Introduction
Notable advancements that have been encountered in
the field of cancer treatment over the preceding decades include
the development of next-generation sequencing (NGS) technologies
and the clinical application of various molecularly-targeting
therapeutics. NGS has enabled the high-speed and low-cost
sequencing of whole genomes in individual patients, which opened
the era of genome-based precision medicine (1). In addition, the development of
numerous molecular targeting agents, such as anti-VEGF antibodies,
multikinase inhibitors, poly (ADP-ribose) polymerase (PARP)
inhibitors and immune checkpoint blockers has improved the efficacy
of systemic cancer therapy by combining with conventional
chemotherapeutics and with different types of targeting agents
(2). This improvement in efficacy
has even been observed for patients with advanced or metastatic
tumors with inflamed phenotypes (2). Accumulating research evidence has led
to the detailed elucidation of a number of oncogenic signaling
pathways, e.g. cell cycle regulation, apoptotic signaling, kinase
signaling, DNA damage response, DNA mismatch repair, and immune
checkpoint signaling, resulting in the identification of a variety
of cancer-associated biomarkers. Cancer-associated biomarkers can
in turn be utilized for a number of specific purposes, including
predicting patient prognosis, predicting tumor response to
chemotherapeutic and molecular targeting agents, early diagnosis or
prevention of cancer and aiding precise diagnoses. In addition,
companion diagnostics have been developed using some of the
biomarker profiles, which can be applied clinically and are now
becoming widely utilized for guiding cancer treatment (3). These include BRACAnalysis (Myriad
Genetics), myChoice (Myriad Genetics) and FoundationOne CDx
(Foundation Medicine) (3).
Selecting the appropriate biomarkers are predicted to improve
treatment efficacy, avoid overtreatment and reduce the cost of
preventing and treating gynecological malignancies, rendering
biomarkers essential tools in precision medicine. In the present
review, current evidence of cancer-related biomarkers in the
gynecological oncology field was summarized. This will be based on
findings extracted from clinical trials, their molecular
interpretations and future perspectives.
Biomarkers for predicting patient
prognosis
Biomarkers used for predicting prognosis prior to
commencing treatment are expected to be beneficial for stratifying
patients based on the recurrence risks, to designate the optimal
combination of treatment modalities and therapeutics.
Endometrial cancer (EC)
ECs were conventionally classified into two groups,
type I and II, based on clinicopathological, epidemiological and
endocrinological features (4). Type
I EC is characterized by low-grade endometrioid histology and tend
to more frequently develop in younger and obese women. In addition,
the pathogenesis of this type of EC is associated with unopposed
estrogen, superficial myometrial invasion, early stage at diagnosis
and favorable prognosis. By contrast, type II EC is characterized
by high-grade, non-endometrioid histology, tend to occur in older
and more slender-shaped women, deep myometrial invasion, advanced
stages and poorer prognosis. Commonly mutated genes in type I
tumors include PTEN, PI3K catalytic subunit α, KRAS,
AT-rich interactive domain-containing protein 1A and β-catenin 1,
whilst TP53 is more frequently mutated in type II tumors
(5). This dualistic classification
system has been pivotal for understanding this entire disease
entity in terms of EC pathogenesis. However, this categorizing
model is considered incomplete, due to intergroup overlapping
caused by tumor diversity and heterogeneity (5).
In 2013, molecular analyses of 232 endometrial
carcinomas by The Cancer Genome Atlas (TCGA) program on the results
of exome sequencing, microsatellite instability (MSI) testing and
microarray of somatic copy number alterations resulted in the
proposal of classifying EC into four genomic categories. They are
POLE-ultramutated [1], microsatellite instability
hypermutated [2], copy-number low [3] and copy-number high [4],
each showing distinct progression-free survival (PFS) profiles
(6). Of note, POLE is a catalytic
subunit of DNA polymerase ε, which is involved in nuclear DNA
replication and repair.
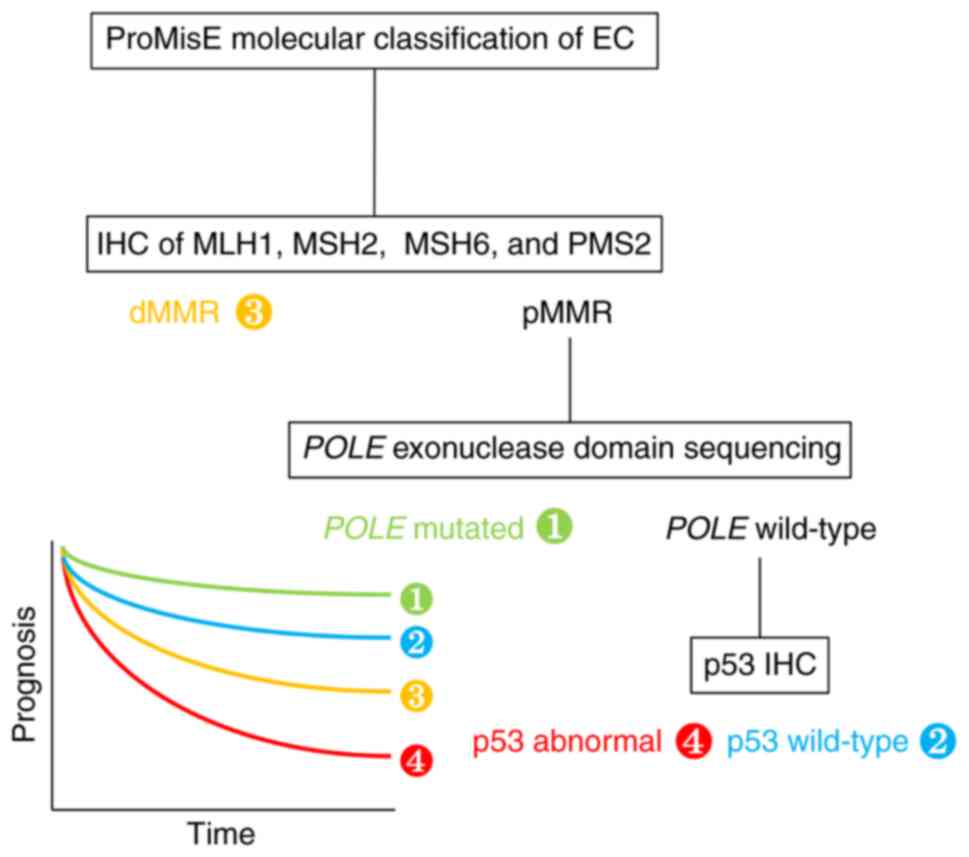
In 2015, a more clinically applicable,
cost-effective classification system, named ‘ProMisE’, was
developed using the same TCGA data and applied it to a new cohort
of cases (7). The four categories
of POLE-mutated [POLE exonuclease domain mutation
(EDM) was determined by sequencing] [1], p53 wild-type [2], DNA
mismatch repair deficiency [determined by immunohistochemistry
(IHC) testing of MutL homolog 1 (MLH1), MutS homolog (MSH)2, MSH6
and postmeiotic segregation increased 2 (PMS2)] [3] and p53
abnormal (p53 status was determined by p53 IHC) [4] revealed
significantly different overall (OS; P=0.0082, n=141),
disease-specific (P=0.0378, n=139) and recurrence-free survival
(RFS; P=0.0358; n=133; Fig. 1).
In 2017, the ProMisE molecular classification system
was confirmed in a large cohort of 319 EC samples, showing distinct
OS (P<0.0001), disease-specific (P<0.0001) and PFS
(P<0.0001) (8). In particular,
the POLE EDM group had the most favorable outcome, whereas
the p53 abnormal group had the worst outcome. To conclude, using
these genome-based risk factors to stratify the patients into the
various adjuvant therapeutic regimens may improve treatment outcome
and reduce overtreatment. However, prospective studies, such as
PORTEC-4a (Molecular Profile-based Versus Standard Adjuvant
Radiotherapy in Endometrial Cancer; NCT03469674) and TAPER
(Tailored Adjuvant Therapy in POLE-mutated and p53-wildtype
Early Stage Endometrial Cancer; NCT04705649), are currently
on-going, from which additional results are expected (9,10).
Biomarkers for predicting tumor response to
chemo-therapeutic and molecular targeting agents
Biomarkers used for predicting the efficacy of
systemic therapeutics are expected to be useful, especially for
advanced or aggressive tumors, where localized therapies alone are
insufficient.
Ovarian cancer
The majority of ovarian cancer cases are diagnosed
already at advanced stages and chemosensitivity is one of its most
important prognostic factors. NGS was previously performed on 393
patients with ovarian carcinoma who received primary surgery. They
were also prospectively followed-up for survival analysis.
Multivariate logistic regression on 281 high-grade serous carcinoma
(HGSC) cases adjusted for germline or somatic BRCA
mutations, age at diagnosis, optimal cytoreduction and neoadjuvant
chemotherapy was performed. It was found that the presence of any
TP53 mutations were associated with platinum sensitivity,
which was defined as time to progression after the completion of
adjuvant platinum chemotherapy (odds ratio, 0.41; 95% confidence
interval, 0.17–0.99; P=0.048) (11). This observation suggests that the
presence of TP53 mutations may predict platinum sensitivity
in patients with HGSC.
The PARP inhibitor olaparib was previously evaluated
in a phase I trial in 60 refractory solid tumors (12). An expansion cohort of this trial was
studied further in patients with ovarian cancer harboring germline
breast cancer gene (BRCA)1/2 mutations, revealing
significant associations between platinum-free interval and the
maximal % tumor response rate after olaparib treatment
(radiological change, P=0.001, n=38; cancer antigen 125 change,
P=0.002, n=46) (13). This finding
suggests that platinum sensitivity of patients with germline
BRCA1/2 mutations may predict the response to PARP
inhibitors.
The efficacy of maintenance olaparib therapy was
also evaluated by the PAOLA-1 phase 3 trial, which compared
olaparib treatment with placebo in patients with newly diagnosed
stage III/IV ovarian cancer who showed a response to first-line
platinum-taxane plus bevacizumab followed by maintenance
bevacizumab (14). The hazard ratio
(HR; 95% CI) for disease progression or death for olaparib vs.
placebo was 0.33 (0.25–0.45) in homologous-recombination deficiency
(HRD)-positive tumors and 1.00 (0.75–1.35) in HRD-negative tumors.
Tumor HRD status was determined by Miriad myChoice CDx testing,
which is designed based on germline/somatic BRCA1/2
mutations and/or positive genomic instability scores using DNA
isolated from formalin-fixed paraffin-embedded (FFPE) tumor
tissues. This finding suggests that HRD positivity can be used to
predict the efficacy of adding maintenance PARP inhibitors into the
primary treatment strategy for advanced ovarian cancer.
The safety and activity of niraparib monotherapy was
evaluated by the QUADRA phase 2 trial in patients with relapsed
high-grade serous ovarian cancers treated with ≥3 chemotherapy
regimens (15). In patients whose
tumors were platinum-sensitive to the most recent line of platinum
therapy (n=105), the overall response rate was 26% in HRD-positive
tumors compared with 4% in HRD-negative or unknown tumors. HRD
status was also determined using the Miriad myChoice CDx testing.
This finding suggests that HRD positivity can also be applied to
predict the effectiveness of PARP inhibitor monotherapy for
patients with recurrent platinum-sensitive serous ovarian
cancer.
EC
The PORTEC-3 is a phase III trial that investigated
the benefit of chemoradiotherapy compared with radiotherapy alone
for high-risk endometrial cancer (endometrioid G3 stage IA with
lymphovascular space invasion; endometrioid G3 stage IB;
endometrioid stage II–III; and non-endometrioid stage I–III)
(16). Using tissue samples from
this trial, the prognostic value of a molecular classification
system similar to ProMisE (7) was
evaluated. Significant improvement in the RFS was found with the
addition of adjuvant chemotherapy to radiotherapy for p53 abnormal
tumors (5-year RFS, 59 vs. 36%; P=0.019; n=93) (17). p53 status was evaluated by IHC and
if applicable, the TP53 mutational status was also analyzed
by NGS. This finding suggests that the p53 mutational status can be
used to predict tumor chemosensitivity or enhancing effect of
radiosensitivity by chemotherapy in patients with high-risk EC.
KEYNOTE-028 is a phase Ib trial in patients with
programmed death ligand 1 (PD-L1)-positive advanced solid tumors,
including EC, who were treated with the anti-programmed cell death
1 (PD-1) monoclonal antibody pembrolizumab. Data from this trial
found that higher response rates and longer PFS are significantly
associated with higher T-cell-inflamed gene-expression profile
(GEP), PD-L1 expression and tumor mutational burden (TMB;
T-cell-inflamed GEP, P=0.012 and 0.017, n=203; PD-L1, P=0.018 and
0.005, n=198; TMB, P=0.018 and 0.051, n=77) (18). T-cell inflamed GEP was evaluated
based on the normalized expression values of 18 selected genes
using RNA extracted from FFPE tumor tissues (19). PD-L1 expression was evaluated by
IHC, which was used to calculate the combined positive score [CPS;
the number of PD-L1-positive cells (tumor cells, lymphocytes,
macrophages) divided by the total number of viable tumor cells
×100]. TMB was assessed by whole-exome sequencing using DNA
isolated from the FFPE tissues. This finding suggests that higher
T-cell-inflamed GEP, PD-L1 expression and/or TMB may predict the
efficacy of pembrolizumab in advanced EC.
The KEYNOTE-158 phase II trial assessed
pembrolizumab monotherapy in previously treated, advanced but
incurable solid tumors (n=790), including EC (n=82). TMB was
evaluated in FFPE tumor tissues using the FoundationOne CDx assay
(20). TMB-high was defined as ≥10
mutations per megabase. The response rates of TMB-high and TMB-low
groups were found to be 29 vs. 6%, suggesting that TMB can be used
to predict the efficacy of pembrolizumab in patients with
previously treated, advanced EC.
The KEYNOTE-775 phase III trial assessed the
efficacy of lenvatinib, a multikinase inhibitor of VEGFR1-3 and
other receptor tyrosine kinases, combined with pembrolizumab or
chemotherapy, in 827 patients with advanced EC who had previously
received ≥ one platinum-based chemotherapy regimen (21). PFS was found to be longer in the
lenvatinib plus pembrolizumab group compared with that in the
lenvatinib plus chemotherapy group in both mismatch repair (MMR)
proficient (HR, 0.60; 95% CI, 0.50–0.72; P<0.001) and in all
patients (HR, 0.56; 95% CI, 0.47–0.66; P<0.001). In addition, OS
was longer with in the lenvatinib plus pembrolizumab group compared
with that in the lenvatinib plus chemotherapy group in both MMR
proficient (HR, 0.68; 95% CI, 0.56–0.84; P<0.001) and all
patients (HR, 0.62; 95% CI, 0.51–0.75; P<0.001). MMR status was
determined by IHC staining of MLH1, MSH2, MSH6 and PMS2 proteins.
These results suggest that lenvatinib plus pembrolizumab is
efficacious for advanced EC irrespective of the MMR status.
However, it should be noted that lenvatinib was discontinued due to
drug-related adverse events in 22.7% of the patients, where the
most frequent grade ≥3 adverse event was hypertension (37.9%) and
was clinically significant for lenvatinib (21). Accordingly, considering the results
of tumor assessment in terms of MSI/MMR status, T-cell-inflamed GEP
and PD-L1 expression, coupled with using TMB for predicting
pembrolizumab efficacy, may still be important even for this
regimen in case of switching to pembrolizumab monotherapy.
Cervical cancer
KEYNOTE-826 phase III trial assessed the efficacy of
pembrolizumab compared with placebo in 617 patients with
persistent, recurrent or metastatic cervical cancer who were also
receiving platinum-based chemotherapy with or without bevacizumab.
PFS (P<0.001) and OS (P<0.001) were found to be significantly
longer with pembrolizumab compared with those in placebo (22). The HR (95% CI) for disease
progression or death were 0.94 (0.52–1.70) for PD-L1 CPS <1,
compared with 0.68 (0.49–0.94) for CPS 1 to <10 and 0.58
(0.44–0.77) for CPS ≥10. Likewise, the HR for death were 1.00
(0.53–1.89) for PD-L1 CPS <1, compared with 0.67 (0.46–0.97) for
CPS 1 to <10 and 0.61 (0.44–0.84) for CPS ≥10. These findings
suggest that PD-L1 expression can be used to predict the efficacy
of adding concurrent pembrolizumab to chemotherapy in persistent,
recurrent or metastatic cervical cancer.
Biomarkers for the early
diagnosis/prevention of cancer
Biomarkers that can facilitate the early diagnosis
or prevention of cancer are expected to enable the provision of an
optimal cost-effective and ideal healthcare plan.
Cervical cancer
High risk (HR)-human papilloma virus (HPV) DNA
genotyping is more sensitive compared with cytology, rendering them
useful for long-term risk prediction. By contrast, cytology has
high specificity (apart from atypical squamous cells of
undetermined significance) and is useful for estimating immediate
risk, but has lower sensitivity and lower negative predictive value
compared with HR-HPV DNA genotyping (23). HPV 16 or 18 infections have the
highest risk of cervical intraepithelial neoplasia (CIN) 3 and
occult cancer development, requiring colposcopy with targeted
biopsy even when cytology results turn out negative (24).
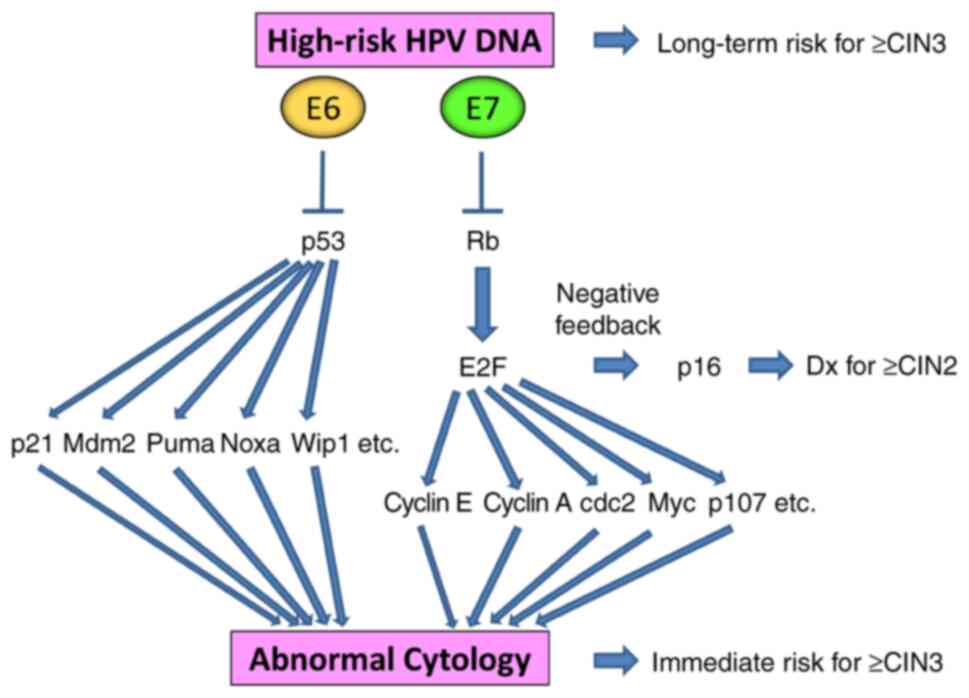
Cyclin-dependent kinase inhibitor 2A (p16 INK4A) is
a cyclin-dependent kinase inhibitor that can inhibit
cyclin-dependent kinases 4 and 6, inducing G1 cell cycle
arrest. Degradation of the tumor suppressor retinoblastoma (Rb)
protein by the HR-HPV oncoprotein E7 and E2F upregulation result in
a feedback loop, leading to the increased expression of p16
(25). p16 IHC staining has been
reported to be 86.7% sensitive and 82.8% specific for ≥CIN 2 (CIN 2
or worse) diagnoses, rendering this useful for distinguishing
high-grade CIN from ≤CIN 1 (26).
When p16 staining is combined with H&E staining, the
sensitivity for high-grade CIN is increased by 13%, decreasing the
false-negative rate by 50% (27).
Ovarian cancer
Hereditary breast and ovarian cancer (HBOC) is an
autosomal dominant hereditary cancer predisposition syndrome that
is caused by pathogenic germline BRCA1/2 variants. The
life-time risk for developing ovarian cancer in individuals
harboring BRCA1 mutations is 39–48%, compared with 11–20% in
those harboring BRCA2 mutations (28–31).
To date, an effective screening method for improving the survival
rate of ovarian cancer has remained elusive (32–36).
Women with HBOC are recommended to receive risk-reducing
salpingo-oophorectomy (RRSO), which has been shown to reduce
mortality according to results from large-population prospective
studies (37,38). Specifically, RRSO reduced mortality
in individuals with BRCA1 mutations aged 35–40 years and in
individuals with BRCA2 mutations aged 40–45 years. This
appeared to be due to later ovarian cancer onset in carriers of
BRCA2 mutations compared with their BRCA1
counterparts (37), after
childbearing age (39). Serous
tubal intra-epithelial carcinoma (STIC) is an early precursor for
high-grade serous carcinoma of fallopian tube origin and is
incidentally found in RRSO specimens (40–42).
Coupling IHC results of p53 and Ki-67 with histological morphology
has been found to improve the reproducibility of successfully
pathologically diagnosing STIC (43). Ki-67 is a nuclear non-histone
protein that is expressed during the G1, S and
G2 phases, with peak expression at the M phase of the
cell cycle but is typically absent at the G0 phase
(44).
EC
Lynch syndrome (LS) is an autosomal dominant
hereditary cancer predisposition condition. It is diagnosed by the
presence of germline pathogenic variants in one of the MMR genes
MLH1, MSH2, MSH6, PMS2 and epithelial cell adhesion molecule
(45). LS is screened by MMR IHC
and/or MSI testing on tumor tissues (46,47),
specifically the loss of MLH1, MSH2, MSH6 and PMS2 expression
(47). Detection of the loss MLH1
expression is followed by MLH1 promoter methylation testing,
where the presence of its hypermethylation would be deemed as a
sporadic tumor instead of LS (47,48).
MSI testing is conducted by comparing the PCR amplicons of
microsatellite repeats in the tumor and those in the corresponding
normal control. The life-time risks of developing colorectal cancer
and EC in women with LS are 30–52 and 28–60%, respectively
(49–53). However, although risk-reducing
surgery for preventing EC in women with LS can reduce the incidence
(54), it has not been reported to
reduce mortality (55).
Molecular interpretations
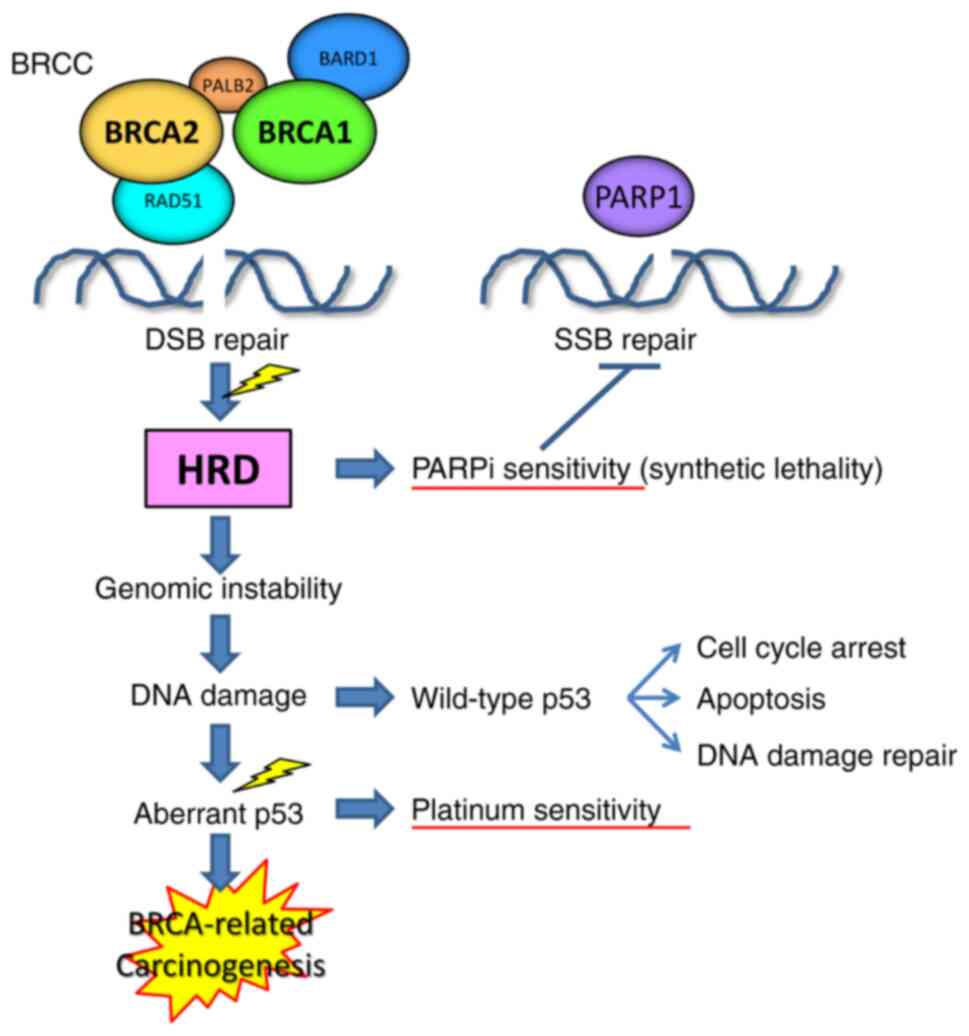
BRCA1, BRCA2, BRCA1-associated RING Domain 1, RAD51,
BRCC36, BRCC45 etc. make up the BRCA1/BRCA2-containing
complex (BRCC), which is involved in the homologous
recombination-mediated repair of DNA double-strand breaks (Fig. 2) (56,57).
Tumors with the loss of heterozygosity in either the BRCA1
or 2 gene correspondingly show defects in repairing
double-strand DNA breaks, and are sensitive to inhibitors of PARP1,
an enzyme that contributes to repairing single-strand DNA breaks,
by causing the synthetic lethality of tumor cells. Tumors with HRD
tend to show genomic instability, accumulate DNA damage, undergo
cell cycle arrest and apoptosis in a wild-type p53-dependent
manner, which is pivotal for the DNA damage response (58–60).
Acquisition of p53 aberrations, which appear to be an early and
requisite event during BRCA-related carcinogenesis (61,62),
overcomes cell cycle arrest and circumvents apoptosis, causing
dysregulated proliferation (58–60).
p53 dysfunction also causes defects in DNA damage repair, leading
to sensitivity to DNA-damaging chemotherapeutics, such as platinum
agents.
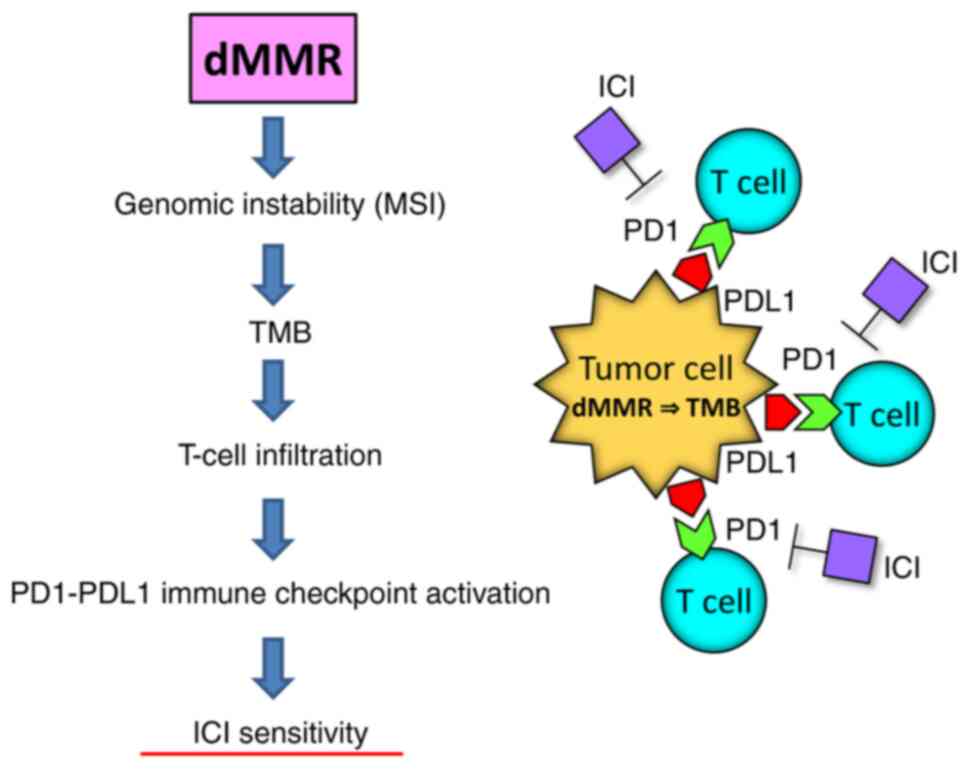
Tumors with MMR deficiency show high MSI and TMB,
which promotes the T-cell inflammatory phenotype and activation of
the PD1/PD-L1-mediated immune checkpoint pathway (Fig. 3) (63). These tumors are sensitive to immune
checkpoint inhibitors, such as anti-PD1 antibodies. Endometrial
carcinomas with POLE mutations can be treated by surgery
alone, leading to favorable prognoses. Surgery alone also avoids
the need of overtreatment to maintain a good quality of life (QOL).
By contrast, endometrial carcinomas with p53 aberrations tend to
have the worst prognosis. They may be treated with conventional
adjuvant therapies based on clinicopathological risk factors or
pembrolizumab plus lenvatinib, combined with surgery.
Persistent infection with high-risk HPVs causes
cervical carcinogenesis due to the chronic overexpression of viral
oncoproteins E6 and E7, which degrade and inactivate tumor
suppressors p53 and Rb, respectively. This in turn regulates a
variety of cellular functions, such as apoptosis, cell cycle
arrest, DNA damage response, immune system response,
differentiation, transformation and immortalization. Therefore,
whilst cytological changes are the results of cellular
transformation, the presence of high-risk HPV DNA can reflect both
the resultant status and future risk of transformation. Degradation
of Rb by E7 and E2F upregulation results in a feedback loop,
leading to p16 overexpression, which then supports the histological
diagnosis for ≥CIN2 (Fig. 4).
Future perspectives
Clinical studies are currently ongoing to
investigate the advantage of risk stratification using molecular
biomarkers over conventional clinicopathological factors for the
treatment of EC (Table I).
PORTEC-4a phase III randomized trial is currently recruiting
patients (9). It compares standard
adjuvant vaginal brachytherapy with adjuvant treatment assignment
(observation, vaginal brachytherapy or external beam radiotherapy)
based on integrated clinicopathological and molecular risk
profiles. It also performs the same evaluations as ProMisE for
stage I–II EC (9). TAPER is a
single-arm prospective cohort study that is also recruiting
patients. It intends to investigate whether early-stage EC with
POLE mutations or wild-type p53 carries a lower risk of
pelvic recurrence at 3 years following no or de-escalated adjuvant
therapy (10). The RAINBO trial
(NCT05255653), which consists of four clinical trials investigating
novel adjuvant therapies, is also recruiting patients (64). Patients are assigned to one of the
following trials according to the molecular profile of their tumor:
i) p53 abnormal to the p53abn-RED trial; ii) MMR deficient to the
MMRd-GREEN trial; iii) no specific molecular profile to the
NSMP-ORANGE trial; and iv) POLE mutant to the
POLEmut-BLUE trial. The p53abn-RED randomized phase III
trial compares adjuvant chemoradiation with/without 2 years of
following treatment with olaparib. The MMRd-GREEN randomized phase
III trial compares adjuvant pelvic external-beam radiotherapy
with/without combined and following durvalumab, a human monoclonal
anti-PD-L1 antibody, for 1 year. The NSPM-ORANGE randomized phase
III trial compares adjuvant pelvic external-beam radiotherapy
with/without 2-year following treatment with progestogens. The
POLEmut-BLUE phase II single-arm trial evaluates the
de-escalation of adjuvant therapy: No adjuvant therapy for stage
I–II and no adjuvant therapy or adjuvant pelvic external-beam
radiotherapy for stage III. The results of these studies are
expected to provide useful evidence for formulating genome-based
therapeutic strategies for EC. In terms of ovarian cancer, the most
compelling evidence on the application of biomarkers is for HGSC.
However, ovarian cancer is comprised of a variety of histological
types. A comprehensive molecular classification system beyond
pathological morphology needs to be constructed, in a manner that
is applicable for risk stratification and therapeutic selection.
Additionally, although the majority of ovarian cancers are
diagnosed at advanced stages at present, an effective surveillance
method for early detection remains elusive. Therefore, biomarkers
for such utility are eagerly anticipated. For cervical cancer,
considering the global effort for the prevalence of HPV
vaccination, biomarkers for increased efficiency and economical
screening instead of those for efficacious treatment will be needed
in the near future.
 | Table I.Selected studies on biomarkers for
patient prognosis and tumor response in gynecological oncology. |
Table I.
Selected studies on biomarkers for
patient prognosis and tumor response in gynecological oncology.
| Biomarker | Target disease | Sample size | Study for
biomarker | ClinicalTrials.gov identifier | Study phase | Published year | (Refs.) |
|---|
| MSI, POLEm,
copy number | Stage I–IV EC | 232 | Observational |
|
| 2013 | (6) |
| dMMR, POLEm,
p53 | Stage I–IV EC | 133, 139, 141 | Observational |
|
| 2015 | (7) |
| dMMR, POLEm,
p53 | Stage I–IV EC | 319 | Observational |
|
| 2017 | (8) |
| dMMR, POLEm,
p53 | Stage I–II EC | recruiting | Interventional | NCT03469674
(POTEC-4a) | 3 |
| (9) |
| POLEm,
p53 | Stage I–II EC | recruiting | Interventional | NCT04705649
(TAPER) |
|
| (10) |
| dMMR, POLEm,
p53 | Stage I–III EC | recruiting | Interventional | NCT05255653
(RAINBO) | 2, 3 |
| (64) |
| p53 | High-risk, stage
IB-III EC | 93 | Observational | NCT00411138
(PORTEC-3) | 3 | 2020 | (17) |
| T-cell-inflamed
GEP, PD-L1, TMB | PD-L1+
advanced solid tumors (including EC) | 203, 198, 77 | Observational | NCT02054806
(KEYNOTE-028) | 1b | 2019 | (18) |
| TMB (FoundationOne
CDx) | Advanced, incurable
solid tumors (including EC) | 790 (82) | Observational | NCT02628067
(KEYNOTE-158) | 2 | 2020 | (20) |
| p53 | HGSC | 281 | Observational |
|
| 2021 | (11) |
| PFI |
gBRCAm+ OC | 38, 46 | Observational |
|
| 2010 | (13) |
| HRD (myChoice
CDx) | Stage III–IV
OC | 667 | Observational | NCT02477644
(PAOLA-1) | 3 | 2019 | (14) |
| HRD (myChoice
CDx) | Recurrent,
platinum-sensitive HGSC | 105 | Observational | NCT02354586
(QUADRA) | 2 | 2019 | (15) |
| PD-L1 |
Persistent/recurrent/metastatic CC | 617 | Observational | NCT03635567
(KEYNOTE-826) | 3 | 2021 | (22) |
Conclusion
The present review provided an overview for the
current evidence on the use of cancer-related biomarkers for
gynecological malignancies. Due to the recent acceleration in the
advancements of human genomics and therapeutic developments, the
knowledge and application of biomarkers are fast becoming essential
for maximizing therapeutic efficacy and patient QOL whilst
minimizing overtreatment and waste of limited resources. Several
biomarkers have been suggested to be viable for guiding therapies,
such as companion diagnostics based on the data from mainly
observational studies (Table I).
However, currently ongoing and future prospective interventional
studies are warranted. They are expected to provide robust evidence
on potentially effective and beneficial biomarkers that are
applicable for the prevention, diagnosis and treatment of
gynecological cancers, to further facilitate genome-directed
precision medicine.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
TM performed the literature search and drafted the
manuscript. AS, AA and TS critically reviewed the manuscript. TS
revised the manuscript. Data authentication is not applicable. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
TS received participant/speaker/advisor/chair
payments from Aska Pharmaceutical, AstraZeneca, Bayer Yakuhin,
Bristol-Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo, Eisai,
Fuji Pharma, GE HealthCare, Johnson & Johnson, Kyowa Kirin,
Merck, Mochida Pharmaceutical, Nippon Kayaku, Nobelpharma, Otsuka
Pharmaceutical, Pfizer, Taiho Pharmaceutical, Takeda
Pharmaceutical, Tsumura and Yakult Honsha. AS received
speaker/chair payments from AstraZeneca, Eisai, Johnson &
Johnson, Medtronic, Merck, Sanofi S.A., Taiho Pharmaceutical and
Takeda Pharmaceutical. AA received speaker payments from MSD and
Takeda Pharmaceutical.
References
|
1
|
Friedman AA, Letai A, Fisher DE and
Flaherty KT: Precision medicine for cancer with next-generation
functional diagnostics. Nat Rev Cancer. 15:747–756. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang W, Lei C, Song S, Jing W, Jin C, Gong
S, Tian H and Guo T: Immune checkpoint blockade in the treatment of
malignant tumor: Current statue and future strategies. Cancer Cell
Int. 21:5892021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Herzog TJ, Vergote I, Gomella LG,
Milenkova T, French T, Tonikian R, Poehlein C and Hussain M:
Testing for homologous recombination repair or homologous
recombination deficiency for poly (ADP-ribose) polymerase
inhibitors: A current perspective. Eur J Cancer. 179:136–146. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bokhman JV: Two pathogenetic types of
endometrial carcinoma. Gynecol Oncol. 15:10–17. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Murali R, Soslow RA and Weigelt B:
Classification of endometrial carcinoma: More than two types.
Lancet Oncol. 15:e268–e278. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cancer Genome Atlas Research Network, .
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Talhouk A, McConechy MK, Leung S, Li-Chang
HH, Kwon JS, Melnyk N, Yang W, Senz J, Boyd N, Karnezis AN, et al:
A clinically applicable molecular-based classification for
endometrial cancers. Br J Cancer. 113:299–310. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Talhouk A, McConechy MK, Leung S, Yang W,
Lum A, Senz J, Boyd N, Pike J, Anglesio M, Kwon JS, et al:
Confirmation of ProMisE: A simple, genomics-based clinical
classifier for endometrial cancer. Cancer. 123:802–813. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
PORTEC-4a. Molecular profile-based versus
standard adjuvant radiotherapy in endometrial cancer (PORTEC-4a).
https://beta.clinicaltrials.gov/study/NCT03469674?cond=NCT03469674&rank=1February
25–2023
|
|
10
|
Tailored Adjuvant Therapy in POLE-mutated
and p53-wildtype Early Stage Endometrial Cancer (TAPER). https://beta.clinicaltrials.gov/study/NCT04705649?cond=NCT04705649&rank=1February
25–2023
|
|
11
|
Ghezelayagh TS, Pennington KP, Norquist
BM, Khasnavis N, Radke MR, Kilgore MR, Garcia RL, Lee M, Katz R,
Leslie KK, et al: Characterizing TP53 mutations in ovarian
carcinomas with and without concurrent BRCA1 or BRCA2 mutations.
Gynecol Oncol. 160:786–792. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fong PC, Boss DS, Yap TA, Tutt A, Wu P,
Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et
al: Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fong PC, Yap TA, Boss DS, Carden CP,
Mergui-Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S,
Messiou C, et al: Poly(ADP)-ribose polymerase inhibition: Frequent
durable responses in BRCA carrier ovarian cancer correlating with
platinum-free interval. J Clin Oncol. 28:2512–2519. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ray-Coquard I, Pautier P, Pignata S, Pérol
D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus Bevacizumab as first-line
maintenance in ovarian cancer. N Engl J Med. 381:2416–2428. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moore KN, Secord AA, Geller MA, Miller DS,
Cloven N, Fleming GF, Hendrickson AEW, Azodi M, DiSilvestro P, Oza
AM, et al: Niraparib monotherapy for late-line treatment of ovarian
cancer (QUADRA): A multicentre, open-label, single-arm, phase 2
trial. Lancet Oncol. 20:636–648. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Boer SM, Powell ME, Mileshkin L,
Katsaros D, Bessette P, Haie-Meder C, Ottevanger PB, Ledermann JA,
Khaw P, Colombo A, et al: Adjuvant chemoradiotherapy versus
radiotherapy alone for women with high-risk endometrial cancer
(PORTEC-3): Final results of an international, open-label,
multicentre, randomised, phase 3 trial. Lancet Oncol. 19:295–309.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Leon-Castillo A, de Boer SM, Powell ME,
Mileshkin LR, Mackay HJ, Leary A, Nijman HW, Singh N, Pollock PM,
Bessette P, et al: Molecular classification of the PORTEC-3 trial
for high-risk endometrial cancer: Impact on prognosis and benefit
from adjuvant therapy. J Clin Oncol. 38:3388–3397. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ott PA, Bang YJ, Piha-Paul SA, Razak ARA,
Bennouna J, Soria JC, Rugo HS, Cohen RB, O'Neil BH, Mehnert JM, et
al: T-cell-inflamed gene-expression profile, programmed death
ligand 1 expression, and tumor mutational burden predict efficacy
in patients treated with pembrolizumab across 20 cancers:
KEYNOTE-028. J Clin Oncol. 37:318–327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ayers M, Lunceford J, Nebozhyn M, Murphy
E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran
V, et al: IFN-γ-related mRNA profile predicts clinical response to
PD-1 blockade. J Clin Invest. 127:2930–2940. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Marabelle A, Fakih M, Lopez J, Shah M,
Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin
JA, Miller WH Jr, et al: Association of tumour mutational burden
with outcomes in patients with advanced solid tumours treated with
pembrolizumab: Prospective biomarker analysis of the multicohort,
open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21:1353–1365.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Makker V, Colombo N, Herraez AC, Santin
AD, Colomba E, Miller DS, Fujiwara K, Pignata S, Baron-Hay S,
Ray-Coquard I, et al: Lenvatinib plus Pembrolizumab for advanced
endometrial cancer. N Engl J Med. 386:437–448. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Colombo N, Dubot C, Lorusso D, Caceres MV,
Hasegawa K, Shapira-Frommer R, Tewari KS, Salman P, Usta EH, Yañez
E, et al: Pembrolizumab for persistent, recurrent, or metastatic
cervical cancer. N Engl J Med. 385:1856–1867. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schiffman M, Kinney WK, Cheung LC, Gage
JC, Fetterman B, Poitras NE, Lorey TS, Wentzensen N, Befano B,
Schussler J, et al: Relative performance of HPV and cytology
components of cotesting in cervical screening. J Natl Cancer Inst.
110:501–508. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perkins RB, Guido RS, Castle PE, Chelmow
D, Einstein MH, Garcia F, Huh WK, Kim JJ, Moscicki AB, Nayar R, et
al: 2019 ASCCP risk-based management consensus guidelines for
abnormal cervical cancer screening tests and cancer precursors. J
Low Genit Tract Dis. 24:102–131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Serrano M, Hannon GJ and Beach D: A new
regulatory motif in cell-cycle control causing specific inhibition
of cyclin D/CDK4. Nature. 366:704–707. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Galgano MT, Castle PE, Atkins KA, Brix WK,
Nassau SR and Stoler MH: Using biomarkers as objective standards in
the diagnosis of cervical biopsies. Am J Surg Pathol. 34:1077–1087.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bergeron C, Ordi J, Schmidt D, Trunk MJ,
Keller T and Ridder R; European CINtec Histology Study Group, :
Conjunctive p16INK4a testing significantly increases accuracy in
diagnosing high-grade cervical intraepithelial neoplasia. Am J Clin
Pathol. 133:395–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kuchenbaecker KB, Hopper JL, Barnes DR,
Phillips KA, Mooij TM, Roos-Blom MJ, Jervis S, van Leeuwen FE,
Milne RL, Andrieu N, et al: Risks of breast, ovarian, and
contralateral breast cancer for BRCA1 and BRCA2 mutation carriers.
JAMA. 317:2402–2416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Antoniou A, Pharoah PD, Narod S, Risch HA,
Eyfjord JE, Hopper JL, Loman N, Olsson H, Johannsson O, Borg A, et
al: Average risks of breast and ovarian cancer associated with
BRCA1 or BRCA2 mutations detected in case series unselected for
family history: A combined analysis of 22 studies. Am J Hum Genet.
72:1117–1130. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen S and Parmigiani G: Meta-analysis of
BRCA1 and BRCA2 penetrance. J Clin Oncol. 25:1329–1333. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen J, Bae E, Zhang L, Hughes K,
Parmigiani G, Braun D and Rebbeck TR: Penetrance of breast and
ovarian cancer in women who carry a BRCA1/2 mutation and do not use
risk-reducing salpingo-oophorectomy: An updated meta-analysis. JNCI
Cancer Spectr. 4:pkaa0292020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pinsky PF, Yu K, Kramer BS, Black A, Buys
SS, Partridge E, Gohagan J, Berg CD and Prorok PC: Extended
mortality results for ovarian cancer screening in the PLCO trial
with median 15years follow-up. Gynecol Oncol. 143:270–275. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj
A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E,
Cruickshank D, et al: Ovarian cancer screening and mortality in the
UK collaborative trial of ovarian cancer screening (UKCTOCS): A
randomised controlled trial. Lancet. 387:945–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marchetti C, De Felice F, Perniola G,
Lecce F, Vertechy L, Monti M, Musio D, Muzii L, Tombolini V and
Panici PB: Screening program in ovarian cancer: A logical step in
clinical management? A meta-analysis. Curr Probl Cancer.
42:235–240. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jacobs IJ, Skates SJ, MacDonald N, Menon
U, Rosenthal AN, Davies AP, Woolas R, Jeyarajah AR, Sibley K, Lowe
DG and Oram DH: Screening for ovarian cancer: A pilot randomised
controlled trial. Lancet. 353:1207–1210. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Henderson JT, Webber EM and Sawaya GF:
Screening for ovarian cancer: Updated evidence report and
systematic review for the US preventive services task force. JAMA.
319:595–606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Finch AP, Lubinski J, Møller P, Singer CF,
Karlan B, Senter L, Rosen B, Maehle L, Ghadirian P, Cybulski C, et
al: Impact of oophorectomy on cancer incidence and mortality in
women with a BRCA1 or BRCA2 mutation. J Clin Oncol. 32:1547–1553.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Domchek SM, Friebel TM, Singer CF, Evans
DG, Lynch HT, Isaacs C, Garber JE, Neuhausen SL, Matloff E, Eeles
R, et al: Association of risk-reducing surgery in BRCA1 or BRCA2
mutation carriers with cancer risk and mortality. JAMA.
304:967–975. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
NCCN Guidelines Genetic/Familial High-risk
Assessment, . Breast, Ovarian, and Pancreatic. NCCN Clinical
Practice Guidelines in Oncology. 2023.https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1503February
25–2023
|
|
40
|
Callahan MJ, Crum CP, Medeiros F,
Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS and
Muto MG: Primary fallopian tube malignancies in BRCA-positive women
undergoing surgery for ovarian cancer risk reduction. J Clin Oncol.
25:3985–3990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Powell CB, Kenley E, Chen LM, Crawford B,
McLennan J, Zaloudek C, Komaromy M, Beattie M and Ziegler J:
Risk-reducing salpingo-oophorectomy in BRCA mutation carriers: Role
of serial sectioning in the detection of occult malignancy. J Clin
Oncol. 23:127–132. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shaw PA, Rouzbahman M, Pizer ES, Pintilie
M and Begley H: Candidate serous cancer precursors in fallopian
tube epithelium of BRCA1/2 mutation carriers. Mod Pathol.
22:1133–1138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vang R and Shih IM: Serous tubal
intra-epithelial carcinoma: What do we really know at this point?
Histopathology. 81:542–555. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Lopez F, Belloc F, Lacombe F, Dumain P,
Reiffers J, Bernard P and Boisseau MR: Modalities of synthesis of
Ki67 antigen during the stimulation of lymphocytes. Cytometry.
12:42–49. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Latham A, Srinivasan P, Kemel Y, Shia J,
Bandlamudi C, Mandelker D, Middha S, Hechtman J, Zehir A,
Dubard-Gault M, et al: Microsatellite instability is associated
with the presence of lynch syndrome pan-cancer. J Clin Oncol.
37:286–295. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hendriks YM, de Jong AE, Morreau H, Tops
CM, Vasen HF, Wijnen JT, Breuning MH and Bröcker-Vriends AH:
Diagnostic approach and management of Lynch syndrome (hereditary
nonpolyposis colorectal carcinoma): A guide for clinicians. CA
Cancer J Clin. 56:213–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Crosbie EJ, Ryan NAJ, Arends MJ, Bosse T,
Burn J, Cornes JM, Crawford R, Eccles D, Frayling IM, Ghaem-Maghami
S, et al: The Manchester international consensus group
recommendations for the management of gynecological cancers in
Lynch syndrome. Genet Med. 21:2390–2400. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Giardiello FM, Allen JI, Axilbund JE,
Boland CR, Burke CA, Burt RW, Church JM, Dominitz JA, Johnson DA,
Kaltenbach T, et al: Guidelines on genetic evaluation and
management of Lynch syndrome: A consensus statement by the US
multi-society task force on colorectal cancer. Gastroenterology.
147:502–526. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Stoffel E, Mukherjee B, Raymond VM, Tayob
N, Kastrinos F, Sparr J, Wang F, Bandipalliam P, Syngal S and
Gruber SB: Calculation of risk of colorectal and endometrial cancer
among patients with Lynch syndrome. Gastroenterology.
137:1621–1627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dunlop MG, Farrington SM, Carothers AD,
Wyllie AH, Sharp L, Burn J, Liu B, Kinzler KW and Vogelstein B:
Cancer risk associated with germline DNA mismatch repair gene
mutations. Hum Mol Genet. 6:105–110. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Barrow E, Robinson L, Alduaij W, Shenton
A, Clancy T, Lalloo F, Hill J and Evans DG: Cumulative lifetime
incidence of extracolonic cancers in Lynch syndrome: A report of
121 families with proven mutations. Clin Genet. 75:141–149. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hampel H, Stephens JA, Pukkala E, Sankila
R, Aaltonen LA, Mecklin JP and de la Chapelle A: Cancer risk in
hereditary nonpolyposis colorectal cancer syndrome: Later age of
onset. Gastroenterology. 129:415–421. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Aarnio M, Sankila R, Pukkala E, Salovaara
R, Aaltonen LA, de la Chapelle A, Peltomäki P, Mecklin JP and
Järvinen HJ: Cancer risk in mutation carriers of
DNA-mismatch-repair genes. Int J Cancer. 81:214–218. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Schmeler KM, Lynch HT, Chen LM, Munsell
MF, Soliman PT, Clark MB, Daniels MS, White KG, Boyd-Rogers SG,
Conrad PG, et al: Prophylactic surgery to reduce the risk of
gynecologic cancers in the Lynch syndrome. N Engl J Med.
354:261–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
NCCN Guidelines, . Genetic/Familial
High-risk Assessment: Colorectal. NCCN Clinical Practice Guidelines
in Oncology 2023. https://www.nccn.org/guidelines/guidelines-detail?category=2&id=1436February
25–2023
|
|
56
|
Dong Y, Hakimi MA, Chen X, Kumaraswamy E,
Cooch NS, Godwin AK and Shiekhattar R: Regulation of BRCC, a
holoenzyme complex containing BRCA1 and BRCA2, by a
signalosome-like subunit and its role in DNA repair. Mol Cell.
12:1087–1099. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Christou CM and Kyriacou K: BRCA1 and its
network of interacting partners. Biology. 2:40–63. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Brugarolas J and Jacks T: Double
indemnity: p53, BRCA and cancer. p53 mutation partially rescues
developmental arrest in Brca1 and Brca2 null mice, suggesting a
role for familial breast cancer genes in DNA damage repair. Nat
Med. 3:721–722. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Patel KJ, Yu VP, Lee H, Corcoran A,
Thistlethwaite FC, Evans MJ, Colledge WH, Friedman LS, Ponder BA
and Venkitaraman AR: Involvement of Brca2 in DNA repair. Mol Cell.
1:347–357. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Xu X, Weaver Z, Linke SP, Li C, Gotay J,
Wang XW, Harris CC, Ried T and Deng CX: Centrosome amplification
and a defective G2-M cell cycle checkpoint induce genetic
instability in BRCA1 exon 11 isoform-deficient cells. Mol Cell.
3:389–395. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kindelberger DW, Lee Y, Miron A, Hirsch
MS, Feltmate C, Medeiros F, Callahan MJ, Garner EO, Gordon RW,
Birch C, et al: Intraepithelial carcinoma of the fimbria and pelvic
serous carcinoma: Evidence for a causal relationship. Am J Surg
Pathol. 31:161–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ahmed AA, Etemadmoghadam D, Temple J,
Lynch AG, Riad M, Sharma R, Stewart C, Fereday S, Caldas C, Defazio
A, et al: Driver mutations in TP53 are ubiquitous in high grade
serous carcinoma of the ovary. J Pathol. 221:49–56. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dudley JC, Lin MT, Le DT and Eshleman JR:
microsatellite instability as a biomarker for PD-1 blockade. Clin
Cancer Res. 22:813–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Refining Adjuvant Treatment IN Endometrial
Cancer Based On Molecular Features (RAINBO). https://beta.clinicaltrials.gov/study/NCT05255653?cond=NCT05255653&rank=1February
25–2023
|