Introduction
As the fifth most common cancer type and the third
leading cause of cancer-related death worldwide, gastric cancer
remains a serious health challenge (1). Cancer patients with poor nutritional
status are not only limited in their choice of treatment strategies
but also often have worse clinical outcomes (2,3). The
nutritional status of cancer patients deserves attention,
particularly that of patients with digestive tract cancers
(4,5).
Pyloric stenosis is a common complication in
patients with gastric cancer, particularly in antral tumors
(6). Mechanical obstruction of the
pyloric duct due to tumor progression causes patients to suffer
from digestive system symptoms such as abdominal pain, bloating and
loss of appetite, which severely affects energy intake. The
increase in energy consumption caused by cancer makes the
nutritional status of patients with pyloric stenosis deteriorate
rapidly, even leading to cachexia (7–9).
Numerous studies have explored that poor nutritional status was an
important negative influence on the development of postoperative
complications and on prognosis (10–12).
Parenteral nutrition is an important treatment strategy for
malnourished cancer patients. Preoperative parenteral nutrition may
not only improve the treatment tolerance of cancer patients but
also reduce the occurrence of postoperative complications and
perioperative mortality (13).
The nutritional risk index (NRI) incorporates
changes in patient weight and serum albumin levels, making it an
accurate scoring system for evaluating the nutritional status of
cancer patients (14). Numerous
studies have revealed a significant association between NRI and
clinical outcomes in a variety of cancer types, including gastric
cancer (15–17). Patients with gastric cancer with
pyloric stricture who received preoperative parenteral nutrition
have a unique nutritional status and the predictive ability of the
NRI for them has remained to be clarified. The present study
analyzed the pathological characteristics and clinical outcomes of
patients with gastric cancer with pyloric stricture in detail and
explored the predictive ability of NRI for the prognosis of
patients with gastric cancer with pyloric stricture who received
preoperative parenteral nutrition. These results provided a
reference for evaluating the severity of the disease and the risk
of postoperative recurrence.
Materials and methods
Patients
The present study included 415 patients with gastric
cancer treated at The Second People's Hospital of Neijiang
(Neijiang, China) between January 2016 and December 2021, including
194 cases with pyloric stenosis who received preoperative
parenteral nutrition and 221 cases without pyloric stenosis. The
inclusion criteria were as follows: i) All subjects were confirmed
by pathological diagnosis; ii) all subjects received surgical
treatment; iii) pyloric stenosis was confirmed by electric
gastroscope or imaging examination; iv) all subjects with pyloric
stenosis received parenteral nutrition for 3–14 days before the
surgery; and v) all subjects had complete clinical information.
Patients with pyloric stenosis received a peripherally inserted
central catheter or central venous catheter within 24 h after
admission. Parenteral nutrient solutions were Kabiven Peripheral
(1,920 ml) or Kabiven Peripheral (1,440 ml) (Fresenius Kabi AG).
This study was based on the Helsinki Declaration as well as its
amendments and the protocol was approved by the Ethics Committee of
the Second People's Hospital of Neijiang (Neijiang, China). This
study was retrospective and did not require informed consent.
Data collection
The observation outcome of the present study was
overall survival (OS), which was defined as the period from the
first day of treatment to death or the last follow-up and was
obtained by telephone follow-up. The NRI was calculated as follows:
NRI=1.519 × albumin (g/l) + 41.7 × personal weight (kg)/ideal
weight (kg) (14). The ideal weight
was calculated by the Lorentz equations, which were as follows:
Male ideal weight (kg)=height (m)-100-[(height (m)-150)/4]; female
ideal weight (kg)=height (m)-100-[(height (m)-150)/2.5]. The
cut-off point for the NRI in patients with pyloric stenosis was
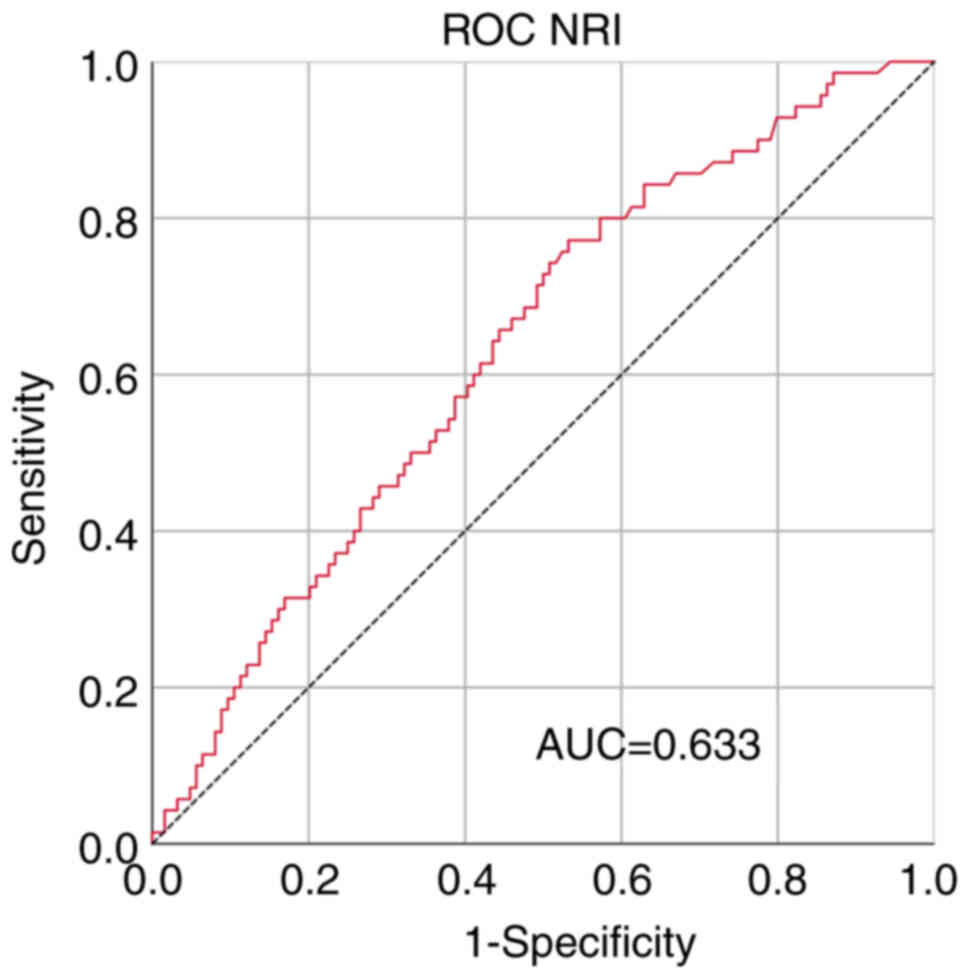
obtained from the receiver operating characteristic (ROC) curve.
The area under curve of the ROC curve at the maximum Youden index
of 0.239 was 0.633, with sensitivity and specificity values of
0.771 and 0.468, respectively (Fig.
1). All patients were divided into the low-value group
(NRI<93.42) and the high-value group (NRI ≥93.42).
Statistical analysis
All analyses were completed by R 4.2.2 and
continuous variables were expressed as the mean ± standard
deviation and compared by Student's t-test and Pearson's
correlation analysis, while categorical variables were reported as
the number of patients and percentage and compared by the
Chi-square test or Fisher's exact test. Kaplan-Meier survival
curves and the log-rank test were used to compare differences in
survival time. Univariate and multivariate Cox's analyses were
conducted to identify significant prognostic markers and the
predictive ability of NRI for OS was further explored through
stratified analyses. Nomograms were also constructed the
calibration curves were drawn to analyze the predictive
effectiveness of the nomograms. A two-sided P<0.05 was
considered to indicate a statistically significant difference.
Results
Patient characteristics
Of the 415 subjects included, 295 (71.1%) were males
and 120 (28.9%) were females, with a mean (± SD) age of 60.83±10.42
years. There were 194 patients (46.7%) with pyloric stenosis, and
it was observed that subjects with pyloric stenosis had a higher
age, larger proportion of males, lower body mass index (BMI),
longer length of stay, lower occurrence of stomachache, a higher
occurrence of abdominal distension, weight loss and sour
regurgitation, a larger proportion of cases with lymph
node-positive status, TNM stage III and lower NRI, as well as
larger tumor size and poorer blood parameters (all P<0.05). In
addition, Fisher's exact test indicated that tumors in patients
with pyloric stenosis tended to be less frequently subjected to
radical resection (P<0.001), and more frequently located in the
lower 1/3 (P=0.025), moderately differentiated (P=0.004) and of the
Borrmann type III (P<0.001) (Table
I).
 | Table I.Patient characteristics grouped by
pyloric stenosis. |
Table I.
Patient characteristics grouped by
pyloric stenosis.
|
|
| Pyloric
stenosis |
|
|---|
|
|
|
|
|
|---|
| Item | Total (n=415) | Yes (n=194) | No (n=221) | P-value |
|---|
| Age, years | 60.83±10.42 | 63.85±9.55 | 58.18±10.46 | <0.001 |
| Sex |
|
|
| 0.048 |
|
Male | 295 (71.1) | 147 (75.8) | 148 (67.0) |
|
|
Female | 120 (28.9) | 47 (24.2) | 73 (33.0) |
|
| BMI,
kg/m2 | 21.97±3.51 | 20.83±3.34 | 22.97±3.34 | <0.001 |
| Length of stay,
days | 18.44±5.67 | 19.06±5.25 | 17.90±5.98 | 0.036 |
| Stomachache |
|
|
| <0.001 |
|
Yes | 254 (61.2) | 98 (50.5) | 156 (70.6) |
|
| No | 161 (38.8) | 96 (49.5) | 65 (29.4) |
|
| Abdominal
distension |
|
|
| <0.001 |
|
Yes | 177 (42.7) | 113 (58.2) | 64 (29.0) |
|
| No | 238 (57.3) | 81 (41.8) | 157 (71.0) |
|
| Black stool |
|
|
| 0.814 |
|
Yes | 92 (22.2) | 44 (22.7) | 48 (21.7) |
|
| No | 323 (77.8) | 150 (77.3) | 173 (78.3) |
|
| Weight loss |
|
|
| <0.001 |
|
Yes | 271 (65.3) | 167 (86.1) | 104 (47.1) |
|
| No | 144 (34.7) | 27 (13.9) | 117 (52.9) |
|
| Fatigue |
|
|
| 0.578 |
|
Yes | 134 (32.3) | 60 (30.9) | 74 (33.5) |
|
| No | 281 (67.7) | 134 (69.1) | 147 (66.5) |
|
| Sour
regurgitation |
|
|
| 0.001 |
|
Yes | 138 (33.3) | 80 (41.2) | 58 (26.2) |
|
| No | 277 (66.7) | 114 (58.8) | 163 (73.8) |
|
| Radical
resection |
|
|
| <0.001 |
|
Yes | 346 (83.4) | 130 (67.0) | 216 (97.7) |
|
| No | 69 (16.6) | 64 (33.0) | 5 (2.3) |
|
| Primary tumor
site |
|
|
| 0.025 |
| Upper
1/3 | 20 (4.8) | 13 (6.7) | 7 (3.2) |
|
| Middle
1/3 | 56 (13.5) | 22 (11.3) | 34 (15.4) |
|
| Lower
1/3 | 320 (77.1) | 155 (79.9) | 165 (74.7) |
|
|
Whole | 19 (4.6) | 4 (2.1) | 15 (6.8) |
|
| Borrmann type |
|
|
| <0.001 |
| I | 34 (8.2) | 1 (0.5) | 33 (14.9) |
|
| II | 106 (25.5) | 30 (15.5) | 76 (34.4) |
|
|
III | 250 (60.2) | 141 (72.7) | 109 (49.3) |
|
| IV | 25 (6.0) | 22 (11.3) | 3 (1.4) |
|
| LNP |
|
|
| <0.001 |
|
Yes | 205 (49.4) | 151 (77.8) | 54 (24.4) |
|
| No | 210 (50.6) | 43 (22.2) | 167 (75.6) |
|
| Tumor size, mm |
|
|
| <0.001 |
|
<50 | 229 (55.2) | 85 (43.8) | 144 (65.2) |
|
|
≥50 | 186 (44.8) | 109 (56.2) | 77 (34.8) |
|
|
Differentiation |
|
|
| <0.001 |
|
Poor | 223 (53.7) | 79 (40.7) | 144 (65.2) |
|
|
Moderate | 189 (45.5) | 112 (57.7) | 77 (34.8) |
|
|
Well | 3 (0.7) | 3 (1.5) | 0 (0.0) |
|
| TNM stage |
|
|
| <0.001 |
| I | 21 (5.1) | 4 (2.1) | 17 (7.7) |
|
| II | 181 (43.6) | 62 (32.0) | 119 (53.8) |
|
|
III | 213 (51.3) | 128 (66.0) | 85 (38.5) |
|
| ALT, U/l | 18.37±12.92 | 14.29±10.42 | 21.94±13.83 | <0.001 |
| AST, U/l | 20.33±8.24 | 18.35±7.79 | 22.07±8.24 | <0.001 |
| ALP, U/l | 76.05±45.07 | 72.39±24.12 | 76.43±21.27 | 0.070 |
| γ-GGT, U/l | 21.23±17.70 | 17.64±11.86 | 24.37±21.09 | <0.001 |
| LDH, U/l | 163.43±52.66 | 162.91±68.65 | 163.90±32.92 | 0.855 |
| TBIL, µmol/l | 12.30±9.19 | 10.82±9.23 | 13.59±8.97 | 0.002 |
| DBIL, µmol/l | 3.62±9.19 | 2.67±1.88 | 4.46±1.90 | <0.001 |
| IDBIL, µmol/l | 8.13±4.58 | 7.73±5.02 | 8.47±4.15 | 0.100 |
| TP, g/l | 64.86±7.42 | 61.21±7.20 | 68.06±6.00 | <0.001 |
| ALB, g/l | 38.37±5.05 | 35.52±4.40 | 40.86±4.21 | <0.001 |
| GLOB, g/l | 26.77±4.55 | 26.34±5.37 | 27.14±3.65 | 0.079 |
| PALB, mg/l | 229.50±83.86 | 171.94±52.37 | 280.02±73.10 | <0.001 |
| Urea, mmol/l | 6.34±5.95 | 6.56±7.18 | 6.14±4.62 | 0.474 |
| CREA, µmol/l | 79.78±31.41 | 74.67±14.09 | 84.27±40.48 | 0.002 |
| UA, µmol/l | 281.37±88.86 | 258.54±91.78 | 301.42±81.28 | <0.001 |
| Glu, mmol/l | 5.45±1.51 | 5.71±1.80 | 5.22±1.16 | 0.001 |
| Hb, g/l | 129.19±27.25 | 119.66±27.12 | 137.56±24.53 | <0.001 |
| Hct, l/l | 39.47±8.39 | 37.23±9.50 | 41.44±6.71 | <0.001 |
| W,
109/l | 6.49±2.09 | 6.14±1.79 | 6.80±2.28 | 0.001 |
| N,
109/l | 4.06±1.87 | 4.04±1.57 | 4.07±2.10 | 0.841 |
| L,
109/l | 1.75±0.70 | 1.45±0.55 | 2.02±0.71 | <0.001 |
| P,
109/l | 256.09±79.89 | 258.37±82.60 | 254.09±77.57 | 0.587 |
| NRI | 97.00±9.67 | 91.46±8.61 | 101.87±7.74 | <0.001 |
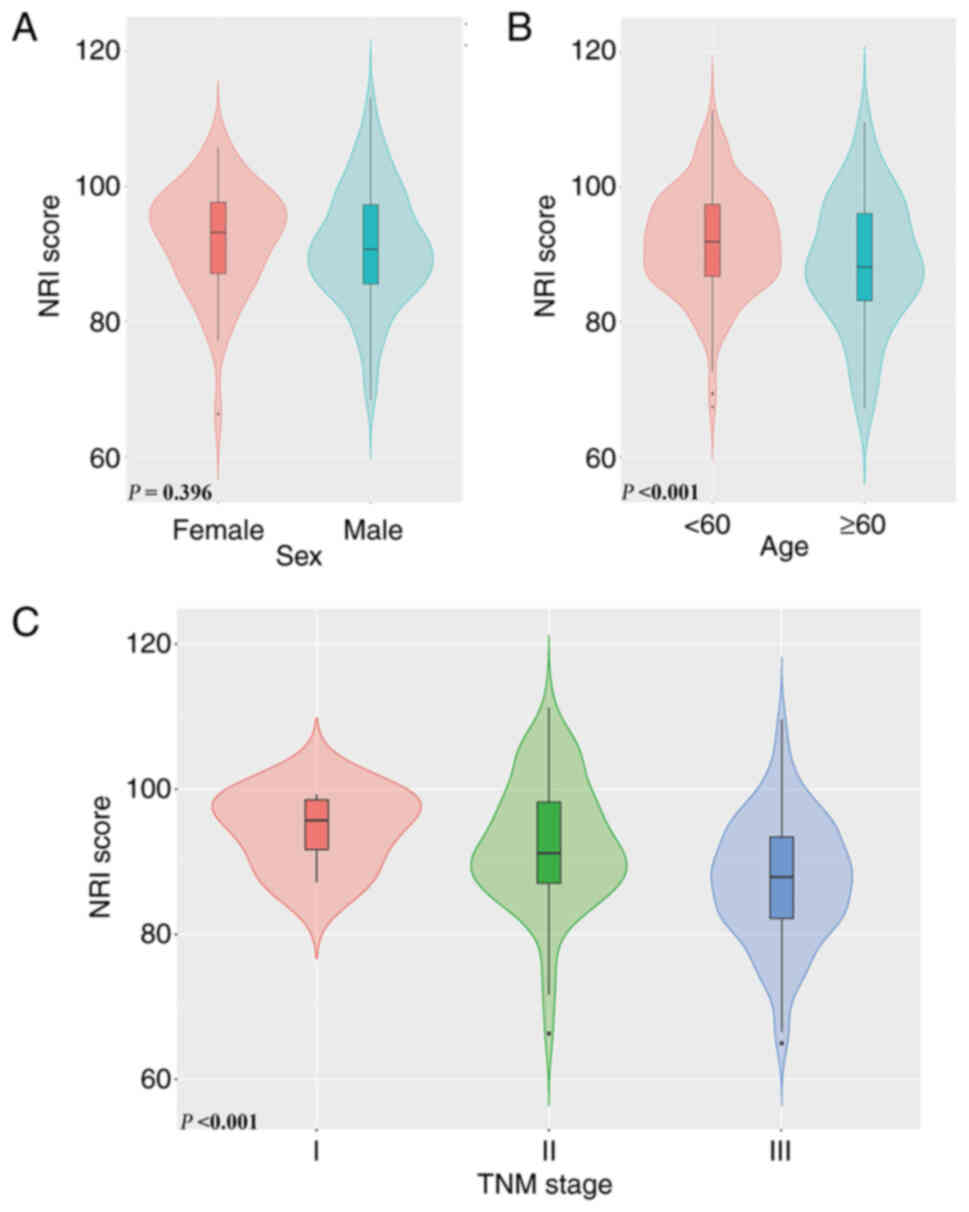
NRI
Comparisons of different groups of patients with
pyloric stenosis revealed that there was no significant difference
in the NRI score among patients with different sex (P=0.396).
However, patients with higher age had a lower NRI score
(P<0.001). Furthermore, a decrease in the NRI score was observed
to be associated with an increase in TNM stage (P<0.001)
(Fig. 2A-C). After conducting a
Gaussian distribution test on the data, Pearson correlation
analysis was performed on all parameters that satisfied the
criteria for a Gaussian distribution. The NRI was found to be
significantly correlated with higher total protein, albumin (ALB),
pre-ALB, hemoglobin and hematocrit (all R>0.3, P<0.05)
(Fig. 3).
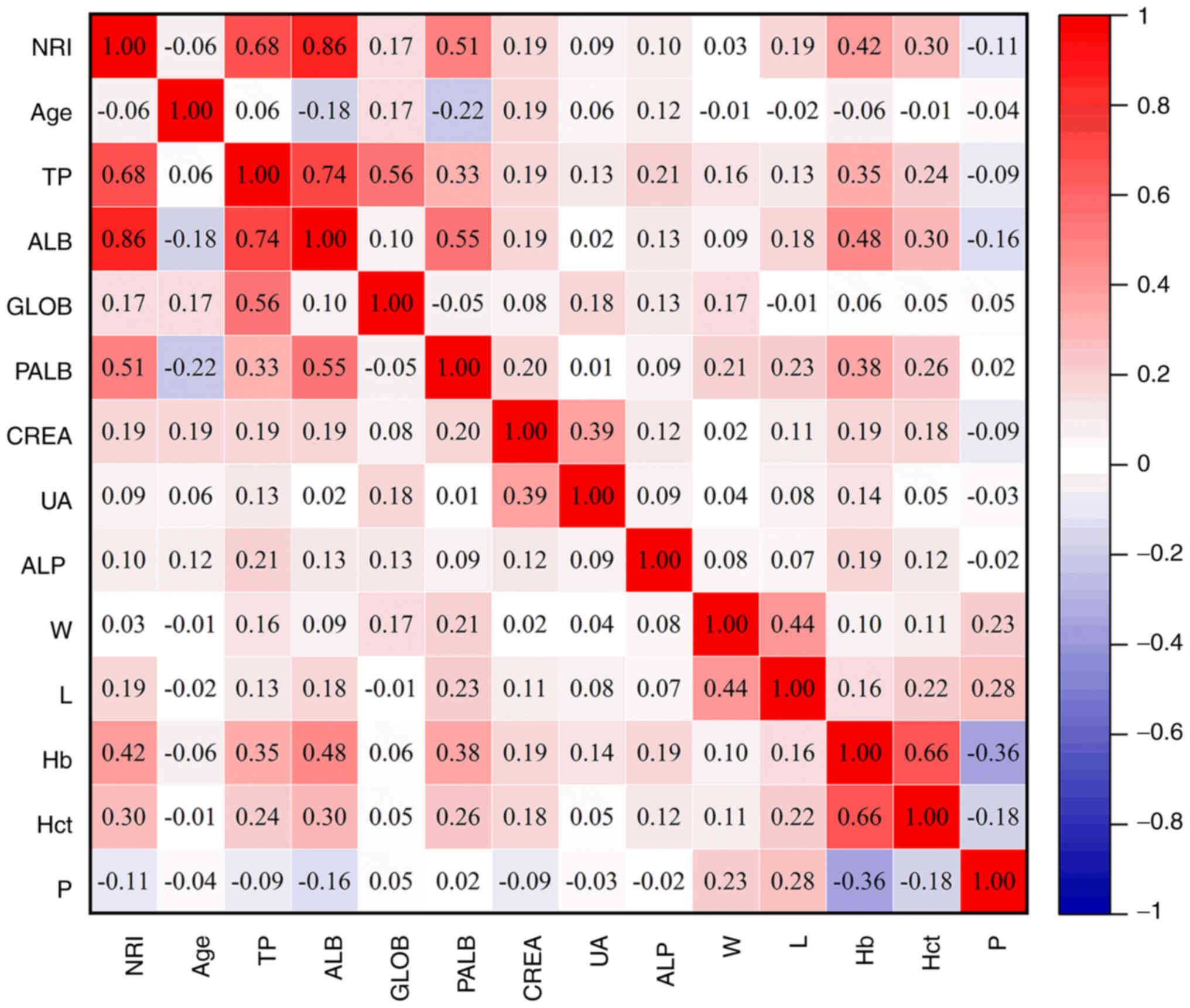 | Figure 3.Person analysis of the correlation of
the NRI with various factors. NRI, nutritional risk index; TP,
total protein; ALB, albumin; GLOB, globulin; PALB, prealbumin;
CREA, creatinine; UA, uric acid; Hb, hemoglobin; W, white blood
cell; L, lymphocyte; Hb, hemoglobin; Hct, hematocrit; P,
platelet. |
All cases were stratified into two groups based on
the cut-off point of the NRI. There were 120 patients (61.9%) with
NRI <93.42 and 74 patients (38.1%) with NRI ≥93.42. The NRI was
related to age, BMI, TNM stage and extensive blood parameters (all
P<0.05) (Table II).
 | Table II.Patient characteristics grouped by
NRI. |
Table II.
Patient characteristics grouped by
NRI.
|
|
| NRI |
|
|---|
|
|
|
|
|
|---|
| Item | Total (n=194) | <93.42
(n=120) | ≥93.42 (n=74) | P-value |
|---|
| Age, years | 63.85±9.55 | 65.54±8.77 | 60.25±9.31 | <0.001 |
| Sex |
|
|
| 0.160 |
|
Male | 147 (75.8) | 95 (79.2) | 52 (70.3) |
|
|
Female | 47 (24.2) | 25 (20.8) | 22 (29.7) |
|
| BMI,
kg/m2 | 20.83±3.34 | 19.70±3.28 | 22.66±2.55 | <0.001 |
| Length of stay,
days | 19.06±5.25 | 19.30±5.21 | 18.68±5.32 | 0.422 |
| Stomachache |
|
|
| 0.855 |
|
Yes | 98 (50.5) | 60 (50.0) | 38 (51.4) |
|
| No | 96 (49.5) | 60 (50.0) | 36 (48.6) |
|
| Abdominal
distension |
|
|
| 0.385 |
|
Yes | 113 (58.2) | 67 (55.8) | 46 (62.2) |
|
| No | 81 (41.8) | 53 (44.2) | 28 (37.8) |
|
| Black stool |
|
|
| 0.529 |
|
Yes | 44 (22.7) | 29 (24.2) | 15 (20.3) |
|
| No | 150 (77.3) | 91 (75.8) | 59 (79.7) |
|
| Weight loss |
|
|
| 0.765 |
|
Yes | 167 (86.1) | 104 (86.7) | 63 (85.1) |
|
| No | 27 (13.9) | 16 (13.3) | 11 (14.9) |
|
| Fatigue |
|
|
| 0.777 |
|
Yes | 60 (30.9) | 38 (31.7) | 22 (29.7) |
|
| No | 134 (69.1) | 82 (68.3) | 52 (70.3) |
|
| Sour
regurgitation |
|
|
| 0.175 |
|
Yes | 80 (41.2) | 54 (45.0) | 26 (35.1) |
|
| No | 114 (58.8) | 66 (55.0) | 48 (64.9) |
|
| Radical
resection |
|
|
| 0.283 |
|
Yes | 130 (67.0) | 77 (64.2) | 53 (71.6) |
|
| No | 64 (33.0) | 43 (35.8) | 21 (28.4) |
|
| Primary tumor
site |
|
|
| 0.106 |
| Upper
1/3 | 13 (6.7) | 12 (10.0) | 1 (1.4) |
|
| Middle
1/3 | 22 (11.3) | 12 (10.0) | 10 (13.5) |
|
| Lower
1/3 | 155 (79.9) | 93 (77.5) | 62 (83.8) |
|
|
Whole | 4 (2.1) | 3 (2.5) | 1 (1.4) |
|
| Borrmann type |
|
|
| 0.554 |
| I | 1 (0.5) | 0 (0.0) | 1 (1.4) |
|
| II | 30 (15.5) | 17 (14.2) | 13 (17.6) |
|
|
III | 141 (72.7) | 89 (74.2) | 52 (70.3) |
|
| IV | 22 (11.3) | 14 (11.7) | 8 (10.8) |
|
| LNP |
|
|
| 0.200 |
|
Yes | 151 (77.8) | 97 (80.8) | 54 (73.0) |
|
| No | 43 (22.2) | 23 (19.2) | 20 (27.0) |
|
| Tumor size, mm |
|
|
| 0.863 |
|
<50 | 85 (43.8) | 52 (43.3) | 33 (44.6) |
|
|
≥50 | 109 (56.2) | 68 (56.7) | 41 (55.4) |
|
|
Differentiation |
|
|
| 0.552 |
|
Poor | 79 (40.7) | 48 (40.0) | 31 (41.9) |
|
|
Moderate | 112 (57.7) | 71 (59.2) | 41 (55.4) |
|
|
Well | 3 (1.5) | 1 (0.8) | 2 (2.7) |
|
| TNM stage |
|
|
| <0.001 |
| I | 4 (2.1) | 1 (0.1) | 3 (4.1) |
|
| II | 62 (32.0) | 25 (20.8) | 37 (50.0) |
|
|
III | 128 (66.0) | 94 (79.1) | 34 (45.9) |
|
| ALT, U/l | 14.29±10.41 | 12.41±7.67 | 17.34±13.27 | 0.004 |
| AST, U/l | 18.35±7.79 | 17.27±5.77 | 20.11±10.05 | 0.029 |
| ALP, U/l | 72.39±24.12 | 70.33±23.17 | 75.73±25.39 | 0.130 |
| γ-GGT, U/l | 17.64±11.86 | 16.17±9.04 | 20.04±15.14 | 0.049 |
| LDH, U/l | 162.91±68.65 | 157.90±43.05 | 171.03±96.60 | 0.197 |
| TBIL, µmol/l | 10.82±9.23 | 9.79±5.42 | 12.49±13.14 | 0.096 |
| DBIL, µmol/l | 2.67±1.88 | 2.56±1.67 | 2.84±2.17 | 0.319 |
| IDBIL, µmol/l | 7.73±5.02 | 7.26±4.46 | 8.48±5.75 | 0.100 |
| TP, g/l | 61.21±7.20 | 58.15±6.00 | 66.18±6.15 | <0.001 |
| ALB, g/l | 35.52±4.40 | 33.14±3.19 | 39.39±3.17 | <0.001 |
| GLOB, g/l | 26.34±5.37 | 26.10±5.91 | 26.73±4.36 | 0.422 |
| PALB, mg/l | 171.94±52.37 | 155.83±45.50 | 198.08±52.50 | <0.001 |
| Urea, mmol/l | 6.56±7.18 | 6.03±2.10 | 7.43±11.31 | 0.188 |
| CREA, µmol/l | 74.67±14.09 | 72.39±12.70 | 78.36±15.49 | 0.004 |
| UA, µmol/l | 258.54±91.78 | 252.38±90.36 | 168.51±93.80 | 0.235 |
| Glu, mmol/l | 5.71±1.80 | 5.73±1.84 | 5.69±1.74 | 0.886 |
| Hb, g/l | 119.66±27.12 | 113.33±25.55 | 129.92±16.61 | <0.001 |
| Hct, l/l | 37.23±9.50 | 35.67±9.26 | 39.75±9.40 | <0.001 |
| W,
109/l | 6.14±1.79 | 6.13±1.93 | 6.16±1.55 | 0.913 |
| N,
109/l | 4.04±1.57 | 4.03±1.67 | 4.06±1.40 | 0.900 |
| L,
109/l | 1.45±0.55 | 1.40±0.54 | 1.53±0.57 | 0.101 |
| P,
109/l | 258.37±82.60 | 263.68±85.54 | 249.74±77.40 | 0.255 |
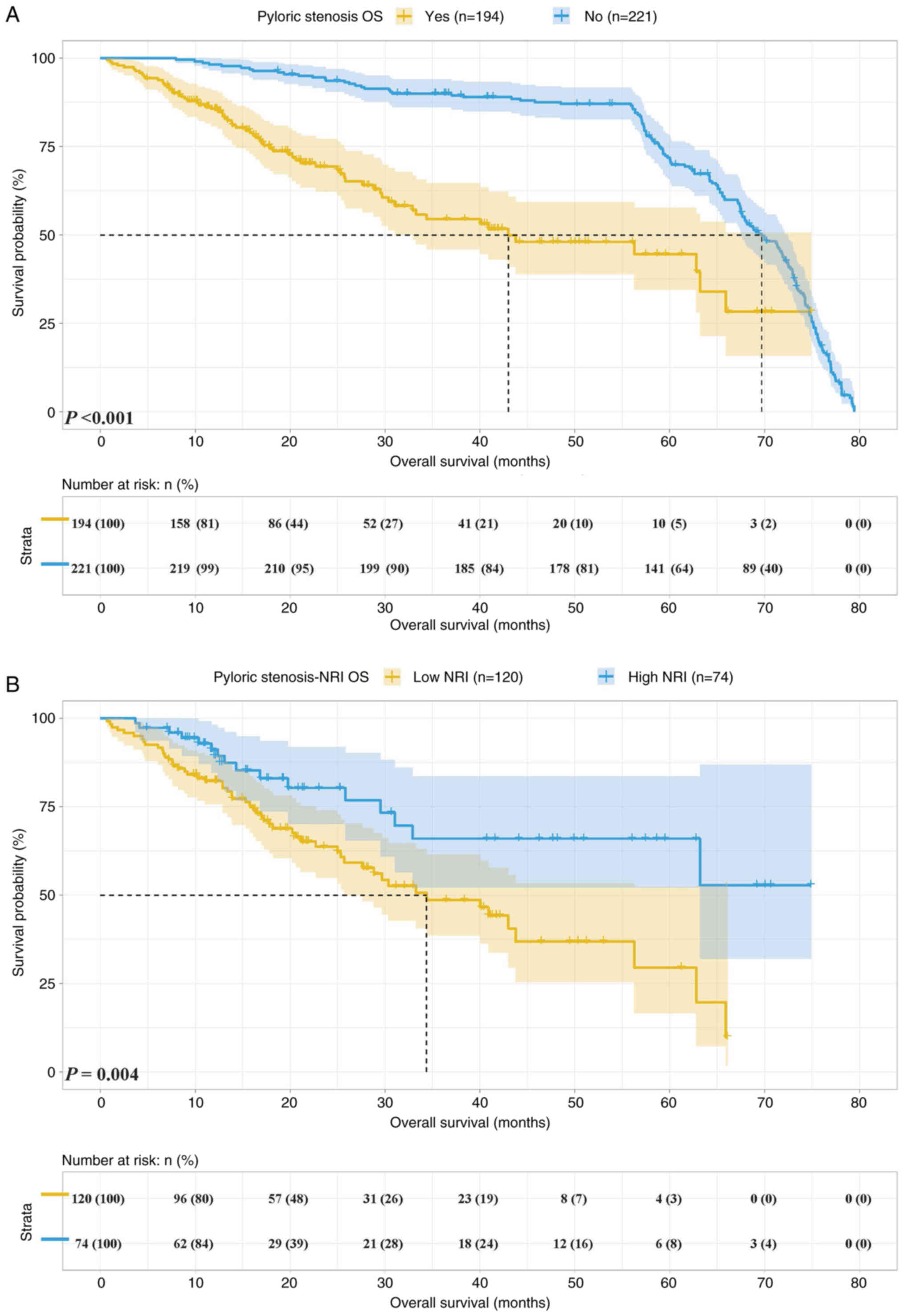
Survival analysis for NRI
The cohort of the present study included 194 cases
(46.7%) with pyloric stenosis and 221 cases (53.3%) with
non-pyloric stenosis. The 1-year survival rate of patients with
pyloric stenosis and non-pyloric stenosis was 85.7 and 98.2%, while
the 3-year survival rate was 54.5 vs. 90.0% and the 5-year survival
rate was 44.6 and 71.9%, respectively. Patients with pyloric
stenosis had a shorter OS [median survival time (MST): 42.71 vs.
69.92 months, P<0.001] (Fig.
4A).
Among the patients with pyloric stenosis, there were
120 cases (61.9%) with NRI <93.42 and 74 cases (38.1%) with NRI
≥93.42. The 1- and 3-year survival rates of patients with NRI
<93.42 and NRI ≥93.42 were 82.2 and 48.7 vs. 91.2 and 66.0%,
respectively. Patients with NRI <93.42 had shorter OS (MST:
34.37 months vs. not reached, P=0.004) (Fig. 4B).
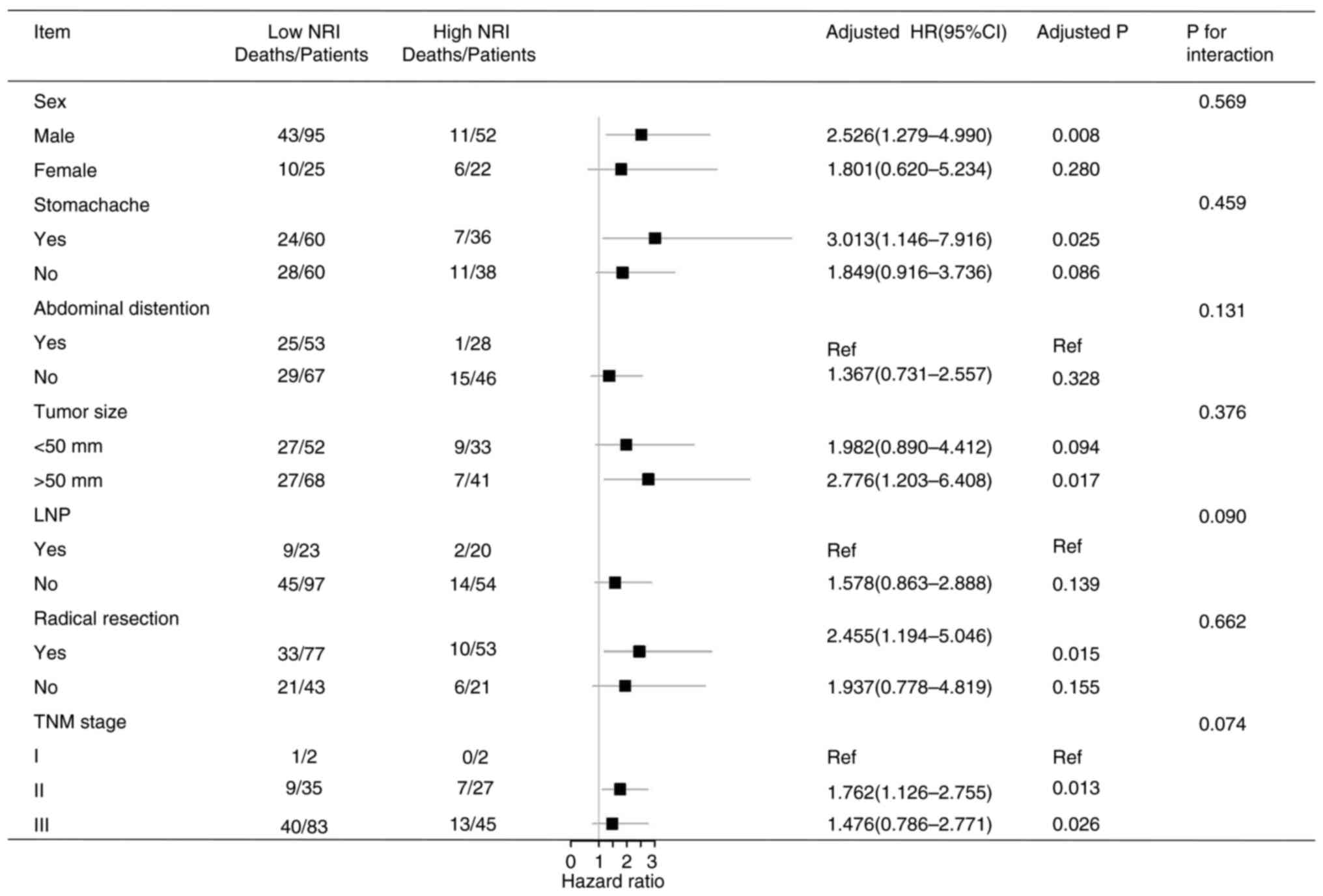
Univariate and multivariate survival
analysis
In the present study, OS was related to age, ALB,
NRI, radical resection, tumor size and TNM stage (all P<0.05).
The independent prognostic factors for OS were age, NRI, radical
resection, tumor size and TNM stage (all P<0.05) (Table III). In addition, a stratified
analysis was performed according to the multivariate analysis. It
was found that a low NRI was associated with a shorter survival
time in patients who were males and those who had a stomachache,
tumor size ≥50 mm, radical resection, TNM stage II and TNM stage
III (all P<0.05) (Fig. 5).
 | Table III.Univariate and multivariate analysis
of factors influencing overall survival. |
Table III.
Univariate and multivariate analysis
of factors influencing overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Item | Hazard ratio
(95%CI) | Crude P-value | Hazard ratio
(95%CI) | Adjusted P |
|---|
| Age, years | 1.011
(0.985–1.038) | 0.018 | 1.009
(1.000–1.015) | 0.049 |
| ALB, g/l | 0.925
(0.875–0.979) | 0.007 |
|
|
| BMI,
kg/m2 | 0.963
(0.890–1.041) | 0.339 |
|
|
| NRI (<93.42 vs.
≥93.42) | 2.305
(1.309–4.058) | 0.004 | 2.048
(1.139–3.811) | 0.017 |
| Sex (female vs.
male) | 1.168
(0.668–2.043) | 0.586 |
|
|
| Stomachache | 1.151
(0.717–1.848) | 0.560 |
|
|
| Abdominal
distension | 1.187
(0.730–1.930) | 0.490 |
|
|
| Black stool | 1.288
(0.735–2.255) | 0.376 |
|
|
| Weight loss | 1.561
(0.713–3.416) | 0.265 |
|
|
| Fatigue | 1.155
(0.708–1.884) | 0.564 |
|
|
| Sour
regurgitation | 1.164
(0.727–4.864) | 0.528 |
|
|
| Radical
resection | 1.615
(0.989–2.636) | 0.035 | 1.637
(0.969–2.765) | 0.046 |
| Primary tumor
site |
|
|
|
|
| Middle
vs. upper 1/3 | 0.402
(0.148–1.094) | 0.074 | 0.722
(0.250–2.084) | 0.547 |
| Lower
vs. upper 1/3 | 0.431
(0.211–0.879) | 0.021 | 0.607
(0.285–1.291) | 0.195 |
| Whole
vs. upper 1/3 | 0.605
(0.131–2.801) | 0.520 | 0.870
(0.151–5.011) | 0.876 |
| LNP | 2.099
(1.100–4.007) | 0.025 | 1.220
(0.425–3.506) | 0.712 |
| Tumor size (≥50 vs.
<50 mm) | 1.454
(0.905–2.336) | 0.022 | 1.775
(1.064–2.960) | 0.028 |
| TNM stage |
|
|
|
|
| II vs.
I | 2.541
(1.214–4.573) | 0.004 | 2.354
(1.687–3.265) | 0.006 |
| III vs.
I | 3.459
(1.789–5.862) | 0.012 | 2.582
(1.848–3.156) | 0.015 |
Nomogram for OS
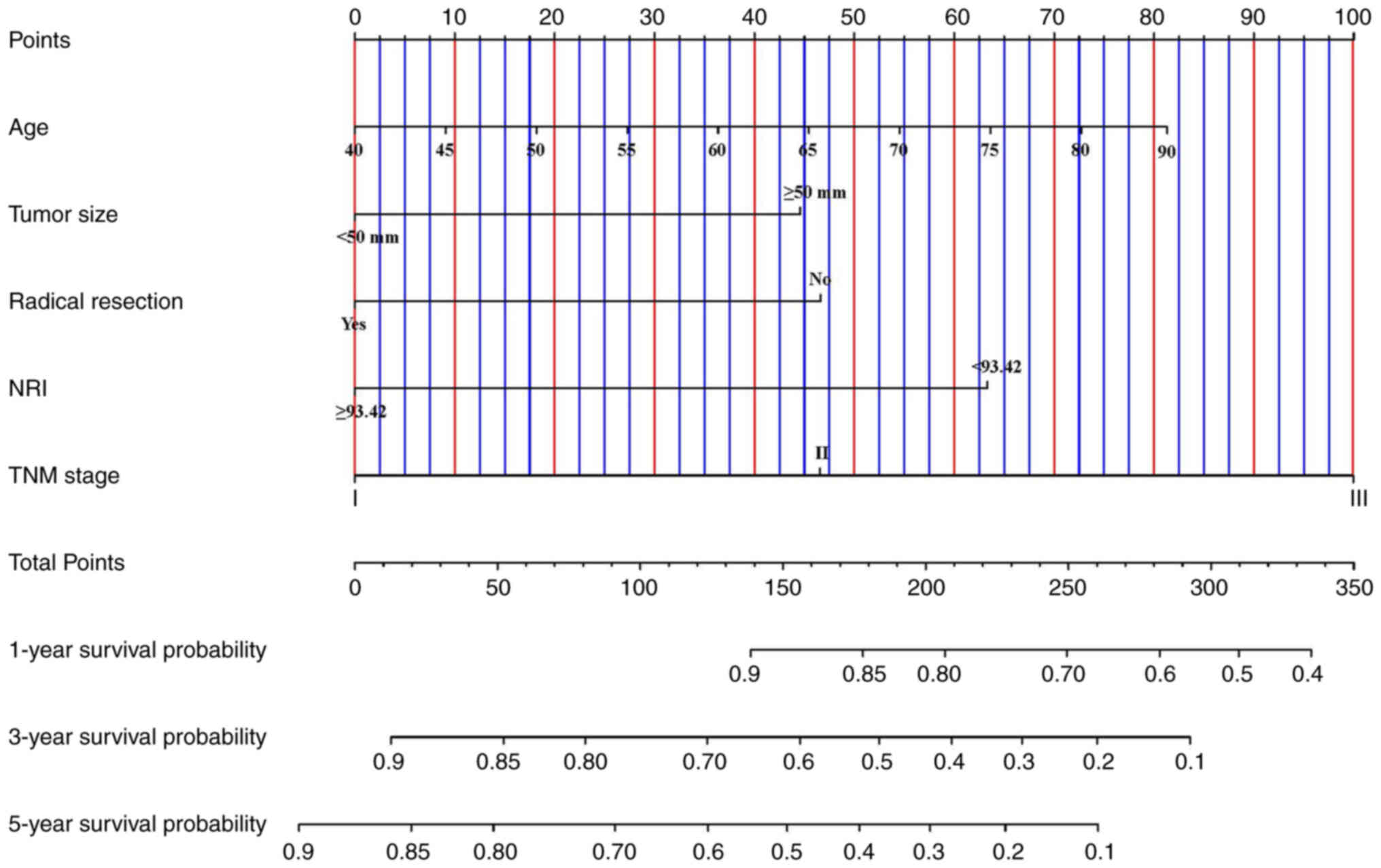
Finally, a nomogram was established to predict the
survival probability of patients with pyloric stenosis based on
independent prognostic factors obtained from the multivariate
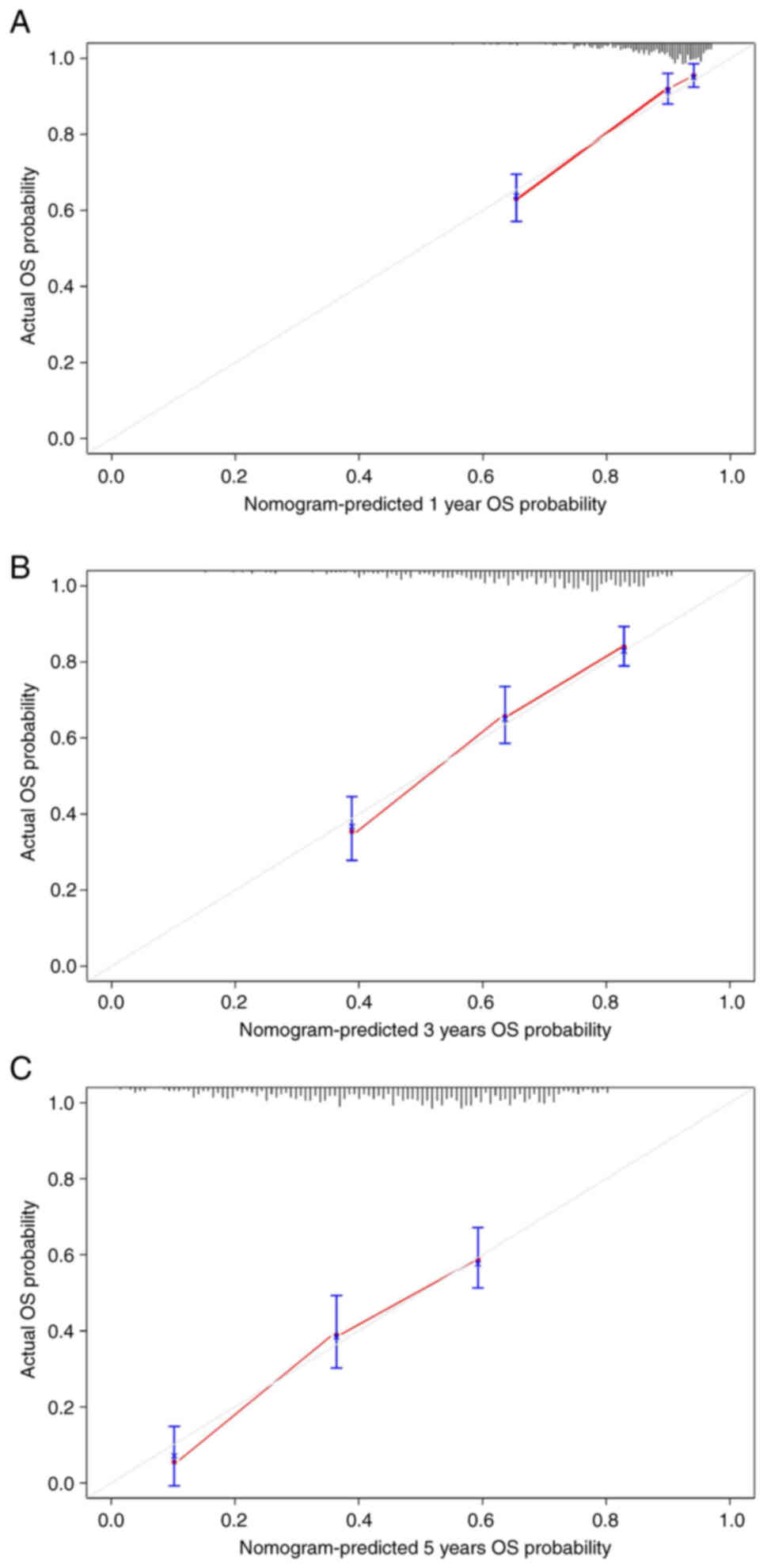
analysis (Fig. 6). To verify the
predictive ability of the nomogram, a bootstrap calibration was
conducted and the calibration curves were plotted (Fig. 7A-C). The C-index of the nomogram was
0.760 (95%CI: 0.688–0.832) and the calibration curves also showed
good predictive ability.
Discussion
Pyloric stenosis is a common complication in
patients with gastric cancer, which is related to the site of tumor
growth and disease progression. However, detailed and comprehensive
studies on the clinical and pathological characteristics and
prognosis of patients with gastric cancer with pyloric stenosis are
still lacking. In addition, patients with pyloric stenosis may
present with malnutrition early. Therefore, preoperative parenteral
nutrition is essential to restore patients' surgical tolerance. The
predictive ability of the NRI regarding the prognosis of such
patients had so far remained elusive.
Certain clinical studies have reported on patients
with gastric cancer with early pyloric stenosis. In 1998, Watanabe
et al (6) analyzed 122
gastric cancer patients with pyloric stenosis and found that those
cases had faster disease progression and a higher probability of
distant metastasis. In addition, they also found that operation was
a significant prognostic factor for patients with pyloric stenosis,
even if the tumor had distant metastasis. Another study by Mizutani
et al (18) also found that
patients with pyloric stenosis were more inclined to be stage IV
and surgery was able to improve their prognosis. Of note, the NRI
has been widely used in cancer for numerous years. Xie et al
(16) combined the NRI and handgrip
strength to predict the survival rate of patients with cancer
cachexia and they found that NRI was an independent prognostic
factor for cancer cachexia. Oh et al (19) analyzed the application of NRI in
patients with head and neck cancer who received concurrent
chemo-radiotherapy. They collected 110 patients and found that the
NRI was able to predict OS and complications of their subjects. The
close relationship between gastric cancer and nutritional status
made the NRI equally widely used in gastric cancer. Song et
al (20) specifically studied
the predictive ability of the NRI regarding the prognosis of
patients with stage III gastric cancer. Their results indicated
that the NRI was related to a shorter survival time. Other studies
on gastric cancer had also reached similar conclusions (21–25).
The present study mainly reported on the clinical
and pathological characteristics of patients with gastric cancer
with pyloric stricture and analyzed the application of the NRI in
patients with gastric cancer with pyloric stricture who received
preoperative parenteral nutrition. Correlation analysis indicated
that pyloric stenosis was significantly associated with faster
disease progression and poorer blood parameters. The NRI also had a
strong predictive ability for OS in patients with pyloric
stricture. In addition, further multivariate analysis of all
patients with gastric cancer found that the NRI was an independent
prognostic marker of OS. Finally, the bootstrap correction for the
nomogram also showed good consistency between the predicted
probability and the actual probability.
The causes of malnutrition in patients with gastric
cancer with pyloric stenosis were multiple, mainly related to
feeding difficulties and disease progression (26–30).
The present study indicated that patients with pyloric stenosis
were more prone to abdominal distension and higher TNM stage, which
were the main reasons for malnutrition and shorter survival
(31–34). The NRI contains ALB levels and body
weight, which are closely related to the prognosis of patients with
gastric cancer (35–37). ALB not only reflects the nutritional
status of patients but also correlates with the systemic
inflammatory status (38).
Inflammatory factors may act on the liver and inhibit the synthesis
of ALB by the liver (39,40). Low serum levels of ALB reflect poor
hepatic functional reserve of patients to a certain extent, leading
to worse treatment tolerance and shorter survival time (41). The body weight also reflects the
nutritional status and it is associated with surgery or
chemotherapy tolerance in cancer patients (42). Several studies have indicated that
body weight was a strong independent prognostic factor for patients
with gastric cancer and the predictive ability of the BMI regarding
the clinical outcomes of patients with gastric cancer who received
immune checkpoint inhibitors has also been confirmed (43,44).
Due to the retrospective nature of the present
study, a certain degree of information bias was inevitable. In
addition, the parenteral nutrition for certain patients with mild
malnutrition was affected by the operation schedule and did not
reach the optimal nutritional status. The present study was only
for patients with pyloric stenosis and did not consider gastric
emptying disorders due to other causes, such as Borrmann IV gastric
cancer. Finally, although the NRI could accurately reflect the
nutritional status of patients, it may be more effective when
combined with other factors that reflect the inflammatory status or
tumor progression. The conclusions of the present study still
require to be further verified by numerous prospective studies.
In conclusion, the NRI was an accurate score
reflecting the nutritional status of patients, which could predict
the clinical outcomes for patients with gastric cancer with pyloric
stricture who received preoperative parenteral nutrition. Patients
with a low NRI had shorter survival times.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Data availability statement
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
GL designed and conducted the study, and drafted the
manuscript. HS and LH were responsible for data collection,
analysis and interpretation. GL and HS confirm the authenticity of
all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
the Second People's Hospital of Neijiang (Neijiang, China)
(approval no. LSY2022015). All patients provided written informed
consent before the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thrift AP and El-Serag HB: Burden of
gastric cancer. Clin Gastroenterol Hepatol. 18:534–542. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bullock AF, Greenley SL, McKenzie GAG,
Paton LW and Johnson MJ: Relationship between markers of
malnutrition and clinical outcomes in older adults with cancer:
Systematic review, narrative synthesis and meta-analysis. Eur J
Clin Nutr. 74:1519–1535. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Viana ECRM, Oliveira IDS, Rechinelli AB,
Marques IL, Souza VF, Spexoto MCB, Pereira TSS and Guandalini VR:
Malnutrition and nutrition impact symptoms (NIS) in surgical
patients with cancer. PLoS One. 15:e02413052020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poisson J, Martinez-Tapia C, Heitz D,
Geiss R, Albrand G, Falandry C, Gisselbrecht M, Couderc AL,
Boulahssass R, Liuu E, et al: Prevalence and prognostic impact of
cachexia among older patients with cancer: A nationwide
cross-sectional survey (NutriAgeCancer). J Cachexia Sarcopeni
Muscle. 12:1477–1488. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pobłocki J, Jasińska A, Syrenicz A,
Andrysiak-Mamos E and Szczuko M: The neuroendocrine neoplasms of
the digestive tract: Diagnosis, treatment and nutrition. Nutrients.
12:14372020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watanabe A, Maehara Y, Okuyama T, Kakeji
Y, Korenaga D and Sugimachi K: Gastric carcinoma with pyloric
stenosis. Surgery. 123:330–334. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu R, Chen XD and Ding Z: Perioperative
nutrition management for gastric cancer. Nutrition. 93:1114922022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Izumi D, Ida S, Hayami M, Makuuchi R,
Kumagai K, Ohashi M, Watanabe M, Sano T and Nunobe S: Increased
rate of serum prealbumin level after preoperative enteral nutrition
as an indicator of morbidity in gastrectomy for gastric cancer with
outlet obstruction. World J Surg. 46:624–630. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gong C, Wan Q, Zhao R, Zuo X, Chen Y and
Li T: Cachexia index as a prognostic indicator in patients with
gastric cancer: A retrospective study. Cancers (Basel).
14:44002022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aprile G, Basile D, Giaretta R, Schiavo G,
La Verde N, Corradi E, Monge T, Agustoni F and Stragliotto S: The
clinical value of nutritional care before and during active cancer
treatment. Nutrients. 13:11962021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saroul N, Puechmaille M, Lambert C, Hassan
AS, Biau J, Lapeyre M, Mom T, Bernadach M and Gilain L: Prognosis
in head and neck cancer: Importance of nutritional and biological
inflammatory status. Otolaryng Head Neck. 166:118–127. 2022.
View Article : Google Scholar
|
|
12
|
Steenhagen E, van Vulpen JK, van
Hillegersberg R, May AM and Siersema PD: Nutrition in
peri-operative esophageal cancer management. Expert Rev
Gastroenterol Hepatol. 11:663–672. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lakananurak N and Gramlich L: The role of
preoperative parenteral nutrition. Nutrients. 12:13202020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin F, Xia W, Chen M, Jiang T, Guo J,
Ouyang Y, Sun H, Chen X, Deng W, Guo L and Lin H: A prognostic
model based on nutritional risk index in operative breast cancer.
Nutrients. 14:37832022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim KW, Lee K, Lee JB, Park T, Khang S,
Jeong H, Ko CS, Yook JH, Kim BS and Lee IS: Preoperative
nutritional risk index and postoperative one-year skeletal muscle
loss can predict the prognosis of patients with gastric
adenocarcinoma: A registry-based study. BMC Cancer. 21:1572021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie H, Ruan G, Zhang Q, Ge Y, Song M,
Zhang X, Liu X, Lin S, Zhang X, Li X, et al: Combination of
nutritional risk index and handgrip strength on the survival of
patients with cancer cachexia: A multi-center cohort study. J
Inflamm Res. 15:1005–1015. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen L, Qi Y, Kong X, Su Z, Wang Z, Wang
X, Du Y, Fang Y, Li X and Wang J: Nutritional risk index predicts
survival in patients with breast cancer treated with neoadjuvant
chemotherapy. Front Nutr. 8:7867422021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mizutani S, Shioya T, Maejima K, Yoshino
M, Komine O, Bou H, Ogata M, Watanabe M, Shibuya T, Tokunaga A and
Tajiri T: Significance of gastrectomy as palliative surgery for
gastric carcinoma with pyloric stenosis. J Nippon Med Sch.
74:241–245. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oh J, Liu A, Tran E, Berthelet E, Wu J,
Olson RA, Chau N, Bowman A and Hamilton SN: Association between
nutritional risk index and outcomes for head and neck cancer
patients receiving concurrent chemo-radiotherapy. Head Neck.
42:2560–2570. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Song H, Sun H, Yang L, Gao H, Cui Y, Yu C,
Xu H and Li L: Nutritional risk index as a prognostic factor
predicts the clinical outcomes in patients with stage III gastric
cancer. Front Oncol. 12:8804192022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ma LX, Taylor K, Espin-Garcia O, Anconina
R, Suzuki C, Allen MJ, Honorio M, Bach Y, Allison F, Chen EX, et
al: Prognostic significance of nutritional markers in metastatic
gastric and esophageal adenocarcinoma. Cancer Med. 10:199–207.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fujiya K, Kawamura T, Omae K, Makuuchi R,
Irino T, Tokunaga M, Tanizawa Y, Bando E and Terashima M: Impact of
malnutrition after gastrectomy for gastric cancer on long-term
survival. Ann Surg Oncol. 25:974–983. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Vicente MA, Barão K, Silva TD and Forones
NM: What are the most effective methods for assessment of
nutritional status in outpatients with gastric and colorectal
cancer? Nutr Hosp. 28:585–591. 2013.PubMed/NCBI
|
|
24
|
Karabulut S, Dogan I, Afsar CU, Karabulut
M, Ak N, Duran A and Tastekin D: Does nutritional status affect
treatment tolerability, chemotherapy response and survival in
metastatic gastric cancer patients? Results of a prospective
multicenter study in Turkey. J Oncol Pharm Pract. 28:127–134. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Seo SH, Kim SE, Kang YK, Ryoo BY, Ryu MH,
Jeong JH, Kang SS, Yang M, Lee JE and Sung MK: Association of
nutritional status-related indices and chemotherapy-induced adverse
events in gastric cancer patients. BMC Cancer. 16:9002016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Przekop Z, Szostak-Węgierek D, Milewska M,
Panczyk M, Zaczek Z and Sobocki J: Efficacy of the nutritional risk
index, geriatric nutritional risk index, BMI, and GLIM-defined
malnutrition in predicting survival of patients with head and neck
cancer patients qualified for home enteral nutrition. Nutrients.
14:12682022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ongaro E, Buoro V, Cinausero M,
Caccialanza R, Turri A, Fanotto V, Basile D, Vitale MG, Ermacora P,
Cardellino GG, et al: Sarcopenia in gastric cancer: When the loss
costs too much. Gastric Cancer. 20:563–572. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martin L, Jia C, Rouvelas I and Lagergren
P: Risk factors for malnutrition after oesophageal and cardia
cancer surgery. Br J Surg. 95:1362–1328. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huang DD, Yu DY, Wang WB, Song HN, Luo X,
Wu GF, Chen XL, Yu Z and Yan JY: Global leadership initiative in
malnutrition (GLIM) criteria using hand-grip strength adequately
predicts postoperative complications and long-term survival in
patients underwent radical gastrectomy for gastric cancer. Eur J
Clin Nutr. 76:1323–1331. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li Q, Zhang X, Tang M, Song M, Zhang Q,
Zhang K, Ruan G, Zhang X, Ge Y, Yang M, et al: Different muscle
mass indices of the Global leadership initiative on malnutrition in
diagnosing malnutrition and predicting survival of patients with
gastric cancer. Nutrition. 89:1112862021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu R, Gu Q, Xiao S, Zhao P and Ding Z:
Patient-reported gastrointestinal symptoms following surgery for
gastric cancer and the relative risk factors. Front Oncol.
12:9514852022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, Zou S, Luo R, Zhu Z, Xu H and
Huang B: Proposal of a novel stage grouping of the eighth edition
of American joint committee on cancer TNM staging system for
gastric cancer: Results from a retrospective study of 30 years
clinical data from a single institute in China. Expert Rev
Gastroenterol Hepatol. 14:55–64. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhuang CL, Dong QT, Chen XL and Wang SL:
ASO author reflections: Comparison of sarcopenia and cachexia for
their prognostic value in gastric cancer patients at different TNM
stages after gastrectomy. Ann Surg Oncol. 29:585–586. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cai W, Yang H, Zheng J, Huang J, Ji W, Lu
Y, Yang X, Zhang W, Shen X and Chen X: Global leaders malnutrition
initiative-defined malnutrition affects long-term survival of
different subgroups of patients with gastric cancer: A propensity
score-matched analysis. Front Nutr. 9:9952952022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Zhu JY, Zhou LN, Tang M, Chen MB
and Tao M: Predicting the prognosis of gastric cancer by
albumin/globulin ratio and the prognostic nutritional index. Nutr
Cancer. 72:635–644. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin GT, Ma YB, Chen QY, Zhong Q, Zheng CH,
Li P, Xie JW, Wang JB, Lin JX and Huang CM: Fibrinogen-albumin
ratio as a new promising preoperative biochemical marker for
predicting oncological outcomes in gastric cancer: A
multi-institutional study. Ann Surg Oncol. 28:7063–7073. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang N, Jiang J, Xi W, Wu J, Zhou C, Shi
M, Wang C, Zhu Z, Liu J and Zhang J: Postoperative BMI loss at one
year correlated with poor outcomes in Chinese gastric cancer
patients. Int J Med Sci. 17:2276–2284. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Evans DC, Corkins MR, Malone A, Miller S,
Mogensen KM, Guenter P and Jensen GL: The use of visceral proteins
as nutrition markers: An ASPEN position paper. Nutr Clin Pract.
36:22–28. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Coffelt SB and de Visser KE: Cancer:
Inflammation lights the way to metastasis. Nature. 507:48–49. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bito R, Hino S, Baba A, Tanaka M, Watabe H
and Kawabata H: Degradation of oxidative stress-induced denatured
albumin in rat liver endothelial cells. Am J Physiol Cell Physiol.
289:C531–C542. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Terashima T, Yamashita T, Arai K,
Kawaguchi K, Kitamura K, Yamashita T, Sakai Y, Mizukoshi E, Honda M
and Kaneko S: Beneficial effect of maintaining hepatic reserve
during chemotherapy on the outcomes of patients with hepatocellular
carcinoma. Liver Cancer. 6:236–249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhang R, Li H, Li N, Shi JF, Li J, Chen
HD, Yu YW, Qin C, Ren JS, Chen WQ and He J: Risk factors for
gastric cancer: A large-scale, population-based case-control study.
Chin Med J (Engl). 134:1952–1958. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ahmed M, von Itzstein MS, Sheffield T,
Khan S, Fattah F, Park JY, Popat V, Saltarski JM, Gloria-McCutchen
Y, Hsiehchen D, et al: Association between body mass index, dosing
strategy, and efficacy of immune checkpoint inhibitors. J
Immunother Cancer. 9:e0023492021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Indini A, Rijavec E, Ghidini M, Tomasello
G, Cattaneo M, Barbin F, Bareggi C, Galassi B, Gambini D and Grossi
F: Impact of BMI on survival outcomes of immunotherapy in solid
tumors: A systematic review. Int J Mol Sci. 22:26282021. View Article : Google Scholar : PubMed/NCBI
|