Introduction
Oesophageal cancer (EC) was the fourth most common
type of cancer in China in 2015 and the sixth most frequent cause
of cancer-associated death worldwide (1). Squamous cell carcinoma is the main
histopathological type of EC in Asia, particularly in China, and
its five-year overall survival (OS) rate is <10% (2).
Hypoxia-inducible factor-1α (HIF-1α) plays a key
role in the maintenance of human oxygen homeostasis. Its expression
increases in a hypoxic atmosphere and is maintained at a normal
level in a normoxic atmosphere. The overexpression of HIF-1α has
been shown to cause the transcription of certain genes associated
with angiogenesis, cell proliferation and glucose metabolism
(3,4). Upregulated expression of HIF-1α has
been detected in various cancers, including brain, breast and
uterine cancers (5). Hypoxic
conditions are known to be common in cancers. HIF-1α is critical
for glucose uptake and glycolysis; Glucose transporter 1 (GLUT1) is
upregulated during glycolysis and regulated by HIF-1α (6). The upregulation of GLUT1 may be an
important mechanism by which cancer cells increase glucose intake
and compensate for the lack of energy triggered by hypoxia
(7,8). Hypoxia plays a major role in radio-
and chemoresistance, which may lead to a poor prognosis for
patients (9). The association
between the expression of GLUT1 and the prognosis of patients with
various cancers has been investigated previously. Specifically,
several studies have shown that the high expression of GLUT1
protein in tumors is associated with poor survival in patients with
various tumors, including, lung, breast and liver cancer (10–13).
However, there have been few reports on HIF-1α and GLUT1 in
oesophageal squamous cell carcinoma (ESCC) and their association
with the prognosis of patients with ESCC (14).
In the present study, the in vitro and in
vivo expression levels of HIF-1α and GLUT1 under hypoxic or
normoxic conditions were investigated and compared. In addition,
the associations between the expression levels of HIF-1α and GLUT1
and chemoresistance were evaluated in vivo. Furthermore, the
relationships between HIF-1α and GLUT1 and the prognosis of ESCC
were also analysed.
Materials and methods
Cell lines
The Eca109, Kyse150, TE-1 and TE-10 human ESCC cell
lines were confirmed by cell morphology and genomic short tandem
repeats. All cell lines were incubated in RPMI-1640 with 10% foetal
bovine serum (Yeasen Biotech Co., Ltd.) and 1%
penicillin-streptomycin (Invitrogen, USA) at 37°C in a humidified
atmosphere with 5% CO2. In the hypoxic experiments, the
cells were treated with 150 µM CoCl2 for 24 h at 37°C
and then cultured in a humidified atmosphere with 5% CO2
at 37°C.
Western blot analysis
All cell lines were separately cultured under
normoxic and hypoxic conditions. Cell lysates were collected.
Proteins extracted from mouse tumor tissues (80 µg) were analyzed
using western blotting. Samples of tissue and cells were
homogenized in radioimmunoprecipitation buffer containing a
protease inhibitor cocktail (Roche Applied Science). The protein
concentration was determined using a bicinchoninic acid kit
(Beyotime Institute of Biotechnology). A total of 50 µg of protein
per lane were resolved on 10% SDS-PAGE gels and transferred to PVDF
membranes (Roche Diagnostics). Membranes were blocked using 5%
skimmed milk for 1 h at room temperature. The membranes were
incubated at 4°C with primary antibodies targeting HIF-1α (1:1,000;
cat. no. sc-13515; Santa Cruz Biotechnology), GLUT1 (1:1,000; cat.
no. sc-377228; Santa Cruz Biotechnology), cleaved caspase 3
(1:1,000; cat. no. ab2302; Abcam), H2A histone family member X
(H2AX) (1:1,000; cat. no. sc-517336; Santa Cruz Biotechnology),
phosphorylated H2AX (γH2AX) (1:100; cat. no. A0264; ABclonal
Biotech Co., Ltd.) and GAPDH (1:1,000; cat. no. sc-47724 Santa Cruz
Biotechnology). Next day, secondary antibody (goat anti-rabbit;
cat. no. SC2004; Santa Cruz Biotechnology) was applied for 2 h at
room temperature. The Clarity™ Western ECL substrate
(Bio-Rad Laboratories, Inc.) was used to detect the
antigen-antibody complexes.
Patient characteristics
A total of 157 tissue specimens from patients with
ESCC were collected from the Cancer Center of Sun Yat-Sen
University between January 2012 and December 2014. All patients
were histologically confirmed to have ESCC before surgery and
received surgery without radiation or chemotherapy. The clinical
information of the patients is presented in Table I. The median age of the patients was
61.75 years (range, 35–90 years). There were 125 males and 32
females; 78 cases had TNM stage I and II tumors, and 79 cases had
TNM stage III and IV tumors according to the TNM staging system of
the World Health Organization published in 2002 (15).
 | Table I.Clinical characteristic of 157
patients with oesophageal squamous cell carcinoma. |
Table I.
Clinical characteristic of 157
patients with oesophageal squamous cell carcinoma.
|
Characteristics | N (%) |
|---|
| Total cases | 157 |
| Age (years) |
|
|
Median | 61.75 |
|
Range | 35-90 |
| Sex |
|
|
Male | 125 (79.6) |
|
Female | 32 (20.4) |
| Degree of
differentiation |
|
| G1 | 48 (30.6) |
| G2 | 69 (43.9) |
| G3 | 40 (25.5) |
| Tumor status |
|
| T1 | 12 (7.6) |
| T2 | 42 (26.8) |
| T3 | 98 (62.4) |
| T4 | 5 (3.2) |
| Lymph node
status |
|
| N0 | 84 (53.5) |
| N1 | 73 (46.5) |
| Distant metastasis
status |
|
| M0 | 149 (94.9) |
| M1 | 8 (5.1) |
| TNM stage |
|
| I | 10 (6.4) |
| II | 68 (43.3) |
|
III | 71 (45.2) |
| IV | 8 (5.1) |
| Death |
|
| No | 42 (26.8) |
|
Yes | 115 (73.2) |
Xenograft tumor models
A total of 16 male 6–8-week-old BALB/c-nude mice
(20–25 g) were provided by Beijing Vital River Laboratory
Technology Co., Ltd. The animals were housed in the Laboratory
Animal Center of Sun Yat-Sen University at 21°C with 50% relative
humidity and a 12 h light/dark cycle. The animal experimentation
ethics committee of Sun Yat-Sen University approved the animal
experimentation protocol (L201501054). The animals were assigned to
two groups: Normoxia Eca109 and hypoxia Eca109 (n=8/group). Hypoxic
and normoxic Eca109 cells (2×106) were each combined
with Matrigel in a 1:5 ratio and subcutaneously inoculated into the
right infra-axillary area of the BALB/c nude mice in the respective
group. When the volumes of the tumours reached 200–300
mm3, treatment with 5-fluorouracil (5-FU) by
intraperitoneal injection was initiated, using a dosage of 20 mg/kg
twice a week for 2 weeks. The mice were anesthetized using 1%
pentobarbital sodium (50 mg/kg of body weight) during the
intraperitoneal injection. The mice were sacrificed by
CO2 inhalation using a 30% vol/min air displacement rate
when they met any of the humane endpoint criteria, namely severe
tumor burden (tumor size >1,500 mm3), prostration,
significant body weight loss, difficulty breathing, rotational
motion and body temperature drop. The volume of the xenograft tumor
and the body weight of each mouse were recorded twice a week. The
tumor volumes were calculated using the following formula: Volume
(mm3)=1/2 × (length × width2). The maximum
tumor diameter measured in this experiment did not exceed 17
mm.
Immunohistochemical (IHC)
staining
The IHC analysis of HIF-1α and GLUT1 was performed
using 4-µm formalin-fixed paraffin-embedded sections of the patient
tumor specimens. The sections underwent deparaffinization using
xylene, followed by hydration with a decreasing ethanol series. To
quench endogenous peroxidases, the sections were immersed in Dako
REAL peroxidase blocking solution (Agilent Technologies) for 5 min
at room temperature and then rinsed in PBS for 1 min using a
magnetic stirrer. Staining was performed overnight at 4°C using
GLUT-1 and HIF-1α mouse/rabbit polyclonal antibodies (1:100; cat.
nos. ab8366 and ab252403; Abcam). Subsequently, the slides were
washed three times for 5 min each with PBS containing 0.2% Triton.
The sections were then incubated with a horseradish
peroxidase-conjugated rabbit anti-mouse Ig antibody or goat
anti-rabbit IgG antibody (1:100; cat. nos. ab6728 and ab288151;
Abcam, USA) at room temperature for 1 h, followed by DAB staining
at room temperature for 15 min. Finally, hematoxylin was applied as
a counterstain at room temperature for 10 min. The sections were
imaged using a Leica microscope (Leica Microsystems GmbH). When
evaluating HIF-1α expression, homogenously and darkly stained
nuclei and >1% positive nuclei were considered positive. GLUT1
was considered as positive when membrane staining was observed in
>1% of the cells. The immunohistochemically stained slides were
scanned, imaged and digitized using a Panoramic Midi digital slide
scanner (3DHISTECH Ltd.). Panoramic Viewer software (version
1.15.2; 3DHISTECH Ltd.) was used to analyse the data. The IHC
scores of HIF-1α and GLUT1 expression were determined by a
semi-quantitative method according to the percentage and intensity
of positively stained cells (15).
The positive staining was scored as follows: 0, <5% positively
stained cells; 1, 5–24% positively stained cells; 2, 25–49%
positively stained cells; 3, 50–74% positively stained cells; and
4, 75–100% positively stained cells. The intensity was scored as
follows: 0, negative staining; 1, weak staining; 2, moderate
staining; and 3, strong staining. The final score was generated by
multiplying the percentage score by the staining intensity score.
Two independent observers blindly evaluated the IHC scores of
HIF-1α and GLUT1 expression in all specimens, and the mean values
were calculated. The cut-off value for high HIF-1α and GLUT1
expression was determined based on the median IHC score, and high
HIF-1α and GLUT1 expression was defined as an IHC score greater
than the cut-off value.
In situ TUNEL staining
An In Situ Cell Death Detection Kit (Roche
Diagnostics GmbH) was used to perform TUNEL staining of the mouse
xenograft tissues. The deparaffinized sections were treated with
Proteinase K solution without DNase I (Sigma-Aldrich; Merck KGaA)
at 37°C for 30 min. The slices were then exposed to terminal
deoxynucleotidyl transferase (TdT) equilibration buffer,
recombinant TdT enzyme and fluorescein isothiocyanate (FITC)-dUTP
Labeling Mix. This reaction processed for 60 min at 37°C in the
dark. The slices were washed twice with 1× PBS and then incubated
with DAPI (Beyotime Institute of Biotechnology) for 5–10 min at
room temperature after the reaction was stopped using 50 ml of 1×
TdT Stop Buffer at room temperature for 5 min. The labelling
solution alone was used to incubate sections as negative controls.
Fluorescent images were captured using an Olympus BX51 microscope.
Twenty-six microscopic fields were examined for each sample.
Statistical analysis
Each experiment was performed in triplicate, at
least three times. Analyses were performed using SPSS (version
19.0; IBM Corp.). Differences in tumour volume and body weight
between mice in the two treatment groups were assessed using
unpaired Student's t-tests. The TUNEL results were also evaluated
using an unpaired Student's t-test. The associations between
clinicopathological features and the expression levels of HIF-1α
and GLUT1 were analysed using the Kruskal-Wallis test. Kaplan-Meier
curves were assessed using the log-rank test to analyse the
relationship of HIF-1α and GLUT1 expression with the clinical
prognosis of the patients. Prognostic factors for progression-free
survival (PFS) and OS were evaluated by multivariate Cox regression
analyses. The relationship between HIF-1α and GLUT1 expression was
analysed by Spearman's correlation analysis and a χ2
test. A receiver operating curve analysis was also performed to
investigate the sensitivity and specificity of HIF-1α and GLUT1
expression in the prediction of death. A two-tailed P<0.05 was
considered to indicate a statistically significant result.
Results
HIF-1α and GLUT1 expression in ESCC
cell lines and xenografts derived from cells cultured under a
normoxic or hypoxic atmosphere
Western blotting revealed that the expression of
HIF-1α and GLUT1 in all four ESCC cell lines cultured with hypoxic
stress was increased compared with that of the respective cells
cultured under normoxic conditions (Fig. 1A). Subsequently, Eca109 cells
cultured under normoxic or hypoxic conditions were used to
establish xenografts in nude mice and investigate their
chemosensitivity to 5-FU. When compared with the hypoxic Eca109
×enografts, the normoxic Eca109 ×enografts were more sensitive to
5-FU; the tumor volume in the normoxia Eca109 group was smaller
than that in the hypoxia Eca109 group (Fig. 1B). After treatment with 5-FU for 2
weeks, the mean tumor volume in the hypoxia Eca109 group reached
~1,800 mm3 at the time of last measurement, while the
tumor volume in the normoxia group was ~750 mm3 at the
same time point. A comparable result was observed for tumor weights
(Fig. 1C). The levels of HIF-1α and
GLUT1 in the two xenograft groups were consistent with those
obtained in vitro as revealed by western blotting (Fig. 1D). In addition, the protein levels
of cleaved caspase 3 and γH2AX were higher in the normoxia Eca109
×enograft group compared with the hypoxia Eca109 ×enograft group
(Fig. 1D). The percentage of TUNEL
positive cells in the normoxia Eca109 ×enograft group was ~25%,
which was significantly higher compared with that in the hypoxia
Eca109 ×enograft group (5%; Fig. 1E and
F). These results indicate that the chemoresistance of the
hypoxia Eca109 ×enograft group to 5-FU was increased compared with
that of the normoxia Eca109 ×enograft group.
Expression of HIF-1α and GLUT1 in
normal and ESCC tissues
To investigate the expression of HIF-1α and GLUT1
protein in ESCC tissues, the expression of HIF-1α and GLUT1 in
tumor tissues and matched adjacent tissues was detected using IHC
staining. As shown in Fig. 2, the
expression of HIF-1α in the tumor tissue was higher than that in
the matched adjacent tissue. Similarly, higher expression of GLUT1
was detected in the tumor tissue compared with the adjacent normal
tissue.
Relationship between HIF-1α and
GLUT1
To determine the relationship between HIF-1α and
GLUT1, IHC scores for HIF-1α were compared with those for GLUT1
(Table II; Fig. 3). HIF-1α expression was
significantly associated with GLUT1 (Chi-square test, P=0.008;
Spearman's r=0.204, P=0.01). The optimal cut-off values for HIF-1α
and GLUT1 expression were investigated for sensitivity and
specificity in the prediction of death by receiver operating curve
analysis (Table III; Fig. 4). Both HIF-1α and GLUT1 had
statistically significant areas under the curve (0.689 and 0.648,
respectively; P<0.001 and P=0.005, respectively). A high
expression level of HIF-1α protein was detected in 51.0% of
patients (80/157, cut-off score 4) and a high expression level of
GLUT1 was observed in 49.7% of patients (78/157, cut-off score
7).
 | Table II.Association between HIF-1α and GLUT1
expression determined by immunohistochemical analysis in patients
with oesophageal squamous cell carcinoma. |
Table II.
Association between HIF-1α and GLUT1
expression determined by immunohistochemical analysis in patients
with oesophageal squamous cell carcinoma.
|
| HIF-1α
expression |
|
|---|
| GLUT1
expression |
|
|
|---|
| High | Low | Total | P-value |
|---|
| High | 46 | 32 | 78 | 0.008 |
| Low | 34 | 45 | 79 |
|
| Total | 80 | 77 | 157 |
|
 | Table III.Optimal cut-off values for high
expression of markers in the prediction of death. |
Table III.
Optimal cut-off values for high
expression of markers in the prediction of death.
|
|
|
|
| Prediction of
death |
|---|
|
|
|
|
|
|
|---|
| Marker | AUROC (95%CI) | P-value | Cut-off score | Sensitivity
(%) | Specificity
(%) |
|---|
| HIF-1α | 0.689
(0.593–0.785) | <0.001 | 4 | 0.722 | 0.619 |
| GLUT1 | 0.646
(0.554–0.739) | 0.005 | 7 | 0.552 | 0.833 |
Clinicopathological characteristics
and their association with HIF-1α and GLUT1 expression
The associations between the expression levels of
HIF-1α and GLUT1 and clinicopathological characteristic were
analysed, based on the protein levels of HIF-1α and GLUT1
determined by IHC in the 157 formalin-fixed paraffin-embedded ESCC
tissues. The associations between clinicopathological features and
the protein expression levels of HIF-1α and GLUT1 are listed in
Table IV. High expression levels
of HIF-1α protein were found to be significantly associated with
advanced ESCC, including tumor status (P=0.007), lymph node status
(P=0.011) and clinical TNM stage (P=0.04), but not with age, sex,
degree of tumour differentiation and distant metastasis. However,
GLUT1 expression levels were only associated with sex (P=0.047),
and not with the other clinical pathological features, namely age,
degree of differentiation, tumour status, lymph node status,
metastasis status and TNM stage.
 | Table IV.Association of HIF-1α and GLUT1
expression with the clinicopathological characteristics of patients
with oesophageal squamous cell carcinoma. |
Table IV.
Association of HIF-1α and GLUT1
expression with the clinicopathological characteristics of patients
with oesophageal squamous cell carcinoma.
|
|
| HIF-1α score | GLUT1 score |
|---|
|
|
|
|
|
|---|
|
Characteristics | N | Median (Q1-Q3) | P-value | Median (Q1-Q3) | P-value |
|---|
| Sex |
|
| 0.096 | | 0.047 |
|
Male | 125 | 7.0 (3.5–8.0) |
| 4.0 (2.0–7.0) |
|
|
Female | 32 | 4.2 (2.9–8.0) |
| 3.0 (1.0–4.2) |
|
| Age (years) |
|
| 0.705 |
| 0.169 |
|
≥61 | 84 | 7.0 (3.4–8.0) |
| 3.0 (1.9–6.0) |
|
|
<61 | 73 | 6.0 (3.0–8.0) |
| 4.0 (2.0–7.0) |
|
| Degree of
differentiation |
|
| 0.139 |
| 0.606 |
| G1 | 48 | 7.0 (3.5–8.0) |
| 4.0 (2.4–7.0) |
|
| G2 | 69 | 7.0 (3.5–8.5) |
| 3.0 (1.5–7.0) |
|
| G3 | 40 | 4.5 (3.0–7.6) |
| 3.5 (2.0–7.2) |
|
| Tumor status |
|
| 0.007 |
| 0.218 |
|
T1-2 | 54 | 4.0 (3.0–7.4) |
| 3.0 (2.0–6.0) |
|
|
T3-4 | 103 | 7.0 (3.5–8.5) |
| 4.0 (2.0–7.0) |
|
| Lymph node
status |
|
| 0.011 |
| 0.576 |
| N0 | 84 | 5.0 (3.0–8.0) |
| 3.5 (2.0–6.2) |
|
| N1 | 73 | 7.0 (4.0–8.5) |
| 3.0 (2.0–8.0) |
|
| Distant metastasis
status |
|
| 0.776 |
| 0.347 |
| M0 | 149 | 7.0 (3.0–8.0) |
| 3.0 (2.0–7.0) |
|
| M1 | 8 | 6.5 (2.8–9.8) |
| 7.5 (1.8–8.0) |
|
| TNM stage |
|
| 0.040 |
| 0.396 |
|
I–II | 89 | 5.0 (3.0–8.0) |
| 3.0 (2.0–6.0) |
|
|
III–IV | 68 | 7.0 (3.5–8.5) |
| 3.8 (2.0–8.0) |
|
Relationship between the levels of
HIF-1α and GLUT1 protein and the survival of patients with
ESCC
The median OS of the 157 patients with ESCC was 25
months (range, 0–133 months). The cumulative 5- and 10-year PFS
rates were 28.8 and 22%, respectively, whereas the cumulative 5-
and 10-year OS rates were 32.8 and 22.3%, respectively. Fig. 5A and B demonstrate a negative
association of HIF-1α expression with PFS and OS (both P<0.001).
In addition, a statistically significant negative association was
also detected for the expression of GLUT1 with PFS and OS (both
P<0.001; Fig. 5C and D). In
addition to sex and nodal status, the multivariate Cox analysis
indicates that HIF-1α and GLUT1 expression levels are independent
unfavourable factors for PFS and OS in patients with ESCC
(P<0.05; Table V).
 | Table V.Multivariate Cox regression analysis
of OS and PFS for 157 patients with oesophageal squamous cell
carcinoma. |
Table V.
Multivariate Cox regression analysis
of OS and PFS for 157 patients with oesophageal squamous cell
carcinoma.
|
| OS | PFS |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Sex
(male/female) | 0.440
(0.250–0.775) | 0.003 | 0.499
(0.287–0.870) | 0.014 |
| Age (≥61/<61
years) | 0.849
(0.580–1.243) | 0.401 | 0.850
(0.581–1.245) | 0.405 |
| Degree of
differentiation (G1/2/3) | 1.226
(0.952–1.579) | 0.115 | 1.250
(0.971–1.609) | 0.083 |
| Tumor status
(T1-2/T3-4) | 0.735
(0.431–1.254) | 0.259 | 0.717
(0.423–1.218) | 0.219 |
| Lymph node status
(N0/N1) | 2.778
(1.440–5.359) | 0.002 | 2.260
(1.180–4.329) | 0.014 |
| Distant metastasis
status (M0/M1) | 1.113
(0.435–2.843) | 0.823 | 1.034
(0.407–5.634) | 0.943 |
| TNM stage
(I–II/III–IV) | 0.800
(0.556–1.151) | 0.228 | 0.937
(0.653–1.345) | 0.725 |
| HIF-1α | 1.745
(1.177–2.588) | 0.006 | 1.629
(1.090–2.435) | 0.017 |
| GLUT1 | 2.341
(1.595–3.435) | 0.001 | 2.114
(1.439–3.105) | 0.001 |
Combined expression levels of HIF-1α
and GLUT1 and the survival of patients with ESCC
The patients were assigned to four groups, according
to whether the HIF-1α and GLUT1 expression levels were low or high.
As shown in Fig. 6, the patients
with combined low expression levels of HIF-1α and GLUT1 had the
longest PFS and OS times compared with those with high expression
of HIF-1α and/or GLUT1. Additionally, the patients with high
expression levels of HIF-1α and GLUT1 had the shortest PFS and OS
times among the four groups. The results presented in Fig. 6 indicate that the combined
expression of HIF-1α and GLUT1 is likely to be a marker for
prognosis in patients with ESCC. The impact of GLUT1 on PFS and OS
may be greater than the effect of HIF-1α. The results also indicate
that HIF-1α and GLUT1 are negatively associated with PFS and OS;
however, GLUT1 was not compared with HIF-1α in this analysis.
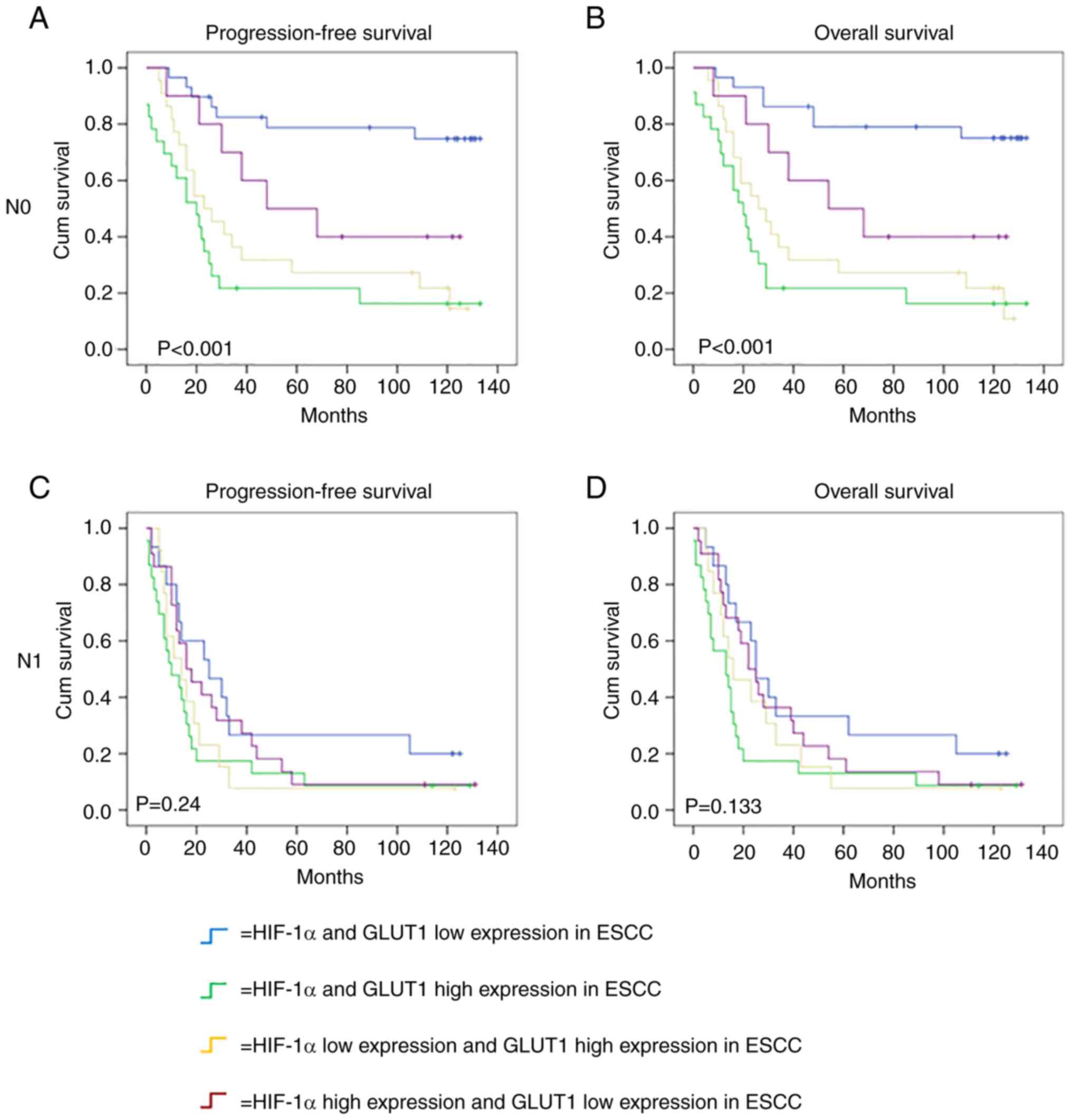
Among the 157 patients with ESCC, there were 84
(53.5) patients without lymph node metastasis and 73 (46.5)
patients with lymph node metastasis. Kaplan-Meier survival analysis
showed that the combined high expression of HIF-1α and GLUT1 was
significantly associated with poor PFS (P<0.001) and OS
(P<0.001) in patients with ESCC without lymph node metastasis
(Fig. 7A and B), but not with
either poor OS (P=0.133) or PFS (P=0.24) in patients with ESCC with
lymph node metastasis (Fig. 7C and
D). The results indicate that the combined expression of HIF-1α
and GLUT1 may be a prognostic marker for patients without lymph
node metastasis, but not those with lymph node metastasis.
Discussion
Locally advanced ESCC may be treated using
radiotherapy; however, ESCC frequently becomes resistant to
radiation (16). The resistance of
tumors to radiotherapy and chemotherapy is associated with hypoxia,
and HIF-1 serves a major role in the regulation of the adaptive
responses of tumors to hypoxic conditions (17). Tumor cells adapt to hypoxia via the
activation of various signaling pathways (18,19),
such as the Wnt/β-catenin signaling pathway (18) and the p-JNK signaling pathway
(20). In addition, HIF-1α
contributes to tumor growth and metastasis. Tumor-associated
vasculature is poorly organized and hyperpermeable compared with
normal blood vessels, which makes effective drug delivery
challenging and creates an abnormal microenvironment that causes
radio- and chemotherapy to be less effective. The upregulation of
HIF-1α and GLUT1 has been shown to be associated with reduced
sensitivity to radiotherapy and chemotherapy in numerous solid
tumors (21,22). Consistently, the in vivo
experiment in the present study demonstrated that the sensitivity
of xenografts to 5-FU generated from hypoxic cells was reduced
compared with those generated from normoxic cells. However,
researchers have demonstrated that anti-angiogenic drugs can
normalize the blood vessels of tumors, causing them to be more
sensitive to chemotherapy and radiotherapy (23).
HIF-1α activates the glucose transporter GLUT1. The
protein expression level of GLUT1 has been reported to be an
important biomarker in a number of different cancers, including
ESCC, breast cancer and gastric cancer (24–26).
Furthermore, a review confirmed that GLUT1 is a valid biomarker in
various types of solid cancers (27); specifically, it firmly established
that the upregulation of GLUT1 is associated with a poor prognosis
in patients with solid tumors. GLUT1 is regulated by numerous
transcription factors, including HIF-1α, which has been shown to
elevate the expression of GLUT1 under a hypoxic atmosphere
(28). In the present study, GLUT1
was only found to be associated with sex among the various
clinicopathological features that were analyzed. The reason may be
that most of the patients were male (125 patients, ~80%), and the
expression of GLUT1 may differ between the sexes. The differential
expression of GLUT1 between males and females has also been
observed in colorectal adenocarcinomas (29).
Previous data also showed that the upregulation of
HIF-1α was closely associated with a poor prognosis and
chemo-radiation effectiveness in patients with ESCC (30). High levels of HIF-1α have previously
been suggested to be a predictive marker of poor prognosis in
patients ESCC and to be significantly associated with invasion and
metastasis (31). In a hypoxic
environment, HIF-1α has been shown to reduce tissue integrity via
the loss of E-cadherin, which is considered as a suppressor of
invasion and metastasis in numerous cancers (32). The cell basement membrane and
extracellular matrix are also undermined by HIF-1α (33). As aforementioned, hypoxia is a
common pathological feature in solid tumors, which results from
insufficient blood supply and rapid tumor growth (34). Under anoxic and hypoxic conditions,
tumor cells produce several different proteins that stimulate cell
invasiveness, promote angiogenesis, and result in chemotherapy or
radiotherapy resistance (35). The
prognostic value of HIF-1α in EC remains unclear. Although a number
of studies have shown that the expression level of HIF-1α in tumor
cells is closely associated with clinical tumor stage (TNM stage)
(32), another study found that
HIF-1α was not a significant independent prognostic factor for PFS
and OS (33). Although HIF-1α may
regulate p53 and VEGF downstream signalling pathway (36,37),
the relationships between these factors remain unclear in patients
with EC.
Since ESCC is a common pathological type of EC, it
is important to identify the clinical significance of HIF-1α and
GLUT1 in patients with ESCC as this may improve upon the current
prognostic system based on TNM staging. Notably, the present study
examined the roles of HIF-1α and GLUT1 in the hypoxic signalling of
ESCC by IHC analysis combined with in vivo and in
vitro experiments. The correlation between HIF-1α and GLUT1 was
confirmed, and both proteins were shown to be associated with the
outcomes of patients with ESCC. In addition, only HIF-1α were found
to be associated with lymph node metastasis. Furthermore, the
results of the multivariate analysis demonstrated that high
expression levels of HIF-1α and GLUT1 are prognostic factors that
indicate poorer OS and PFS in patients with ESCC.
Further studies of HIF-1α and GLUT1 may focus on
their use as targets for therapeutic intervention. In addition,
their use as molecular biomarkers to identify the cancer patients
who would respond best to radiation therapy and chemotherapy merits
further investigation, as it may improve the clinical treatment
outcomes of patients with ESCC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the fund of the National
Natural Science Foundation of China (grant no. 82003268) and by the
Guangdong Province Natural Science Foundation (grant no.
2018A030310260) and by The Science and Technology Plan Project of
Jiangxi Provincial Health Commission (grant no. 20203263).
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
HY and YH performed the main experiments and drafted
the manuscript. QL, RL, JZ and YY collected the data and analyzed
the statistical analysis. XW and LZ conceived and designed the
experiments. HY, YH and LZ confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The animal studies were performed under the guidance
of Sun Yat-Sen University Committee for Use and Care of Laboratory
Animals and approved by the animal experimentation ethics committee
of Sun Yat-Sen University (L201501054). The use of clinical
materials was performed with the written informed consent of all
patients and approved by the Institutional Research Ethics
Committee of Sun Yat-Sen University Cancer Center
(GZR2015-093).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zheng RS, Zhang SW, Sun KX, Chen R, Wang
SM, Li L, Zeng HM, Wei WW and He J: Cancer statistics in China,
2016. Zhonghua Zhong Liu Za Zhi. 45:212–220. 2023.(In Chinese).
PubMed/NCBI
|
|
2
|
Kamangar F, Dores GM and Anderson WF:
Patterns of cancer incidence, mortality, and prevalence across five
continents: Defining priorities to reduce cancer disparities in
different geographic regions of the world. J Clin Oncol.
24:2137–2150. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brahimi-Horn MC, Chiche J and Pouyssegur
J: Hypoxia and cancer. J Mol Med (Berl). 85:1301–1307. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JW, Bae SH, Jeong JW, Kim SH and Kim
KW: Hypoxia-inducible factor (HIF-1)alpha: Its protein stability
and biological functions. Exp Mol Med. 36:1–12. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen C, Pore N, Behrooz A, Ismail-Beigi F
and Maity A: Regulation of glut1 mRNA by hypoxia-inducible
factor-1. Interaction between H-ras and hypoxia. J Biol Chem.
276:9519–9525. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Airley RE and Mobasheri A: Hypoxic
regulation of glucose transport, anaerobic metabolism and
angiogenesis in cancer: Novel pathways and targets for anticancer
therapeutics. Chemotherapy. 53:233–256. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Song K, Li M, Xu XJ, Xuan L, Huang GN,
Song XL and Liu QF: HIF-1α and GLUT1 gene expression is associated
with chemoresistance of acute myeloid leukemia. Asian Pac J Cancer
Prev. 15:1823–1829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coleman CN: Modulating the radiation
response. Stem Cells. 14:10–15. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaira K, Murakami H, Endo M, Ohde Y, Naito
T, Kondo H, Nakajima T, Yamamoto N and Takahashi T: Biological
correlation of 18F-FDG uptake on PET in pulmonary
neuroendocrine tumors. Anticancer Res. 33:4219–4228.
2013.PubMed/NCBI
|
|
10
|
Chen B, Tang H, Liu X, Liu P, Yang L, Xie
X, Ye F, Song C, Xie X and Wei W: miR-22 as a prognostic factor
targets glucose transporter protein type 1 in breast cancer. Cancer
Lett. 356:410–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim BW, Cho H, Chung JY, Conway C, Ylaya
K, Kim JH and Hewitt SM: Prognostic assessment of hypoxia and
metabolic markers in cervical cancer using automated digital image
analysis of immunohistochemistry. J Transl Med. 11:1852013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Osugi J, Yamaura T, Muto S, Okabe N,
Matsumura Y, Hoshino M, Higuchi M, Suzuki H and Gotoh M: Prognostic
impact of the combination of glucose transporter 1 and ATP citrate
lyase in node-negative patients with non-small lung cancer. Lung
Cancer. 88:310–318. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tohma T, Okazumi S, Makino H, Cho A,
Mochizuki R, Shuto K, Kudo H, Matsubara K, Gunji H, Matsubara H and
Ochiai T: Overexpression of glucose transporter 1 in esophageal
squamous cell carcinomas: A marker for poor prognosis. Dis
Esophagus. 18:185–189. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiba I, Ogawa K, Morioka T, Shimoji H,
Sunagawa N, Iraha S, Nishimaki T, Yoshimi N and Murayama S:
Clinical significance of GLUT-1 expression in patients with
esophageal cancer treated with concurrent chemoradiotherapy. Oncol
Lett. 2:21–28. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Waters JK and Reznik SI: Update on
management of squamous cell esophageal cancer. Curr Oncol Rep.
24:375–385. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He S, Xu J, Liu X and Zhen Y: Advances and
challenges in the treatment of esophageal cancer. Acta Pharm Sin B.
11:3379–3392. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ajduković J: HIF-1-a big chapter in the
cancer tale. Exp Oncol. 38:9–12. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang K, Toyozumi T, Murakami K, Sakata H,
Kano M, Endo S, Matsumoto Y, Suito H, Takahashi M, Sekino N, et al:
HIF-1α stimulates the progression of oesophageal squamous cell
carcinoma by activating the Wnt/β-catenin signalling pathway. Br J
Cancer. 127:474–487. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dhani N, Fyles A, Hedley D and Milosevic
M: The clinical significance of hypoxia in human cancers. Semin
Nucl Med. 45:110–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Zhang Z, Zhou S, Liu X, Li G, Song
B and Xu W: Claudin-1/4 as directly target gene of HIF-1α can
feedback regulating HIF-1α by PI3K-AKT-mTOR and impact the
proliferation of esophageal squamous cell though Rho GTPase and
p-JNK pathway. Cancer Gene Ther. 29:665–682. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Moreno-Acosta P, Vallard A, Carrillo S,
Gamboa O, Romero-Rojas A, Molano M, Acosta J, Mayorga D, Rancoule
C, Garcia MA, et al: Biomarkers of resistance to radiation therapy:
A prospective study in cervical carcinoma. Radiat Oncol.
12:1202017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen SW, Shen WC, Lin YC, Chen RY, Hsieh
TC, Yen KY and Kao CH: Correlation of pretreatment
18F-FDG PET tumor textural features with gene expression
in pharyngeal cancer and implications for radiotherapy-based
treatment outcomes. Eur J Nucl Med Mol Imaging. 44:567–580. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Batchelor TT, Sorensen AG, di Tomaso E,
Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M,
et al: AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor,
normalizes tumor vasculature and alleviates edema in glioblastoma
patients. Cancer Cell. 11:83–95. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu M, Yongzhi H, Chen S, Luo X, Lin Y,
Zhou Y, Jin H, Hou B, Deng Y, Tu L and Jian Z: The prognostic value
of GLUT1 in cancers: A systematic review and meta-analysis.
Oncotarget. 8:43356–43367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carvalho KC, Cunha IW, Rocha RM, Ayala FR,
Cajaíba MM, Begnami MD, Vilela RS, Paiva GR, Andrade RG and Soares
FA: GLUT1 expression in malignant tumors and its use as an
immunodiagnostic marker. Clinics (Sao Paulo). 66:965–972. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Brown RS and Wahl RL: Overexpression of
Glut-1 glucose transporter in human breast cancer. An
immunohistochemical study. Cancer. 72:2979–2985. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang J, Ye C, Chen C, Xiong H, Xie B, Zhou
J, Chen Y, Zheng S and Wang L: Glucose transporter GLUT1 expression
and clinical outcome in solid tumors: A systematic review and
meta-analysis. Oncotarget. 8:16875–16886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ping W, Sun W, Zu Y, Chen W and Fu X:
Clinicopathological and prognostic significance of
hypoxia-inducible factor-1α in esophageal squamous cell carcinoma:
A meta-analysis. Tumour Biol. 35:4401–4409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jun YJ, Jang SM, Han HL, Lee KH, Jang KS
and Paik SS: Clinicopathologic significance of GLUT1 expression and
its correlation with Apaf-1 in colorectal adenocarcinomas. World J
Gastroenterol. 17:1866–1873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun G, Hu W, Lu Y and Wang Y: A
meta-analysis of HIF-1α and esophageal squamous cell carcinoma
(ESCC) risk. Pathol Oncol Res. 19:685–693. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Esteban MA, Tran MG, Harten SK, Hill P,
Castellanos MC, Chandra A, Raval R, O'brien TS and Maxwell PH:
Regulation of E-cadherin expression by VHL and hypoxia-inducible
factor. Cancer Res. 66:3567–3575. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fillies T, Werkmeister R, van Diest PJ,
Brandt B, Joos U and Buerger H: HIF1-alpha overexpression indicates
a good prognosis in early stage squamous cell carcinomas of the
oral floor. BMC Cancer. 5:842005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shao N, Han Y, Song L and Song W: Clinical
significance of hypoxia-inducible factor 1α, and its correlation
with p53 and vascular endothelial growth factor expression in
resectable esophageal squamous cell carcinoma. J Cancer Res Ther.
16:269–275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zimna A and Kurpisz M: Hypoxia-inducible
factor-1 in physiological and pathophysiological angiogenesis:
Applications and therapies. Biomed Res Int. 2015:5494122015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Ye SB, Li ZL, Ma G, Chen SP, He
J, Liu WL, Xie D, Zeng YX and Li J: Increased HIF-1alpha expression
in tumor cells and lymphocytes of tumor microenvironments predicts
unfavorable survival in esophageal squamous cell carcinoma
patients. Int J Clin Exp Pathol. 7:3887–3897. 2014.PubMed/NCBI
|
|
36
|
Cheng J, Yang HL, Gu CJ, Liu YK, Shao J,
Zhu R, He YY, Zhu XY and Li MQ: Melatonin restricts the viability
and angiogenesis of vascular endothelial cells by suppressing
HIF-1α/ROS/VEGF. Int J Mol Med. 43:945–955. 2019.PubMed/NCBI
|
|
37
|
Xie L, Wang Y, Li Q, Ji X, Tu Y, Du S, Lou
H, Zeng X, Zhu L, Zhang J and Zhu M: The
HIF-1α/p53/miRNA-34a/Klotho axis in retinal pigment epithelial
cells promotes subretinal fibrosis and exacerbates choroidal
neovascularization. J Cell Mol Med. 25:1700–1711. 2021. View Article : Google Scholar : PubMed/NCBI
|