Introduction
Cervical cancer is a common gynecological cancer
worldwide (1–3) and squamous cell carcinoma (SCC)
accounts for ~75% of cervical cancer cases (4). SCC of the cervix usually develops from
cervical intraepithelial neoplasia (CIN); high-grade CIN (CIN2 or
CIN3) is considered to be a precursor (5–7). To
date, screening for CIN2, CIN3 and SCC (CIN2+) has been widely
performed using cytological tests (8,9). For
several years, cytology has been the first choice for screening.
The presence of high-risk human papillomavirus (hrHPV) is also
associated with CIN2+ because CIN and cancer can develop due to
hrHPV infection (10,11). HPV16 and HPV18 are two major
carcinogenic subtypes of hrHPV (12). Moreover, several other hrHPV
subtypes, including 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and
68, are associated with a high frequency of cervical cancer
development (13). Therefore, tests
for detecting hrHPV infections are widely used for screening
(13); however, hrHPV infection
does not always indicate CIN or cervical cancer. Therefore, hrHPV
status should be correlated with cytological findings.
Screening for high-grade squamous intraepithelial
lesion (HSIL) and cervical cancer is associated with a controversy
regarding the age at which it should be performed and variation in
screening content with age. In Japan, SCC is frequently observed
even in individuals >60 years of age. Several reports have
described different distribution patterns of hrHPV subtypes and
cytologies among different age groups (14–17).
If the distribution of hrHPV and/or cytology results differs among
age groups, the interpretation of cytological results and hrHPV
status combinations should vary among age groups. However, few
reports have comprehensively observed the cytological background
and hrHPV status of patients with CIN2+, and examined the clinical
impact of screening among different age groups from younger to
older patients (18,19).
The present study aimed to elucidate the
significance of cytological results and hrHPV status in patients
with CIN2+ across a wide age group. In addition, the clinical
impact of HPV vaccination was evaluated. In Japan, a public HPV
vaccination program for 13–16-year-olds began in 2010. By 2013, the
vaccination rate in this generation was ~70% (20,21).
However, due to repeated media reports of various symptoms
following HPV vaccination, the government announced the suspension
of active vaccine recommendations in April 2013, which led to a
decline in vaccination rates to <1% (22). Therefore, individuals born between
1994 and 1999 are called the ‘vaccination generation’, as
vaccination rates in earlier generations were 0% and those in the
later generations are also very low (23). In the present study, the
‘vaccination generation’ corresponds to individuals in the
18–24-year age group.
Materials and methods
Study design and patients
The present case-control study aimed to investigate
the relationship among cytological results, hrHPV status and
clinicopathological findings. The patient examination flow chart is
shown in Fig. 1. Patients who
underwent cytological tests at the Department of Obstetrics and
Gynecology, Toyooka Public Hospital (Toyooka, Japan) between April
2016 and January 2019 were first included, which revealed the
presence of atypical squamous cells (ASC) of undetermined
significance (ASC-US), low-grade squamous intraepithelial lesion
(LSIL), ASC not excluding HSIL (ASC-H) or HSIL. For patients with
ASC-US cytology, a qualitative hrHPV test was performed. If the
test results were positive, hrHPV typing and colposcopy-directed
biopsy were performed. For patients with LSIL, ASC-H or HSIL
cytologies, hrHPV typing and colposcopy-directed biopsy were
performed simultaneously. The biopsy results were categorized as
malignancy (SCC), CIN3, CIN2, CIN1 or no malignancy. CIN2, CIN3 and
SCC were then grouped as CIN2+.
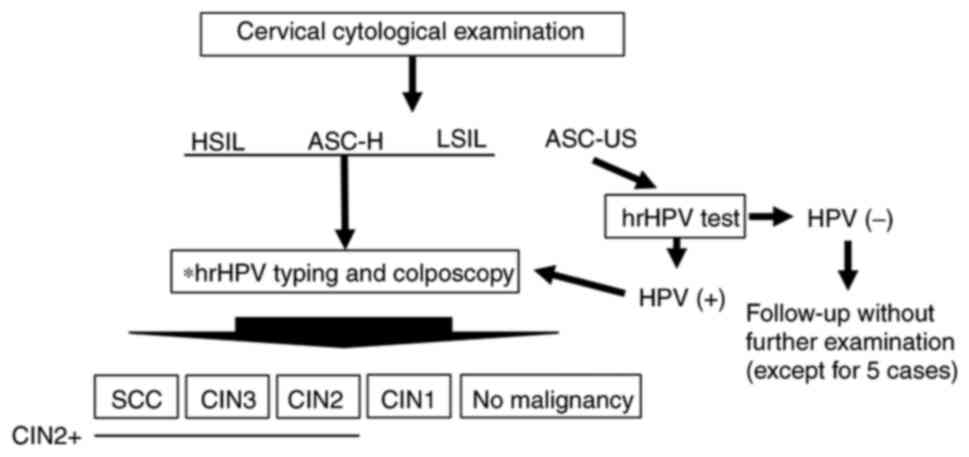 | Figure 1.Schematic diagram of the present
study. A total of 446 patients underwent cervical cytological
examination. For patients with HSIL, ASC-H, and LSIL cytology,
colposcopy-directed biopsy and hrHPV typing were performed. For
patients with ASC-US cytology, an hrHPV test was performed. If
positive, colposcopy-directed biopsy and hrHPV typing were
performed. Additionally, for clinical reasons, five patients with
ASC-US and hrHPV-underwent colposcopy-directed biopsy and hrHPV
typing. *HPV typing test: 16, 18, 31, 33, 35, 39, 45, 51, 52, 56,
58, 59, 66 and 68 types were examined. ASC-US, atypical squamous
cells of undetermined significance; LSIL, low-grade squamous
intraepithelial lesion; ASC-H, atypical squamous cells not
excluding HSIL; HSIL, high-grade squamous intraepithelial lesion;
hrHPV, high-risk human papillomavirus; hrHPV-, hrHPV-negative; SCC,
squamous celll carcinoma; CIN, cervical intraepithelial neoplasia
grade; CIN2+, SCC, CIN3 and CIN2. |
Collection of clinical data, and
cytological and hrHPV results
Data pertaining to cytological results, hrHPV status
and histopathological diagnoses were extracted from the medical
records of the patients. Patient age, and history of pregnancy and
childbirth at the time of examination were confirmed from the
medical records or by interviewing the patient. Cervical cytology
was performed using a cervix brush (Rovers Medical Devices B.V.,
Netherlands) with the conventional method of mounting cells
directly on a glass slide. Cytological results were analyzed and
classified according to the Bethesda system (24).
Qualitative hrHPV assessment of patients with ASC-US
cytology was performed using the Cobas HPV test (Roche Diagnostics
K.K.). Quantitative PCR was used to determine the subtype of HPV
(16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66 and 68). hrHPV
typing was performed using the PCR-rSSO method (MEVGEN HPV kit;
cat. no. GS-B0702; Medical & Biological Laboratories Co.,
Ltd.). The test results were reported as ‘positive for HPV16/18’ or
‘positive for an HPV subtype other than 16/18’.
The pathological diagnoses of the biopsy specimens
were determined in consultation with a gynecological oncologist and
a pathologist specializing in gynecology.
Statistical analysis
For continuous variables, one-way analysis of
variance was first performed to assess the presence of a
significant difference in the overall distribution. When it was
present, Bonferroni correction for multiple comparisons was applied
to compare two groups. For categorical data, Fisher's exact test
was first used to determine whether there was a significant
difference overall, after which the Bonferroni correction was
performed to compare two groups. For calculation of relative risk
(RR) and 95% confidence interval (CI), Fisher's exact test was
used. P<0.05 was considered to indicate a statistically
significant difference. GraphPad Prism version 9.0 (Dotmatics) was
used for statistical analysis.
Results
Association between age, different
cytological findings, hrHPV status and pathology in all
patients
In total, 446 patients with the following
cytological findings were included in the present study: ASC-US,
n=310; LSIL, n=44; ASC-H, n=38; and HSIL, n=54. hrHPV status was
categorized into three groups: hrHPV-negative (hrHPV-), n=263;
hrHPV positive for subtypes other than 16/18 (others+), n=137; and
HPV 16/18-positive (16/18+), n=46. The age of patients and
frequency of multiparity in each group are shown in Table I.
 | Table I.Clinical background of patients. |
Table I.
Clinical background of patients.
| Patient
characteristic | Number | Median age, years
(IQR) | Multiparity
(%) |
|---|
| Total | 446 | 44.0
(36.0–53.0) | 82.10 |
| Cytology |
|
|
|
|
ASC-US | 310 | 46.0
(39.0–56.0) | 84.20 |
|
LSIL | 44 | 35.5
(30.0–46.5) | 72.70 |
|
ASC-H | 38 | 46.0
(38.0–58.3) | 76.30 |
|
HSIL | 54 | 39.0
(29.0–45.0) | 81.50 |
| hrHPV status |
|
|
|
|
Negative | 263 | 47.0
(40.0–57.5) | 84.80 |
|
Others+ | 137 | 43.0
(35.0–50.0) | 78.10 |
|
16/18+ | 46 | 37.0
(32.75–43.0) | 80.40 |
The present study first examined the age
distribution of patients with a particular cytological and hrHPV
status. Significant differences were observed with respect to both
cytological results and hrHPV status. Specifically, patients with
ASC-US and ASC-H were significantly older, whereas those with LSIL
and HSIL were significantly younger (Fig. S1A). Regarding hrHPV status,
patients with 16/18+ were significantly younger than those with
others+ and hrHPV-(Fig. S2A).
There were no significant differences in age distribution among
patients with different histopathological findings (Table SI; Fig. S1B). Next, the influence of the HPV
vaccine was compared among all patients (n=446) by dividing the
entire group into nine groups based on age (18–24 years, n=12;
25–29 years, n=36; 30–34 years, n=46; 35–39 years, n=52; 40–44
years, n=84; 45–49 years, n=70; 50–54 years, n=42; 55–59 years,
n=31; ≥60 years, n=73; Tables SII
and SIII; Fig. S2B). Regarding the influence of the
HPV vaccine, the occurrence of 16/18+ was considerably lower at
8.3% in the 18–24-year age group, which corresponded to the
‘vaccination generation’ (Table
SII; Fig. S2B). By contrast,
the occurrence of others+ was considerably higher in the
‘vaccination generation’ at 66.7% (Table SIII; Fig. S2B). The details of the age
distribution of patients with a particular cytological and hrHPV
status, and the results of the influence of the HPV vaccine are
described in Appendix SI.
Comparison of hrHPV status among
different age groups in patients with CIN2+
Next, the present study focused on patients with
CIN2+ (n=107). The age distribution and parity for each
pathological status are described in Table SI. The present study first examined
the hrHPV status and found significant differences in age
distribution among the three groups (P=0.0157; Table II; Fig.
2A). Patients with 16/18+ were relatively younger than those
with others+ and hrHPV-(16/18+ vs. others+, P=0.0092; 16/18+ vs.
hrHPV-, P=0.056; Table II;
Fig. 2A).
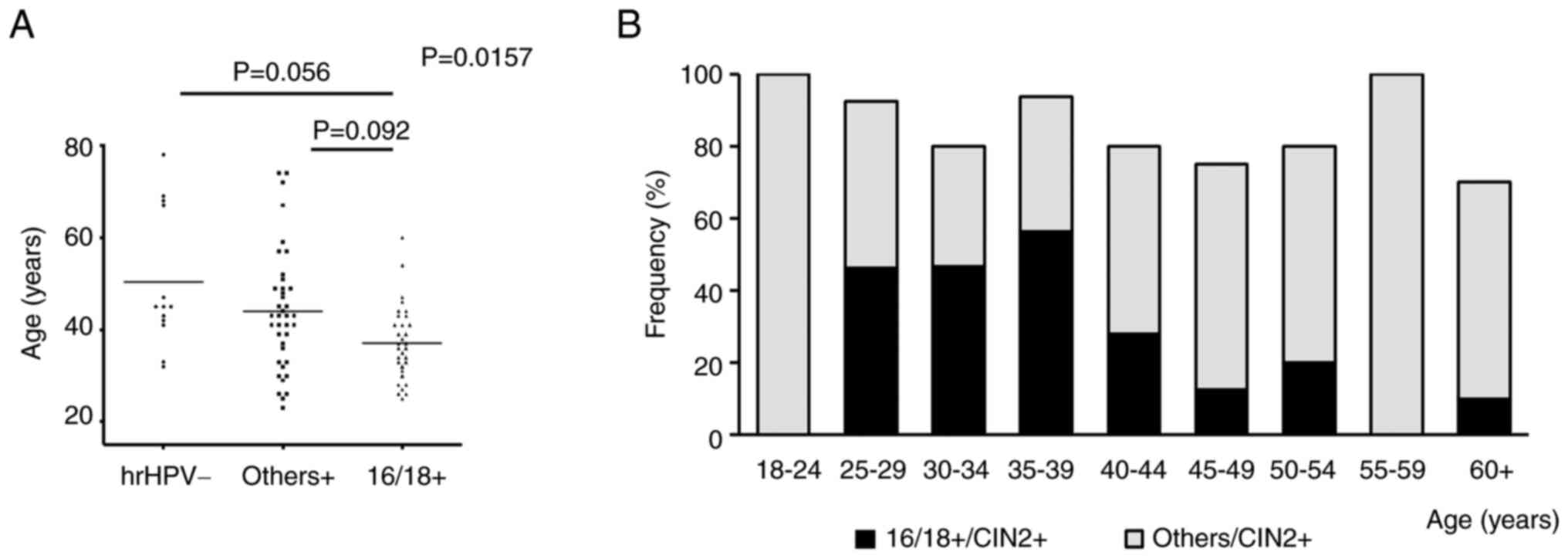 | Figure 2.Comparison of hrHPV findings with
respect to age in patients with CIN2+ (n=107). (A) Comparison among
different hrHPV statuses. One-way analysis of variance was
performed, and in case of a significant difference, the results
were compared between the two groups. If the difference was
significant, an adjusted P-value was calculated. (B) Frequency of
occurrence of 16/18+ and others+ in each age group. hrHPV,
high-risk human papillomavirus; hrHPV-, hrHPV-negative; others+,
hrHPV positive for subtypes other than 16/18; 16/18+,
HPV16/18-positive; CIN2+, squamous cell carcinoma, CIN3 and CIN2;
CIN, cervical intraepithelial neoplasia. |
 | Table II.Age of patients with CIN2+ according
to cytology and hrHPV status. |
Table II.
Age of patients with CIN2+ according
to cytology and hrHPV status.
| CIN2+ patients | Number | Median age, years
(IQR) |
|---|
| Patient
characteristic | 107 | 41.0
(33.0–47.0) |
| Cytology |
|
|
|
ASC-US | 29 | 43.0
(39.0–49.0) |
|
LSIL | 12 | 34.5
(32.25–45.0) |
|
ASC-H | 23 | 44.0
(38.0–58.0) |
|
HSIL | 43 | 39 (29.0–44.0) |
| hrHPV status |
|
|
|
Negative | 18 | 44
(38.75–46.5) |
|
Others+ | 56 | 43 (36.0–49.5) |
|
16/18+ | 33 | 37 (32.0–41.0) |
The present study evaluated the effects of the HPV
vaccine. As aforementioned, patients with CIN2+ were categorized
into nine groups by age (18–24 years, n=2; 25–29 years, n=13; 30–34
years, n=15; 35–39 years, n=16; 40–44 years, n=25; 45–49 years,
n=16; 50–54 years, n=5; 55–59 years, n=5; ≥60 years, n=10) and the
occurrence of 16/18+ and others+ was calculated for each age group
(Tables SII and SIII; Fig.
2B). In the ‘vaccination generation’, the occurrence of 16/18+
was considerably lower at 0%, whereas that of others+ was 100%
(Tables SII and SIII; Fig.
2B). In addition, the occurrence of others+ tended to be higher
in the ≥40-years age groups (Table
SIII; Fig. 2B).
Comparison of cytology among different
age groups in patients with CIN2+
The present study examined the differences in
cytological findings and age distribution, and significant
differences in age distribution were revealed among patients with
different cytological findings (P=0.028; Table II; Fig.
3A). Subsequently, the distribution of cytological findings
were compared among the 107 patients with CIN2+ by dividing the
entire group into four groups based on age (age groups, 18–29
years, n=15; 30–39 years, n=31; 40–49 years, n=41; ≥50 years, n=20;
Fig. 3B). The distribution pattern
differed significantly among the four age groups (P=0.0037). In the
18–29-year age group, the occurrence of HSIL was considerably
higher (80.0%) and was much lower in the 50-year age group (25.0%)
(Fig. 3B). By contrast, the
occurrence of ASC-US was high in the ≥40-years age groups (18–29
years, 6.7%; 30–39 years, 22.6%; 40–49 years, 36.6%; 50 years,
30.0%; Fig. 3B).
 | Figure 3.Comparison of cytology findings with
respect to age, and distribution of cytology and hrHPV status
combinations in patients with CIN2+ (n=107). (A) Comparison among
different cytology findings. (B) Comparison of distribution of
cytology in patients with CIN2+ in different age groups (age
groups, 18–29 years: n=15; 30–39 years: n=31; 40–49 years: n=41;
≥50 years: n=20). (C-F) Distribution of combination of cytological
results and hrHPV status in each age group. There were 12
combinations of cytological results and hrHPV status, and all
patients with the 11 combinations (with the exception of ASC-US and
hrHPV-) underwent a biopsy. The numerator/denominator indicates the
number of patients included in each combination/total patients with
CIN2+, respectively. (C) 18–29-year age group. The combination of
HSIL and 16/18+ accounted for 40.0% of all CIN2+ cases, followed by
that of HSIL and others+ at 33.3%. (D) 30–39-year age group. (E)
40–49-year age group. The combination of ASC-US and others+
accounted for >25.0% of all CIN2+ cases. (F) ≥50-year age group.
The combination of ASC-US and others+ accounted for 25.0% of all
CIN2+ cases. There were no cases of HSIL and 16/18+. ASC-US,
atypical squamous cells of undetermined significance; LSIL,
low-grade squamous intraepithelial lesion; ASC-H, atypical squamous
cells not excluding HSIL; HSIL, high-grade squamous intraepithelial
lesion; hrHPV, high-risk human papillomavirus; hrHPV-,
hrHPV-negative; others+, hrHPV positive for subtypes other than
16/18; 16/18+, HPV16/18-positive; CIN2+, squamous cell carcinoma,
CIN3 and CIN2; CIN, cervical intraepithelial neoplasia. |
Comparison of cytology and hrHPV
combinations in different age groups among patients with CIN2+
As both background cytology and hrHPV findings in
patients with CIN2+ differed significantly among the different age
groups, the present study examined the association between
cytological findings and hrHPV status in each age group. There were
12 groups based on cytology and hrHPV combinations, and all
patients with 11 combinations (with the exception of ASC-US and
hrHPV-) underwent biopsy. The occurrence of CIN2+ was calculated in
each age group (Fig. 3C-F).
In the 18–29-year age group, the most common
combination was HSIL and 16/18+, followed by HSIL and others+;
these two combinations accounted for 73% of total CIN2+ cases. Only
one case of hrHPV-was detected with HSIL cytology (Fig. 3C).
This definite contrast was not observed in any other
age group. More than half of patients with CIN2+ in the 30–39-year
group exhibited 16/18+, but their cytological findings were not
highly skewed (ASC-H and 16/18+, n=6; HSIL and 16/18+, n=4; LSIL
and 16/18+, n=4; ASC-US and 16/18+, n=2; Fig. 3D).
In the 40–49 and 50-year age groups, the majority of
patients with CIN2+ had a hrHPV status of others+; the most common
combination was ASC-US and others+, with a proportion of 31.7% in
the 40–49-year age group and 25% in the 50-year age group (Fig. 3E and F). In the 50-year age group,
no patient had a combination of HSIL and 16/18+ (Fig. 3F).
A screening perspective: Different
clinical impacts of cytology and hrHPV combinations for each age
group
Subsequently, the present study investigated
perspectives on screening for CIN2+. The occurrence of CIN2+,
according to cytological findings and hrHPV status, was compared in
all participants with the exception of the population whose
cytological findings and hrHPV combination was ASC-US/hrHPV-. As a
result, 232 cases were included in the analysis. As expected, the
occurrence of CIN2+ differed significantly depending on the
cytological findings and hrHPV status (Fig. S3A and B). In addition, CIN2+
occurrence was more precisely reflected by a combination of
cytological findings and hrHPV status (Fig. S3C). The details are described in
the Appendix SI.
The present study investigated the occurrence of
CIN2+ in the 12 groups in which cytological findings and hrHPV
status were assessed according to each age group (Fig. 4). In the 18–29-year age group, the
occurrence of CIN2+ was very high in patients with HSIL and very
low in patients with ASC-US (Fig.
4A). However, this definite distinction was not observed in
patients aged ≥30 years. Nonetheless, in the 30–39 and 40–49-year
age groups, CIN2+ was detected at a relatively high frequency in
patients with 16/18+, regardless of cytological findings, and in
those with HSIL, regardless of hrHPV status (Fig. 4B and C). However, this distinction
was unclear in the 50-year age group (Fig. 4D). In this age group, CIN2+ was
detected to some extent in all hrHPV and cytological combinations.
The details are described in Appendix
SI.
 | Figure 4.Frequency of occurrence of CIN2+ was
calculated for each combination of cytological results and hrHPV
status among the different age groups (age groups, 18–29 years:
n=48; 30–39 years: n=98; 40–49 years: n=153; ≥50 years: n=147). The
numerator/denominator indicates patients with CIN2+/number of
patients included in each combination. (A) 18–29-year age group.
The occurrence of CIN2+ was predominant in patients with HSIL,
whereas it was limited in patients with ASC-US. (B) 30–39-year age
group. The frequency of occurrence of CIN2+ was relatively high in
patients with HSIL and/or 16/18+. (C) 40–49-year age group. The
frequency of occurrence of CIN2+ was relatively high in patients
with HSIL and/or 16/18+. (D) ≥50 year age group. The distribution
of CIN2+ was unclear. ASC-US, atypical squamous cells of
undetermined significance; LSIL, low-grade squamous intraepithelial
lesion; ASC-H, atypical squamous cells not excluding HSIL; HSIL,
high-grade squamous intraepithelial lesion; hrHPV, high-risk human
papillomavirus; hrHPV-, hrHPV-negative; others+, hrHPV positive for
subtypes other than 16/18; 16/18+, HPV16/18-positive; CIN2+,
squamous cell carcinoma, CIN3 and CIN2; CIN, cervical
intraepithelial neoplasia. |
Finally, the occurrence of CIN2+ in these
cytological and hrHPV status combinations was compared among
different generations. While it would be ideal to validate all
combinations, due to the limited number of cases, there were not
enough cases to analyze every combination in every age group;
therefore, several classifications were combined. In patients with
ASC-H/HSIL and 16/18+, CIN2+ was detected at a high frequency in
all generations (Table IIIA). By
contrast, in patients with a combination of ASC-US and others+,
although the difference was not significant, CIN2+ was more
frequently detected in other age groups than in the 18–29-year age
group, especially in the 40–49-year age group, with a very high
relative risk (RR) of 4.33 [95% confidence interval (CI):
0.98–24.97] (Table IIIB).
Additionally, in patients with a combination of LSIL/ASC-H and
hrHPV-, CIN2+ was more frequently detected in other age groups than
in the 18–29-year age group, especially in the 40–49-year age group
with a very high RR of 8.00 (95% CI: 0.99–82.9) (Table IIIC).
 | Table III.Comparison of CIN2+ detection in the
cytology and hrHPV status combinations among different age
groups. |
Table III.
Comparison of CIN2+ detection in the
cytology and hrHPV status combinations among different age
groups.
| A, Analysis of
ASC-H, HSIL and 16/18+ combinations. |
|---|
|
|---|
| Age group,
years | CIN2+/Total | RR | 95% CI |
|---|
| 18-29 | 6/6 | Ref. | Ref. |
| 30-39 | 10/11 | 0.91 | 0.62–1.51 |
| 40-49 | 7/7 | 1.0 | 0.65–1.64 |
| 50 | 1/2 | 0.50 | 0.095–1.06 |
|
| B, Analysis of
ASC-US and others+ combination |
|
| Age group,
years |
CIN2+/Total | RR | 95% CI |
|
| 18-29 | 1/10 | Ref. | Ref. |
| 30-39 | 5/21 | 2.38 | 0.46–14.70 |
| 40-49 | 13/30 | 4.33 | 0.98–24.97 |
| 50 | 5/22 | 2.27 | 0.43–14.05 |
|
| C, Analysis of
LSIL, ASC-H and hrHPV-combinations |
|
| Age group,
years |
CIN2+/Total | RR | 95%CI |
|
| 18-29 | 0/7 | Ref. | Ref. |
| 30-39 | 1/8 | 2.00 | 0.16–26.6 |
| 40-49 | 4/8 | 8.00 | 0.99–82.9 |
| 50 | 2/12 | 2.67 | 0.26–30.4 |
Discussion
The present study comprehensively analyzed the
pathological status of cervical lesions for combinations of
cytological results and hrHPV status in different age groups. To
the best of our knowledge, the present study is the first in Japan
to examine the background cytology and hrHPV status of patients
with CIN2+ among different age groups.
First, it was confirmed that the background hrHPV
types differed significantly among age groups in patients with
CIN2+. Specifically, a greater number of younger patients had
HPV16/18 types, whereas more older patients had others+ or hrHPV-.
Regarding the clinical impact of HPV vaccination, the occurrence of
16/18+ was considerably lower at 0% in patients of the ‘vaccination
generation’ with CIN2+.
Notably, cytological findings were significantly
different among the age groups and hrHPV statuses, even in patients
with CIN2+. Younger patients tended to be HSIL-dominant, whereas
older patients tended to be ASC-US-dominant.
Furthermore, the distribution patterns of cytology
and hrHPV status combinations varied significantly among age groups
in patients with CIN2+. The combinations of HSIL and 16/18+, as
well as HSIL and others+ were predominant in the <29-year age
groups, whereas this definite distinction was not seen in other age
groups. The analysis in terms of screening perspective also
reflected this result, with the cytology and hrHPV combination
being more important at younger ages, especially in the <29-year
age groups.
It was hypothesized that this difference may be due
to the hrHPV type and time to carcinogenesis. Previous reports have
indicated that the CIN2+ background hrHPV type differs among
different age groups. Onuki et al (25) reported that the prevalence of
HPV16/18 in patients with CIN2-3 or SCC was highest in the
20–29-year age group (SCC: 90% and CIN2-3: 53.9%), and the
occurrence of SCC decreased with age to 56.3% and of CIN2-3 to
25.0% in the 60+ age group. Giannella et al (17) also reported that the occurrence of
CIN3 with hrHPV types other than HPV16/18 increased with age, with
a significant difference observed when the age range was 30–45
years (<30: 23.6%, 30–44: 32.1%, >45: 38.0%; P=0.0004). This
previous study reported that younger patients had a greater
probability of HPV16/18 infection, whereas older patients had a
higher probability of others+ or HPV-. The present results are
consistent with those of previous studies. CIN2+ cases in the
<29-year age groups, which are mainly caused by persistent
HPV16/18 infection, followed by others+, may require a relatively
short time for carcinogenesis, resulting in uniform characteristics
(HSIL-dominant). By contrast, in older patients with CIN2+, the
time required for carcinogenesis differs significantly (16). Therefore, the carcinogenic
background of patients with CIN2+ is not as consistent as that of
younger patients, which may be reflected in the heterogeneous
combination pattern of cytology and hrHPV status in older age
groups. Several reports have referred to the association between
age and prognosis in cervical cancer (16,26–29),
and have indicated differences in features of cervical cancer
between older and younger patients. The present findings of a more
heterogeneous cytology and hrHPV status in older patients with
CIN2+ may reflect its unique characteristics, which require a
longer time from HPV infection to carcinogenesis.
Since the present study was a retrospective study
conducted at a single center, the limitations, including the
limited number of cases and patient backgrounds, should be
considered. Additionally, it has been reported that
colposcopy-directed biopsies do not extract all CIN lesions
(30,31); therefore, pathological assessments
may be insufficient. However, because this was a single-center
study, the diagnostic criteria were consistent.
The influence of HPV vaccination on hrHPV infection
status cannot be ignore, thus the present study also evaluated this
factor. There are a number of reports that refer to the marked
reduction in the occurrence of CIN2-3 and SCC due to HPV
vaccination in foreign countries, as well as in Japan (32,33).
In Japan, large-scale vaccination (either bivalent or quadrivalent)
at public expense started in 2010, mainly for 12–16-year-old women.
That generation is called the ‘vaccination generation’ and
corresponds to the 18–24-year age group in the present study.
Epidemiological studies have estimated that ~70% of this population
is vaccinated (18,19). Although the direct influence of HPV
vaccination cannot be verified in each patient, it was hypothesized
that large-scale vaccination had a considerable influence on the
apparent change in the occurrence of 16/18+ in the 18–24-year
group.
Finally, the present study determined the definite
frequency of occurrence of CIN2+ for each combination of
cytological results and hrHPV status. It was hypothesized that the
results of the present study may be useful in clinical practice.
Based on the results, further investigations should be conducted
for each age group. First, cytology was revealed to be relatively
more important in younger patients; there was a very high frequency
of occurrence of CIN2+ in patients with HSIL; conversely, the
frequency of occurrence of CIN2+ was very low in patients with
ASC-US. When considering the indications of conization in young
patients, their cytological results and hrHPV status should be
considered because conization is significantly related to a high
incidence of preterm birth (34,35).
However, the presence of CIN2+ should be considered if cytological
results or hrHPV status are abnormal in older patients. In case of
a clinical suspicion of CIN2+, diagnostic conization should be
considered in older patients.
In Japan, where the application of the HPV vaccine
has not progressed due to social factors and the rapidly aging
population, early detection of cervical cancer is extremely
important. The current study determined the specific frequency of
occurrence of CIN2+ for every combination of cytology and hrHPV
status. In addition, the distribution of cytology and hrHPV status
varied widely among different age groups. Based on these results,
we propose screening protocols based on age that can detect CIN2+
more efficiently. In patients with CIN2+, the clinical impact of
the combination of cytology and hrHPV status can vary among age
group and age-specific perspectives are important for screening
CIN2+.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KY, MiS, NM, TO, HS, TK, YI, SS and MaS were
involved in the conception and design, case enrollment, data
interpretation and manuscript writing. MiS collected the data, and
KY and MiS analyzed the data. KY and MiS prepared the manuscript.
KY and MiS confirm the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Patients were not required to provide informed
consent for the present study because anonymous clinical data were
obtained after each patient agreed to treatment by written consent.
The study design was approved by the Toyooka Public Hospital's
Ethics Committee (approval no. 226).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nagase S, Ohta T, Takahashi F and Yaegashi
N; Board members of the 2020 Committee on Gynecologic Oncology of
the Japan Society of Obstetrics and Gynecology, : Annual report of
the committee on gynecologic oncology, the japan society of
obstetrics and gynecology: Annual patient report for 2017 and
annual treatment report for 2012. J Obstet Gynaecol Res.
47:1631–1642. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vizcaino AP, Moreno V, Bosch FX, Muñoz N,
Barros-Dios XM, Borras J and Parkin DM: International trends in
incidence of cervical cancer: II. Squamous-cell carcinoma. Int J
Cancer. 86:429–435. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quinn M, Babb P, Jones J and Allen E:
Effect of screening on incidence of and mortality from cancer of
cervix in England: Evaluation based on routinely collected
statistics. BMJ. 318:904–908. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Soutter WP, de Barros Lopes A, Fletcher A,
Monaghan JM, Duncan ID, Paraskevaidis E and Kitchener HC: Invasive
cervical cancer after conservative therapy for cervical
intraepithelial neoplasia. Lancet. 349:978–980. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arbyn M, Xu L, Simoens C and Martin-Hirsch
PP: Prophylactic vaccination against human papillomaviruses to
prevent cervical cancer and its precursors. Cochrane Database Syst
Rev. 5:CD0090692018.PubMed/NCBI
|
|
8
|
Lees BF, Erickson BK and Huh WK: Cervical
cancer screening: Evidence behind the guidelines. Am J Obstet
Gynecol. 214:438–443. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yuri S, Osamu I, Ichiro A, Takuya M,
Yoshiki M, Kuniko I and Ryo K: Cervical cancer screening with human
papillomavirus DNA and cytology in Japan. Int J Gynecol Cancer.
27:523–529. 2017. View Article : Google Scholar
|
|
10
|
Hashiguchi M, Nakao Y, Honda A, Kawaguchi
A, Hanashima K, Nishiyama S and Yokoyama M: What has changed since
the introduction of human papillomavirus testing with the
cytology-based cervical cancer screening system in Japan a social
experiment. Acta Cytol. 63:385–390. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Barroeta JE, Adhikari-Guragain D and
Grotkowski CE: Cervical cancer screening in the era of HPV
vaccination: A review of shifting paradigms in cytopathology. Diagn
Cytopathol. 45:903–914. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bosch FX and de Sanjosé S: Chapter 1:
Human papillomavirus and cervical cancer-burden and assessment of
causality. J Natl Cancer Inst Monogr. 2003:3–13. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huh WK, Ault KA, Chelmow D, Davey DD,
Goulart RA, Garcia FA, Kinney WK, Massad LS, Mayeaux EJ, Saslow D,
et al: Use of primary high-risk human papillomavirus testing for
cervical cancer screening: Interim clinical guidance. Gynecol
Oncol. 136:178–182. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tao X, Zhang H, Wang L, Pan Q, Ji S, Zhou
X and Zhao C: Atypical squamous cells of undetermined significance
cervical cytology in the Chinese population: Age-stratified
reporting rates, high-risk HPV testing, and immediate histologic
correlation results. Cancer Cytopathol. 129:24–32. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Massad LS, Einstein MH, Huh WK, Katki HA,
Kinney WK, Schiffman M, Solomon D, Wentzensen N and Lawson HW; 2012
ASCCP Consensus Guidelines Conference, : 2012 updated consensus
guidelines for the management of abnormal cervical cancer screening
tests and cancer precursors. Obstet Gynecol. 121:829–846. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aro K, Nieminen P, Louvanto K, Jakobsson
M, Virtanen S, Lehtinen M, Dillner J and Kalliala I: Age-specific
HPV type distribution in high-grade cervical disease in screened
and unvaccinated women. Gynecol Oncol. 154:354–359. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giannella L, Delli Carpini G, Di Giuseppe
J, Prandi S, Tsiroglou D and Ciavattini A: Age-related changes in
the fraction of cervical intraepithelial neoplasia grade 3 related
to HPV genotypes included in the nonavalent vaccine. J Oncol.
2019:71378912019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bao H, Ma L, Zhao Y, Song B, Di J, Wang L,
Gao Y, Ren W, Wang S, Wu J and Wang HJ: Age-specific effectiveness
of primary human papillomavirus screening versus cytology in a
cervical cancer screening program: A nationwide cross-sectional
study. Cancer Commun (Lond). 42:191–204. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agorastos T, Chatzistamatiou K,
Katsamagkas T, Koliopoulos G, Daponte A, Constantinidis T and
Constantinidis TC; HERMES study group, : Primary screening for
cervical cancer based on high-risk human papillomavirus (HPV)
detection and HPV 16 and HPV 18 genotyping, in comparison to
cytology. PLoS One. 10:e01197552015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yagi A, Ueda Y, Nakagawa S, Ikeda S,
Tanaka Y, Sekine M, Miyagi E, Enomoto T and Kimura T: Potential for
cervical cancer incidence and death resulting from Japan's current
policy of prolonged suspension of its governmental recommendation
of the HPV vaccine. Sci Rep. 10:159452020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yagi A, Ueda Y, Kakuda M, Nakagawa S,
Hiramatsu K, Miyoshi A, Kobayashi E, Kimura T, Kurosawa M,
Yamaguchi M, et al: Cervical cancer protection in Japan: Where are
we? Vaccines (Basel). 9:12632021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sekine M, Kudo R, Adachi S, Yamaguchi M,
Ueda Y, Takata T, Morimoto A, Tanaka Y, Yagi A, Miyagi E and
Enomoto T: Japanese crisis of HPV vaccination. Int J Pathol Clin
Res. 2:392016. View Article : Google Scholar
|
|
23
|
Yagi A, Ueda Y, Ikeda S, Miyagi E, Sekine
M, Enomoto T and Kimura T: The looming health hazard: A wave of
HPV-related cancers in Japan is becoming a reality due to the
continued suspension of the governmental recommendation of HPV
vaccine. Lancet Reg Health West Pac. 18:1003272021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Solomon D, Davey D, Kurman R, Moriarty A,
O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T Jr, et
al: The 2001 Bethesda system: Terminology for reporting results of
cervical cytology. JAMA. 287:2114–2119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Onuki M, Matsumoto K, Satoh T, Oki A,
Okada S, Minaguchi T, Ochi H, Makao S, Someya K, Yamada N, et al:
Human papillomavirus infections among Japanese women: Age-related
prevalence and type-specific risk for cervical cancer. Cancer Sci.
100:1312–1316. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vänskä S, Luostarinen T, Lagheden C,
Eklund C, Kleppe SN, Andrae B, Sparén P, Sundström K, Lehtinen M
and Dillner J: Differing age-specific cervical cancer incidence
between different types of human papillomavirus: Implications for
predicting the impact of elimination programs. Am J Epidemiol.
190:506–514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de Sanjose S, Wheeler CM, Quint WGV, Hunt
WC, Joste NE, Alemany L, Bosch FX; Retrospective International
Survey and HPV Time Trends Study Group, ; Myers ER and Castle PE:
Age-specific occurrence of HPV16- and HPV18-related cervical
cancer. Cancer Epidemiol Biomarkers Prev. 22:1313–1318. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hammer A, Rositch A, Qeadan F, Gravitt PE
and Blaakaer J: Age-specific prevalence of HPV16/18 genotypes in
cervical cancer: A systematic review and meta-analysis. Int J
Cancer. 138:2795–2803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Burger EA, Kim JJ, Sy S and Castle PE: Age
of acquiring causal human papillomavirus (HPV) infections:
Leveraging simulation models to explore the natural history of
HPV-induced cervical cancer. Clin Infect Dis. 65:893–899. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baasland I, Hagen B, Vogt C, Valla M and
Romundstad PR: Colposcopy and additive diagnostic value of biopsies
from colposcopy-negative areas to detect cervical dysplasia. Acta
Obstet Gynecol Scand. 95:1258–1263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tatiyachonwiphut M, Jaishuen A, Sangkarat
S, Laiwejpithaya S, Wongtiraporn W, Inthasorn P, Viriyapak B and
Warnnissorn M: Agreement between colposcopic diagnosis and cervical
pathology: Siriraj hospital experience. Asian Pac J Cancer Prev.
15:423–426. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rebolj M, Pesola F, Mathews C, Mesher D,
Soldan K and Kitchener H: The impact of catch-up bivalent human
papillomavirus vaccination on cervical screening outcomes: An
observational study from the English HPV primary screening pilot.
Br J Cancer. 127:278–287. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hiramatsu K, Ueda Y, Yagi A, Morimoto A,
Egawa-Takata T, Nakagawa S, Kobayashi E, Kimura T, Kimura T,
Minekawa R, et al: The efficacy of human papillomavirus vaccination
in young Japanese girls: The interim results of the OCEAN study.
Hum Vaccin Immunother. 18:19510982022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kyrgiou M, Athanasiou A, Paraskevaidi M,
Mitra A, Kalliala I, Martin-Hirsch P, Arbyn M, Bennett P and
Paraskevaidis E: Adverse obstetric outcomes after local treatment
for cervical preinvasive and early invasive disease according to
cone depth: Systematic review and meta-analysis. BMJ.
354:i36332016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arbyn M, Kyrgiou M, Simoens C, Raifu AO,
Koliopoulos G, Martin-Hirsch P, Prendiville W and Paraskevaidis E:
Perinatal mortality and other severe adverse pregnancy outcomes
associated with treatment of cervical intraepithelial neoplasia:
Meta-analysis. BMJ. 337:a12842008. View Article : Google Scholar : PubMed/NCBI
|


















