Introduction
Hepatocellular carcinoma (HCC) is a common type of
cancer originating from liver cells, typically occurring in
patients with liver cirrhosis or chronic hepatitis (1). HCC is a significant global burden and
is ranked as the sixth most common cancer and the fourth leading
cause of cancer-related death worldwide (2). The primary risk factors for HCC
include viral hepatitis, liver cirrhosis, alcohol abuse and
non-alcoholic fatty liver disease (3). The early diagnosis of HCC is difficult
as symptoms such as jaundice, abdominal pain and weight loss are
not always apparent until the advanced stages of the disease. As a
result, the treatment of HCC remains a challenge, despite the
availability of certain treatment modalities, such as surgical
resection, liver transplantation, chemotherapy and radiation
therapy (4). Surgery is the primary
treatment modality for HCC; however, for patients with a high risk
of postoperative recurrence, such as those with larger tumor
volumes (diameter >5 cm), multiple tumor nodules, the presence
of satellite lesions, elevated preoperative α-fetoprotein levels
and active chronic viral hepatitis, adjuvant therapy can be
considered after curative resection. Adjuvant therapy commonly used
for patients with HCC includes targeted therapy and immunotherapy
(5).
Immune checkpoint inhibitors (ICIs) are a novel
class of cancer treatment drugs that restore the ability of T cells
to attack tumor cells by blocking inhibitory signaling molecules,
such as cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) and
programmed cell death protein 1 (PD-1)/PD ligand 1 (PD-L1), on the
surface of T cells (6–8). ICIs have been reported to be effective
in treating certain solid tumors, including HCC (9–11).
Previous studies have also reported that ICIs may improve treatment
efficacy and the survival rate of patients with HCC (12,13).
However, the application of ICIs also presents certain challenges,
including adverse reactions and treatment resistance in some
patients. Studies focusing on ICIs have shown that the objective
response rates (ORR) for nivolumab and pembrolizumab are only 20
and 16.9%, respectively (14,15).
Existing biomarkers that indicate the use of ICIs, such as PD-L1
expression levels and microsatellite instability, are difficult to
assess and may not be applicable to all patients (16,17).
Therefore, it is still crucial to identify convenient and effective
biomarkers to determine which patients will benefit from ICI
treatment.
Non-invasive biomarkers have attracted attention
since they are easy to assess without the need for tissue biopsy or
other invasive procedures. The effectiveness of non-invasive
biomarkers for determination of the use of ICIs has also been
widely reported (18–20). These biomarkers are also more
practical for patients with HCC with lower surgical and biopsy
rates. The efficacy of ICIs is dependent on the immune function of
the patient, which is influenced by inflammation and nutritional
status (21). Numerous studies have
shown that certain inflammatory and nutritional biomarkers such as
prognostic nutrition index (PNI) and systemic immune-inflammation
index (SII) could predict the prognosis of patients with solid
tumors who receive ICIs (22–25).
However, to the best of our knowledge, the value of these
biomarkers in HCC remains unclear.
Therefore, the present study comprehensively
evaluated the predictive ability of the PNI, nutritional risk index
(NRI), geriatric NRI (GNRI), SII, systemic inflammation response
index (SIRI) and advanced lung cancer inflammation index (ALI) for
the determination of the prognosis of patients with HCC who
received ICIs.
Materials and methods
Patients
The present study included 151 patients with HCC who
received ICIs at Harbin Medical University Cancer Hospital (Harbin,
China) from January 2019 to December 2021. The age range was 37–81
years old. The clinical and pathological data of the patients were
collected through the electronic medical record system. All
patients had a confirmed diagnosis of HCC through pathological
assessment, and complete clinical and pathological data were
available. Clinical data loss or treatment abandonment were
exclusion criteria for enrollment in the present study. The
Barcelona Clinic Liver Cancer (BCLC) stages system, which combines
tumor burden, liver function and performance status, is the most
commonly used staging system for HCC (26,27).
BCLC stage has been indicated as the primary reference for the
selection of treatment modalities for patients with HCC in numerous
guidelines (28,29). Therefore, both BCLC and
Tumor-Node-Metastasis (TNM) stage information was collected from
the patients and the main subgroup analyses were established based
on the BCLC stages (30). The data
collection and statistical analysis process adhered to the
principles of The Declaration of Helsinki and its subsequent
amendments, and the present study was approved by The Ethics
Committee of Harbin Medical University Cancer Hospital (approval
no. ALTN-AK105-III-06). Due to the retrospective nature of the
present investigation, The Ethics Committee of Harbin Medical
University Cancer Hospital waived the requirement for informed
patient consent.
Data collection and follow-up
The progression-free survival (PFS) and overall
survival (OS) time were determined following routine patient
follow-up via telephone. PFS was determined as the period from the
start of treatment to the occurrence of tumor progression, with
evidence of progression obtained through imaging and pathological
examination. In addition, PFS for patients without evidence of
tumor progression was determined as the period from the start of
treatment to death or the last follow-up. OS was defined as the
period from the start of treatment to death or the last follow-up.
The clinical information, pathological characteristics and blood
parameters of the patients were obtained from the electronic
medical record system and subsequently analyzed.
Treatment methods
Due to the unsatisfactory outcomes of using targeted
therapy or immunotherapy alone, combination therapy is the main
approach in current HCC treatment (31). Among the 151 patients included in
the present study, 51 patients (33.8%) underwent curative
resection, with 29 of them (56.9%) receiving atezolizumab combined
with bevacizumab treatment due to poor pathological results or
postoperative recurrence. The remaining patients participated in a
clinical trial and received camrelizumab combined with apatinib
treatment (trial registration number: CTR20211710). A total of 100
patients (66.2%) did not undergo surgical treatment due to disease
progression or poor liver function. Among them, 48 patients (48.0%)
received atezolizumab combined with bevacizumab treatment, while
the rest participated in the same clinical trial and received
camrelizumab combined with apatinib treatment.
Nutritional and inflammatory
markers
The nutritional and inflammatory markers evaluated
in the present study were calculated based on the blood parameters
of the patients. The calculation formulas of PNI, GNRI, NRI, SII,
SIRI and ALI are presented in Table
I. Death-based survival receiver operating characteristic (ROC)
curves were plotted and cut-off points for biomarkers in the
present study were determined by calculating the maximum Youden
index (Fig. 1). The area under the
curve (AUC), maximum Youden index and cut-off points are presented
in Table II.
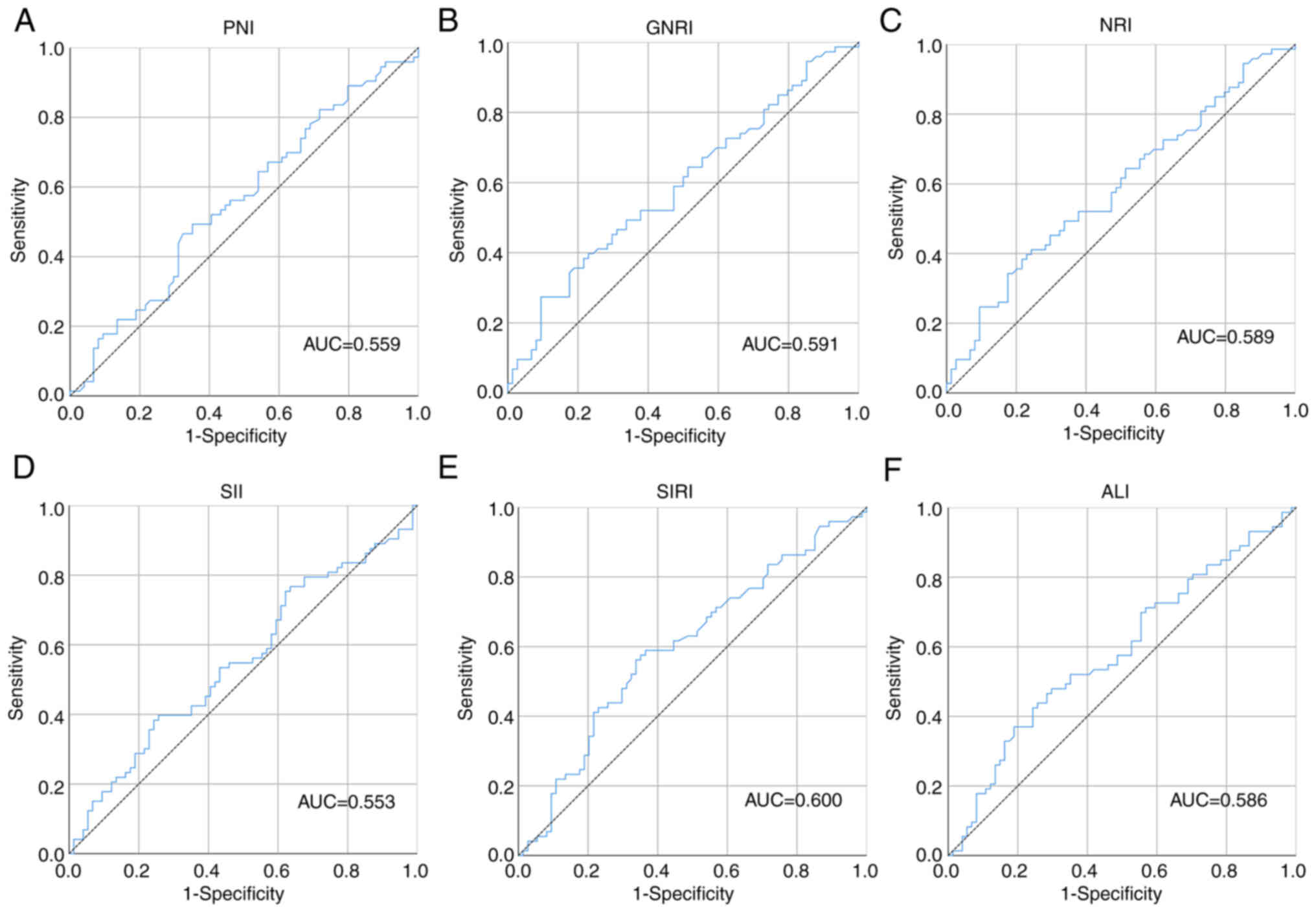 | Figure 1.ROC curves of the inflammation and
nutritional markers. The ROC curves for (A) PNI, (B) GNRI, (C) NRI,
(D) SII, (E) SIRI and (F) ALI. AUC, area under the curve; ROC,
receiver operating characteristic; PNI, prognostic nutrition index;
NRI, nutritional risk index; GNRI, geriatric NRI; SII, systemic
immune-inflammation index; SIRI, systemic inflammation response
index; ALI, advanced lung cancer inflammation index. |
 | Table I.Calculation formulas for the
inflammation and nutritional markers. |
Table I.
Calculation formulas for the
inflammation and nutritional markers.
| Marker | Calculation
formula |
|---|
| PNI | Albumin (g/l) + 5 ×
lymphocyte (109/l) |
| GNRI | [1.489 × albumin
(g/l)] + [41.7 × (weight/ideal weighta)] |
| NRI | [1.519 × albumin
(g/l)] + [41.7 × (weight/ideal weighta)] |
| SII | platelet
(109/l) × neutrophil (109/l)/lymphocyte
(109/l) |
| SIRI | monocyte
(109/l) × neutrophil (109/l)/lymphocyte
(109/l) |
| ALI | BMI
(kg/m2) × albumin (g/dl) × lymphocyte
(109/l)/neutrophil (109/l) |
 | Table II.Cut-off points for inflammation and
nutritional markers. |
Table II.
Cut-off points for inflammation and
nutritional markers.
| Marker | AUC | 95% CI | Youden index | Cut-off point |
|---|
| PNI | 0.559 | 0.466–0.652 | 0.142 | 43.37 |
| GNRI | 0.591 | 0.499–0.683 | 0.179 | 88.92 |
| NRI | 0.589 | 0.497–0.681 | 0.168 | 92.22 |
| SII | 0.553 | 0.460–0.646 | 0.141 | 377.03 |
| SIRI | 0.600 | 0.508–0.692 | 0.224 | 1.02 |
| ALI | 0.586 | 0.494–0.679 | 0.182 | 30.31 |
Statistical analysis
The one-sample Kolmogorov-Smirnov test was used to
assess whether continuous variables followed a Gaussian
distribution. Continuous variables following a Gaussian
distribution are presented as the mean ± SD and were analyzed using
unpaired independent-sample t-test. Continuous variables not
following a Gaussian distribution are presented as the median and
interquartile range and were analyzed using the Mann-Whitney U
test. Categorical variables are presented as the number and
percentage of patients. Survival analysis was performed using the
Kaplan-Meier curve and evaluated the differences in patient
survival through log-rank test. Prognostic markers were assessed
using Cox regression analysis and are presented as risk hazard
ratios and 95% confidence intervals. In addition, time-ROC curves
were plotted to evaluate the prognostic value of inflammation and
nutritional markers. Nomograms were constructed to predict the
survival probability of patients with HCC who received ICIs, and
the accuracy of the nomograms was evaluated by drawing calibration
curves. All statistical analyses were performed using R 4.2.2
(r-project.org; ‘ggplot2’, ‘survival’, ‘survminer’, ‘rms’, ‘pROC’,
and ‘timeROC’). P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
Among the 151 patients who received ICIs, there were
124 (82.1%) male and 27 (17.9%) female patients, with a mean age of
57.41 (SD, 9.14) years. All patients received standard treatment
prior to receiving ICIs. Among the patients, 123 (81.5%) patients
had tumor ≥5 cm. The number of patients in BCLC stage A, stage B
and stage C were 4 (2.6%), 62 (41.1%) and 85 (56.3%), respectively.
TNM staging indicated that 4 (2.6%) patients were in stage I, 55
(36.4%) patients were in stage II, 70 (46.4%) patients were in
stage III and 22 (14.6%) patients were in stage IV. In addition,
due to the markedly skewed distribution of carcinoembryonic
antigen, α-fetoprotein and carbohydrate antigen 199, patients were
grouped based on the median values of these factors. The detailed
patient characteristics are presented in Table III. The detailed blood parameters
from patients before treatment were also collected (Table IV). Because BCLC stage A patients
usually experience a greater survival advantage compared with stage
B or C patients, and the limited number of cases (n=4) did not
support conducting a subgroup analysis for BCLC stage A patients,
the BCLC stage A patients were excluded from all subsequent
analyses to prevent the introduction of bias into the results
(32).
 | Table III.Patient characteristics (n=151). |
Table III.
Patient characteristics (n=151).
| Patient
characteristic | Value |
|---|
| Sex, n (%) |
|
|
Male | 124 (82.10) |
|
Female | 27 (17.90) |
| Mean age, years
(SD) | 57.41 (9.14) |
| Mean BMI,
kg/m2 (SD) | 23.34 (3.57) |
| Smoking, n (%) |
|
|
Yes | 31 (20.5) |
| No | 120 (79.5) |
| Alcohol
consumption, n (%) |
|
|
Yes | 19 (12.6) |
| No | 132 (87.4) |
| ECG, n (%) |
|
|
Normal | 66 (43.7) |
|
Abnormal | 85 (56.3) |
| ABO blood type, n
(%) |
|
| A | 42 (27.8) |
| B | 45 (29.8) |
| AB | 22 (14.6) |
| O | 42 (27.8) |
| Surgery, n (%) |
|
|
Yes | 51 (33.8) |
| No | 100 (66.2) |
| Tumor number, n
(%) |
|
|
Single | 62 (41.1) |
|
Multiple | 89 (58.9) |
| Tumor size, n
(%) |
|
| <5
cm | 28 (18.5) |
| ≥5
cm | 123 (81.5) |
| Liver cirrhosis, n
(%) |
|
|
Yes | 45 (29.8) |
| No | 106 (70.2) |
| BCLC stage, n
(%) |
|
| A | 4 (2.6) |
| B | 62 (41.1) |
| C | 85 (56.3) |
| TNM stage, n
(%) |
|
| I | 4 (2.6) |
| II | 55 (36.4) |
|
III | 70 (46.4) |
| IV | 22 (14.6) |
| CEA, n (%) |
|
|
<2.38 ng/ml | 75 (49.7) |
| ≥2.38
ng/ml | 76 (50.3) |
| AFP, n (%) |
|
|
<151.4 ng/ml | 75 (49.7) |
| ≥151.4
ng/ml | 76 (50.3) |
| CA199, n (%) |
|
|
<22.64 U/ml | 74 (49.0) |
| ≥22.64
U/ml | 77 (51.0) |
 | Table IV.Patient blood parameters (n=151). |
Table IV.
Patient blood parameters (n=151).
| Parameter | Median
(interquartile range) |
|---|
| ALT, U/l | 31.00 (22.00,
49.00) |
| AST, U/l | 47.00 (31.00,
47.00) |
| γ-GGT, U/l | 97.00 (47.00,
237.00) |
| ALP, U/l | 120.00 (91.00,
204.00) |
| TBIL, µmol/l | 20.00 (14.50,
32.10) |
| DBIL, µmol/l | 5.00 (3.20,
10.50) |
| IDBIL, µmol/l | 15.30 (11.10,
22.50) |
| TP, g/l | 72.00 (68.10,
77.10) |
| ALB, g/l | 38.30 (34.10,
41.70) |
| GLOB, g/l | 32.90 (29.30,
39.40) |
| A/G | 1.10 (0.90,
1.40) |
| PALB, mg/l | 146.00 (101.00,
200.00) |
| Urea, mmol/l | 5.40 (4.40,
6.90) |
| CREA, µmol/l | 73.00 (63.00,
83.00) |
| UA, µmol/l | 298.00 (232.00,
364.00) |
| CYS-C, mg/l | 0.97 (0.76,
1.07) |
| CO2-CP,
mmol/l | 25.6 (23.6,
27.8) |
| LDH, U/l | 225.00 (184.00,
295.00) |
| Glu, mmol/l | 5.20 (4.70,
5.90) |
| K, mmol/l | 4.00 (3.70,
4.30) |
| Na, mmol/l | 139.00 (137.00,
140.00) |
| Cl, mmol/l | 103.00 (101.00,
105.00) |
| Ca, mmol/l | 2.30 (2.10,
2.40) |
| PHOS, mmol/l | 1.00 (0.90,
1.14) |
| Mg, mmol/l | 0.86 (0.77,
0.95) |
| WBC,
109/l | 6.06 (4.82,
7.64) |
| NEU,
109/l | 4.08 (2.98,
5.18) |
| LYM,
109/l | 1.30 (0.90,
1.70) |
| MON,
109/l | 0.45 (0.31,
0.62) |
| EOS,
109/l | 0.08 (0.04,
0.13) |
| BAS,
109/l | 0.02 (0.01,
0.03) |
| RBC,
109/l | 4.49 (4.05,
4.85) |
| HGB,
109/l | 140.00 (126.00,
155.00) |
| HCT,
×109/l | 41.80 (37.90,
46.60) |
| PLT,
×109/l | 158.00 (109.00,
207.00) |
| PT, sec | 12.20 (11.60,
13.10) |
| INR, | 1.07 (1.01,
1.15) |
| Fbg, g/l | 2.97 (2.39,
3.90) |
| TT, sec | 16.90 (16.20,
17.50) |
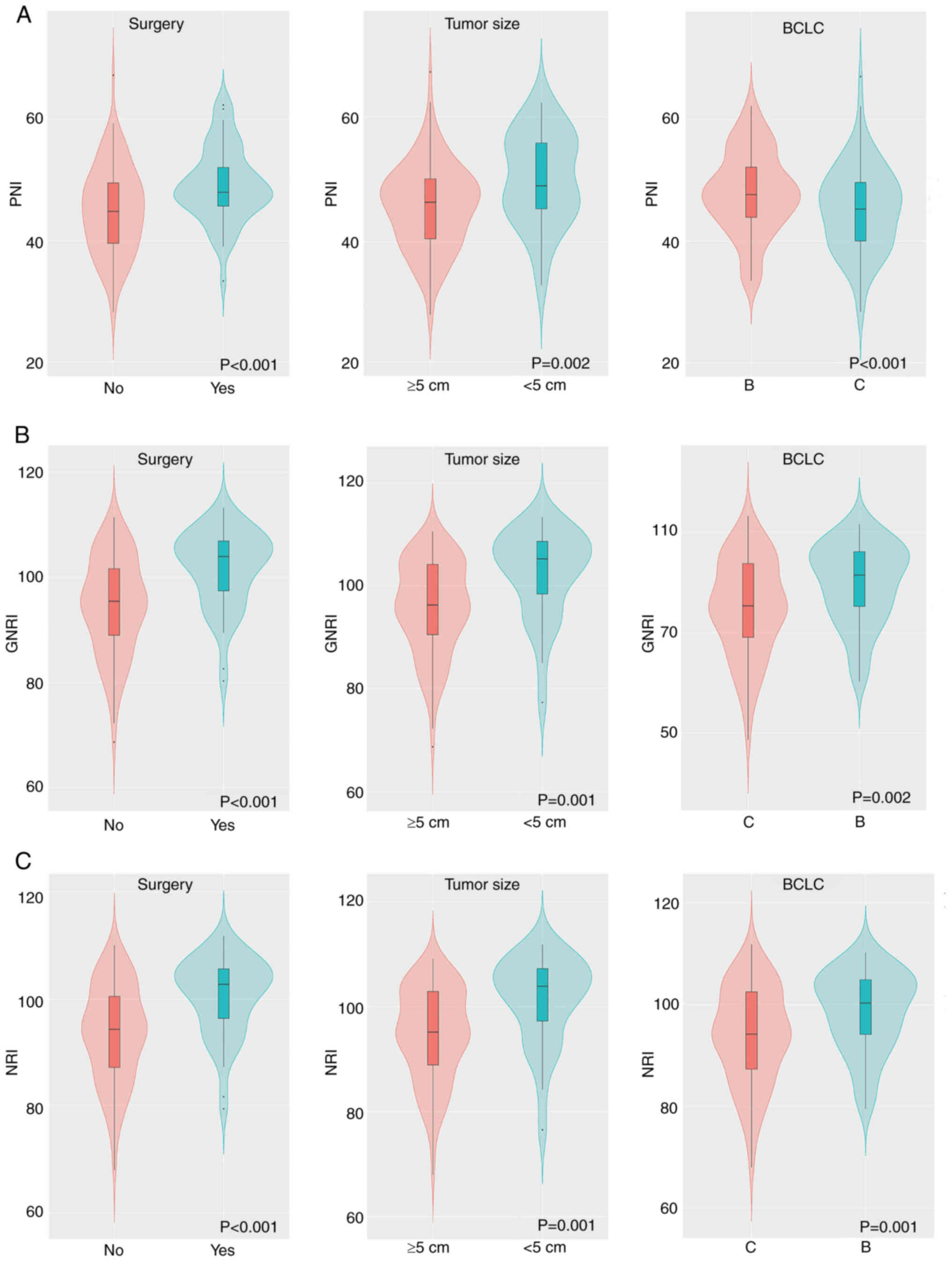
Distribution differences in
inflammation and nutritional marker scores
Differences in the inflammatory and nutritional
marker scores among different surgery, tumor size and BCLC stage
groups were assessed. The nutritional markers (PNI, GNRI and NRI)
all followed a Gaussian distribution. The unpaired independent
samples t-test demonstrated significant differences in these
biomarkers among different surgery, tumor size and BCLC stage
groups (all P<0.05; Fig. 2). The
inflammatory markers (SII, SIRI and ALI) did not demonstrate a
Gaussian distribution and so significant differences between their
maximum and minimum values, the data characteristics and
distributions of these markers could not be evaluated using violin
plots combined with box plots. The median values of SII, SIRI, and
ALI for patients who underwent surgery were 653.64 (313.46,
1165.24), 0.87 (0.56, 3.34), and 45.03 (18.15, 71.64),
respectively, while for patients who did not undergo surgery, the
respective values were 554.55 (355.89, 838.45), 0.97 (0.63, 1.72),
and 34.74 (23.36, 53.25). Furthermore, the median values of SII,
SIRI, and ALI for patients with tumor size <5 cm were 533.28
(311.35, 1033.29), 0.77 (0.45, 1.38), and 35.55 (25.31, 73.70),
respectively, while for patients with tumor size ≥5 cm, the
respective values were 590.21 (350.39, 859.96), 1.01 (0.64, 2.06),
and 36.93 (19.69, 56.51). Median SII, SIRI, and ALI for patients
with BCLC B stage were 592.41 (333.91, 918.23), 0.89 (0.64, 1.74),
and 37.65 (24.68, 64.32), respectively, while for patients with
BCLC C stage, the respective values were 554.55 (349.67, 873.63),
1.03 (0.58, 1.97), and 35.00 (19.83, 55.93). The Mann-Whitney U
test demonstrated significant differences in SII, SIRI and ALI
scores among different surgery, tumor size and BCLC stage groups
(all P<0.05; Table V). These
results suggested a possible significant association between
inflammation and nutritional markers, and disease progression.
 | Table V.SII, SIRI, and ALI scores. |
Table V.
SII, SIRI, and ALI scores.
| A, SII |
|---|
|
|---|
| Characteristic | Rank mean | Sum of ranks | U-value | Z-value | P-value |
|---|
| Surgery |
|
| 2254.500 | −4.428 | <0.001 |
|
Yes | 62.77 | 7204.50 |
|
|
|
| No | 86.53 | 3673.50 |
|
|
|
| Tumor size, cm |
|
| 1517.500 | −3.513 | <0.001 |
|
<5 | 50.20 | 1895.50 |
|
|
|
| ≥5 | 74.85 | 8982.50 |
|
|
|
| BCLC stage |
|
| 2534.500 | −3.394 | <0.001 |
| B | 52.38 | 4487.50 |
|
|
|
| C | 75.18 | 6390.50 |
|
|
|
|
| B, SIRI |
|
|
Characteristic | Rank
mean | Sum of
ranks | U-value | Z-value | P-value |
|
| Surgery |
|
| 2371.000 | −4.350 | <0.001 |
|
Yes | 63.90 | 3547.00 |
|
|
|
| No | 84.05 | 7331.00 |
|
|
|
| Tumor size, cm |
|
| 2280.000 | −2.701 | <0.001 |
|
<5 | 61.41 | 1658.00 |
|
|
|
| ≥5 | 86.83 | 9220.00 |
|
|
|
| BCLC stage |
|
| 2545.400 | −3.710 | <0.001 |
| B | 61.08 | 4407.00 |
|
|
|
| C | 76.13 | 6471.00 |
|
|
|
|
| C, ALI |
|
|
Characteristic | Rank
mean | Sum of
ranks | U-value | Z-value | P-value |
|
| Surgery |
|
| 2095.500 | −2.362 | <0.001 |
|
Yes | 51.17 | 7045.50 |
|
|
|
| No | 79.84 | 3832.50 |
|
|
|
| Tumor size, cm |
|
| 1755.500 | −3.323 | <0.001 |
|
<5 | 61.80 | 8615.50 |
|
|
|
| ≥5 | 93.80 | 2262.50 |
|
|
|
| BCLC stage |
|
| 1336.500 | −3.171 | <0.001 |
| B | 50.49 | 5991.50 |
|
|
|
| C | 88.81 | 4886.50 |
|
|
|
Univariate and multivariate Cox
regression analysis
Cox regression analysis was performed on the disease
characteristics, and the inflammation and nutritional markers of
patients. The univariate results demonstrated that both the PFS and
OS of patients were significantly related to surgery, tumor number,
tumor size, liver cirrhosis, BCLC stage, TNM stage. and all
inflammatory and nutritional markers (all P<0.05; Table VI). In addition, sex was also a
significant prognostic factor for OS. Moreover, the multivariate
analysis found that GNRI, PNI, BCLC stage and TNM stage were
independent prognostic markers for PFS, and GNRI, BCLC stage and
TNM stage were independent prognostic markers for OS.
 | Table VI.Univariate and multivariate analyses
for PFS and OS. |
Table VI.
Univariate and multivariate analyses
for PFS and OS.
|
| PFS | OS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
|
Male | Ref |
|
|
| Ref |
| Ref |
|
|
Female | 1.565 | 0.094 |
|
| 1.760 | 0.035 | 1.041 | 0.900 |
|
| (0.927–2.643) |
|
|
| (1.042–2.973) |
| (0.556–1.947) |
|
| Age | 0.995 | 0.732 |
|
| 0.995 | 0.723 |
|
|
|
| (0.970–1.022) |
|
|
| (0.970–1.021) |
|
|
|
| BMI | 0.939 | 0.070 |
|
| 0.957 | 0.213 |
|
|
|
| (0.877–1.005) |
|
|
| (0.893–1.026) |
|
|
|
| Smoking |
|
|
|
|
|
|
|
|
| No | Ref |
|
|
| Ref |
|
|
|
|
Yes | 1.358 | 0.317 |
|
| 1.346 | 0.332 |
|
|
|
| (0.745–2.475) |
|
|
| (0.739–2.452) |
|
|
|
| Drinking |
|
|
|
|
|
|
|
|
| No | Ref |
|
|
| Ref |
|
|
|
|
Yes | 1.125 | 0.719 |
|
| 1.105 | 0.761 |
|
|
|
| (0.592–2.139) |
|
|
| (0.581–2.100) |
|
|
|
| CEA, U/ml |
|
|
|
|
|
|
|
|
|
<2.38 | Ref |
|
|
| Ref |
|
|
|
|
≥2.38 | 1.360 | 0.191 |
|
| 1.448 | 0.116 |
|
|
|
| (0.858–2.155) |
|
|
| (0.913–2.297) |
|
|
|
| AFP, U/ml |
|
|
|
|
|
|
|
|
|
<151.4 | Ref |
|
|
| Ref |
|
|
|
|
≥151.4 | 1.176 | 0.489 |
|
| 1.290 | 0.278 |
|
|
|
| (0.743–1.862) |
|
|
| (0.814–2.045) |
|
|
|
| CA199, U/ml |
|
|
|
|
|
|
|
|
|
<22.64 | Ref |
|
|
| Ref |
|
|
|
|
≥22.64 | 1.291 | 0.278 |
|
| 1.161 | 0.524 |
|
|
|
| (0.814–2.047) |
|
|
| (0.733–1.838) |
|
|
|
| Surgery |
|
|
|
|
|
|
|
|
|
Yes | Ref |
| Ref |
| Ref |
| Ref |
|
| No | 2.481 | 0.001 | 1.169 | 0.629 | 2.683 | <0.001 | 1.324 | 0.369 |
|
| (1.457–4.222) |
| (0.621–2.202) |
| (1.578–4.562) |
| (0.718–2.439) |
|
| Tumor number |
|
|
|
|
|
|
|
|
|
Single | Ref |
| Ref |
| Ref |
| Ref |
|
|
Multiple | 1.721 | 0.029 | 1.259 | 0.402 | 1.761 | 0.022 | 1.434 | 0.202 |
|
| (1.058–2.800) |
| (0.734–2.161) |
| (1.084–2.860) |
| (0.824–2.493) |
|
| Tumor size, cm |
|
|
|
|
|
|
|
|
|
<5 | Ref |
| Ref |
| Ref |
| Ref |
|
| ≥5 | 3.225 | 0.002 | 1.881 | 0.137 | 3.284 | 0.002 | 2.069 | 0.086 |
|
| (1.537–6.770) |
| (0.818–4.321) |
| (1.568–6.877) |
| (0.901–4.752) |
|
| Liver
cirrhosis |
|
|
|
|
|
|
|
|
| No | Ref |
| Ref |
| Ref |
| Ref |
|
|
Yes | 1.874 | 0.013 | 1.742 | 0.050 | 1.655 | 0.045 | 1.645 | 0.066 |
|
| (1.141–3.076) |
| (1.013–2.998) |
| (1.011–2.708) |
| (0.967–2.797) |
|
| BCLC stage |
|
|
|
|
|
|
|
|
| B | Ref |
| Ref |
| Ref |
| Ref |
|
| C | 2.726 | <0.001 | 1.769 | 0.045 | 3.178 | <0.001 | 2.353 | 0.006 |
|
| (1.667–4.457) |
| (0.999–3.133) |
| (1.938–5.209) |
| (1.276–4.340) |
|
| TNM stage |
|
|
|
|
|
|
|
|
| II | Ref |
| Ref |
| Ref |
| Ref |
|
|
III | 2.314 | <0.001 | 1.345 | 0.037 | 2.782 | <0.001 | 2.017 | 0.004 |
|
| (1.117–3.028) |
| (0.893–2.461) |
| (1.564–4.792) |
| (1.325–4.660) |
|
| IV | 3.406 | <0.001 | 1.970 | 0.025 | 3.749 | <0.001 | 2.807 | 0.001 |
|
| (2.040–5.688) |
| (1.091–3.559) |
| (2.246–6.258) |
| (1.487–5.298) |
|
| PNI |
|
|
|
|
|
|
|
|
|
<43.37 | Ref |
| Ref |
| Ref |
| Ref |
|
|
≥43.37 | 4.189 | <0.001 | 2.069 | 0.046 | 2.606 | <0.001 | 1.212 | 0.572 |
|
| (2.497–7.029) |
| (1.013–4.226) |
| (1.628–4.170) |
| (0.623–2.358) |
|
| GNRI |
|
|
|
|
|
|
|
|
|
<88.92 | Ref |
| Ref |
| Ref |
| Ref |
|
|
≥88.92 | 7.855 | <0.001 | 2.841 | 0.019 | 5.889 | <0.001 | 2.654 | 0.031 |
|
| (4.443–13.919) |
| (1.183–6.821) |
| (3.441–10.078) |
| (1.094–6.438) |
|
| NRI |
|
|
|
|
|
|
|
|
|
<92.22 | Ref |
| Ref |
| Ref |
| Ref |
|
|
≥92.22 | 3.170 | <0.001 | 1.077 | 0.852 | 3.021 | <0.001 | 1.541 | 0.292 |
|
| (1.959–5.131) |
| (0.494–2.348) |
| (1.880–4.853) |
| (0.689–3.447) |
|
| SII |
|
|
|
|
|
|
|
|
|
<377.03 | Ref |
| Ref |
| Ref |
| Ref |
|
|
≥377.03 | 1.849 | 0.027 | 1.071 | 0.857 | 2.006 | 0.012 | 1.004 | 0.992 |
|
| (1.073–3.186) |
| (0.510–2.249) |
| (1.163–3.460) |
| (0.473–2.130) |
|
| SIRI |
|
|
|
|
|
|
|
|
|
<1.02 | Ref |
| Ref |
| Ref |
| Ref |
|
|
≥1.02 | 1.962 | 0.005 | 1.703 | 0.157 | 2.134 | 0.002 | 1.796 | 0.124 |
|
| (1.228–3.134) |
| (0.815–3.555) |
| (1.332–3.421) |
| (0.852–3.789) |
|
| ALI |
|
|
|
|
|
|
|
|
|
<30.31 | Ref |
| Ref |
| Ref |
| Ref |
|
|
≥30.31 | 1.884 | 0.007 | 1.149 | 0.714 | 1.956 | 0.004 | 1.502 | 0.280 |
|
| (1.188–2.987) |
| (0.547–2.416) |
| (1.232–3.105) |
| (0.718–3.142) |
|
Survival analysis for inflammatory and
nutritional markers
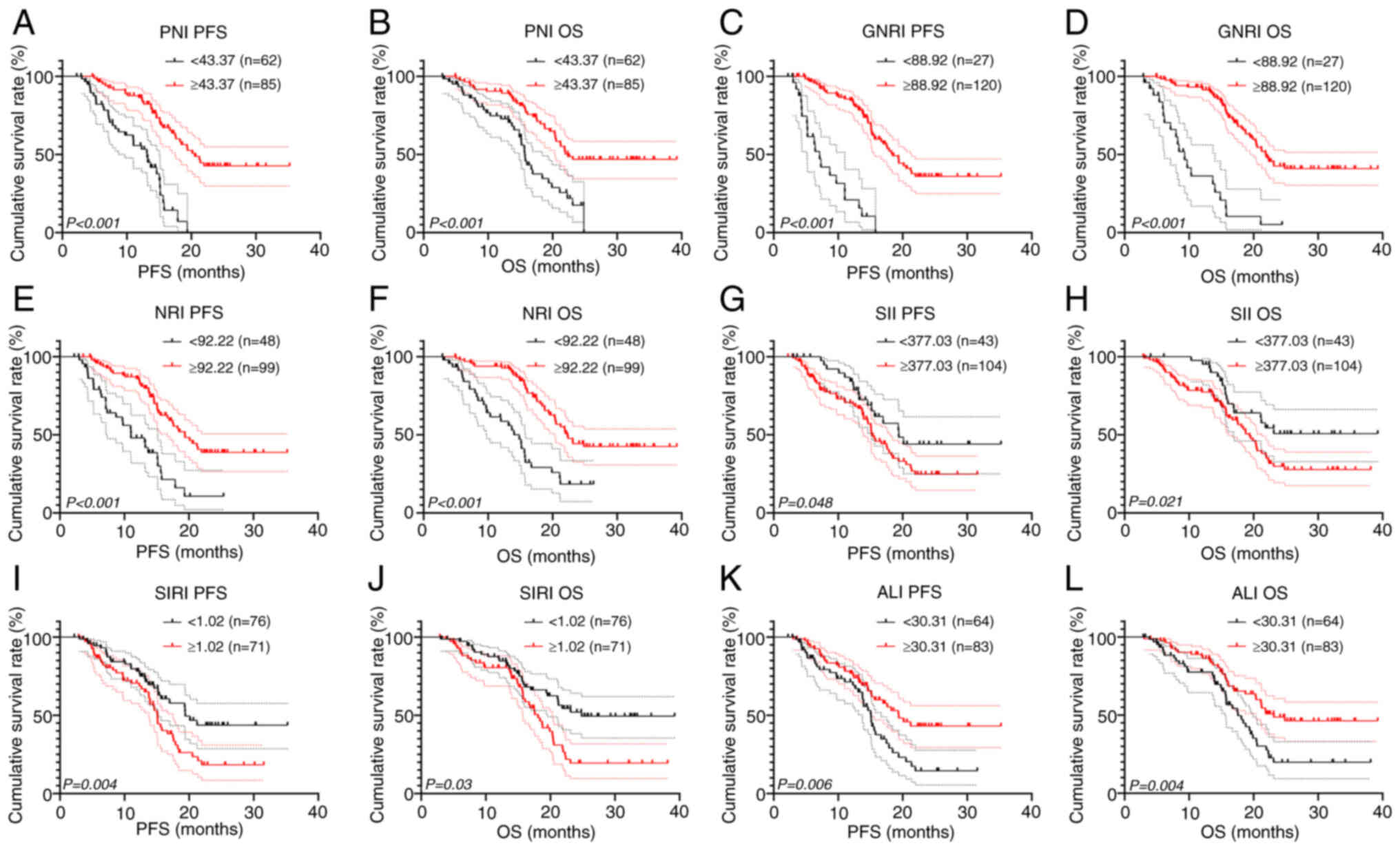
In the present study, survival analysis was
performed for inflammation and nutritional markers after grouping
and survival curves were plotted. There were 62 cases with a PNI
<43.37 and 85 cases with a PNI ≥43.37. Patients with a low PNI
had significantly shorter PFS (13.14 vs. 20.53 months; P<0.001)
and OS (15.70 vs. 22.37 months; P<0.001) times compared with
patients with a high PNI (Fig. 3A and
B). Furthermore, there were 27 patients with a GNRI <88.92
and 120 patients with a GNRI ≥88.92. Patients with a GNRI <88.92
had significantly shorter PFS (7.13 vs. 18.43 months; P<0.001)
and OS (9.30 vs. 21.87 months; P<0.001) times compared with
patients with a GNRI ≥88.92 (Fig. 3C
and D). There were 48 patients with a NRI <92.22 and 99
cases with a NRI ≥92.22. Patients with a NRI <92.22 had
significantly shorter PFS (11.02 vs. 19.39 months; P<0.001) and
OS (14.01 vs. 22.27 months; P<0.001) times compared with
patients with NRI ≥92.22 (Fig. 3E and
F).
For the inflammatory markers, there were 43 cases
with a SII <377.03 and 104 cases with SII ≥377.03. Patients with
a SII ≥377.03 had significantly shorter PFS (20.13 vs. 15.24
months; P=0.048) and OS (not reached vs. 18.77 months; P=0.021)
times compared with patients with a SII <377.03 (Fig. 3G and H). Furthermore, 76 patients
had a SIRI <1.02 and 71 patients had a SIRI ≥1.02. Patients with
a SIRI ≥1.02 had significantly shorter PFS (20.13 vs. 15.07 months;
P=0.004) and OS (not reached vs. 17.57 months; P=0.003) times
compared with patients with a SIRI <1.02 (Fig. 3I and J). Finally, there were 64
cases with an ALI <30.31 and 83 cases with an ALI ≥30.31.
Patients with an ALI <30.31 had significantly shorter PFS (15.10
vs. 19.39 months; P=0.006) and OS (17.57 vs. 23.13 months; P=0.004)
times compared with patients with an ALI ≥30.31 (Fig. 3K and L).
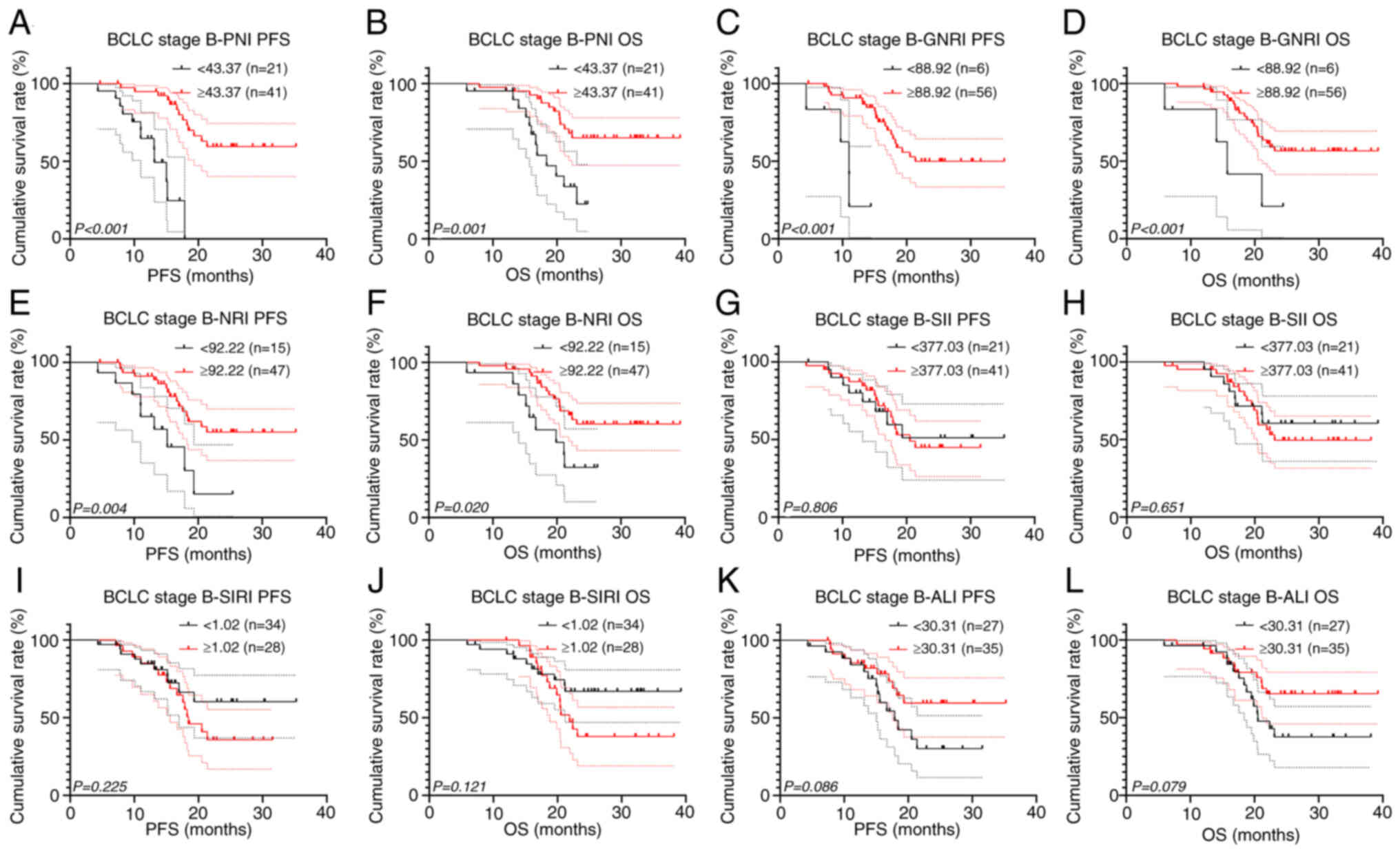
Subgroup survival analysis
To further investigate the prognostic value of
inflammatory and nutritional biomarkers, subgroup survival analyses
among patients with different BCLC stages were performed. There
were 62 patients (42.2%) in BCLC stage B, among whom 30 patients
(48.4%) underwent surgery and targeted therapy combined with
immunotherapy, while 32 patients (51.6%) only received targeted
therapy combined with immunotherapy. After analyzing all BCLC stage
B patients, the results revealed significant associations between
PNI, GNRI and NRI, and survival among patients with BCLC stage B
(all P<0.001; Fig. 4). However,
it is worth noting that the results for GNRI may be biased due to a
small sample size of only 6 patients in the low GNRI group.
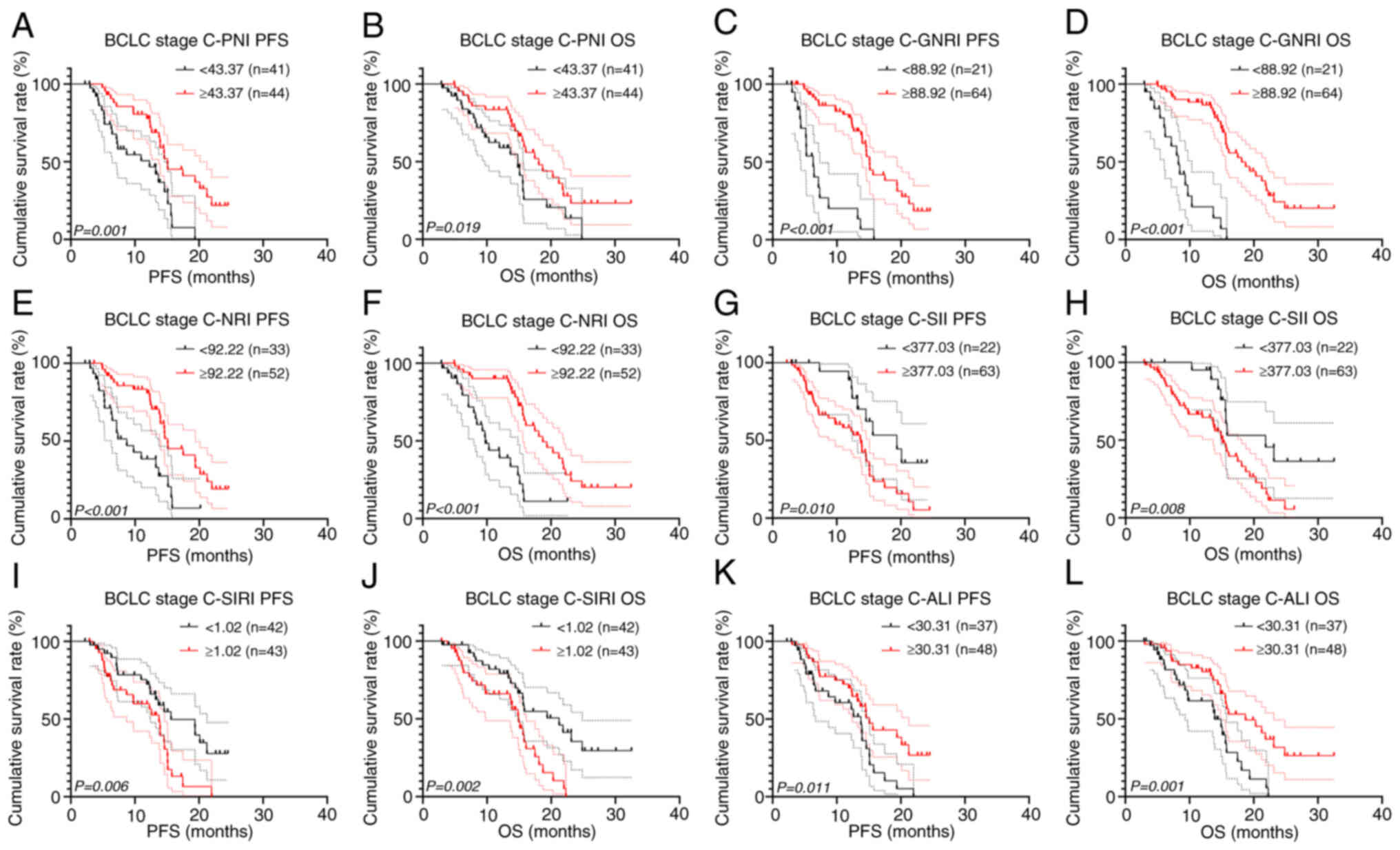
Furthermore, there were 85 patients (57.8%) with BCLC stage C,
among whom 18 patients (21.2%) underwent surgery and targeted
therapy combined with immunotherapy, while 67 patients (78.8%) only
received targeted therapy combined with immunotherapy. After
analyzing all BCLC stage C patients, we found that all inflammatory
and nutritional indicators demonstrated significant prognostic
value in patients with BCLC stage C (all P<0.05; Fig. 5).
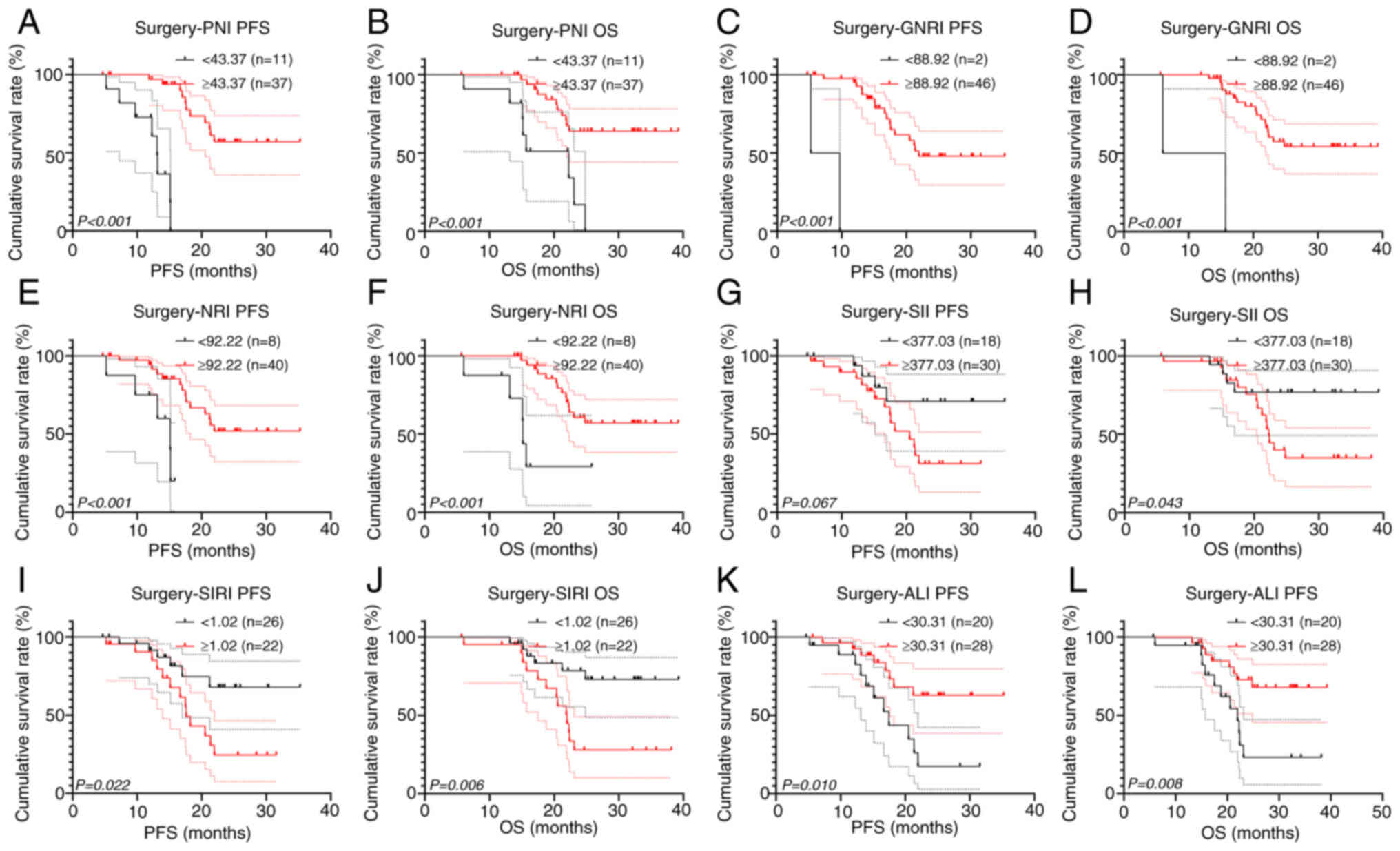
Subgroup survival analysis was also performed on
surgical and non-surgical patients. Among patients who underwent
surgery, all inflammatory and nutritional markers demonstrated
significant prognostic value for OS, and all except SII
demonstrated significant prognostic value for PFS (all P<0.05;
Fig. 6). However, the results of
GNRI were also limited in their reference value due to the small
sample size of the GNRI <88.92 group (n=2). Furthermore, PNI,
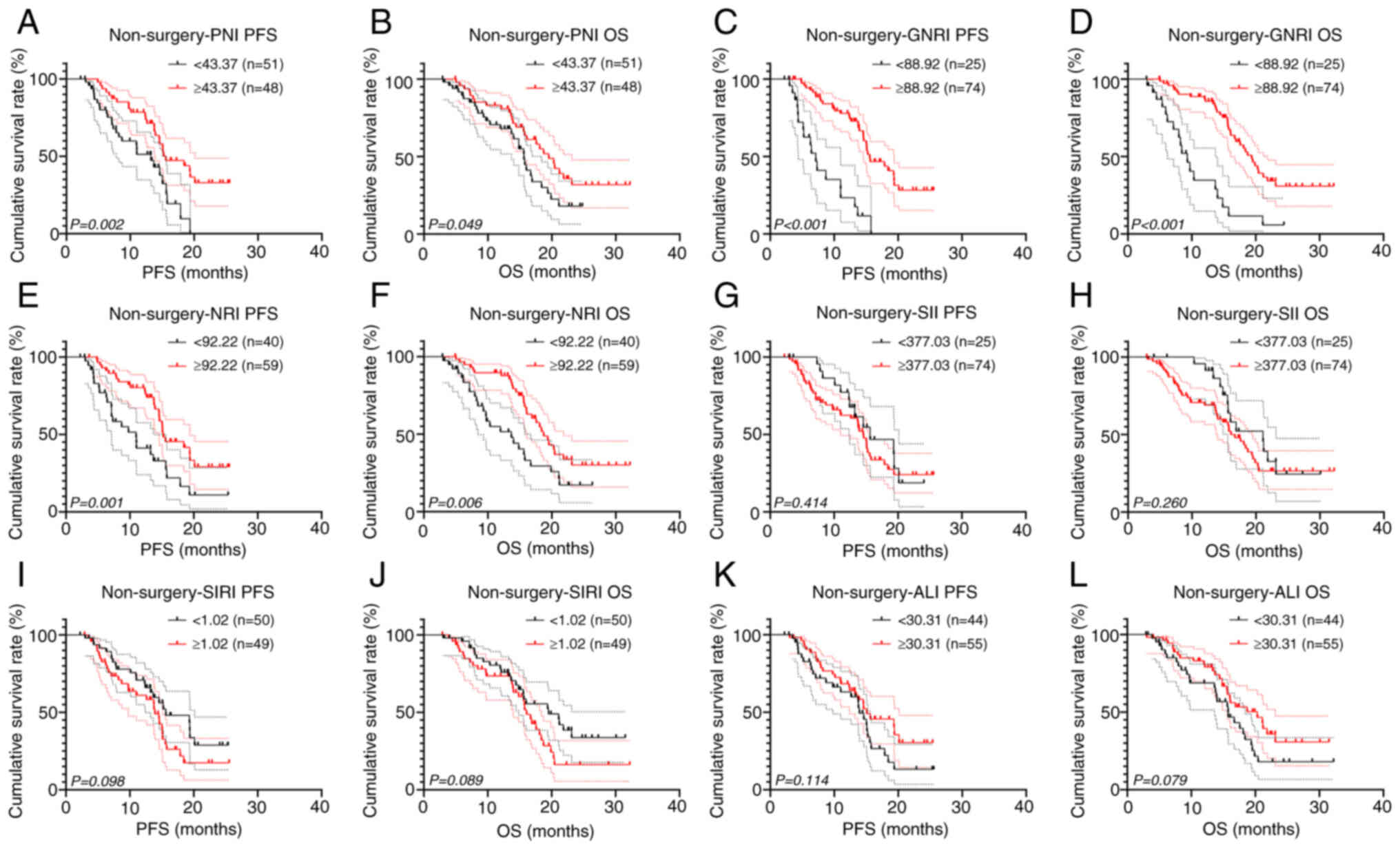
GNRI, and NRI also demonstrated significant prognostic value in
non-surgical patients (all P<0.05; Fig. 7).
Prognostic value of inflammation and
nutritional markers
In the ROC curves based on death, it was
demonstrated that SIRI and ALI had a markedly higher Youden index
and AUC than inflammation and nutritional markers in this study
(Fig. 1; Table II). To evaluate the prognostic
value of the inflammation and nutritional markers, time-ROC curves
based on PFS and OS were plotted (Fig.
8), the results demonstrated that the prognostic values of
GNRI, NRI and PNI were higher than those for SII, SIRI and ALI, at
all times, with the prognostic value of GNRI being the highest at
all times.
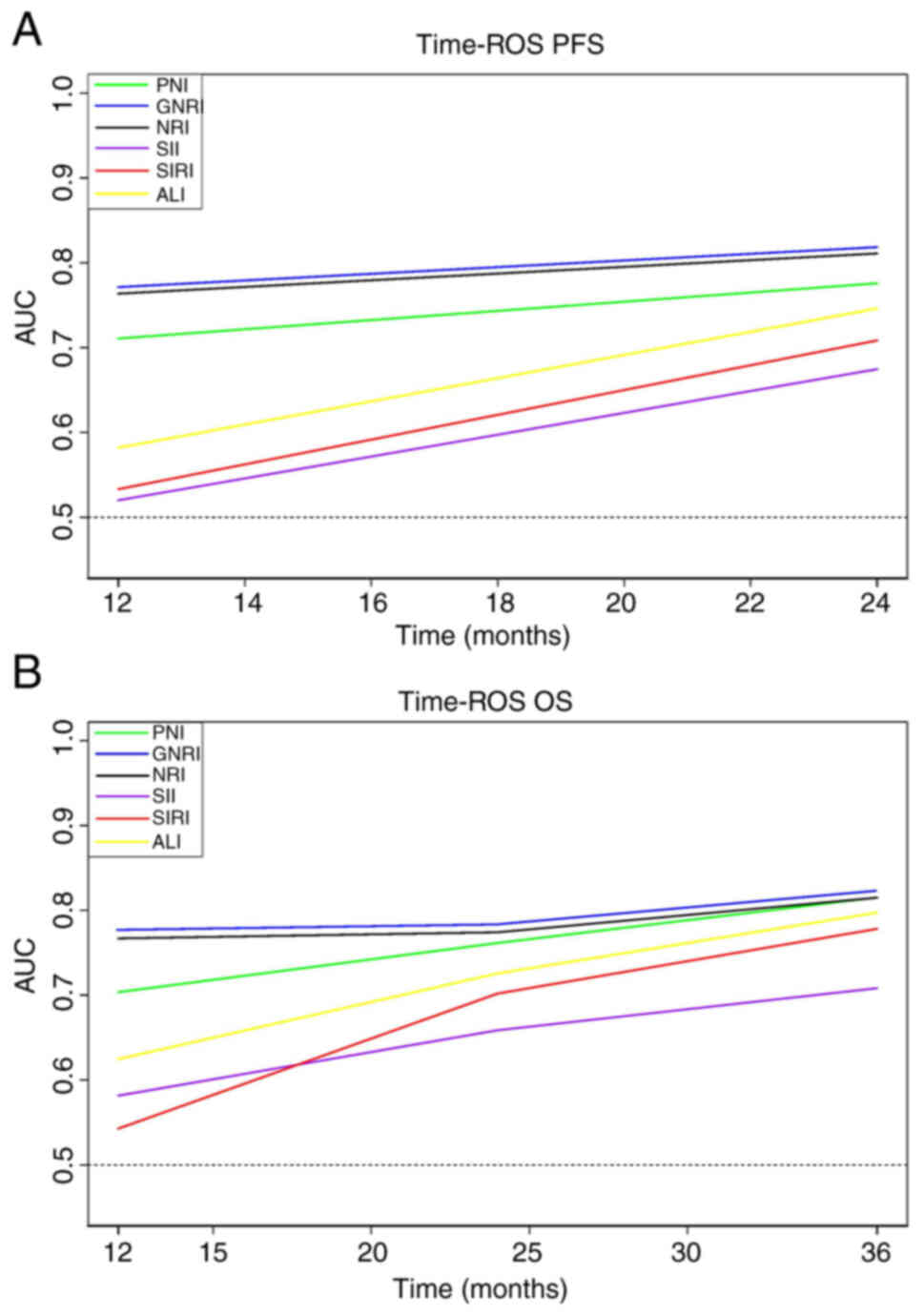 | Figure 8.Inflammation and nutritional
marker-related time-ROC curves of PFS and OS. Time-ROC curves of
(A) PFS and (B) OS. AUC of GNRI was consistently higher than that
of other indicators at all time points, indicating its superior
predictive value. AUC, area under the curve; ROC, receiver
operating characteristic; PFS, progression-free survival; OS,
overall survival; PNI, prognostic nutrition index; NRI, nutritional
risk index; GNRI, geriatric NRI; SII, systemic immune-inflammation
index; SIRI, systemic inflammation response index; ALI, advanced
lung cancer inflammation index. |
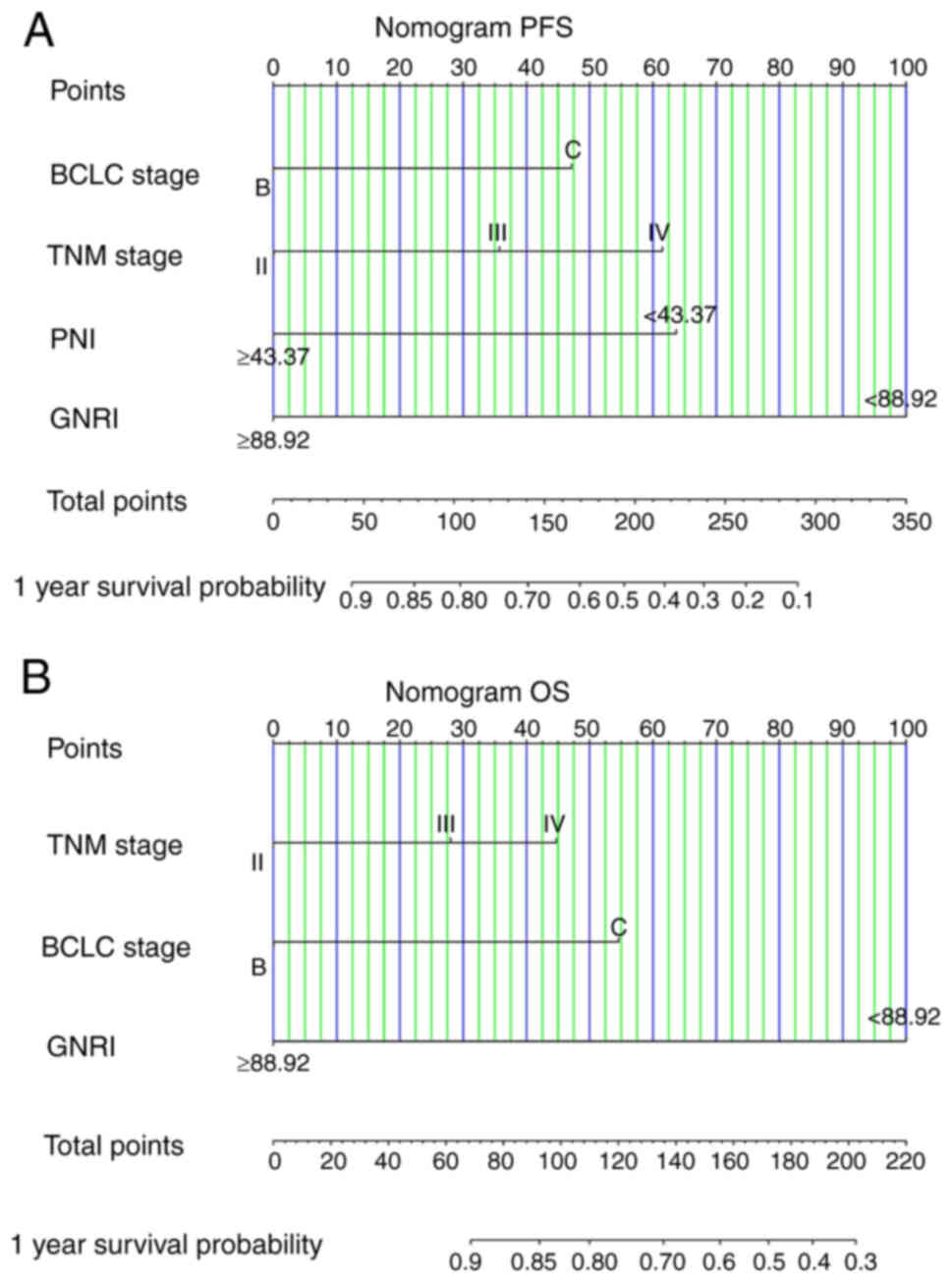
Nomograms predict survival
probability
Due to the identification of PNI and GNRI as
independent prognostic factors according to the Cox regression
analysis, predictive models for patients with HCC who received ICIs
were constructed to further evaluate their prognostic value
(Fig. 9). The C-index (95% CI) of
the nomograms for PFS and OS were 0.801 (0.746–0.877) and 0.823
(0.761–0.898), reflecting the high predictive accuracy of the
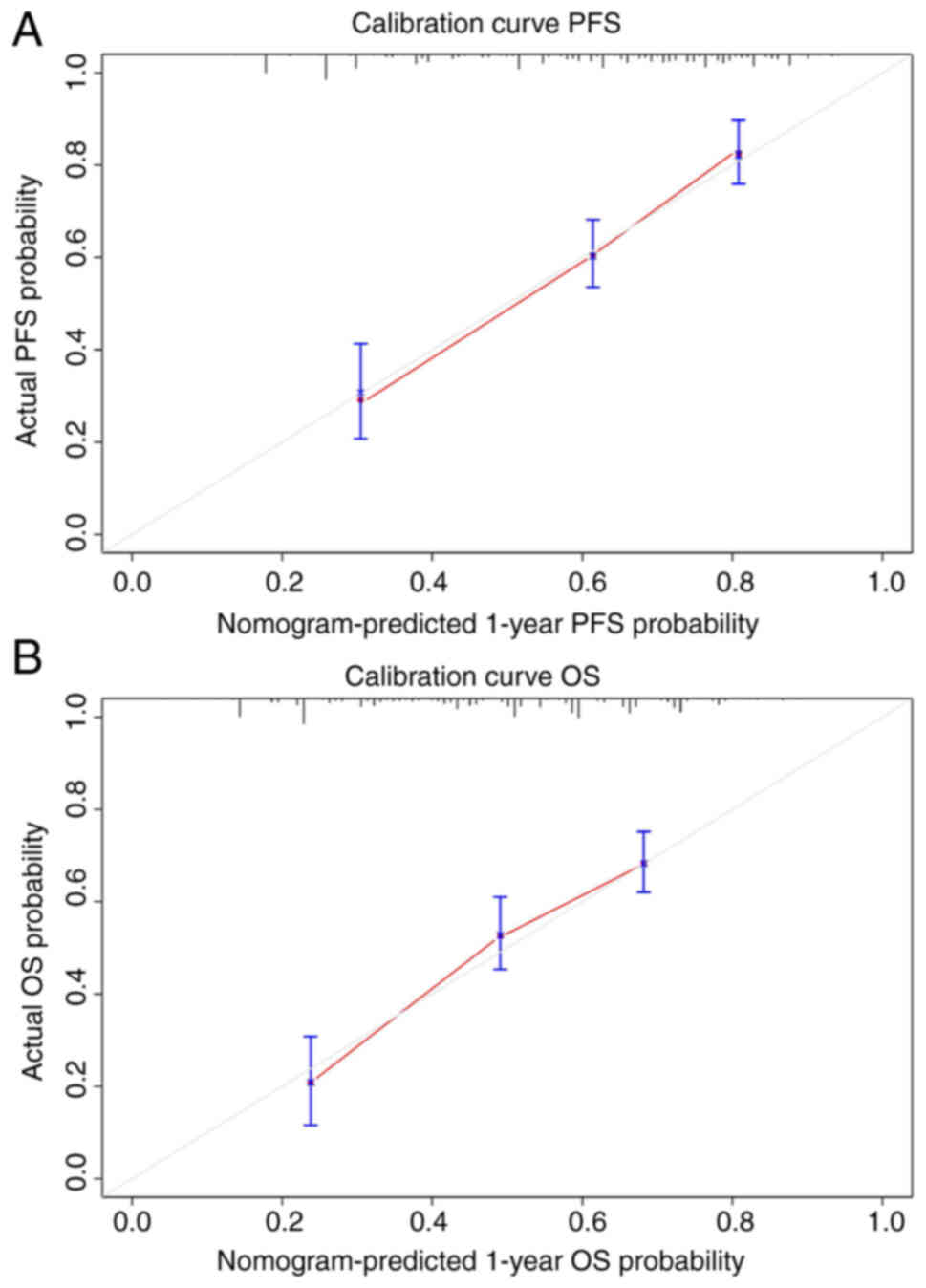
nomograms. Due to limited number of patients, bootstrap validation
was performed on the nomograms and calibration curves were plotted
(Fig. 10), which demonstrated the
high predictive performance of the nomograms.
Discussion
The emergence of ICIs has changed the cancer
treatment landscape, increasing patient survival (33). However, patients with solid tumors
have a low responsiveness to ICIs and only a subset of patients may
benefit from ICI treatment, including patients with HCC (34,35).
Existing biomarkers are costly to assess and may not be applicable
to all patients, making it difficult to further promote their use
(36). Non-invasive biomarkers
based on the inflammatory and nutritional status of patients have
gained widespread attention due to their ease of acquisition and
accuracy and have been used to predict the efficacy of ICIs in
certain solid tumors with satisfactory results. Mezquita et
al (37) conducted a
multicenter study on lung cancer in 2018 to validate the accuracy
of non-invasive indicators in predicting the efficacy of ICIs. They
established a lung immune prognostic index by combining the derived
neutrophil-to-lymphocyte ratio (dNLR) and lactate dehydrogenase
(LDH) and found a significant correlation between this index and
adverse outcomes of ICIs (37). The
present study evaluated the predictive ability of commonly used
inflammatory and nutritional markers on the prognosis of patients
with HCC who received ICIs, offering broader references for
selecting treatment strategies for these patients.
Nutritional status is closely related to immune
function, and nutritional indicators have been widely studied in
the application of ICIs. In 2022, Sun et al (38) collected the data of 146 patients
with gastric cancer who received ICIs or chemotherapy and analyzed
the predictive efficacy of PNI in these patients. The results
demonstrated that PNI was not only a prognostic indicator for ICIs
and chemotherapy in patients, but also an independent prognostic
biomarker for disease-free survival. It is worth noting that, since
ICIs were still not a standard treatment and were expensive, only a
few patients with advanced gastric cancer considered using them,
resulting in a significantly higher survival rate for patients who
received chemotherapy than those who received ICIs (38). Certain studies focusing on GNRI
validated the association between nutritional status and the
efficacy of ICIs (39–41). Sonehara et al (42) assessed the survival time of 85
patients with advanced non-small cell lung cancer who were treated
with ICIs and reported that those with a low GNRI had a shorter
survival time. Other studies on nutritional indicators in patients
receiving ICIs have reported similar findings (23,43,44).
Inflammation is another of the factors that affect immune function.
A study on renal cell carcinoma found that SII was a significant
factor in disease progression and prognosis after analyzing its
application in 49 patients who received ICIs combined with targeted
therapy (45). Qi et al
(46) extensively studied the
application of inflammatory biomarkers in patients with small-cell
lung cancer receiving ICIs. The survival status of 53 patients was
prospectively analyzed and it was found that inflammatory markers
were related to prognosis, in particular the platelet-lymphocyte
ratio. ALI is also an accurate indicator of the inflammatory status
of patients. Mountzios et al (47) collected the data of 672 patients
with non-small cell lung cancer who received ICIs and analyzed the
application of ALI. The results demonstrated that ALI was a
significant prognostic factor for patients who received ICIs, and a
high ALI was associated with a longer survival time. Subsequent
studies also reported the value of ALI in predicting the prognosis
of other tumors (48–50). In summary, certain inflammatory and
nutritional markers have been reported to be related to the
prognosis of patients with cancer.
The present study analyzed the data of 151 patients
with HCC who received ICIs, to evaluate the prognostic value of
classic nutritional and inflammatory markers with a larger sample
size of patients than previous studies (51–53).
As with previous studies, ICIs were not the preferred treatment for
solid tumors and patients who received ICIs had a poor clinical and
pathological status (38,54). Only one-third of patients in the
present study received surgical treatment, and more than one-half
of these patients had BCLC stage C and TNM stage III + IV. Survival
analysis demonstrated that PNI, GNRI, NRI, SII, SIRI and ALI were
all significantly associated with patient prognosis. Subgroup
survival analysis also indicated that nutritional markers
maintained significant prognostic value in all patients.
Furthermore, although the death-based ROC curves had higher AUCs
for SIRI and ALI, both the time-ROC curve and multivariate Cox
regression analysis indicated predictive advantages for PNI, GNRI
and NRI. Moreover, GNRI had the highest prognostic value in the
present study. The nomograms indicated that the prognostic value of
GNRI exceeded the value of the BCLC and TNM stage, which might be
due to the uneven distribution of patients in different stages in
the present study.
ICIs are a novel type of cancer treatment that
inhibit receptors, such as PD-1, PD-L1 and CTLA-4, on the surface
of tumor cells, thus enhancing the ability of immune cells to
attack tumors (55). Therefore, the
effectiveness of ICIs relies on normal immune function. The
nutritional and inflammatory status could affect the immune system
of the patient, thereby affecting its cytotoxicity against tumors,
and consequently influencing the effectiveness of immunotherapy
(56,57). Firstly, malnutrition could affect
the growth and function of immune cells, thereby reducing the
immune response to tumors. For example, a lack of protein and
energy could lead to a decrease in the number and activity of T
cells and B cells, and a decrease in the phagocytic function of
macrophages, thereby decreasing the antitumor ability of the body
(58,59). Secondly, inflammatory status can
have a negative impact on the immune system (60). Inflammation can deplete the nutrient
reserves in the body, and lead to persistent activation of immune
cells and inflammatory responses, thereby inhibiting the immune
response to tumors and enhancing tumor escape mechanisms (61,62).
Albumin levels and weight not only reflect the nutritional status
of patients, but also indicate their liver function reserve and
treatment tolerance (63,64). Furthermore, 'a previous study
reported that low levels of serum albumin are associated with
systemic inflammation (65).
Lymphocytes are a major component of both cellular and humoral
immunity and serve key roles in the antitumor process (66,67).
Low levels of lymphocytes can restrict the ability of the immune
system to fight tumors, leading to accelerated tumor progression
and metastasis. Moreover, the levels of neutrophils, monocytes and
platelets can also reflect the inflammatory status of the patient
and can promote tumor progression and metastasis (68–71).
This may enable classic inflammatory and nutritional markers to
accurately predict the prognosis of patients with HCC who receive
ICIs. Furthermore, GNRI includes changes in patient weight after
illness, which dynamically reflects the patient condition compared
to other indicators and may more accurately reflect the condition
of the patient. Albumin is synthesized by the liver and may more
accurately reflect the liver status of patients with HCC. This may
give GNRI a significant advantage in the prediction of clinical
outcomes in patients with HCC.
The present study had certain limitations. First,
the information bias inherent to retrospective studies could not be
avoided. Especially as ICIs have not yet been routinely used in
treating HCC and the number of patients who received ICIs in the
present study was still relatively small. Second, the cut-off
values of the biomarkers considered in the present study need to be
further evaluated in studies with a larger sample size. Finally,
the prognostic value of GNRI requires further validation through
prospective studies.
In conclusion, the present study found that PNI,
GNRI, NRI, SII, SIRI and ALI were all associated with the efficacy
of ICIs in HCC and could serve as non-invasive biomarkers for ICI
effectiveness. In addition, nutritional markers had greater
predictive ability than inflammatory markers in the present study,
with GNRI being the biomarker with the best prognostic value.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Beijing Medical and
Health Foundation (grant no. YWJKJJHKYJJ-LC19009) and The Beijing
Medical Award Foundation (grant no. YXJL-2022-1350-0312).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL and HZ performed the study and wrote the
manuscript. Data curation and investigation was performed by RZ and
ZG. PW was responsible for data analysis and interpretation. ZQ
designed and performed the study and reviewing and editing the
manuscript. All authors read and approved the final manuscript. CL
and ZQ confirm the authenticity of all the raw data.
Ethics approval and consent to
participate
This study was approved by The Ethics Committee of
Harbin Medical University Cancer Hospital (Harbin, China; approval
no., ALTN-AK105-III-06.). Due to the retrospective nature of this
investigation, The Ethics Committee of Harbin Medical University
Cancer Hospital waived the requirement for informed patient
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wang Y and Deng B: Hepatocellular
carcinoma: Molecular mechanism, targeted therapy, and biomarkers.
Cancer Metastasis Rev. Feb 2–2023.(Epub ahead of print). View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang JD, Hainaut P, Gores GJ, Amadou A,
Plymoth A and Roberts LR: A global view of hepatocellular
carcinoma: Trends, risk, prevention and management. Nat Rev
Gastroenterol Hepatol. 16:589–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang C, Zhang H, Zhang L, Zhu AX, Bernards
R, Qin W and Wang C: Evolving therapeutic landscape of advanced
hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol.
20:203–222. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bagchi S, Yuan R and Engleman EG: Immune
checkpoint inhibitors for the treatment of cancer: Clinical impact
and mechanisms of response and resistance. Annu Rev Pathol.
16:223–249. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Naimi A, Mohammed RN, Raji A, Chupradit S,
Yumashev AV, Suksatan W, Shalaby MN, Thangavelu L, Kamrava S,
Shomali N, et al: Tumor immunotherapies by immune checkpoint
inhibitors (ICIs); the pros and cons. Cell Commun Signal.
20:442022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yap TA, Parkes EE, Peng W, Moyers JT,
Curran MA and Tawbi HA: Development of immunotherapy combination
strategies in cancer. Cancer Discov. 11:1368–1397. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Campos Bragagnoli A, et al: First-line nivolumab plus chemotherapy
versus chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bote H, Mesas A, Baena J, Herrera M and
Paz-Ares L: Emerging immune checkpoint inhibitors for the treatment
of non-small cell lung cancer. Expert Opin Emerg Drugs. 27:289–300.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jácome AA, Castro ACG, Vasconcelos JPS,
Silva MHCR, Lessa MAO, Moraes ED, Andrade AC, Lima FMT, Farias JPF,
Gil RA, et al: Efficacy and safety associated with immune
checkpoint inhibitors in unresectable hepatocellular carcinoma: A
meta-analysis. JAMA Netw Open. 4:e21361282021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng Y, Wang S, Cai J, Ke A and Fan J:
The progress of immune checkpoint therapy in primary liver cancer.
Biochim Biophys Acta Rev Cancer. 1876:1886382021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bai J, Liang P, Li Q, Feng R and Liu J:
Cancer immunotherapy-immune checkpoint inhibitors in hepatocellular
carcinoma. Recent Pat Anticancer Drug Discov. 16:239–248. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Finn RS, Ryoo BY, Merle P, Kudo M,
Bouattour M, Lim HY, Breder VV, Edeline J, Chao Y, Ogasawara S, et
al: Results of KEYNOTE-240: Phase 3 study of pembrolizumab (Pembro)
vs best supportive care (BSC) for second line therapy in advanced
hepatocellular carcinoma (HCC). J Clin Oncol. 37 (15
Suppl):S40042019. View Article : Google Scholar
|
|
15
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doroshow DB, Bhalla S, Beasley MB, Sholl
LM, Kerr KM, Gnjatic S, Wistuba II, Rimm DL, Tsao MS and Hirsch FR:
PD-L1 as a biomarker of response to immune-checkpoint inhibitors.
Nat Rev Clin Oncol. 18:345–362. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rizzo A, Ricci AD and Brandi G: PD-L1,
TMB, MSI, and other predictors of response to immune checkpoint
inhibitors in biliary tract cancer. Cancers (Basel). 13:5582021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Valero C, Lee M, Hoen D, Weiss K, Kelly
DW, Adusumilli PS, Paik PK, Plitas G, Ladanyi M, Postow MA, et al:
Pretreatment neutrophil-to-lymphocyte ratio and mutational burden
as biomarkers of tumor response to immune checkpoint inhibitors.
Nat Commun. 12:7292021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wei J, Feng J, Weng Y, Xu Z, Jin Y, Wang
P, Cui X, Ruan P, Luo R, Li N and Peng M: The prognostic value of
ctDNA and bTMB on immune checkpoint inhibitors in human cancer.
Front Oncol. 11:7069102021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nabet BY, Esfahani MS, Moding EJ, Hamilton
EG, Chabon JJ, Rizvi H, Steen CB, Chaudhuri AA, Liu CL, Hui AB, et
al: Noninvasive early identification of therapeutic benefit from
immune checkpoint inhibition. Cell. 183:363–376.e13. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Alwarawrah Y, Kiernan K and MacIver NJ:
Changes in nutritional status impact immune cell metabolism and
function. Front Immunol. 9:10552018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xia H, Zhang W, Zheng Q, Zhang Y, Mu X,
Wei C, Wang X and Liu Y: Predictive value of the prognostic
nutritional index in advanced non-small cell lung cancer patients
treated with immune checkpoint inhibitors: A systematic review and
meta-analysis. Heliyon. 9:e174002023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ni L, Huang J, Ding J, Kou J, Shao T, Li
J, Gao L, Zheng W and Wu Z: Prognostic nutritional index predicts
response and prognosis in cancer patients treated with immune
checkpoint inhibitors: A systematic review and meta-analysis. Front
Nutr. 9:8230872022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tian BW, Yang YF, Yang CC, Yan LJ, Ding
ZN, Liu H, Xue JS, Dong ZR, Chen ZQ, Hong JG, et al: Systemic
immune-inflammation index predicts prognosis of cancer
immunotherapy: Systemic review and meta-analysis. Immunotherapy.
14:1481–1496. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kou J, Huang J, Li J, Wu Z and Ni L:
Systemic immune-inflammation index predicts prognosis and
responsiveness to immunotherapy in cancer patients: A systematic
review and meta-analysis. Clin Exp Med. Mar 26–2023.(Epub ahead of
print). View Article : Google Scholar
|
|
26
|
Llovet JM, Brú C and Bruix J: Prognosis of
hepatocellular carcinoma: The BCLC staging classification. Semin
Liver Dis. 19:329–338. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Colombo M and Sangiovanni A: Treatment of
hepatocellular carcinoma: Beyond international guidelines. Liver
Int. 35 (Suppl 1):S129–S138. 2015. View Article : Google Scholar
|
|
29
|
Chonprasertsuk S and Vilaichone RK:
Epidemiology and treatment of hepatocellular carcinoma in Thailand.
Jpn J Clin Oncol. 47:294–297. 2017.PubMed/NCBI
|
|
30
|
Huang J, Zhang Y, Peng Z, Gao H, Xu L,
Jiao LR and Chen M: A modified TNM-7 staging system to better
predict the survival in patients with hepatocellular carcinoma
after hepatectomy. J Cancer Res Clin Oncol. 139:1709–1719. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xing R, Gao J, Cui Q and Wang Q:
Strategies to improve the antitumor effect of immunotherapy for
hepatocellular carcinoma. Front Immunol. 12:7832362021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tsilimigras DI, Bagante F, Sahara K, Moris
D, Hyer JM, Wu L, Ratti F, Marques HP, Soubrane O, Paredes AZ, et
al: Prognosis after resection of barcelona clinic liver cancer
(BCLC) stage 0, A, and B hepatocellular carcinoma: A comprehensive
assessment of the current BCLC classification. Ann Surg Oncol.
26:3693–3700. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Hu H, Yuan X, Fan X and Zhang C:
Advances in immune checkpoint inhibitors for advanced
hepatocellular carcinoma. Front Immunol. 13:8967522022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang Z, Wang Y, Gao P and Ding J: Immune
checkpoint inhibitor resistance in hepatocellular carcinoma. Cancer
Lett. 555:2160382023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schoenfeld AJ and Hellmann MD: Acquired
resistance to immune checkpoint inhibitors. Cancer Cell.
37:443–455. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li N, Hou X, Huang S, Tai R, Lei L, Li S,
Abuliz A, Wang G and Yang S: Biomarkers related to immune
checkpoint inhibitors therapy. Biomed Pharmacother. 147:1124702022.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mezquita L, Auclin E, Ferrara R, Charrier
M, Remon J, Planchard D, Ponce S, Ares LP, Leroy L,
Audigier-Valette C, et al: Association of the lung immune
prognostic index with immune checkpoint inhibitor outcomes in
patients with advanced non-small cell lung cancer. JAMA Oncol.
4:351–357. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun H, Chen L, Huang R, Pan H, Zuo Y, Zhao
R, Xue Y and Song H: Prognostic nutritional index for predicting
the clinical outcomes of patients with gastric cancer who received
immune checkpoint inhibitors. Front Nutr. 9:10381182022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Haas M, Lein A, Fuereder T, Brkic FF,
Schnoell J, Liu DT, Kadletz-Wanke L, Heiduschka G and Jank BJ: The
geriatric nutritional risk index (GNRI) as a prognostic biomarker
for immune checkpoint inhibitor response in recurrent and/or
metastatic head and neck cancer. Nutrients. 15:8802023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang Y and Ni Q: Prognostic and
clinicopathological significance of systemic immune-inflammation
index in cancer patients receiving immune checkpoint inhibitors: A
meta-analysis. Ann Med. 55:808–819. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee JH, Hyung S, Lee J and Choi SH:
Visceral adiposity and systemic inflammation in the obesity paradox
in patients with unresectable or metastatic melanoma undergoing
immune checkpoint inhibitor therapy: A retrospective cohort study.
J Immunother Cancer. 10:e0052262022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sonehara K, Tateishi K, Araki T, Komatsu
M, Yamamoto H and Hanaoka M: Prognostic value of the geriatric
nutritional risk index among patients with previously treated
advanced non-small cell lung cancer who subsequently underwent
immunotherapy. Thorac Cancer. 12:1366–1372. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Shoji F, Takeoka H, Kozuma Y, Toyokawa G,
Yamazaki K, Ichiki M and Takeo S: Pretreatment prognostic
nutritional index as a novel biomarker in non-small cell lung
cancer patients treated with immune checkpoint inhibitors. Lung
Cancer. 136:45–51. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ren B, Shen J, Qian Y and Zhou T:
Sarcopenia as a determinant of the efficacy of immune checkpoint
inhibitors in non-small cell lung cancer: A meta-analysis. Nutr
Cancer. 75:685–695. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Stühler V, Herrmann L, Rausch S, Stenzl A
and Bedke J: Role of the systemic immune-inflammation index in
patients with metastatic renal cell carcinoma treated with
first-line ipilimumab plus nivolumab. Cancers (Basel). 14:29722022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Qi WX, Xiang Y, Zhao S and Chen J:
Assessment of systematic inflammatory and nutritional indexes in
extensive-stage small-cell lung cancer treated with first-line
chemotherapy and atezolizumab. Cancer Immunol Immunother.
70:3199–3206. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mountzios G, Samantas E, Senghas K, Zervas
E, Krisam J, Samitas K, Bozorgmehr F, Kuon J, Agelaki S, Baka S, et
al: Association of the advanced lung cancer inflammation index
(ALI) with immune checkpoint inhibitor efficacy in patients with
advanced non-small-cell lung cancer. ESMO Open. 6:1002542021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang X, Wang D, Sun T, Li W and Dang C:
Advanced lung cancer inflammation index (ALI) predicts prognosis of
patients with gastric cancer after surgical resection. BMC Cancer.
22:6842022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Barth DA, Brenner C, Riedl JM, Prinz F,
Klocker EV, Schlick K, Kornprat P, Lackner K, Stöger H, Stotz M, et
al: External validation of the prognostic relevance of the advanced
lung cancer inflammation index (ALI) in pancreatic cancer patients.
Cancer Med. 9:5473–5479. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Pian G, Hong SY and Oh SY: Prognostic
value of advanced lung cancer inflammation index in patients with
colorectal cancer liver metastases undergoing surgery. Tumori.
108:56–62. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Xiong B, Fu B, Wu Y, Gao F and Hou C: Body
composition predicts prognosis of hepatocellular carcinoma patients
undergoing immune checkpoint inhibitors. J Cancer Res Clin Oncol.
Jul 4–2023.(Epub ahead of print). View Article : Google Scholar
|
|
52
|
Zhu HF, Feng JK, Xiang YJ, Wang K, Zhou
LP, Liu ZH, Cheng YQ, Shi J, Guo WX and Cheng SQ: Combination of
alpha-fetoprotein and neutrophil-to-lymphocyte ratio to predict
treatment response and survival outcomes of patients with
unresectable hepatocellular carcinoma treated with immune
checkpoint inhibitors. BMC Cancer. 23:5472023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang L, Feng J, Kuang T, Chai D, Qiu Z,
Deng W, Dong K, Zhao K and Wang W: Blood biomarkers predict
outcomes in patients with hepatocellular carcinoma treated with
immune checkpoint Inhibitors: A pooled analysis of 44 retrospective
sudies. Int Immunopharmacol. 118:1100192023. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chen L, Sun H, Zhao R, Huang R, Pan H, Zuo
Y, Zhang L, Xue Y, Song H and Li X: Controlling nutritional status
(CONUT) predicts survival in gastric cancer patients with immune
checkpoint inhibitor (PD-1/PD-L1) outcomes. Front Pharmacol.
13:8369582022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
van de Donk PP, Kist de Ruijter L, Lub-de
Hooge MN, Brouwers AH, van der Wekken AJ, Oosting SF, Fehrmann RS,
de Groot DJA and de Vries EG: Molecular imaging biomarkers for
immune checkpoint inhibitor therapy. Theranostics. 10:1708–1718.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Collins N and Belkaid Y: Control of
immunity via nutritional interventions. Immunity. 55:210–223. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Di Giosia P, Stamerra CA, Giorgini P,
Jamialahamdi T, Butler AE and Sahebkar A: The role of nutrition in
inflammaging. Ageing Res Rev. 77:1015962022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Childs CE, Calder PC and Miles EA: Diet
and immune function. Nutrients. 11:19332019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Swarbrick GM, Gela A, Cansler ME, Null MD,
Duncan RB, Nemes E, Shey M, Nsereko M, Mayanja-Kizza H, Kiguli S,
et al: Postnatal expansion, maturation, and functionality of MR1T
cells in humans. Front Immunol. 11:5566952020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Divangahi M, Aaby P, Khader SA, Barreiro
LB, Bekkering S, Chavakis T, van Crevel R, Curtis N, DiNardo AR,
Dominguez-Andres J, et al: Trained immunity, tolerance, priming and
differentiation: Distinct immunological processes. Nat Immunol.
22:2–6. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Barrea L, Di Somma C, Muscogiuri G,
Tarantino G, Tenore GC, Orio F, Colao A and Savastano S: Nutrition,
inflammation and liver-spleen axis. Crit Rev Food Sci Nutr.
58:3141–3158. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Iddir M, Brito A, Dingeo G, Fernandez Del
Campo SSF, Samouda H, La Frano MR and Bohn T: Strengthening the
immune system and reducing inflammation and oxidative stress
through diet and nutrition: Considerations during the COVID-19
crisis. Nutrients. 12:15622020. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Dong J, Zhang W, Zhang T, Chen X, Zhao J,
Zeng Y, Chen Y, Wei X, Lei T, Wang P, et al: Baseline nutritional
status could be a predictor for radiation esophagitis in esophageal
cancer patients undergoing radiotherapy. Ann Transl Med.
8:11482020. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kaymak Cerkesli ZA, Ozkan EE and Ozseven
A: The esophageal dose-volume parameters for predicting Grade I–II
acute esophagitis correlated with weight loss and serum albumin
decrease in lung cancer radiotherapy. J Cancer Res Ther. 17:94–98.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zheng C, Liu S, Feng J and Zhao X:
Prognostic value of inflammation biomarkers for survival of
patients with neuroblastoma. Cancer Manag Res. 12:2415–2425. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Golovtchenko AM and Raichvarg D:
Lymphocytes. Roles in cellular immunity and humoral immunity. Ann
Biol Clin (Paris). 33:63–74. 1975.(In French). PubMed/NCBI
|
|
67
|
Cancro MP and Tomayko MM: Memory B cells
and plasma cells: The differentiative continuum of humoral
immunity. Immunol Rev. 303:72–82. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Papayannopoulos V: Neutrophil
extracellular traps in immunity and disease. Nat Rev Immunol.
18:134–147. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Quail DF, Amulic B, Aziz M, Barnes BJ,
Eruslanov E, Fridlender ZG, Goodridge HS, Granot Z, Hidalgo A,
Huttenlocher A, et al: Neutrophil phenotypes and functions in
cancer: A consensus statement. J Exp Med. 219:e202200112022.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Junqueira C, Crespo Â, Ranjbar S, de
Lacerda LB, Lewandrowski M, Ingber J, Parry B, Ravid S, Clark S,
Schrimpf MR, et al: FcγR-mediated SARS-CoV-2 infection of monocytes
activates inflammation. Nature. 606:576–584. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kapellos TS, Bonaguro L, Gemünd I, Reusch
N, Saglam A, Hinkley ER and Schultze JL: Human monocyte subsets and
phenotypes in major chronic inflammatory diseases. Front Immunol.
10:20352019. View Article : Google Scholar : PubMed/NCBI
|