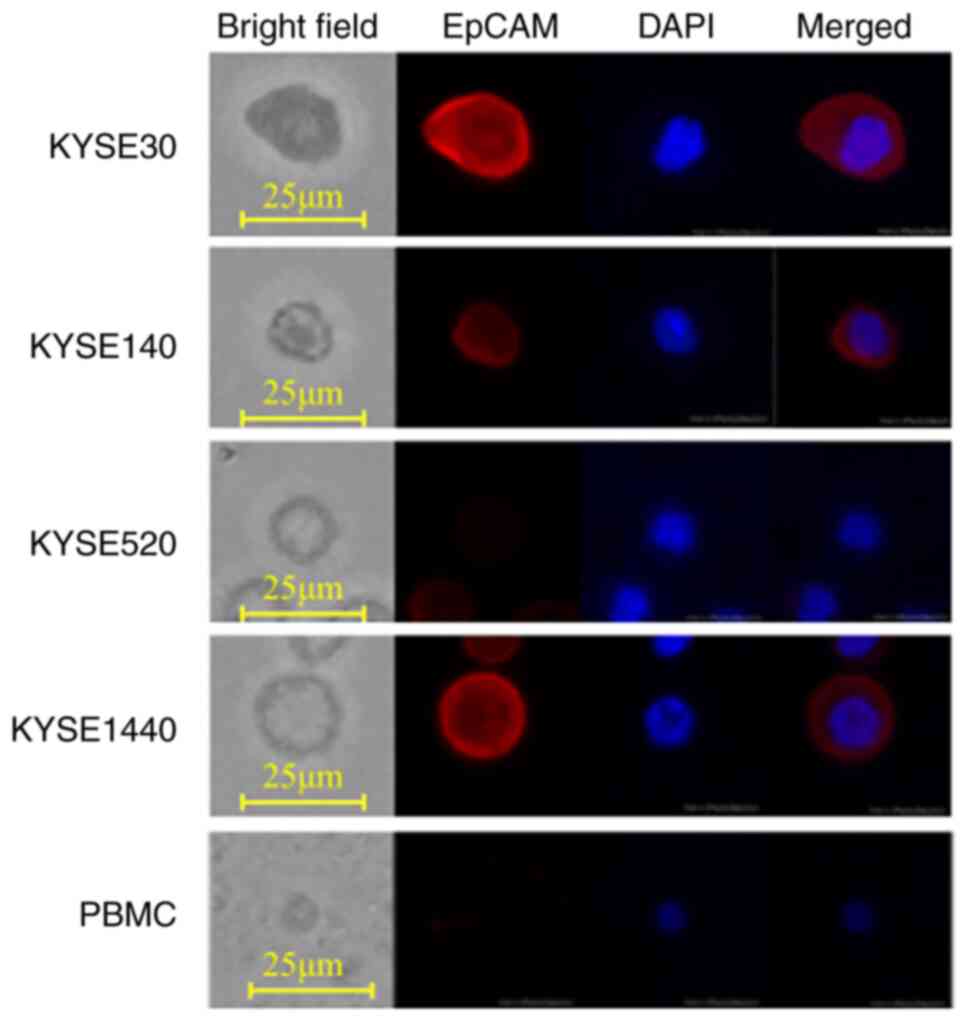
tasked to identify which was the KYSE. Representative images of
four ESCC cell lines (KYSE30, KYSE140, KYSE520, and KYSE1440) and
PBMCs are shown in Fig. 2. PBMCs
had no EpCAM expression and were small in both cell size and
nucleus. KYSE520 did not express EpCAM, both KYSE30 and KYSE1440
strongly expressed EpCAM, while KYSE140 weakly expressed EpCAM. The
AI differentiated KYSE30, KYSE140, KYSE520, and KYSE1440 from PBMCs
with an accuracy of 99.9, 99.8, 99.8, and 100%, respectively, when
trained using the same cell lines (Table I). Interestingly, even using KYSEs
not used for training, the specificity in distinguishing KYSEs from
PBMCs was greater than 99.6%, regardless of the KYSE combination
used in training and examination. On the other hand, sensitivity
varied from 20.4 to 100% depending on the KYSE combination used in
training and examination (Table I).
Among these four ESCC cell lines, we further validated the
efficiency of the AI trained with KYSE140 by comparing it to human
manual CTC detection.
 | Table I.Accuracy of image recognition. |
Table I.
Accuracy of image recognition.
| Cell line used for
training | Cell line used for
evaluation | Accuracy, (%) | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|
| KYSE30 | KYSE30 | 99.9 | 99.8 | 100 | 100 | 99.8 |
|
| KYSE140 | 69.9 | 39.8 | 100 | 100 | 62.4 |
|
| KYSE520 | 80.7 | 61.4 | 100 | 100 | 72.2 |
|
| KYSE1440 | 99.9 | 99.8 | 100 | 100 | 99.8 |
| KYSE140 | KYSE30 | 96.0 | 92.4 | 99.6 | 99.6 | 92.9 |
|
| KYSE140 | 99.8 | 100.0 | 99.6 | 99.6 | 100.0 |
|
| KYSE520 | 60.0 | 20.4 | 99.6 | 98.1 | 55.6 |
|
| KYSE1440 | 98.8 | 98.0 | 99.6 | 99.6 | 98.0 |
| KYSE520 | KYSE30 | 99.8 | 100.0 | 99.6 | 99.6 | 100.0 |
|
| KYSE140 | 70.6 | 41.6 | 99.6 | 99.0 | 63.0 |
|
| KYSE520 | 99.8 | 100.0 | 99.6 | 99.6 | 100.0 |
|
| KYSE1440 | 99.8 | 100.0 | 99.6 | 99.6 | 100.0 |
| KYSE1440 | KYSE30 | 99.9 | 99.8 | 100.0 | 100.0 | 99.8 |
|
| KYSE140 | 83.3 | 66.6 | 100.0 | 100.0 | 75.0 |
|
| KYSE520 | 82.9 | 65.8 | 100.0 | 100.0 | 74.5 |
|
| KYSE1440 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
The efficiency of AI image processing
compared to manual counting
Secondly, the AI and three researchers each counted
the number of DAPI-stained cells in three identical images of
KYSE140 and PBMCs. AI image processing and manual counting detected
the same number of KYSE140 cells and PBMCs (n=1,335.3±168.6 and
1,246.1±113.0 for KYSE140 cells by AI and manual detection,
respectively, P=0.71 Fig. 3A;
n=387.7±45.6 and 425.6±29.1 for PBMCs, P=0.58, Fig. 3B).
Whereas, using KYSE140, AI image processing and
manual counting took 4.9±0.3 and 591.4±62.4 sec, respectively, with
a significant difference (P=0.016, Fig.
3C). Using PBMCs, AI image processing and manual counting took
4.9±0.3 and 243.3±18.8 sec, respectively, with a significant
difference (P=0.016, Fig. 3D).
These results showed no significant difference in
the number of cells detected between AI and humans, but yielded a
significantly shorter AI analysis time.
Comparison of image recognition
accuracy between AI and humans
To compare AI and human image recognition accuracy
in distinguishing cancer cells from PBMCs, the trained AI and four
researchers were tasked to identify KYSE140 from PBMCs using images
of 100 EpCAM/DAPI stained cells (50 of KYSE140 and 50 of PBMCs)
with the answers withheld. After evaluating the three sets of 100
images, the AI completely distinguished KYSE140 from PBMCs with
both a sensitivity and specificity of 100%, while the researchers
distinguished them with a sensitivity and specificity of 86 and
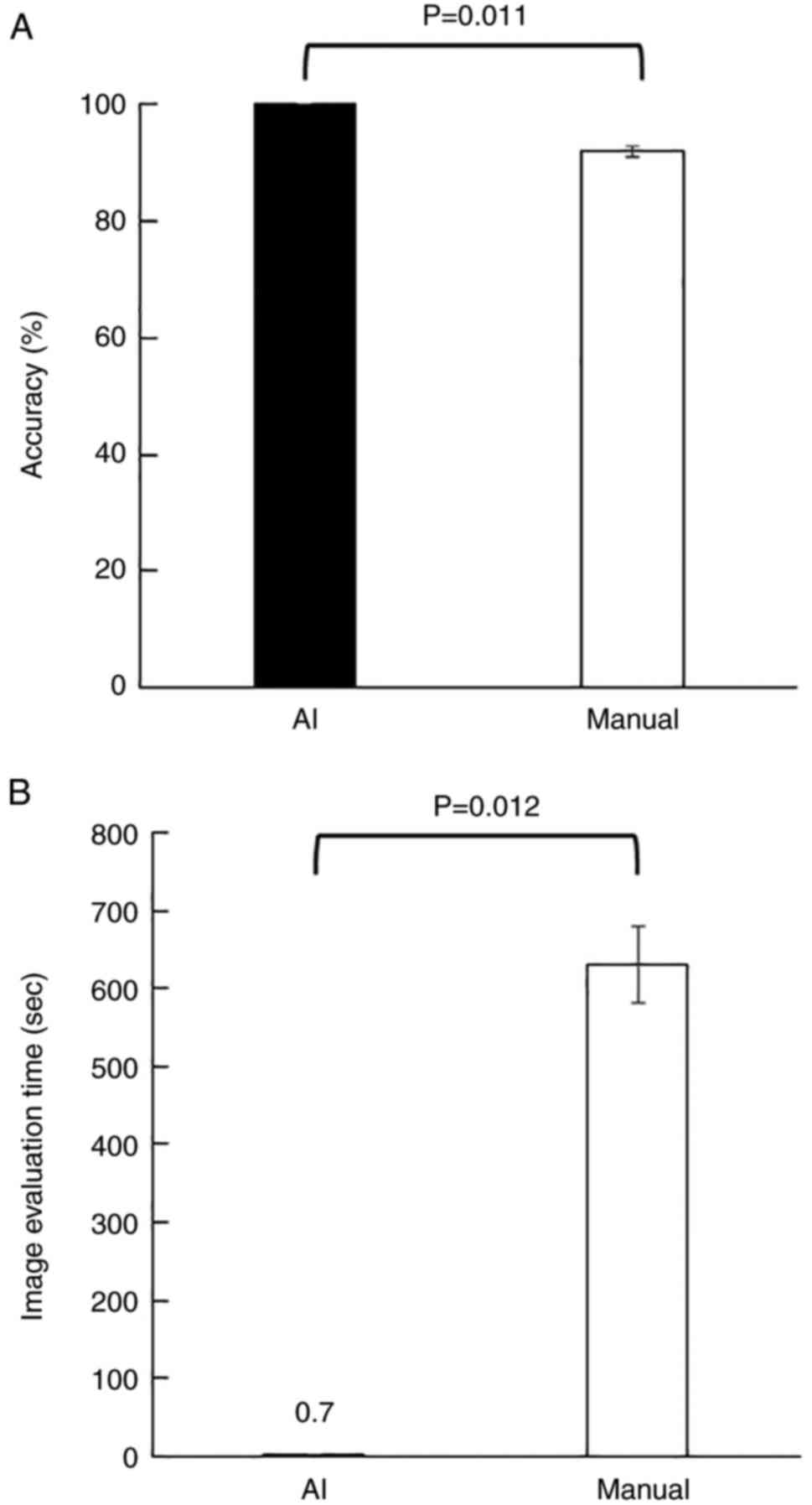
97.5%, respectively (Table II).
The average accuracies of the AI and researchers were 100 and 91.8%
with a significant difference (P=0.011, Fig. 4A). The average times taken to
classify 100 images for the AI and researchers were 0.7±0.01 and
630.4±49.5 sec, with a significant difference (P=0.012, Fig. 4B).
 | Table II.Comparison of image recognition
accuracy between manual and AI methods, using KYSE140 and PBMC
images. |
Table II.
Comparison of image recognition
accuracy between manual and AI methods, using KYSE140 and PBMC
images.
| Method | Accuracy, % | Sensitivity, % | Specificity, % | PPV, % | NPV, % |
|---|
| AI | 100 | 100 | 100 | 100 | 100 |
| Manual | 91.8 | 86.0 | 97.5 | 86.0 | 90.0 |
Detection of CTCs in blood samples of
ESCC patients using the AI algorithm
Finally, CTCs from the peripheral blood of 10 ESCC
patients were enriched and processed using the image recognition AI
algorithm to evaluate its clinical application. The
clinicopathological characteristics of the patients are summarized
in Table III. The patient
population consisted of 5 men and 5 women, with a median age of
71.9 years (range, 54–79 years). Two patients presented with stage
I disease and 8 patients with stage III. Blood samples from 5
healthy volunteers with a median age of 35.3 years (range, 30–39
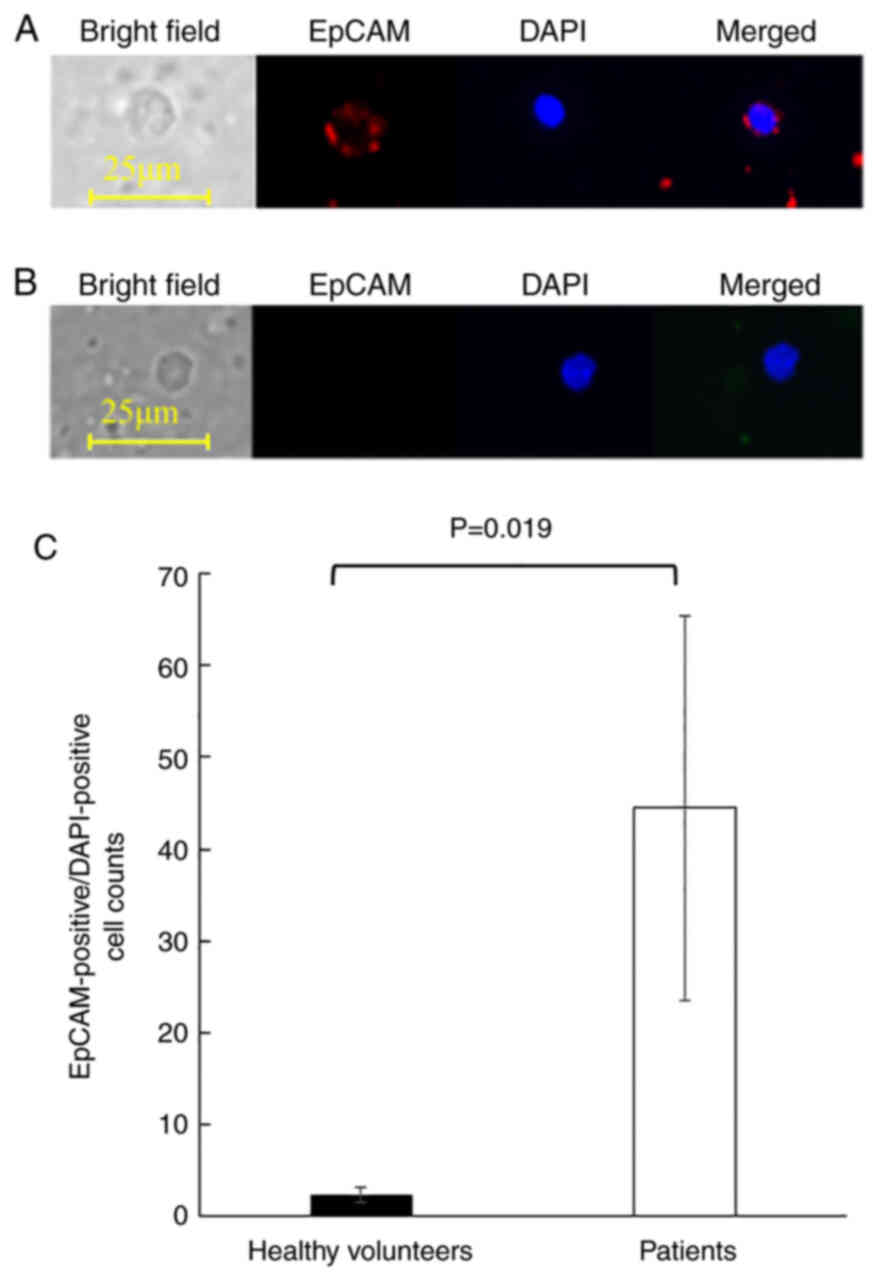
years) were used as negative controls. Representative images of
EpCAM-positive/DAPI-positive cells detected from patients were
shown in Fig. 5A. The combination
of nuclear DAPI staining and cell surface expression of EpCAM
indicated that the cells were mononuclear cells of epithelial
origin. On the other hand, PBMCs detected from healthy volunteers
were small, had round nuclei, and did not express EpCAM, indicating
that they were lymphocytes (Fig.
5B). Although EpCAM-positive/DAPI-positive cells were detected
in all examined samples, ESCC patients yielded significantly more
EpCAM-positive/DAPI-positive cells than the healthy volunteers
(mean cell counts of 2.4±0.8 and 44.5±20.9, respectively, P=0.019,
Fig. 5C).
 | Table III.Patient characteristics and number of
EpCAM-positive cells detected. |
Table III.
Patient characteristics and number of
EpCAM-positive cells detected.
| Patient no. | Age, years | Sex | T | N | M | Stage | Chemotherapy before
blood collection | Number of
EpCAM-positive cells |
|---|
| 1 | 69 | F | 3 | 1 | 0 | III | No | 23 |
| 2 | 54 | M | 3 | 1 | 0 | III | Yes | 60 |
| 3 | 73 | M | 1b | 0 | 0 | I | No | 1 |
| 4 | 71 | F | 4a | 2 | 0 | III | No | 8 |
| 5 | 79 | M | 3 | 1 | 0 | III | No | 221 |
| 6 | 78 | F | 3 | 2 | 0 | III | No | 3 |
| 7 | 69 | M | 1b | 0 | 0 | I | Yes | 41 |
| 8 | 73 | F | 3 | 1 | 0 | III | Yes | 18 |
| 9 | 70 | M | 3 | 1 | 0 | III | Yes | 64 |
| 10 | 73 | F | 3 | 1 | 0 | III | Yes | 6 |
Discussion
Though the presence of CTCs in ESCC patients is
widely accepted, methods of CTC identification with high accuracy
and efficiency are still under investigation. The performance of
recent CNN-based diagnostic support tools is reaching a level
comparable to experts in various medical fields (20–22).
In this study, we established a CNN-based image
processing algorithm and validated its performance with ESCC cell
lines and blood from ESCC patients. These results demonstrated that
AI distinguished cancer cells from PMBC by factors other than EpCAM
expression, a reliable clinical marker. This AI algorithm
distinguished each type of ESCC cell line from PBMCs with an
accuracy of more than 99.8% when the AI was trained with the same
KYSE. Regardless of the combination of KYSEs used for training and
examination, specificity in distinguishing KYSEs from PBMCs was
more than 99.6%. On the other hand, sensitivity in distinguishing
KYSEs from PBMCs varied between 20.4 and 100%. This indicates that
some cancer cells are misidentified as PBMCs depending on the
combination of KYSE used for training and examination. The lower
differentiation sensitivity in the identification of KYSE520 after
training on KYSE30, as well as in the identification of KYSE520
after training on KYSE1440, is partly explained by differences in
EpCAM expression levels. However, KYSE30 was interestingly well
distinguished after training on KYSE520 with an accuracy of 99.8%,
despite marked differences in EpCAM expression, indicating that the
AI algorithm distinguishes cells using factors other than EpCAM
expression, such as cell morphology and nuclear staining. One
strength of a diagnostic system that uses deep learning is that the
AI can discover previously unknown features that are invisible to
the human eye, such as minute differences in nucleus structure
(16,23).
AI differentiated ESCC cell lines from PBMCs better
than humans. Our AI algorithm was both faster and more accurate
than humans. This may be due to as-yet unidentified hierarchical
features that help AI distinguish cancer cells from PMBCs. The AI
algorithm counted almost the same number of cells but was
significantly faster than humans. Additionally, the AI algorithm
distinguished KYSE from PBMC perfectly, unlike humans. Sensitivity
was also lower in humans compared to AI. Researchers recognized
KYSE140 and PBMCs based solely on EpCAM-positive/DAPI-stained cells
and EpCAM-negative/DAPI-stained cells, respectively. Therefore,
EpCAM expression heterogeneity in individual KYSE140 cells, as well
as non-specific PBMC staining, may contribute to errors in the
determination of EpCAM positivity by researchers. Also, it is
possible that the AI algorithm accurately recognized the EpCAM
expression cut-off value through pre-training, or that features
were recognized that were independent of EpCAM expression (24). It is of interest to evaluate whether
diagnosis by a human will approach that of the AI when manual
recognition includes additional cytological details, such as cell
size, shape, and nucleus-to-cytoplasmic ratio alongside EpCAM
expression (25). Nevertheless,
recognition accuracy among researchers varies, with trained
pathologists continuing to use subjective criteria in cytology
(25). It is possible that humans
are unable to match AI's recognition capabilities.
AI counted and classified cells up to 850 times
faster than humans. A full range search (X, Y, and Z axis) is
required for humans to recognize a cell as slides have
three-dimensional structure, despite their flat, two-dimensional
appearance. In fact, this step requires the most time during the
CTC detection process. However, AI performs rapid image acquisition
and analysis. Reducing analysis time greatly improves efficiency,
enabling accelerated AI algorithm evolution through training with a
large library of images. In this study, the AI algorithm was
preliminarily applied to detect EpCAM-positive/DAPI-positive cells
in ESCC patients. EpCAM-positive/DAPI-positive cells were detected
in blood samples from ESCC patients using the AI algorithm,
suggesting potential clinical applications. The average number of
EpCAM-positive cells in the patients was 44.5 cells while in
healthy volunteers it was 2.4 cells, agreeing with previous reports
(6,10).
In a recent report on CNN-based detection of CTC in
cancer patients, Guo et al processed the enriched CTC
fraction for immunofluorescence in situ hybridization against
chromosome 8 centromere, considering a cell as a CTC if it were
CD45-/DAPI+/with more than two centromeres (26). After pre-training with segmented
images of 555 CTCs and 10777 non-CTCs, their CNN model identified
CTCs with a sensitivity and specificity of 97.2 and 94.0%,
respectively (26). With a similar
number of cell images used for pre-training, the sensitivity and
specificity on the test set were comparable to our results. This
demonstrates the usefulness of the CNN-based algorithm for CTC
detection. Further research is required to determine optimal
markers, in terms of accuracy and convenience, to define CTCs for
pre-training the AI algorithms.
Immunological detection of EpCAM expression is a
robust method for CTC identification. However, certain limitations
are being identified. Epithelial-to-mesenchymal transition (EMT) is
reported during CTC detachment from the primary tumor, along with
transformation to mesenchymal and stem-like properties (27–29).
As a result of EMT, downregulation of epithelial markers such as
EpCAM and upregulation of interstitial markers such as cell surface
vimentin (CSV) are observed (27–32).
Previous evaluations of EpCAM-based positive enrichment reported
CTC detection rates in the range of 18–50% (33). Given reports on the involvement of
EMT in treatment resistance (33)
and tumor stem cell maintenance (34), the clinical significance of
EpCAM-negative CTCs is suggested (33,34).
Therefore, methods based on the combination of epithelial and
mesenchymal markers may improve the clinical relevance of CTC
detection.
In addition, using higher resolution images and
setting cutoff values over the number of cases also improved
detection rates. Taking advantage of AI's ability to autonomously
identify hierarchical features (23), it is possible to establish an AI
algorithm upon accumulated cases which identify via currently
unknown marker-independent features. Further improvements in
efficiency and system evolution automation may provide quick and
accurate diagnoses based on simple sample preparation, ideally
requiring only bright field image acquisition.
In this study, a small number of ESCC patients and
healthy volunteers were compared by CTCs detection methods to
assess the potential of our AI algorithm. CTC detection impacts
prognostic value in ESCC patients, as indicated by several reports
(32). The prognostic significance
of AI-based CTC detection compared to conventional CTC detection
remains to be evaluated in large prospective studies.
In addition, molecular mechanisms regulating the
malignant potential of CTCs are still being elucidated (35,36).
Future investigation of the correlation between the unknown
features referenced by the AI algorithm in CTC detection and the
molecular characteristics of CTCs may provide a basis for the
development of novel diagnostic and therapeutic strategies against
ESCC.
This study has certain limitations. First, a small
number of EpCAM positive cells were detected in PBMCs prepared from
healthy volunteers, meaning that not all the EpCAM positive cells
were CTCs in ESCC patients. EpCAM positive cells may correspond to
contamination of skin cells or immature blood cells (5,37).
However, when many EpCAM positive cells are detected in ESCC
patients, the majority are considered CTCs. Detection improvements
include using high-resolution images, understanding EpCAM positive
cells in healthy volunteers, and setting appropriate cutoff values
over several cases. Second, the criteria that AI applied to
distinguish ESCC cells from PBMC are a black box. This makes it
unclear as to whether the present conditions are applicable to
other cancers. Finally, CTCs were detected in ESCC patients by
image processing under the conditions used in the cell lines to
establish a prototype AI and preliminarily applied to the patient's
samples. To establish better AI, supervised learning algorithms are
best performed with many ESCC cell lines. Also, our future goal is
to establish AI trained by peripheral blood samples from ESCC
patients. However, this AI training faces the problem of setting a
positive standard. Preparation of a true CTC to effectively and
robustly train an AI for the correct answer is not trivial.
In conclusion, our results demonstrated that the
CNN-based image processing algorithm for CTC detection provides
higher reproducibility and a shorter analysis time compared to
manual detection by the human eye. In addition, the AI algorithm
appears to distinguish CTCs based on unknown features, independent
of marker expressions.
Acknowledgements
The authors would like to thank Mr. Yusuke Kishi and
Mr. Kisuke Tanaka (Department of Mechanical and Intellectual
Systems Engineering, Faculty of Engineering, University of Toyama)
for their assistance with the experiment.
Funding
This work was partly supported by JSPS KAKENHI (grant nos.
21K08729, 22K07185 and 22K08730).
Availability of data and materials
The datasets used and/or analyzed during the current
study are not publicly available due to individual participants'
privacy but are available from the corresponding author on
reasonable request.
Authors' contributions
TA, TO, HT TY, YN, TaF, TMa, HB and TsF were
involved in the conception and design, experiments, data analysis,
data interpretation and manuscript writing. KT, YY, AY and TS were
involved in the conception and design, data analysis using machine
learning classifiers, data interpretation and manuscript writing.
TMi, TW, KH, TI, SS, IH, KS, SH, IY and KM were involved in the
conception and design, case enrollment, informed consent, blood
sample collection, data interpretation and manuscript writing. TA,
TO and KT confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The protocol of this study was approved by the
ethics review board of University of Toyama Hospital (approval no.
R2021042) and written informed consent was obtained from all
participants.
Patient consent for publication
The patients have provided written informed consent
for publication of the data in this manuscript.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Watanabe M, Otake R, Kozuki R, Toihata T,
Takahashi K, Okamura A and Imamura Y: Recent progress in
multidisciplinary treatment for patients with esophageal cancer.
Surg Today. 50:12–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Castro-Giner F and Aceto N: Tracking
cancer progression: From circulating tumor cells to metastasis.
Genome Med. 12:312020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ganesh K and Massague J: Targeting
metastatic cancer. Nat Med. 27:34–44. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rodrigues P and Vanharanta S: Circulating
tumor cells: Come together, right now, over metastasis. Cancer
Discov. 9:22–24. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ujiie D, Matsumoto T, Endo E, Okayama H,
Fujita S, Kanke Y, Watanabe Y, Hanayama H, Hayase S, Saze Z, et al:
Circulating tumor cells after neoadjuvant chemotherapy are related
with recurrence in esophageal squamous cell carcinoma. Esophagus.
18:566–573. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu HT, Miao J, Liu JW, Zhang LG and Zhang
QG: Prognostic value of circulating tumor cells in esophageal
cancer. World J Gastroenterol. 23:1310–1318. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ohnaga T, Shimada Y, Takata K, Obata T,
Okumura T, Nagata T, Kishi H, Muraguchi A and Tsukada K: Capture of
esophageal and breast cancer cells with polymeric microfluidic
devices for CTC isolation. Mol Clin Oncol. 4:599–602. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Watanabe T, Okumura T, Hirano K, Yamaguchi
T, Sekine S, Nagata T and Tsukada K: Circulating tumor cells
expressing cancer stem cell marker CD44 as a diagnostic biomarker
in patients with gastric cancer. Oncol Lett. 13:281–288. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yamaguchi T, Okumura T, Hirano K, Watanabe
T, Nagata T, Shimada Y and Tsukada K: Detection of circulating
tumor cells by p75NTR expression in patients with esophageal
cancer. World J Surg Oncol. 14:402016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kojima H, Okumura T, Yamaguchi T, Miwa T,
Shimada Y and Nagata T: Enhanced cancer stem cell properties of a
mitotically quiescent subpopulation of p75NTR-positive cells in
esophageal squamous cell carcinoma. Int J Oncol. 51:49–62. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Correnti M and Raggi C: Stem-like
plasticity and heterogeneity of circulating tumor cells: Current
status and prospect challenges in liver cancer. Oncotarget.
8:7094–7115. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Semaan A, Bernard V, Kim DU, Lee JJ, Huang
J, Kamyabi N, Stephens BM, Qiao W, Varadhachary GR, Katz MH, et al:
Characterisation of circulating tumour cell phenotypes identifies a
partial-EMT sub-population for clinical stratification of
pancreatic cancer. Br J Cancer. 124:1970–1977. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shimizu H and Nakayama KI: Artificial
intelligence in oncology. Cancer Sci. 111:1452–1460. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elemento O, Leslie C, Lundin J and
Tourassi G: Artificial intelligence in cancer research, diagnosis
and therapy. Nat Rev Cancer. 21:747–752. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeune LL, Boink YE, van Dalum G, Nanou A,
de Wit S, Andree KC, Swennenhuis JF, van Gils SA, Terstappen LWMM
and Brune C: Deep learning of circulating tumour cells. Nat Machine
Intelligence. 2:124–133. 2020. View Article : Google Scholar
|
|
17
|
Russakovsky O, Deng J, Su H, Krause J,
Bernstein M, Berg A and Fei-Fei L: ImageNet large scale visual
recognition challenge. Int J Computer Vision. 115:211–252. 2015.
View Article : Google Scholar
|
|
18
|
Sobin LH, Gospodarowicz M and Wittekind C:
TNM Classification of Malignant Tumours, 7th edition, UICC
International Union Against Cancer 2010. Hoboken, NJ:
Wiley-Blackwell; 2010
|
|
19
|
Nobuyuki O: A Threshold selection method
from gray-level histograms. IEEE Transactions on Systems, Man, and
Cybernetics. 9:pp62–66. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu X, Faes L, Kale AU, Wagner SK, Fu DJ,
Bruynseels A, Mahendiran T, Moraes G, Shamdas M, Kern C, et al: A
comparison of deep learning performance against health-care
professionals in detecting diseases from medical imaging: A
systematic review and meta-analysis. Lancet Digit Health.
1:e271–e297. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kamba S, Tamai N, Saitoh I, Matsui H,
Horiuchi H, Kobayashi M, Sakamoto T, Ego M, Fukuda A, Tonouchi A,
et al: Reducing adenoma miss rate of colonoscopy assisted by
artificial intelligence: A multicenter randomized controlled trial.
J Gastroenterol. 56:746–757. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Esteva A, Kuprel B, Novoa RA, Ko J,
Swetter SM, Blau HM and Thrun S: Dermatologist-level classification
of skin cancer with deep neural networks. Nature. 542:115–118.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou LQ, Wang JY, Yu SY, Wu GG, Wei Q,
Deng YB, Wu XL, Cui XW and Dietrich CF: Artificial intelligence in
medical imaging of the liver. World J Gastroenterol. 25:672–682.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zheng S, Lin HK, Lu B, Williams A, Datar
R, Cote RJ and Tai YC: 3D microfilter device for viable circulating
tumor cell (CTC) enrichment from blood. Biomed Microdevices.
13:203–213. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moore MJ, Sebastian JA and Kolios MC:
Determination of cell nucleus-to-cytoplasmic ratio using imaging
flow cytometry and a combined ultrasound and photoacoustic
technique: A comparison study. J Biomed Opt. 24:1–10. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Guo Z, Lin X, Hui Y, Wang J, Zhang Q and
Kong F: Circulating tumor cell identification based on deep
learning. Front Oncol. 12:8438792022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Habli Z, AlChamaa W, Saab R, Kadara H and
Khraiche ML: Circulating tumor cell detection technologies and
clinical utility: Challenges and opportunities. Cancers (Basel).
12:19302020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miki Y, Yashiro M, Kuroda K, Okuno T,
Togano S, Masuda G, Kasashima H and Ohira M: Circulating
CEA-positive and EpCAM-negative tumor cells might be a predictive
biomarker for recurrence in patients with gastric cancer. Cancer
Med. 10:521–528. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han D, Chen K, Che J, Hang J and Li H:
Detection of epithelial-mesenchymal transition status of
circulating tumor cells in patients with esophageal squamous
carcinoma. Biomed Res Int. 2018:76101542018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao Y, Fan WH, Song Z, Lou H and Kang X:
Comparison of circulating tumor cell (CTC) detection rates with
epithelial cell adhesion molecule (EpCAM) and cell surface vimentin
(CSV) antibodies in different solid tumors: A retrospective study.
PeerJ. 9:e107772021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chaw SY, Abdul Majeed A, Dalley AJ, Chan
A, Stein S and Farah CS: Epithelial to mesenchymal transition (EMT)
biomarkers-E-cadherin, beta-catenin, APC and Vimentin-in oral
squamous cell carcinogenesis and transformation. Oral Oncol.
48:997–1006. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shi Y, Ge X, Ju M, Zhang Y, Di X and Liang
L: Circulating tumor cells in esophageal squamous cell
carcinoma-Mini review. Cancer Manag Res. 13:8355–8365. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fischer KR, Durrans A, Lee S, Sheng J, Li
F, Wong ST, Choi H, El Rayes T, Ryu S, Troeger J, et al:
Epithelial-to-mesenchymal transition is not required for lung
metastasis but contributes to chemoresistance. Nature. 527:472–476.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng Z, Wu S, Wang Y and Shi D:
Circulating tumor cell isolation for cancer diagnosis and
prognosis. EBioMedicine. 83:1042372022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gkountela S, Castro-Giner F, Szczerba BM,
Vetter M, Landin J, Scherrer R, Krol I, Scheidmann MC, Beisel C,
Stirnimann CU, et al: Circulating tumor cell clustering shapes DNA
methylation to enable metastasis seeding. Cell. 176:98–112. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Allard WJ, Matera J, Miller MC, Repollet
M, Connelly MC, Rao C, Tibbe AG, Uhr JW and Terstappen LW: Tumor
cells circulate in the peripheral blood of all major carcinomas but
not in healthy subjects or patients with nonmalignant diseases.
Clin Cancer Res. 10:6897–6904. 2004. View Article : Google Scholar : PubMed/NCBI
|