Introduction
In recent years, the incidence rate of digestive
system tumors has increased in China, which accounts for 38.71% of
the total new cases of tumors. In addition, around the world,
digestive system tumors account for 23.48% of new cases of tumors.
These tumors have recently occurred in a younger population (aged
<49 years) (1,2). The global cancer statistics report,
containing cancer estimates from GLOBOCAN 2020 and population
estimates from the United Nations, demonstrated that the incidence
rate of cancer in China is lower than that in other countries;
however, the fatality rate is higher (3). Of note, 45.03% of cancer-associated
deaths are due to gastrointestinal tumors and the prognosis is
relatively poor in China, which is higher than the global mortality
rate (30.91%) (4). This may be due
to lack of early diagnosis and inconsistent clinical treatment
strategies in different regions of China. Its treatment strategies
mainly include surgery, radiation therapy, chemotherapy and
endoscopic treatment (5). Digestive
system tumors exhibit no notable adverse symptoms in the early
stages, making early diagnosis difficult. Thus, the majority of
these tumors are diagnosed at intermediate or late stages, at which
point symptoms, such as sudden weight loss, alternating occurrence
of constipation and diarrhea and haematemesis, are aggravated with
increased risk of metastasis and recurrence (6). Therefore, earlier diagnosis of
digestive system tumors is required. Compared with gastroscopy,
enteroscopy, barium meal imaging and other examination techniques,
detection of serum tumor markers (alpha-fetoprotein,
carcinoembryonic antigen, etc.) using a serum biochemical analyzer
exhibits numerous advantages. Detection of serum tumor markers is
simple, fast, non-invasive and accurate (1). Serum tumor markers may be used to
assess occurrence, progression and treatment of tumors and they may
exhibit different levels of specificity in different tumors. Serum
tumor markers may detect and differentiate between different types
of gastrointestinal cancer and improve the positive detection rate
of tumors (7–9). Therefore, further investigation into
effective early diagnostic markers is required for the treatment of
digestive system tumors.
Secreted phosphoprotein 1 (SPP1), also known as
osteopontin, is an integrin-binding protein secreted by various
types of cell, such as macrophages, endothelial cells and
osteoclasts (10). In humans, SPP1
consists of six introns and seven exons, and it is encoded on
chromosome 4q13 (11). SPP1
involves multiple physiological and pathological processes, such as
tumor growth, adhesion and invasion (12–15).
Of note, SPP1 expression is increased in lung (12), colon (13), breast (14), prostate (15) and pancreatic cancer (16) and hepatocellular carcinoma (17). The expression levels of SPP1 are
associated with the stage and degree of malignancy of tumors,
highlighting that SPP1 may serve as a biomarker for the diagnosis
and prognosis of numerous cancers. However, the potential of SPP1
as a marker of digestive system tumors is yet to be fully
elucidated.
Thus, the present study aimed to further explore the
expression and potential clinical values of SPP1 in the plasma,
serum and tissues of patients with colorectal cancer (CRC), gastric
cancer (GC) and esophageal cancer (EC).
Patients and methods
Bioinformatics analysis
The microarray datasets GSE104836 (18), GSE189830 (19) and GSE103236 (20) were downloaded from the Gene
Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). The online tool
GEO2R (version 2.40.0; http://www.ncbi.nlm.nih.gov/geo/geo2r/) was used to
analyze differentially expressed genes (DEGs) in patients with CRC,
GC or EC based on an adjusted P-value<0.01 and |log2 fold
change|>2. DEGs were displayed as a heatmap and volcano map
using R software (version 3.6.1; MathSoft). Kyoto Encyclopedia of
Genes and Genomes (KEGG) pathway enrichment analysis was performed
using KOBAS (version 3.0; kobas.cbi.pku.edu.cn/). The Gene
Expression Profiling Interactive Analysis (GEPIA) online database
(http://gepia.cancer-pku.cn/) was used to
analyzed the expressions of SPP1 in colon, rectum and stomach
adenocarcinoma and EC patients. STRING online database (https://string-db.org/) was used to analyze the
protein-protein-interaction (PPI) networks of upregulated genes in
CRC, GC and EC.
Clinical resources
A total of 42 patients with pathologically confirmed
CRC, GC or EC who were admitted to Hubei No. 3 People's Hospital of
Jianghan University (Wuhan, China) were recruited between January
2022 and January 2023. CRC and GC were confirmed to be
adenocarcinoma and EC was confirmed to be squamous cell carcinoma.
The clinicopathological characteristics of the patients are
presented in Table I. A total of 21
healthy volunteers were included as the control group from January
2022 to January 2023 for CRC, GC or EC, respectively. Tumor tissue
was collected from patients in the tumor groups and serum and
plasma samples were collected from both the control and tumor
groups. Supernatant was collected following low-speed
centrifugation (1,370 × g, 5 min) at 4°C and all samples were
stored at −80°C for future use. The present study was approved by
the Ethics Committee of Hubei No. 3 People's Hospital of Jianghan
University (Wuhan, China; approval no. 013). Written informed
consent was obtained from all participants prior to inclusion in
the study.
 | Table I.Clinicopathological characteristics of
healthy volunteers (n=21) and patients with CRC (n=42), GC (n=42)
or EC (n=42). |
Table I.
Clinicopathological characteristics of
healthy volunteers (n=21) and patients with CRC (n=42), GC (n=42)
or EC (n=42).
|
| CRC | GC | EC |
|---|
|
|
|
|
|
|---|
| Parameter | Healthy | Cancer | Healthy | Cancer | Healthy | Cancer |
|---|
| Age, years |
|
|
|
|
|
|
|
<65 | 8 (38.1) | 11 (26.2) | 10 (47.6) | 14 (33.3) | 9 (42.9) | 15 (35.7) |
| ≥65 | 13 (61.9) | 31 (73.8) | 11 (52.4) | 28 (66.7) | 12 (57.1) | 27 (64.3) |
| Sex |
|
|
|
|
|
|
|
Male | 12 (57.1) | 26 (61.9) | 14 (66.7) | 29 (69.0) | 15 (71.4) | 25 (59.5) |
|
Female | 9 (42.9) | 16 (38.1) | 7 (33.3) | 13 (31.0) | 6 (28.6) | 17 (40.5) |
| Smoking status |
|
|
|
|
|
|
|
Never | 16 (76.2) | 32 (76.2) | 13 (61.9) | 21 (50.0) | 14 (66.7) | 25 (59.5) |
|
Ever | 3 (14.3) | 3 (7.1) | 5 (23.8) | 7 (16.7) | 4 (19.0) | 11 (26.2) |
|
Current | 2 (9.5) | 7 (16.7) | 3 (14.3) | 14 (33.3) | 3 (14.3) | 6 (14.3) |
| Alcohol drinking
status |
|
|
|
|
|
|
|
Never | 16 (76.2) | 36 (85.7) | 15 (71.4) | 13 (30.9) | 11 (52.4) | 21 (50.0) |
|
Ever | 3 (14.3) | 2 (4.8) | 3 (14.3) | 18 (42.9) | 3 (14.3) | 6 (14.3) |
|
Current | 2 (9.5) | 4 (9.5) | 3 (14.3) | 11 (26.2) | 7 (33.3) | 15 (35.7) |
| Tumor
differentiation |
|
|
|
|
|
|
|
Well | - | 3 (7.1) | - | 5 (11.9) | - | 8 (19.0) |
|
Moderate | - | 28 (66.7) | - | 24 (57.1) | - | 22 (52.4) |
|
Poor | - | 11 (26.2) | - | 13 (31.0) | - | 12 (28.6) |
| AJCC stage |
|
|
|
|
|
|
| I | - | 13 (31.0) | - | 16 (38.1) | - | 12 (28.6) |
| II | - | 15 (35.7) | - | 11 (26.2) | - | 18 (42.9) |
|
III | - | 9 (21.4) | - | 9 (21.4) | - | 7 (16.6) |
| IV | - | 5 (11.9) | - | 6 (14.3) | - | 5 (11.9) |
Inclusion criteria were as follows: i) Malignant
tumor located in the digestive tract of patients in the tumor group
confirmed via pathological examination; ii) no digestive tract
disease diagnosed in patients in the control group and iii) all
patients volunteered to participate.
Exclusion criteria were as follows: i) Patients with
tumors located in other parts of the body or gastrointestinal
cancer patients with metastasis to other parts of the body; ii)
patients with serious acute or chronic infections or autoimmune
disease; iii) patients who use antibiotics, proton pump inhibitors,
anticoagulants or other drugs that may affect the examination
results and iv) patients who received radiotherapy and chemotherapy
in the past 6 months.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was used to determine mRNA expression levels
of SPP1 and carcinoembryonic antigen (CEA). Total RNA was isolated
from tissues and serum of control subjects and cancer patients
using TRIzol® reagent (Beyotime Institute of
Biotechnology) and cDNA was synthesized using a RT kit (Takara Bio,
Inc.). Reaction conditions were as follows: 37°C for 15 min, 85°C
for 5 sec. qPCR was performed using a Power SYBR™ Green
RNA-to-CT™ 1-Step kit (Takara Bio, Inc.).
Reaction conditions were as follows: 94°C for 30 sec, 45 cycles of
(95°C for 5 sec, 60°C for 30 sec, 72°C for 30 sec). Expression
levels were normalized to GAPDH and calculated using the
2−ΔΔCq method (21). The
primer sequences (5′-3′) were as follows: SPP1 forward,
CTCCATTGACTCGAACGACTC and reverse, CAGGTCTGCGAAACTTCTTAGAT; CEA
forward, ATTCAAGCAAATATCCCAGGGG and reverse,
GGCATTTATGGTTCGTAGGGTG; GAPDH forward TGTGGGCATCAATGGATTTGG and
reverse, ACACCATGTATTCCGGGTCAAT.
Statistical analysis
Results were analyzed using SPSS software (version
22.0; IBM Corp.) and GraphPad Prism (version 5.01; Dotmatics). Data
are presented as the mean ± standard deviation. All experiments
were independently repeated three times. Differences between two
groups were analyzed using an unpaired Student's t-test.
Differences between >2 groups were assessed by one-way ANOVA
followed by Tukey's post-hoc test. Clinicopathological
characteristics were compared by χ2 test. Moreover,
correlation was determined using Pearson's correlation coefficient.
Receiver operating curve (ROC) was used to analyze clinical
significance of SPP1 with area under the curve (AUC) as the
evaluation index. Z-score in R software: (expressions
levels-average value)/standard deviation. P<0.05 was considered
to indicate a statistically significant difference.
Results
DEGs determined in CRC, GC and EC
Using GSE104836, GSE189830 and GSE103236 datasets, a
total of 24 DEGs were determined in patients with CRC, GC or EC.
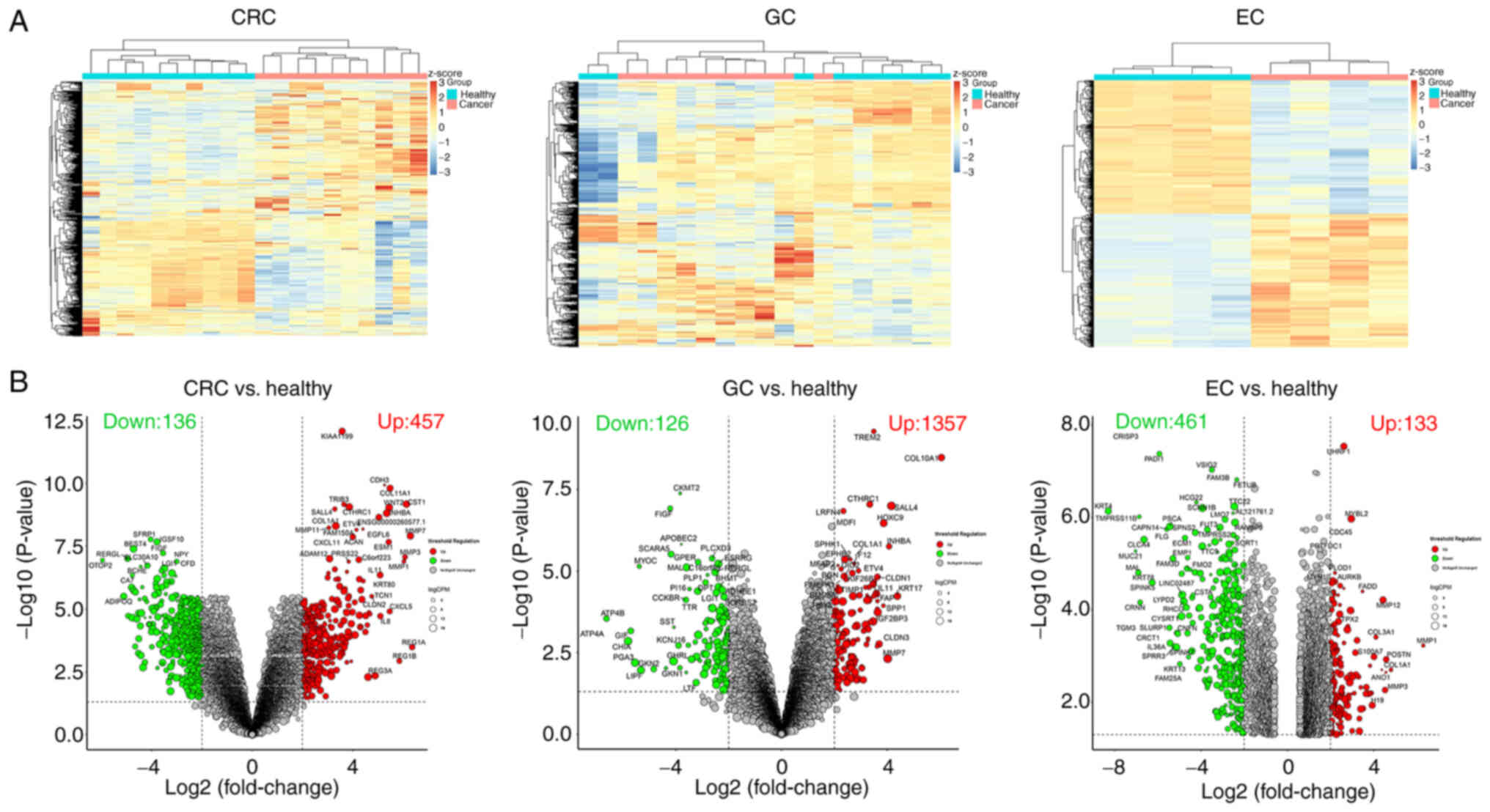
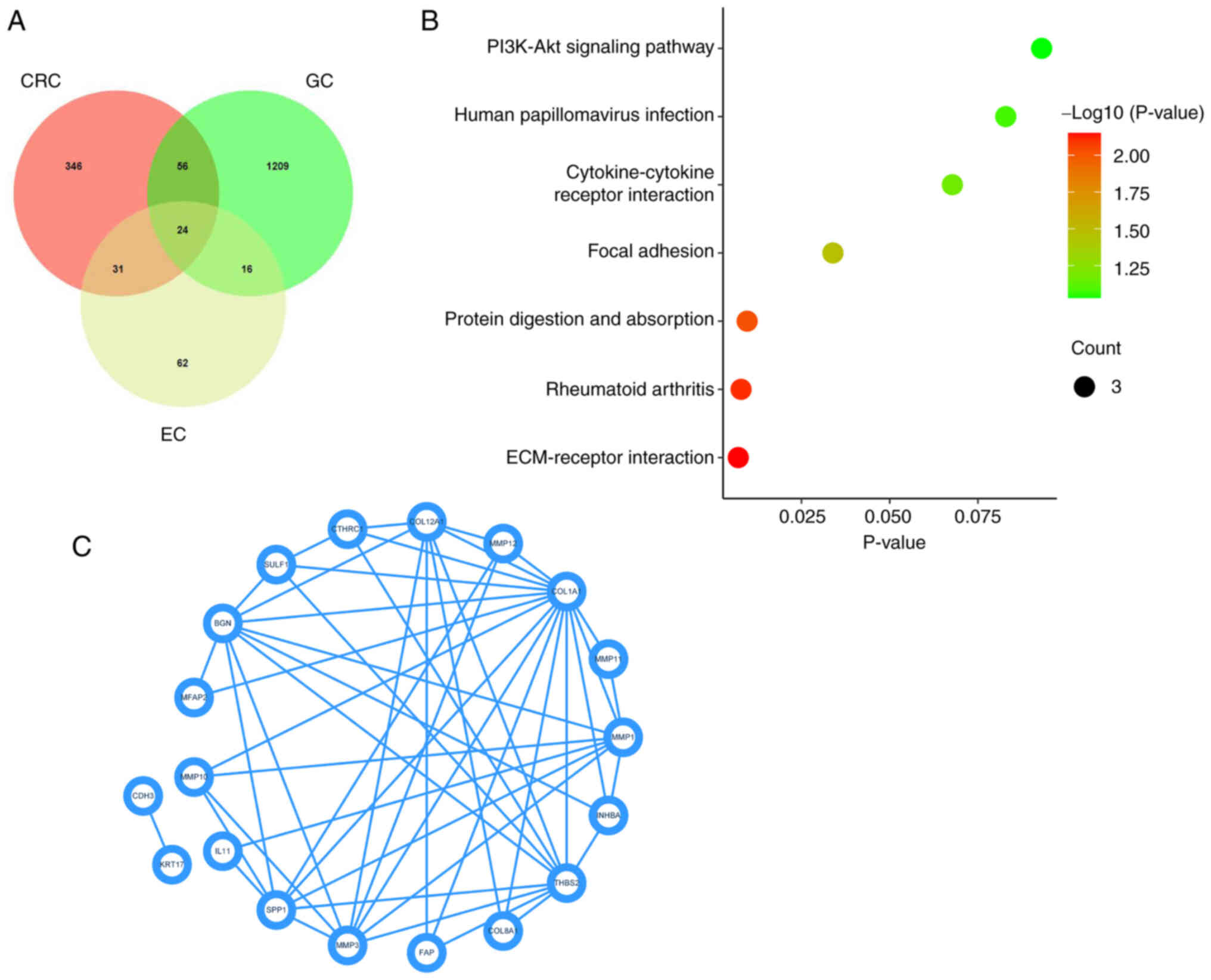
DEGs in CRC, GC and EC were displayed as a heat map (Fig. 1A) and volcano plot (Fig. 1B). Upregulated genes in CRC (n=346),
GC (n=1209) and EC (n=62) were selected through the GSE104836,
GSE189830 and GSE103236 datasets and displayed using a Venn
diagram. A total of 24 genes were highly expressed in CRC, GC and
EC (Fig. 2A). KEGG pathway
enrichment analysis demonstrated that 24 genes were enriched in
‘ECM-receptor interaction’, ‘rheumatoid arthritis’, ‘protein
digestion and absorption’, ‘focal adhesion’, ‘cytokine-cytokine
receptor interaction’, ‘human papillomavirus infection’ and
‘PI3K-Akt signaling pathway’ (Fig.
2B). The Protein-protein interaction of 24 DEGs is displayed in
Fig. 2C. SPP1 was enriched in
‘ECM-receptor interaction’, ‘focal adhesion’, ‘cytokine-cytokine
receptor interaction’ and the ‘PI3K-Akt signaling pathway’. All
these pathways were closely related to the cancer development.
Hence, SPP1 was selected for use in subsequent experiments.
SPP1 is upregulated in patients with
CRC, GC or EC
Subsequently, patients and healthy controls were
recruited for the next experiments. The clinicopathological
characteristics, including age, sex, smoking status, alcohol
consumption status, tumor differentiation and American Joint
Committee on Cancer stage (22)
were recorded. In the CRC group, 31 patients were aged >65 years
(mean age, 68.00 years); in the GC group, 28 patients were aged
>65 years (mean age, 66.40 years); and in the EC group, 27 were
aged >65 years (mean age, 65.57 years). In addition, there were
26 males and 16 females in the CRC group, 29 males and 13 females
in the GC group, and 25 males and 17 females in the EC group.
Generally speaking, patients with gastrointestinal cancer were
older and had a higher proportion of males. In addition, there was
no significant difference in sex and age between the healthy group
and the cancer group (Table I).
Using the GEPIA online database, the present study demonstrated
that SPP1 was significantly upregulated in colon, rectum and
stomach adenocarcinoma and EC (Fig.
3A). In addition, SPP1 expression levels were determined in
patients with CRC, GC and EC. SPP1 was significantly upregulated in
the plasma (Fig. 3B), serum
(Fig. 3C) and tissue (Fig. 3D) of patients with CRC, GC and EC at
stages I, II, III and IV.
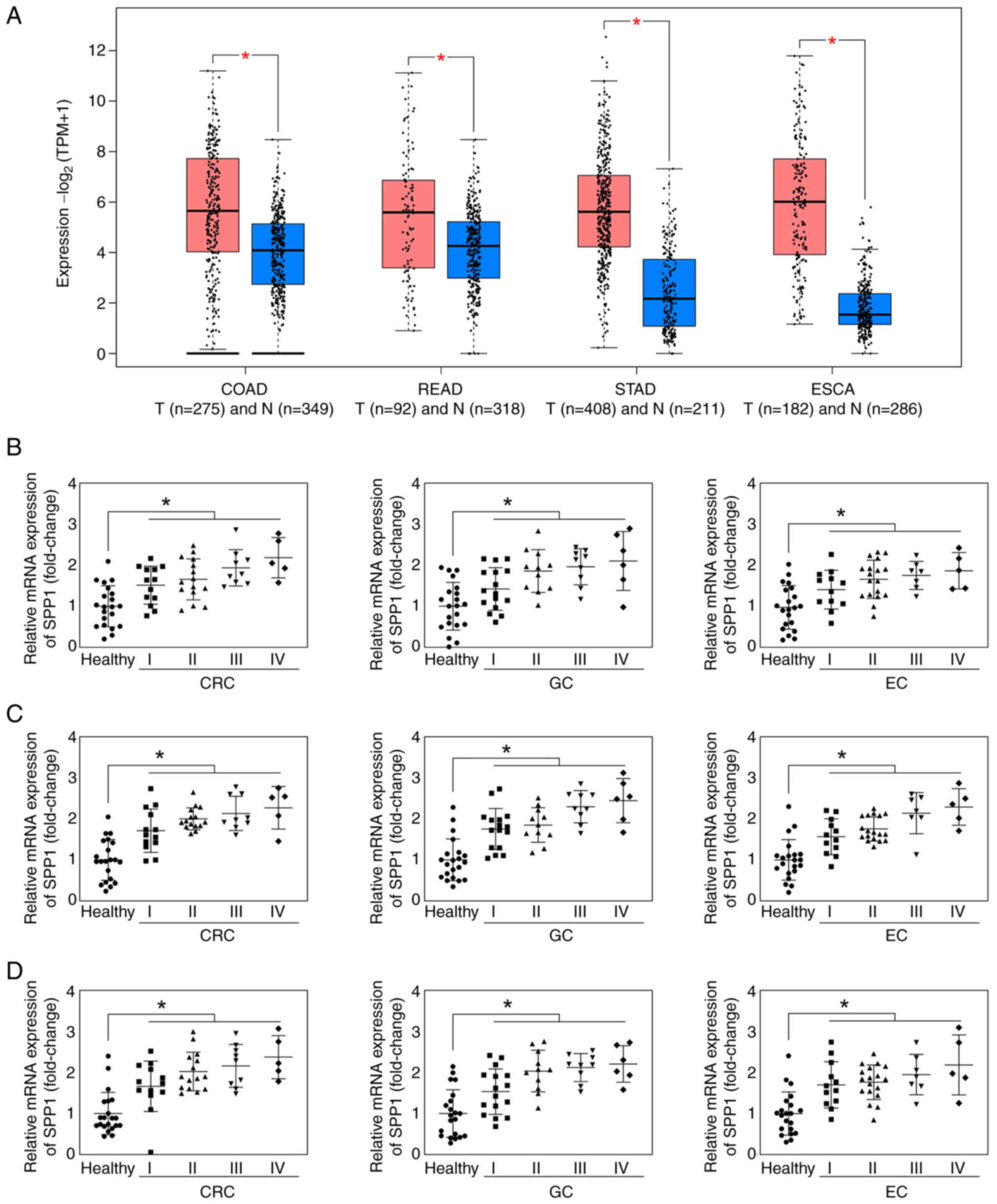 | Figure 3.SPP1 is upregulated in patients with
CRC, GC and EC. (A) SPP1 expression levels in COAD, READ, STAD and
ESCA from Gene Expression Profiling Interactive Analysis online
database. Red colour represents the tumor group; blue colour
represents the normal group. SPP1 expression levels in (B) plasma,
(C) serum and (D) tissue of patients with CRC, GC and EC were
detected using reverse transcription-quantitative PCR. *P<0.05.
T, tumor; N, normal; SPP1, secreted phosphoprotein 1; CRC,
colorectal cancer; GC, gastric cancer; EC, esophageal cancer; COAD,
colon adenocarcinoma; READ, rectum adenocarcinoma; STAD, stomach
adenocarcinoma; ESCA, esophageal carcinoma; TPM, transcripts per
kilobase million. |
SPP1 expression levels in plasma of
patients with CRC, GC and EC exhibit diagnostic value
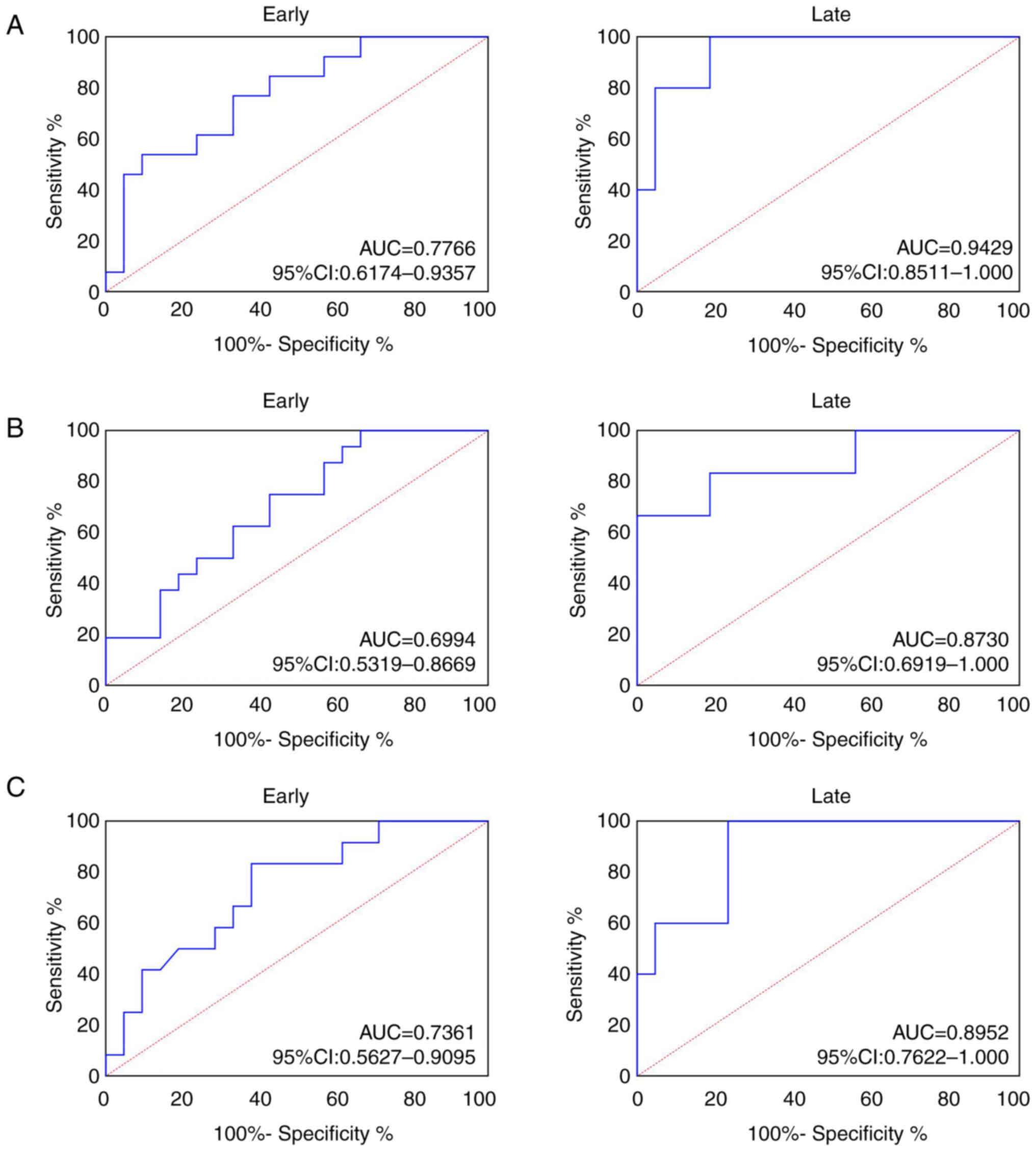
ROC analysis was performed to explore the role of
SPP1 in patients with CRC, GC and EC. The AUC of plasma SPP1 was
0.7766 (95% confidence interval, 0.6174–0.9351) in early and 0.9429
(95% confidence interval, 0.8511–1.0000; Fig. 4A) in late CRC. AUC of plasma SPP1 in
patients with early GC was 0.6994 (95% confidence interval,
0.5319–0.8669) and 0.8730 (95% confidence interval, 0.6919–1.000;
Fig. 4B) in patients with late GC.
AUC of plasma SPP1 in patients with early EC was 0.7361 (95%
confidence interval, 0.5627–0.9095) and 0.8952 (95% confidence
interval, 0.7622–1.000; Fig. 4C) in
patients with late EC. These results revealed that SPP1 exhibited
notable diagnostic significance for CRC, GC and EC.
SPP1 expression levels in serum of
patients with CRC, GC and EC exhibit diagnostic value
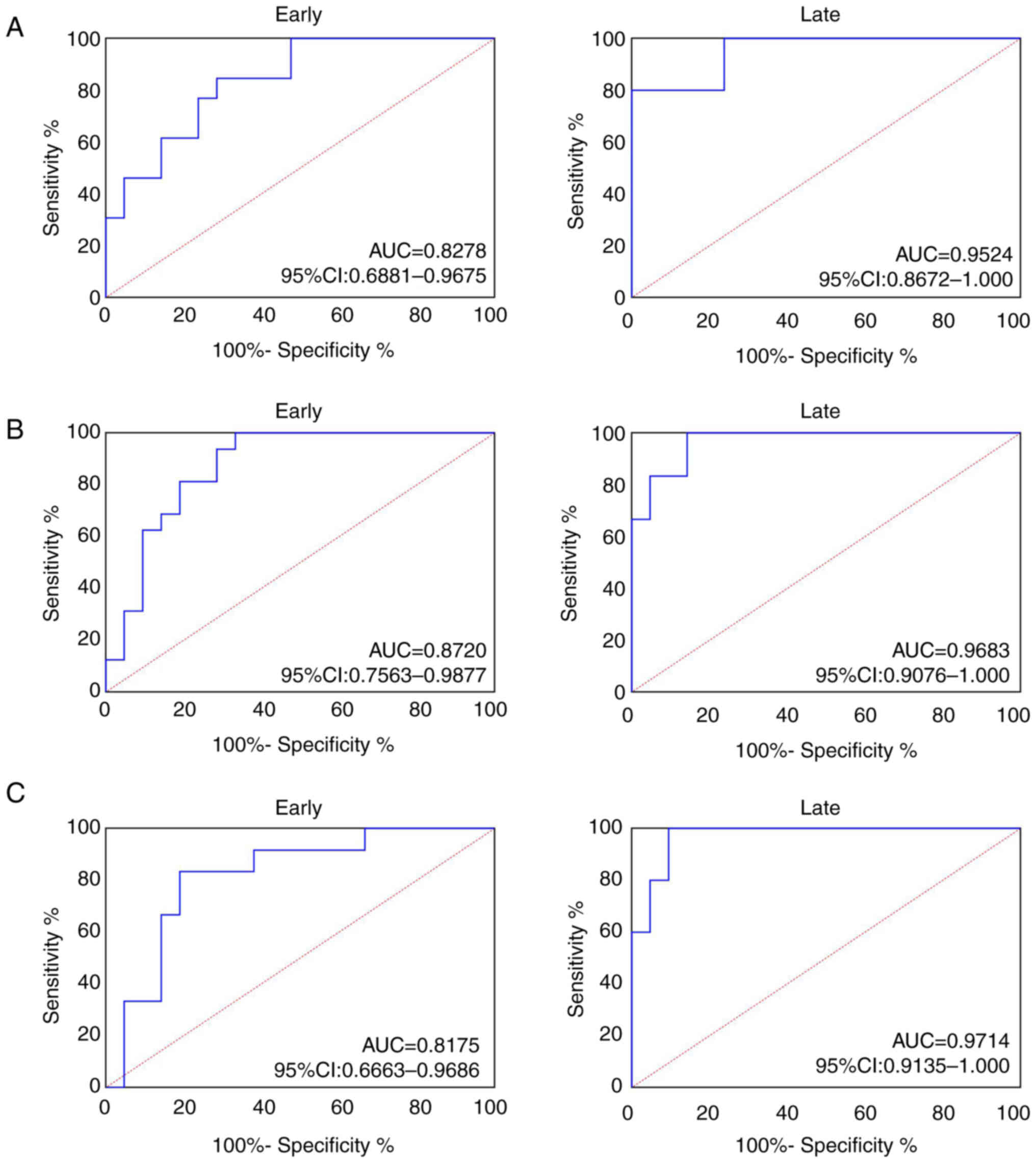
AUC of serum SPP1 in patients with early CRC was
0.8278 (95% confidence interval, 0.6881–0.9675) and 0.9524 (95%
confidence interval, 0.8672–1.000; Fig.
5A) in patients with late CRC. AUC of serum SPP1 in patients
with early GC was 0.8720 (95% confidence interval, 0.7563–0.9877)
and 0.9683 (95% confidence interval, 0.9076–1.000; Fig. 5B) in patients with late GC. AUC of
serum SPP1 in patients with early EC was 0.8175 (95% confidence
interval, 0.6663–0.9686) and 0.9714 (95% confidence interval,
0.9135–1.0000; Fig. 5C) in patients
with late EC.
SPP1 expression levels in cancer
tissue of patients with CRC, GC and EC exhibit diagnostic
value
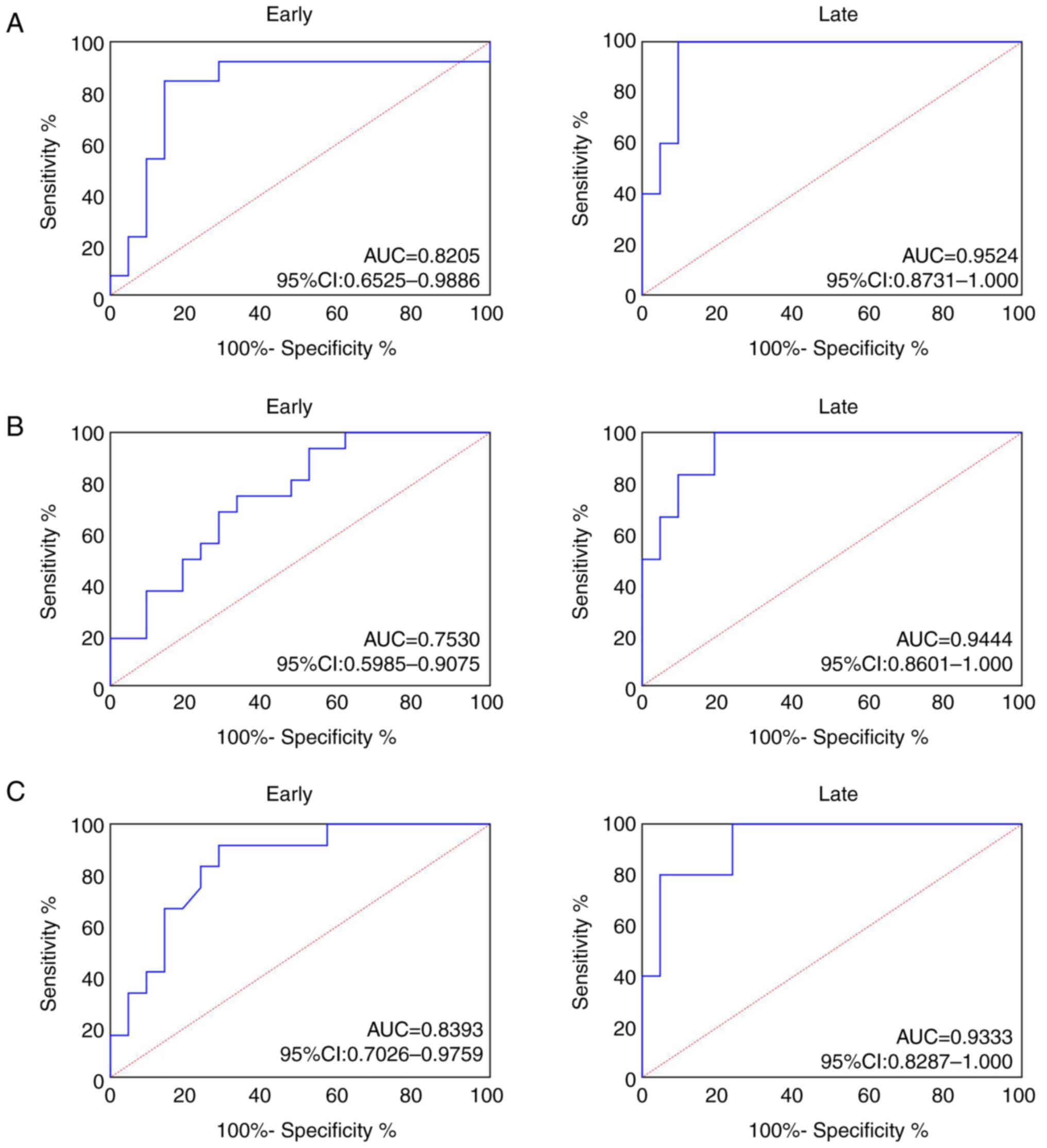
AUC of tissue SPP1 was 0.8205 in patients with early
CRC (95% confidence interval, 0.6525–0.9886) and 0.9524 (95%
confidence interval, 0.8731–1.000; Fig.
6A) in patients with late CRC. AUC of tissue SPP1 in patients
with early GC was 0.7530 (95% confidence interval, 0.5985–0.9075)
and 0.9444 (95% confidence interval, 0.8601–1.000; Fig. 6B) in patients with late GC. AUC of
tissue SPP1 in patients with early EC was 0.8393 (95% confidence
interval, 0.7026–0.9759) and 0.9333 (95% confidence interval,
0.8287–1.000; Fig. 6C) in patients
with late EC.
SPP1 and CEA are positively correlated
in patients with CRC, GC and EC
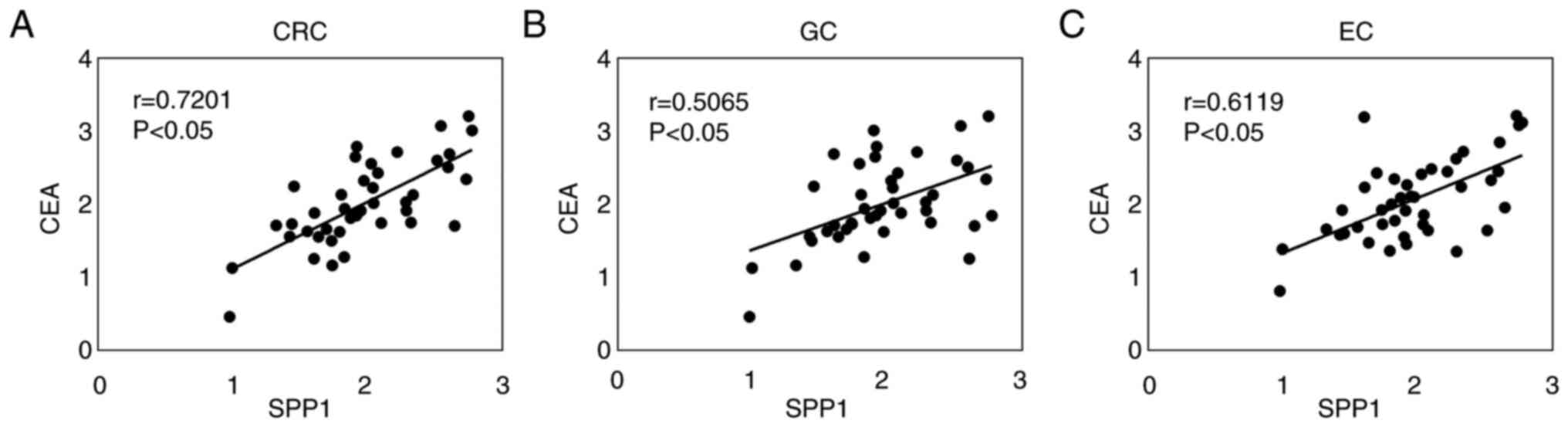
Pearson's correlation coefficient revealed that SPP1
and CEA were positively correlated in the serum of patients with
CRC (r=0.7201; Fig. 7A), GC
(r=0.5065; Fig. 7B) and EC
(r=0.6119; Fig. 7C). These results
indicated that SPP1 may be a potential diagnostic marker for CRC,
GC and EC.
Discussion
Malignant tumors pose a serious threat to human
health and tumors with no specific symptoms in early or
intermediate stages of disease may metastasize, leading to missed
diagnosis (23,24). Patients with digestive tract
malignant tumors may also exhibit dysphagia, abdominal pain,
gastrointestinal discomfort and other symptoms, and the clinical
manifestations differ between different types of digestive tract
malignant tumor. Therefore, early diagnosis of digestive tract
malignant tumors and early symptomatic treatment may improve
prognosis and 5-year survival rate (25,26).
The present study demonstrated that SPP1 expression was upregulated
in patients with CRC, GC and EC. ROC analysis revealed that SPP1
exhibited notable diagnostic significance for CRC, GC and EC, which
highlighted the potential of SPP1 as a molecular biomarker.
To the best of our knowledge, research into the
specific mechanisms and roles of SPP1 in cancer development and
metastasis is lacking. Thus, the present study aimed to analyze the
prognostic and diagnostic value and expression levels of SPP1 in
CRC, GC and EC. To the best of our knowledge, the specific role of
SPP1 as a tumor promoter or suppressor is yet to be fully
elucidated and this cannot be determined using only expression
levels. However, extensive carcinogenic databases may provide
understanding of the molecular mechanisms underlying SPP1. Results
of previous studies demonstrated that SPP1 is upregulated in
breast, prostate, colon, head and neck, liver, lung and esophageal
cancer (12,27–30).
Notably, high SPP1 expression levels are associated with poor
prognosis of the aforementioned cancers. Previous studies
demonstrated that SPP1 may enhance survival, angiogenesis and
inflammatory response of cancer cells and promote
epithelial-to-mesenchymal transition (31–33).
The aforementioned studies indicated that SPP1 may promote tumor
progression. Therefore, further investigations into the role of
SPP1 in digestive tract malignant tumors are required.
KEGG pathway enrichment analysis indicated that SPP1
was enriched in ‘tumor development-related pathway’. Subsequently,
through detecting SPP1 expression levels in cancer tissue, serum
and plasma of patients with CRC, GC and EC, the present study
revealed that SPP1 was significantly upregulated in different
stages of disease. In stages III and IV, the upregulation of SPP1
was more significant than in stages I and II. In addition, ROC
analysis demonstrated that SPP1 expression levels in cancer tissue,
serum and plasma were of diagnostic value for early and advanced
CRC, GC and EC, which was indicated by a high AUC for SPP1 in
cancer tissue, serum and plasma. However, the present study had
certain limitations. For instance, only the expression levels of
SPP1 were detected in patients with CRC, GC and EC, and the sample
size was small. Further in vitro and in vivo
experiments are required to determine the specific mechanisms of
SPP1 in digestive tract malignant tumors.
In conclusion, the present study demonstrated that
SPP1 expression was upregulated in CRC, GC and EC. Thus, SPP1 may
exhibit potential as a novel serological marker for the auxiliary
diagnosis of digestive tract malignant tumors. SPP1 expression may
also exhibit potential in early diagnosis of digestive tract
malignant tumors and provide a reference for tumor staging and
treatment strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LJ, SL and NJ made substantial contributions to
conception and design of the study and acquisition of data. All
authors read and approved the final manuscript. LJ, SL and NJ
confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study protocol was approved by the
Ethics Committee of Hubei No.3 People's Hospital of Jianghan
University (Wuhan, China; approval no. 013). Written informed
consent was obtained from all participants prior to inclusion in
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ma ZY, Gong YF, Zhuang HK, Zhou ZX, Huang
SZ, Zou YP, Huang BW, Sun ZH, Zhang CZ, Tang YQ, et al: Pancreatic
neuroendocrine tumors: A review of serum biomarkers, staging and
management. World J Gastroenterol. 26:2305–2322. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Y, Sun J, Song Y, Gao P, Wang X,
Chen M, Li Y and Wu Z: Roles of fusion genes in digestive system
cancers: Dawn for cancer precision therapy. Crit Rev Oncol Hematol.
171:1036222022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xia C, Dong X, Li H, Cao M, Sun D, He S,
Yang F, Yan X, Zhang S, Li N and Chen W: Cancer statistics in China
and United States, 2022: Profiles, trends, and determinants. Chin
Med J (Engl). 135:584–590. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Feng RM, Zong YN, Cao SM and Xu RH:
Current cancer situation in China: good or bad news from the 2018
global cancer statistics? Cancer Commun (Lond). 39:222019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang H, An J, He S, Liao C, Wang J and Tuo
B: Chloride intracellular channels as novel biomarkers for
digestive system tumors (review). Mol Med Rep. 24:6302021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li HS, He T and Yang LL: Adenosquamous
carcinoma of the digestive system: A literature review. Scand J
Gastroenterol. 55:1268–1276. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Xia D, Wang RX, Zhang YT, Zhang
SY, Yang C, Pan XR and Tong JH: Identification of potential
biomarkers for digestive system cancers from serum-derived
extracellular vesicle RNA. Clin Chim Acta. 531:36–47. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wen R, Lin P, Wu Y, Yin H, Huang W, Guo D,
Peng Y, Liu D, He Y and Yang H: Diagnostic value of CEUS LI-RADS
and serum tumor markers for combined
hepatocellular-cholangiocarcinoma. Eur J Radiol. 154:1104152022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang Z, Miao L, Wang S, Zhao Y, Xie Y,
Yun H, Ren Z, Wang G, Teng M and Li Y: Study on the expression of
c-Met in gastric cancer and its correlation with preoperative serum
tumor markers and prognosis. World J Surg Oncol. 20:1–204.
2022.
|
|
10
|
Wu G, Li X, Seo H, Mclendon BA, Kramer AC,
Bazer FW and Johnson GA: Osteopontin (OPN)/secreted phosphoprotein
1 (SPP1) binds integrins to activate transport of ions across the
porcine placenta. Front Biosci (Landmark Ed). 27:1172022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li S, Yang R, Sun X, Miao S, Lu T, Wang Y,
Wo Y and Jiao W: Identification of SPP1 as a promising biomarker to
predict clinical outcome of lung adenocarcinoma individuals. Gene.
679:398–404. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Du W, Chen Z and Xiang C:
Upregulation of PD-L1 by SPP1 mediates macrophage polarization and
facilitates immune escape in lung adenocarcinoma. Exp Cell Res.
359:449–457. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wei R, Wong J, Lyu P, Xi X, Tong O, Zhang
SD, Yuen HF, Shirasawa S and Kwok HF: In vitro and clinical data
analysis of Osteopontin as a prognostic indicator in colorectal
cancer. J Cell Mol Med. 22:4097–4105. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Changchien CY, Chang HH, Dai MS, Tsai WC,
Tsai HC, Wang CY, Shen MS, Cheng LT, Lee HS, Chen Y, et al:
Distinct JNK/VEGFR signaling on angiogenesis of breast
cancer-associated pleural fluid based on hormone receptor status.
Cancer Sci. 112:781–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pang X, Xie R, Zhang Z, Liu Q, Wu S and
Cui Y: Identification of SPP1 as an extracellular matrix signature
for metastatic castration-resistant prostate cancer. Front Oncol.
9:9242019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang L, Holtzinger A, Jagan I, Begora M,
Lohse I, Ngai N, Nostro C, Wang R, Muthuswamy LB, Crawford HC, et
al: Ductal pancreatic cancer modeling and drug screening using
human pluripotent stem cell- and patient-derived tumor organoids.
Nat Med. 21:1364–1371. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lao L, Shen J, Tian H, Zhong G, Murray SS
and Wang JC: Secreted phosphoprotein 24kD (Spp24) inhibits growth
of hepatocellular carcinoma in vivo. Environ Toxicol Pharmacol.
51:51–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Leng X, Yang J, Liu T, Zhao C, Cao Z, Li
C, Sun J and Zheng S: A bioinformatics framework to identify the
biomarkers and potential drugs for the treatment of colorectal
cancer. Front Genet. 13:10175392022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ren L, Fang X, Shrestha SM, Ji Q, Ye H,
Liang Y, Liu Y, Feng Y, Dong J and Shi R: LncRNA SNHG16 promotes
development of oesophageal squamous cell carcinoma by interacting
with EIF4A3 and modulating RhoU mRNA stability. Cell Mol Biol Lett.
27:892022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang C and Gong A: Integrated
bioinformatics analysis for differentially expressed genes and
signaling pathways identification in gastric cancer. Int J Med Sci.
18:792–800. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Li H, Zhou L, Yu L, Che F and
Heng X: Modified nodal stage of esophageal cancer based on the
evaluation of the hazard rate of the negative and positive lymph
node. BMC Cancer. 20:12002020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsuda T, Ohashi M, Tsujiura M, Ida S,
Kumagai K, Nunobe S, Sano T and Hiki N: Shorter survival of
patients with upper-third gastric cancer preoperatively diagnosed
as stage IA compared with those with middle to lower lesions. Ann
Surg Oncol. 27:276–283. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang Y and Liu Z: Oncolytic virotherapy
for malignant tumor: Current clinical status. Curr Pharm Des.
25:4251–4263. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jeong O, Jung MR, Kang JH and Ryu SY:
Prognostic performance of preoperative staging: Assessed by using
multidetector computed tomography-between the new clinical
classification and the pathological classification in the eighth
American joint committee on cancer classification for gastric
carcinoma. Ann Surg Oncol. 27:545–551. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pobłocki J, Jasińska A, Syrenicz A,
Andrysiak-Mamos E and Szczuko M: The neuroendocrine neoplasms of
the digestive tract: Diagnosis, treatment and nutrition. Nutrients.
12:14372020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gothlin EA, Lagergren K, Othman L,
Montgomery S, Andersson G and Tina E: Evaluation of
SPP1/osteopontin expression as predictor of recurrence in tamoxifen
treated breast cancer. Sci Rep. 10:14512020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bie T and Zhang X: Higher expression of
SPP1 predicts poorer survival outcomes in head and neck cancer. J
Immunol Res. 2021:85695752021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang M, Sun X, Xin H, Wen Z and Cheng Y:
SPP1 promotes radiation resistance through JAK2/STAT3 pathway in
esophageal carcinoma. Cancer Med. 11:4526–4543. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang H, Chen J, Han X, Feng Y and Wang F:
Upregulation of SPP1 is a marker for poor lung cancer prognosis and
contributes to cancer progression and cisplatin resistance. Front
Cell Dev Biol. 9:6463902021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi J, Sun H, Zhang Y, Wang Z, Xun Z, Li Z,
Ding X, Bao R, Hong L, Jia W, et al: Single-cell and spatial
analysis reveal interaction of FAP+ fibroblasts and
SPP1+ macrophages in colorectal cancer. Nat Commun.
13:17422022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu C, Sun L, Jiang C, Zhou H, Gu L, Liu Y
and Xu Q: SPP1, analyzed by bioinformatics methods, promotes the
metastasis in colorectal cancer by activating EMT pathway. Biomed
Pharmacother. 91:1167–1177. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pang X, Zhang J, He X, Gu Y, Qian BZ, Xie
R, Yu W, Zhang X, Li T, Shi X, et al: SPP1 promotes enzalutamide
resistance and epithelial-mesenchymal-transition activation in
castration-resistant prostate cancer via PI3K/AKT and ERK1/2
pathways. Oxid Med Cell Longev. 2021:58066022021. View Article : Google Scholar : PubMed/NCBI
|