Introduction
Hepatoid carcinoma (HC) is a rare extrahepatic
epithelial malignancy exhibiting morphological and
immunohistochemical features similar to hepatocellular carcinoma
(1). HC most frequently occurs in
the ovaries, colon or stomach, with only a few cases of pancreatic
adenocarcinoma (PAC) of the hepatoid subtype having been reported
(2–6). However, several metastatic lesions,
including the poorly differentiated adenocarcinoma of the pancreas
and stomach, have been reported to exhibit hepatoid morphology
(2,7). The clinical course and prognosis of
patients developing hepatoid or high-grade adenocarcinoma are
always poor, as they rapidly develop metastatic lesions (2). The positive and differential diagnosis
of these entities may be challenging, always requiring ancillary
studies and good knowledge of the clinical history of the patient
(1,7,8).
The pathological entity with which pancreatic HC is
related is PAC, which is one of the leading causes of
cancer-related mortality worldwide, mainly presenting at advanced
stages, frequently metastatic, with limited conventional treatment
efficacy (9,10). As the fourth most lethal form of
cancer in the USA, PAC is associated with high mortality and poor
survival rates. The incidence of pancreatic carcinoma is increasing
worldwide and in western countries (9,11),
with its annual incidence having been reported as high as 50,000
patients (12). PAC is estimated to
be the eleventh most reported cancer in 2018 (13) or the fourteenth most common cancer,
as reported in 2021 (14); however,
it is the third or the seventh leading cause of cancer-related
mortality, as reported in 2018 (13) and 2021 (14), respectively, mainly affecting older
adults. The incidence of PAC is expected to increase further
(9), and it is predicted to become
the second leading cause of cancer-related mortality in western
countries by the year 2030. The median age of diagnosis is 71 years
in the USA, with only <1% of diagnoses performed prior to the
age of 50. Inherited pancreatic cancer (PC) syndromes and familial
PC comprise ≤10% of PAC cases. Despite having been reported as one
of the most lethal solid tumors (15), early diagnosis is not frequently
achieved, and reliable diagnostic biomarkers are currently
lacking.
Identifying modifiable and non-modifiable risk
factors for PAC is essential for developing effective preventive
strategies and improving patient outcomes. At least 20 possible
risk factors for PAC have been identified in prospective cohort
studies, with lifestyle and metabolic factors being the most
common. These include tobacco smoking, obesity, a sedentary
lifestyle, alcohol consumption, an increased fat and red meat
intake, a decreased fruit and vegetable intake (16), populations of African descent
(17), cadmium, arsenic and lead
exposure (18). Other risk factors
include a family history (14,19),
genetic predispositions (19) or
disorders (mutational status of several genes such as BRCA2 or
PRSS1, but not only associated with a familial component) (18), long-term diabetes (14,16,17,19–21),
chronic pancreatitis (14,16,18,19),
certain infectious diseases (involving Helicobacter pylori,
Hepatitis B virus and human immunodeficiency virus) (18) and intraductal papillary mucinous
neoplasms (14). Inherited PC
syndromes (hereditary pancreatitis, familial atypical multiple mole
melanoma syndrome, and Peutz-Jeghers syndrome) and familial PC
comprise 10% of PAC cases (22).
Risk prediction models have demonstrated good discrimination and
calibration, providing the possibility of early identification and
prevention of the disease (16). A
recent review (23) proposed a
model about how the dysbiosis of microbial and mycobial species may
contribute to the development of PAC. The model suggested that
bacterial and fungal species in the oral cavity and gut microbiome,
including Porphyromonas gingivalis, Fusobacterium nucleatum,
the Proteobacteria, Helicobacter spp., and Malassezia
globosa can cause inflammation and chronic pancreatitis,
ultimately leading to PC.
A significant metabolic heterogeneity has been
observed in PAC, arising within the ducts of the pancreas (24) and has been linked to the highest
rate of cancer-associated venous thromboembolic disease (25). In total, >50% of PAC patients
develop liver metastases (26,27).
Tumor initiation leading to PC occurs through acinar to ductal cell
metaplasia (28). Previous studies
have suggested several causes for pancreatic tumorigenesis. The
dysbiosis of micro- and mycobiota contribute to tumorigenesis in
PAC (23), while the intratumor
microbiome modulating carcinogenesis, has been recently introduced
as a new component of the tumor microenvironment (11). Dickkopf-1 (Dkkl), a protein that
inhibits the Wnt/β-catenin pathway and induces apoptosis, exhibits
an increased expression in specimens from patients with PAC, and
the serum Dkkl-cytoskeleton associated protein 4 (CKAP4)-PI3K/AKT
signaling pathway in serum also participates in PC cell
proliferation (29). Retinoids play
a critical role in maintaining normal pancreatic functions, and the
dysregulation of retinoid functions is observed in PAC (30).
Several genetic predispositions and molecular
alterations (31) have been found
to be associated with this highly lethal entity. The most common
mutated oncogenes in PAC and that were among the first to be
reported in the literature are KRAS (32) and Bcl-2 (21), while p53 (33–35),
deleted in pancreatic cancer 4 (21), cyclin-dependent kinase inhibitor 2
(CDKN2A) (17,35,36),
retinoblastoma protein (17,21,33,34,36)
are the most frequently deleted tumor suppressor genes. CDKN2A is a
gene that encodes proteins involved in regulating the cell cycle,
and mutations in this gene are commonly detected in PAC (36). Pathogenic germline alterations are
common in individuals with PAC, and ~10% of patients have familial
inheritance (17). DNA repair
dysfunction involving breast cancer BRCA mutations (19,34,35,37–42),
and the polyADP-ribose polymerase (PARP) enzyme (31,34,35)
play a crucial role in the pathogenesis of PAC. Next-generation
sequencing (26,31) is a technology that allows for the
rapid sequencing of DNA or RNA, providing the opportunity for
targeted therapy of other mutated genes, including neurotrophic
receptor tyrosine kinase (26,33),
anaplastic lymphoma kinase (26)
and HER2 (31). The TGF-β signaling
pathway is frequently altered in PC and acts as an inhibitor and
inducer of tumor progression (43).
TGF-β signaling was identified as a potent inducer of
epithelial-to-mesenchymal transition (EMT), a significant factor in
PAC progression and the development of metastases (43). The downstream effects of this
duality are critical in the development of metastatic disease,
immunologic response, and EMT (44–46).
The PI3K pathway (29,47) modifies the tumor microenvironment,
and some components of this signaling cascade (such us subunit α of
phosphoinositide 3-kinase, phosphatase and tensin homolog, protein
kinase B or subunits of PI3K such as p85α or p110α) are frequently
mutated in PAC. Understanding the genetic and molecular alterations
in PAC can lead to targeted therapies and improved treatment
outcomes for this highly lethal disease.
Pancreatic HC is a distinct subtype of PAC, hence
similar to that of PAC, its pathology is expected to have the same
poor prognosis, poor long-term survival (48) and limited availability of
therapeutic strategies (49). Late
symptoms and treatment resistance contribute to its highly fatal
nature, and despite recent improvements, the median overall of
patients survival remains as low as ~11 months (43), while the 5-year survival rate ranges
from 5 to 17% (25,50–58).
Several factors including a lack of screening (54,59,60)
and peritoneal metastases also affect prognosis (58). With a relatively better prognosis,
pancreatic acinar cell carcinoma has a 5-year survival rate of ~15%
(28). Prognostic factors include
tumor size (58), differentiation
(58,61), margins (58,62),
lymph node invasion (58,62) and high-grade tumor budding (63). Exercise during treatment is crucial
for the optimization of the quality of life; however, well-designed
trials are required (64). The low
socio-economic status of patients in Europe and North America is
associated with a reduced access to surgery or an increased
likelihood of refusing surgery, which is influenced by a low
income, poor levels of education, insurance coverage and rural
areas (65).
Surgical resection is the only curative option
limited to a limited number of candidates (56). Multidisciplinary therapy (12,66,67) is
required for locally advanced disease, and suboptimal chemotherapy
response is a major concern. Recent chemotherapy, radiation, and
immunotherapy advancements have improved short-term survival, and
new therapeutic regimens are being developed (68). Artificial intelligence can
potentially improve diagnosis and treatment, and novel approaches
are undergoing preclinical and early clinical evaluation (54).
Case report
A 72-year-old female patient presented to the
Emergency Clinical Unit and further hospitalized in the Second
Department of Surgery, University Emergency Hospital of Bucharest
(Bucharest, Romania), where laboratory tests and radiology
investigations, including ultrasonography and CT scan, indicated
the presence of hepatic and ovarian tumor masses and aroused
suspicion for peritoneal carcinomatosis. Therefore, the affected
peritoneum and omentum were surgically excised and submitted to the
Department of Pathology. The samples were fixed with 10% neutrally
buffered formalin at 4–8°C overnight (20 h) and then processed by
conventional histopathological methods using paraffin embedding,
sectioning (3–5 mm) and hematoxylin-eosin staining at room
temperature (5–10 min for hematoxylin and 1–5 min for eosin). The
slides were observed using light microscopy. Afterwards, the
sections were deparaffinized in toluene and alcohol, washed in PBS
(phosphate saline buffer), incubated with normal serum, and then
incubated with primary antibody overnight. Subsequently, washing in
carbonate buffer and developing in 3,3′-diaminobenzidine
hydrochloride/hydrogen peroxide (MilliporeSigma) and nuclear
counterstain with Meyer's hematoxylin (MilliporeSigma) was
performed according to the provided manufacturer's protocol, at
room temperature for 1–5 min. Normal human serum (MilliporeSigma)
at a concentration of 10% was used as the blocking reagent,
performed at room temperature. Overall, the following
immunohistochemical markers and corresponding antibodies were used:
Cytokeratin (CK)7, CK20 and CK8/18, α-fetoprotein (AFP), paired box
gene 8 (PAX8), caudal-type homeobox transcription factor 2 (CDX2),
GATA-binding protein 3, mucin 5AC (MUC 5AC) and Wilms tumor 1 (WT1)
(Table I). Staining was performed
either manually using a biotin-streptavidin-peroxidase complex
technique or automatically with the Leica Biosystems Bond™ or Roche
Ventana™ immunohistochemistry staining systems. The staining method
is specified along with the automatic staining system in the second
column of Table I. The antibody
clone information as listed by the manufacturers is also
stated.
 | Table I.Immunohistochemistry antibody
information. |
Table I.
Immunohistochemistry antibody
information.
| Antibody type | Staining
method | Manufacturer and
catalog information | Catalog number | Clone number,
clonality | Dilution |
|---|
| AFP | Automatic BOND | MilliporeSigma | A8452-100UL | C3, MMab | 1:500 |
|
| Leica
Biosystems™ |
|
|
|
|
| CK 8.18 | Automatic BOND | Thermo Fisher | MA5-14088 | 5D3, MMab | 1:50 |
|
| Leica
Biosystems™ | Scientific,
Inc. |
|
|
|
| WT1 | Automatic BOND | GeneTex, Inc. | GTX01958 | WT49, MMab | 1:100 |
|
| Leica
Biosystems™ |
|
|
|
|
| CEA | Automatic
VENTANA | Abcam | ab193372 | CEA31, MMab | 1:100 |
|
| Roche™ |
|
|
|
|
| PAX8 | Automatic
VENTANA | MilliporeSigma | 363M-18 | MRQ-50, | Ready- |
|
| Roche™ |
|
| MMab | to-use |
| MUC 5AC | Manual BSB | MilliporeSigma | MAB2011 | CLH2, MMab | 1:50 |
| GLYPICAN | Manual BSB | MilliporeSigma | 261M-9 | 1G12, MMab | 1:200 |
Furthermore, inserting the search query
‘[pancreas(Title) OR pancreatic(Title)]) AND hepatoid
carcinoma[Title]’ into the PubMed database, all English language
cases published until February, 2023 were reviewed, by also
including all reported patients of any age and sex developing
metastatic pancreatic carcinoma confirmed by histopathologic
examination and immunohistochemical analysis, further investigating
the cases with hepatoid morphology. All of the reported cases which
referred to metastatic HC of ovarian, gastric and colonic origin
were excluded.
The case of a 72-year-old female patient with a
medical history of hypertension under treatment and grade II
obesity was investigated in the present study, who presented to the
Emergency Clinical Unit of the University Emergency Hospital of
Bucharest with complaints of severe abdominal pain and abdominal
distension. The patient was further hospitalized in the Second
Department of Surgery. Blood biochemistry later revealed that the
patient had chronic high glucose levels with HbA1c levels at 7.1%,
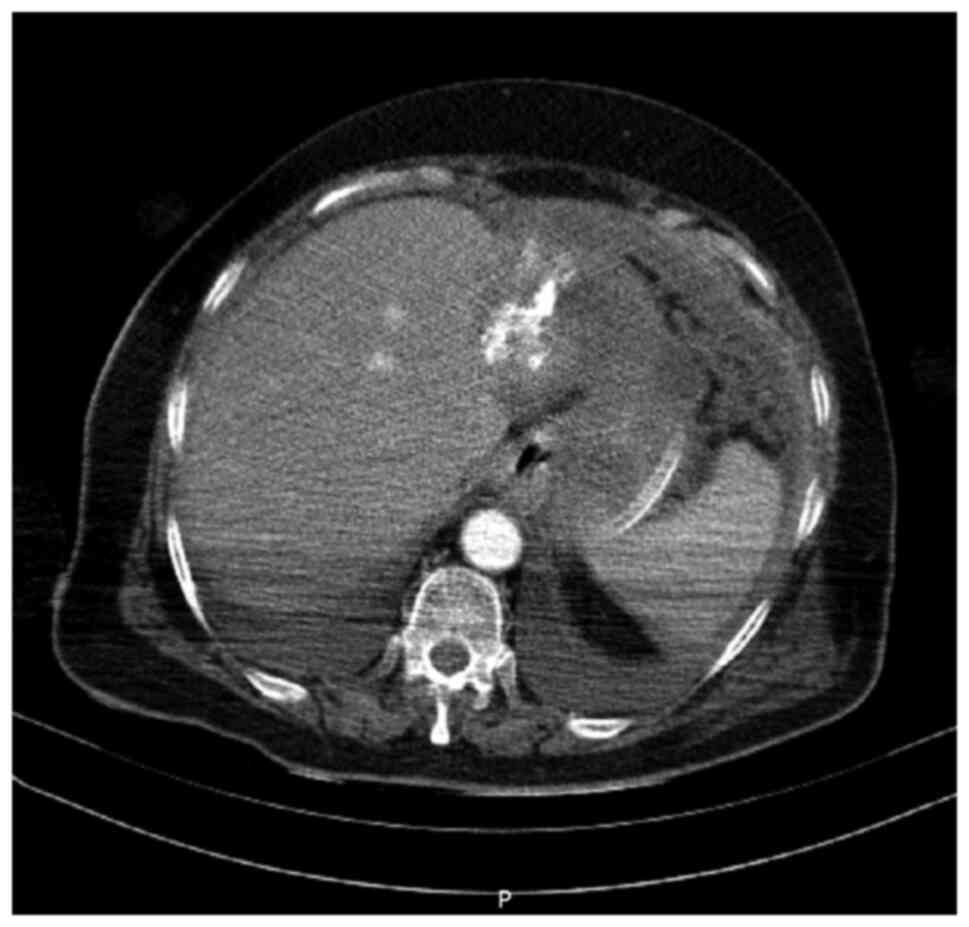
which is a diagnostic criterion of type 2 diabetes (69). A computed tomography (CT) scan
(Fig. 1) was performed, revealing
hepatic and ovarian tumor masses and multiple nodular lesions of
the peritoneum and greater omentum, raising concern about an
extensive abdominal neoplasm. The hepatic lesion involved segments
II and III presented with multiple calcifications. On the CT scan,
the clinical image of the pancreas appeared to be within normal
limits.
Ascitic fluid was observed, and a sample was
submitted for cytological analysis upon which malignant epithelial
cells and acute inflammatory infiltrate were identified. The serum
tumoral markers analysis revealed the following values: CA125,
280.9 U/ml; CEA, 18.8 ng/ml; CA19-9, >1,972 U/ml; and AFP, 3.11
ng/ml. The peritoneum and greater omentum were surgically excised
during open surgery and submitted to the Department of Pathology.
The gross examination of the specimens revealed multiple
infiltrative, solid, firm, grey-white nodules involving the entire
adipose tissue.
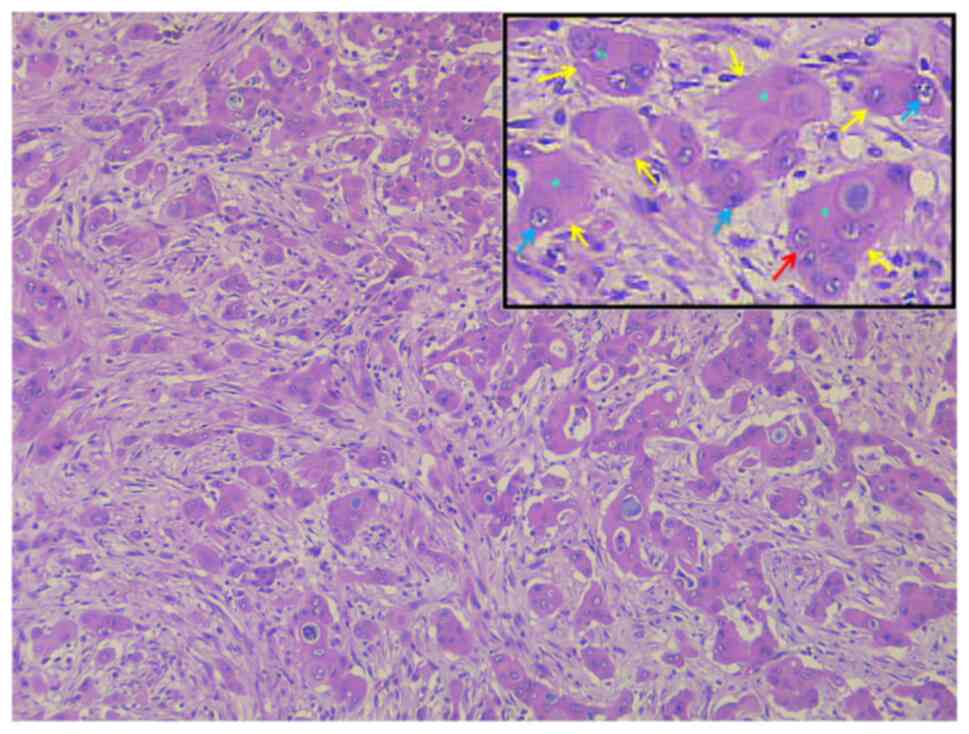
The histological examination of the fragments
revealed a malignant epithelial proliferation exhibiting a diffuse
sheet-like growth pattern, occasionally forming cords and
pseudoglandular structures (Fig.
2). The neoplastic cells demonstrated a particularly polygonal
morphology, with marked nuclear pleomorphism, loss of nuclear
polarity, atypical mitoses and prominent nucleoli, and abundant
eosinophilic cytoplasm containing mucin granules (Fig. 2). The malignant tumor exhibited a
deeply infiltrative growth pattern and marked desmoplastic stromal
proliferation. The angiolymphatic neoplastic invasion was also
documented. As the histopathological aspect of the lesion and
clinical data were highly suggestive of HC, several
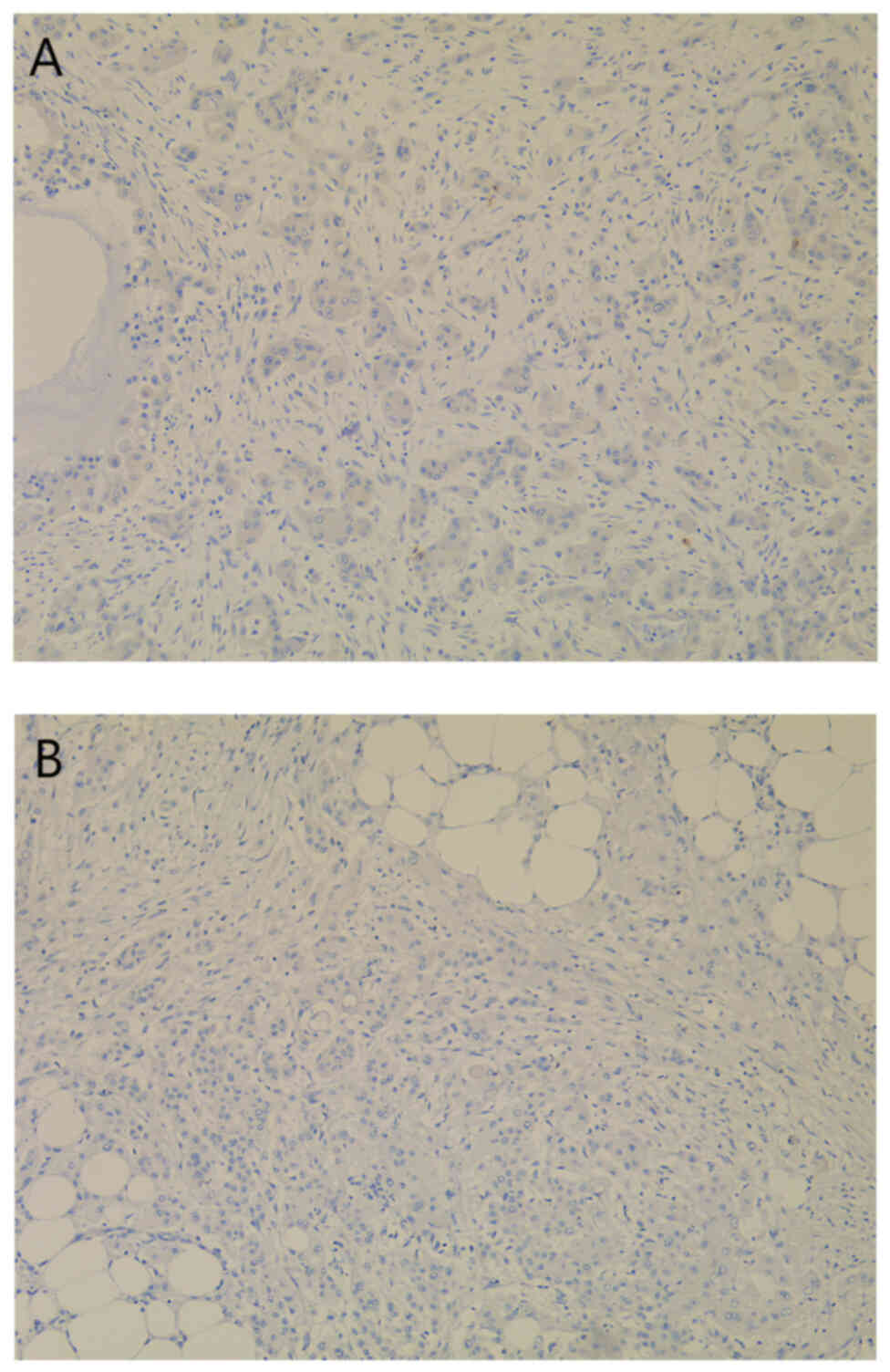
immunohistochemical examinations were performed. Firstly, AFP, PAX8
and WT1 expression analysis revealed negative staining of the
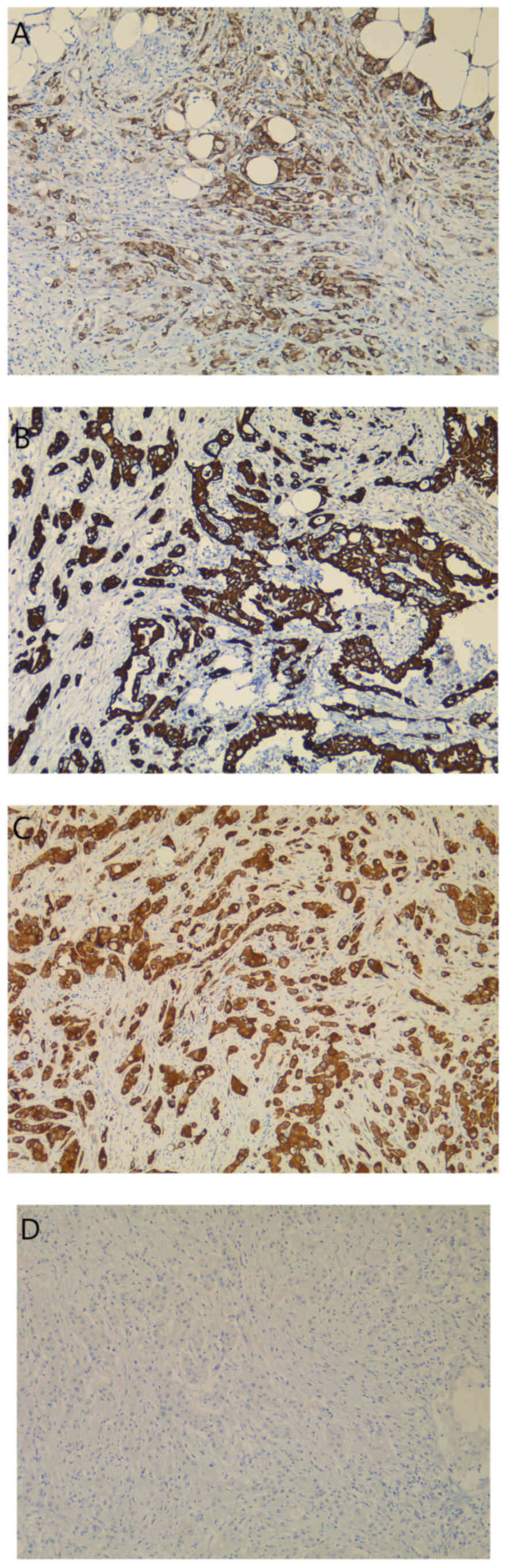
neoplastic cells, excluding an ovarian HC origin (Fig. 3). The absence of CDX2 expression
within the tumor cells excluded a presumptive colonic origin of the
metastatic lesion (Fig. 4). The
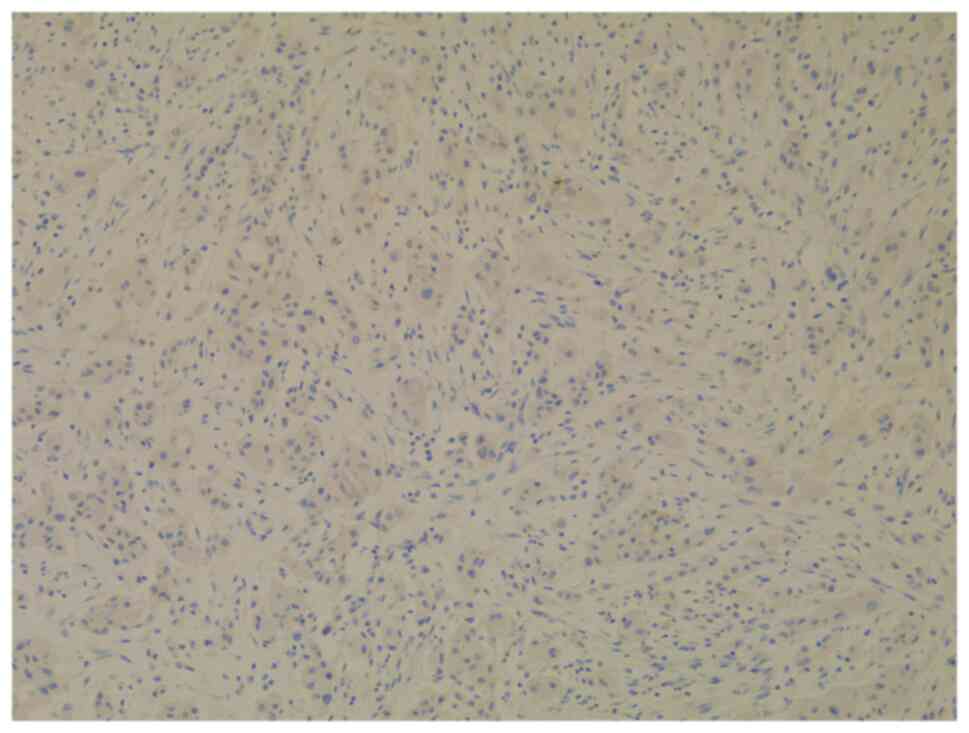
possible pancreatic-biliary origin of the malignant lesion was
later studied by using low molecular weight MUC 5AC (Fig. 5A) and CKs (Fig. 5B and C). The tumor cells
demonstrated CK7-positive (Fig. 5B)
and CK20-negative (Fig. 5D)
staining. The intense expression of MUC 5AC (Fig. 5A) and CK18 (Fig. 5C) within the neoplastic cells
eventually established the diagnosis of metastatic PAC.
Due to advanced metastatic neoplastic condition, the
patient passed away shortly after the surgery, and no specific
oncological treatment had been established due to the fulminant
lethal course of her disease. The histopathological diagnosis was
finalized at approximately the same time. No non-surgical treatment
was initiated due to the fulminant lethal course of the
disease.
Discussion
HC is a rare epithelial neoplasm with an uncertain
histogenesis, which most frequently involves the ovaries or the
digestive tract, particularly the colon and stomach (8). Although very few cases have been
reported, the described tumor can also develop in the pancreatic
head (2,8).
Rare tumors are classified a priori, within
the predictive, preventive and personalized treatment spectrum;
however, since they are only detected with a reduced frequency in
the total population, their susceptibility to adverse disease
progression and the response to targeted therapeutic strategies
remains to be investigated further with the use of genomics.
Immunohistochemical analysis provide results that, considered that
they provide information concerning pathological mechanisms, can be
incorporated in genetic tests with a predictive role for tumor
development and with a possible supporting role in decision-making,
in relation to therapeutic strategies targeted at specific patient
groups.
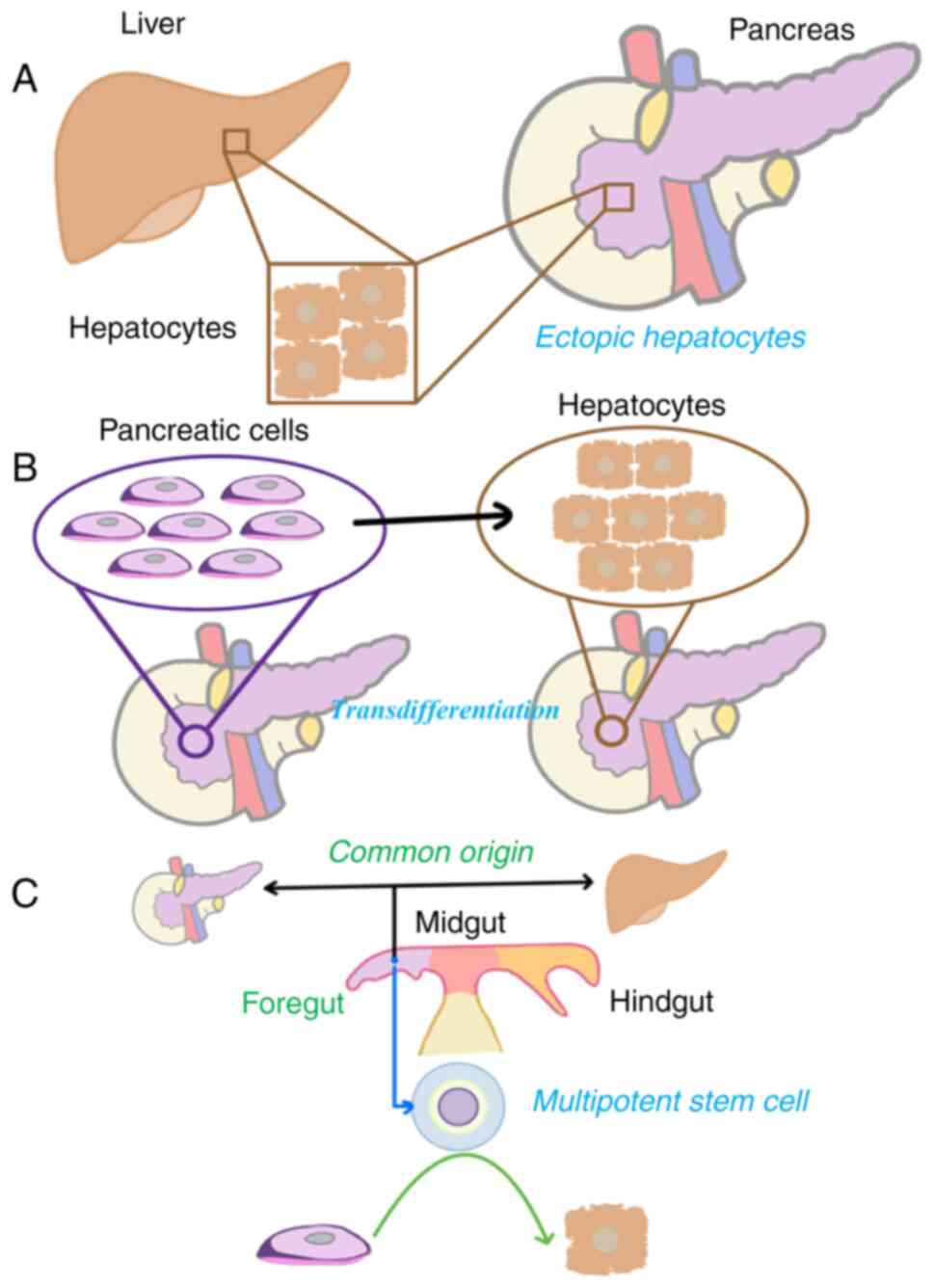
Although not well defined, the pathogenesis of
pancreatic HC can be summarized into three hypotheses (70) (Fig.
6). The first one is the ectopic tissue hypothesis, according
to which HC may have origins in ectopic hepatic tissue in the
pancreas (71,72). Secondly, the pancreas to hepatic
transdifferentiation hypothesis explores the possibility of the
pancreatic cells being able to transdifferentiate into hepatocytes.
Lastly, the pancreatic multipotent stem cell hypothesis states that
the liver and pancreas have the exact embryological origin in the
foregut endoderm and share activating genes that control
differentiation during carcinogenesis (70–72).
Considering the morphological and immunohistochemical aspect,
pancreatic HC can be further classified as a pure or mixed type,
and the latter can be associated with other component types,
including ductal adenocarcinoma and acinar cell carcinoma (73). Using the PubMed search query,
‘[Pancreatic(Title) OR pancreas(Title)] AND adenocarcinoma[Title]’,
9,800 studies related to PAC were discovered (on February 19, 2023)
in the literature, of which 39 were pure-type and 16 were
mixed-type pancreatic HCs (6,70).
Regardless of the affected organ, this malignancy is always
associated with aggressive clinical behavior, as the patients
either decease due to the rapid disease progression or develop
multiple recurrences and distant metastases (2,74).
PACs are rapidly progressive epithelial neoplasms which may exhibit
hepatoid features upon microscopic examination (75,76).
This diagnosis is associated with a poor prognosis, due to early
micrometastatic spread with a limited 5-year survival of <3%
(77).
The poorly-differentiated variants of PAC can
present as deeply infiltrating lesions, composed of large,
polygonal neoplastic cells with abundant eosinophilic cytoplasm and
pleomorphic nuclei with open chromatin, prominent nucleoli,
mimicking hepatoid tumors and even hepatocellular carcinoma
(75,78,79).
Some patients developing this highly lethal malignancy are
frequently diagnosed with a metastatic lesion at the time of first
diagnosis (80). Although PAC and
HC are rapidly progressive tumors associated with metastases,
including peritoneal carcinomatosis, patients developing hepatoid
lesions may exhibit elevated serum levels of AFP (1,2,6,79,81).
In the case described in the present study, the initial
presentation implied the identification of peritoneal
carcinomatosis, with no increase in serum AFP levels. However, the
histopathological examination of the specimens revealed malignant
epithelial proliferation, mainly composed of large, polygonal
cohesive neoplastic cells with abundant eosinophilic cytoplasm,
displaying a sheet-like growth pattern, with occasional forming of
cellular cords and pseudoglandular structures. The most peculiar
aspect identified within the tumor was intracytoplasmic basophilic
granules, raising concern about HC with an ovarian origin,
particularly considering that an ovarian mass was detected by the
CT scan. The neoplastic cells within hepatoid ovarian carcinoma
usually exhibit AFP expression; however, they are also positive for
PAX8 and WT1, with their origin being the surface ovarian
epithelium (2,8,82). In
the case described herein, no expression of PAX8 or AFP was
detected in the tumor cells, simultaneously excluding the
possibility for the ovarian origin of the lesion and an hepatoid
phenotype. However, as mentioned earlier, the microscopic
examination of the fragments obtained from the peritoneum and
omentum revealed a deeply infiltrative tumor proliferation,
predominantly composed of atypical cells, exhibiting mucus
secretion and pseudoglandular structures, distorted by a thick
fibrous and inflammatory stroma.
Hepatoid tumors are often negative for CK7 and
positive for the pan-cytokeratin markers, AE1/AE3 (92%), CK19
(94–100%), glypican-3 (78%), arginase-1 (75%), CK20 (25–47%) and
CK18 (70,81,83).
The possibility of the tumor being classified as a poorly
differentiated adenocarcinoma with a gastric or pancreatic origin
was also considered; thus, further concomitant analyses were
performed. Firstly, CK7, CK20 and MUC 5AC staining was performed,
in order to investigate the hypothesis of the pancreato-biliary or
gastric origin of the neoplasm. Furthermore, CK18 was considered as
a valuable marker for the confirmation of the pancreatic origin of
the peritoneal metastases, as several studies previously suggested
that it can be detected in metastatic PAC and cholangiocarcinoma
(84,85). It is considered that positive CK18
staining of neoplastic cells has a more optimal diagnostic value
when CK20 is not expressed within the tumor (85,86).
MUC 5AC expression in the neoplastic cells has also been analyzed,
since there is significant evidence of the critical role of mucin
expression in colorectal and pancreatic carcinogenesis (86,87).
Eventually, positive CK7, CK18 and MUC 5AC staining, correlated
with the lack of CK20 expression within the patient examined or the
present case report, confirmed the diagnosis of metastatic poorly
differentiated PAC.
In simple, pseudostratified, ductal epithelium,
mesothelium, and urothelium, CK7 has a similar, although more
restricted expression than CK8 and CK18. It is a cytoplasmic or
membranous marker that is detected in both normal epithelia and
generally expressed in a broad spectrum of malignancies, albeit
with considerable variation. CK18 is predominantly expressed in
simple epithelial cells, eccrine glands (88), small dimension vessels (89) and trophoblasts (90). MUC 5AC is expressed in various parts
of the digestive system, particularly in the gastric antrum,
superior airways and metaplastic endometrium (91).
Despite the widespread distribution of CK7
expression, it is a valuable component of a panel for diagnosing
the primary location of metastatic cancer. Its expression is
usually concurrently analyzed with CK20 in tumors of various types
and localizations. Similarly, CK7 and CK18 are expressed in various
tumors, as presented in Table
II.
 | Table II.Common immunochemical features (CK7,
CK18, CK20 and MUC 5AC) of tumors with various localizations,
according to the literature. |
Table II.
Common immunochemical features (CK7,
CK18, CK20 and MUC 5AC) of tumors with various localizations,
according to the literature.
| Tumor | CK7 | CK18 | CK20 | MUC 5AC | (Refs.) |
|---|
| Gastrointestinal
tumors |
|
|
|
|
|
|
Esophageal adenocarcinoma | + | NA | - | NA | (159) |
| Gastric
adenocarcinoma | + | + | + | NA | (160) |
| Diffuse
carcinoma | NA | NA | NA | + | (161) |
| Lymph
nodes of gastric carcinoma | NA | + | NA | NA | (161) |
| Small
intestinal adenocarcinoma (67%) | + |
| + | NA | (162) |
| Colon
carcinoma (in lymph nodes) | NA | + | NA | NA | (163) |
|
Pancreatic adenocarcinoma |
|
|
|
| (8,70) |
|
Poor
prognosis | + | NA | + | NA |
|
|
Good
prognosis | + | NA | - |
|
|
|
Hepatocellular
adenocarcinoma |
| + |
| NA | (164) |
|
Hepatoid carcinoma
mimicking |
|
|
|
|
|
|
hepatocellular
adenocarcinoma |
| + |
| NA | (165) |
|
Carcinoma of the bile
duct |
|
|
|
| (166) |
|
Extrahepatic | + | NA | + | NA |
|
|
Intrahepatic | + |
| - | NA |
|
|
Gynecological tumors |
|
|
|
|
|
|
Endometrial
adenocarcinoma | + | NA | - | NA | (167) |
| Ovarian
mucinous tumors | + | NA | + | + | (80,81,168) |
|
Cervical and uterine
cancer | + | NA | + | NA | (169,170) |
| Breast
cancer |
|
|
|
| (171,172) |
|
Invasive
disease | + | + | - | NA |
|
|
Other |
|
|
|
|
|
| Head
and neck cancer |
|
|
|
| (70,173,174) |
|
Sinonasal | + | NA | + | NA |
|
| Lung
adenocarcinoma | + | NA | - | NA | (175) |
| Urinary
tract cancer | + | + | + | NA | (176) |
Several other immunochemical biomarkers are
mentioned in the literature; however, relevant techniques for their
identification were unfortunately unavailable for the present
study. The lack of the evaluation of the aforementioned markers
would constitute a limitation of this diagnostic and treatment
strategy of the present case report. For instance, a recent study
proposed that cadherin-1 (CDH1), cadherin-2 (CDH2), Vimentin (VIM),
Zinc finger E-box-binding homeobox 1 (ZEB1), and Snail family
transcriptional repressor 1 (SNAI1) could be potential diagnostic,
prognostic, and therapeutic targets for PAC (92).
The most frequently mentioned, CDH1, is a protein
associated with cell adhesion and known be involved in tumor
progression, invasion and metastasis (93,94).
Moreover, the complete or partial loss of CDH1 expression plays a
predictive role in the development of the disease, as it is an
independent predictor of poor outcomes among patients with PAC
undergoing pancreaticoduodenectomy (PD) (95), being primarily related to a worse
median survival than in patients with a uniformly intact CDH1
expression, but also to frequent lymph node metastasis and an
advanced clinical stage (96). More
precisely, the CDH1 gene polymorphism is associated with an
increased risk of PC in the Chinese population; however, larger
samples are required to confirm these findings (97). In particular, a higher histological
grade PAC is related to CDH1 methylation promoter encountered in
long-term diabetes patients with pancreatic cancer (98). A similar effect in tumorigenic
transformation through EMT is exerted by CDH2 (99,100).
However, CDH1 is not PAC-specific, being also a
biomarker involved in the diagnosis of hereditary diffuse gastric
cancer, and may not always initiate the development of PAC when
both pathologies are present in the same patient (101). In the absence of conclusive
imaging results regarding the damage to abdominal organs, CDH1
positivity would support the hypothesis concerning the gastric
origin of the tumor.
Along with the investigation of ZEB1, VIM, or SNAI1,
the detection of CDH1 could have contributed to finalizing the
diagnosis and disease development prediction. ZEB1 is an
EMT-related transcription factor. ZEB1 levels have been negatively
associated with PC, through its involvement with
inositol-3-phosphate synthase 1 into a pathway that enhances cancer
cell migration and invasion (102). Additionally, ZEB1, in combination
with CDH1, has already been confirmed as a negative prognostic
biomarker by previous studies (99,103),
ZEB1 acting as a repressor of both CDH1 (104) which is a controller and of
epithelial cell adhesion molecule, which is a regulator of the
migrating cells adhesiveness (105). Metastatic tumors with a larger
diameter exhibit an increased expression of CDH1 and a decreased
expression of ZEB1 and SNAI1, as compared with smaller metastases
(104). VIM has been suggested as
a predictive biomarker for the PC evolution (106) in parallel with CDH1 (107). Additionally, CDH1, VIM and ZEB1
have been suggested as diagnostic and predictive biomarkers for
intraductal papillary mucinous neoplasm (108).
Following surgery survival or in the absence of
surgical indication, the evaluation of those biomarkers could have
allowed the administration of targeted treatment since they have
been proposed as possible therapeutic targets. Blocking CDH1
inhibits PAC progression by facilitating its expression through the
enforced expression of miRNA-101 (93,96).
The metastasis-associated with colon cancer protein 1/SNAI1 complex
in EMT, a therapeutic target in PC, has been reported to
downregulate CDH1. CDH1 is downregulated when sineoculis homeobox
homolog 1 (Six1) is inhibited, and tumors with impaired Six1
expression exhibit loss of the CD24+/CD44+
phenotype. Therefore, Six1 may be a potential therapeutic target
for PC (109). The downregulation
of VIM inhibits cancer cell migration and may affect the response
to chemotherapy (106). Along with
ZEB1, SNAI1, SNAI2 and CDH2, VIM is involved in the NF-κB signaling
pathway, inducing EMT and promoting lymphovascular and neural
invasion; thus, over the past decade, these markers have been
proposed as potential therapeutic targets for inhibiting PC
progression (110). However, the
positive results of such targeted treatments are not supported by
extensive studies, being otherwise currently unavailable in the
literature.
Early PC detection is hindered by the lack of a
strategy to identify high-risk individuals (14). It has been suggested that a more
targeted screening approach based on modifiable and non-modifiable
risk factors might be more efficient, particularly for individuals
with inherited and familial PC syndromes (22). Early detection of PAC is crucial for
improving therapeutic outcome, and current screening methods are
primarily imaging-based. The interest in detecting PAC and
precursor lesions at an early stage has led to the developing PC
screening programs (56).
The accurate staging of PAC is essential, due to the
metastatic nature and course of treatment of the disease (111). Therefore, imaging techniques are
essential in differentiating metastatic disease from other entities
(56). Novel imaging techniques
(56), including dual-energy CT,
diffusion-weighted MRI and positron emission tomography/MRI, are
being developed to improve the accuracy of diagnosing PC.
Multimodality imaging (pancreatic protocol CT and magnetic
resonance cholangiopancreatography) and interventional endoscopy
[endoscopic retrograde cholangiopancreatography and endoscopic
ultrasound (US)] are currently used for diagnosis and staging
(50); however, monitoring
treatment response remains challenging. Complete surgical resection
should be mandatory whenever possible, followed by adjuvant
chemotherapy and depending on the case of metastatic disease,
hyperthermic intraperitoneal chemotherapy (HIPEC) can be considered
(76,112,113). A review of four studies evaluating
the use of HIPEC in PAC after surgical resection demonstrated
overall survival rates ranging from 2 to 62 months with an 8.5%
hospital mortality rate (114).
However, due to a small sample size and low-quality evidence, no
valid conclusions could be drawn. In a study in which three
therapeutic strategies were used [cytoreductive surgery (CRS) with
HIPEC, prophylactic HIPEC, and neoadjuvant intraperitoneal
chemotherapy], it was concluded that the use CRS and HIPEC in
peritoneal carcinomatosis of pancreatic origin was considered not
useful and unsafe (115).
Several studies have demonstrated the benefits of
surgery even for isolated recurrences of PAC (116). However, <20% of patients are
candidates for curative resection (117). Attempted resection rates, margin
negative resection rates and pathological response are the outcomes
measured for surgical resection (118). Palliative local treatments are
preferred alternatives to surgery (26). However, although only 10–20% of
patients are candidates for curative resection, vascular bypass
graft techniques and neoadjuvant treatment regimens have increased
the curative resection rate (56,117).
Attempted resection rates, margin negative resection rates, and
pathologic response are the outcomes measured for surgical
resection (118). Radical
resections increase margin negativity and life expectancy (48).
Conversion surgery for initially unresectable tumors
is associated with improved survival, and no significant difference
in survival was observed between patients with locally advanced
disease and those with distant metastases after conversion surgery
(119). A systematic review of
published evidence on locally advanced PC treatment strategies with
curative intent (120) reported
that the median resection rate was 25%, with 33.5% of patients
proceeding to surgery after completion of the neoadjuvant pathway
(121). Median progression-free
and overall survival for resected patients may reach 12.9 (122) and 30 (123) months, respectively. The extent of
surgery applied is controversial; lymphadenectomy, nerve plexus,
retroperitoneal tissue, vascular and multi-visceral resections,
total pancreatectomy, and liver metastases are discussed in a
review of the basic underlying concepts and the roles of radical
surgery (48). In a systematic
review comparing PD with and without vascular resection (VR) in
pancreatic head adenocarcinoma, it was reported that the PD+VR
group demonstrated lower 1-, 3- and 5-year overall survival rates.
The PD+VR group presented with larger tumors, positive lymph nodes,
and higher R1 resection. The reported 30-day mortality was higher
in the PD+VR group, and no differences were observed between groups
in post-operative complications (124). Hepatic resection for patients with
PC with hepatic metastases is a safe procedure and provides an
additional survival benefit in the medium term (<3 years);
however, further randomized, controlled trials are urgently
required (27).
Laparoscopic pancreatic surgery is a safe and
feasible option for carefully selected patients and has been
suggested to improve surgical outcomes; however, further
confirmation is necessary through randomized controlled studies
(125). Irreversible
electroporation is being explored as a potential treatment for
locally advanced PC, and while some preliminary evidence is
promising, IRE should only be used after conventional treatments
and within the research context (126). Minimally invasive distal
pancreatectomy is safer than open distal pancreatectomy for
patients with PAC, with lower positive surgical margin rates, less
blood loss, a shorter hospital stay, and lower morbidity and
mortality (127). High-intensity
focused US (HIFUS) is an emerging therapeutic modality for PC,
inducing mechanical effects for targeted drug delivery and pain
management in palliative care and for downstaging borderline
resectable tumors (53). With the
advancement of emerging therapeutic modalities including
sonodynamic therapy and immunomodulation, HIFUS may be a promising
option for improving outcomes.
However, due to its aggressive nature and the
possibility of early hepatic metastasis, some authors present the
utility of neoadjuvant chemotherapy, surgery and adjuvant
chemotherapy with results that are yet unclear and remain to be
elucidated further (41,128–130). The standard treatment care for PC
in clinical practice is the application of combination chemotherapy
(131,132). Conventional chemotherapy offers a
low 5-year survival rate due to its limited efficacy and suboptimal
response (133). Patients with DNA
repair dysfunction, including BRCA mutations, benefit from platinum
chemotherapy and PARP inhibitors (31). Limited success has been reported
concerning the use of therapies, including gemcitabine (134). The use of folinic acid,
5-fluorouracil, irinotecan and oxaliplatin (FOLFIRINOX) has been
reported to exhibit comparably increased efficiency, although
presenting with an increased toxicity (135). FOLFIRINOX and nanoparticle
albumin-bound paclitaxel (nab-paclitaxel) plus gemcitabine have
demonstrated benefit (134), while
a modest improvement in survival has been observed with
gemcitabine-erlotinib and FOLFIRINOX (135). The median overall survival for
patients with metastatic pancreatic adenocarcinoma (mPDA) treated
with first-line regimens remains as low as 1 year, indicating a
significant need for effective later-line options, nab-paclitaxel
being one such example (31).
The global analyses of gene expression in PC led to
the discovery of several potential new PC markers (52). Database searches in a previously
published study identified 76 independent prognostic and predictive
molecular markers implicated in pancreatic tumor growth, apoptosis,
angiogenesis, invasion and resistance to chemotherapy. Researchers
are investigating these biomarkers, in order to capture phenotypic
variability and identify PC earlier, including small extracellular
vesicles, microbial signatures, proteins, metabolites, genetic and
epigenetic markers (52). CA19-9 is
the most commonly used biomarker, although with limited specificity
for PC and sensitivity in the early PC stages (136); thus, new biomarkers clinically
validated by prospective studies are needed for early detection and
subsequent death rate reduction (137). Of these, 11 markers (Ki-67, p27,
p53, TGF-β1, Bcl-2, survivin, VEGF, cyclo-oxygenase 2, CD34, S100
calcium-binding protein A4 and human equilibrative nucleoside
transporter 1) provided independent prognostic or predictive
information in two or more separate studies. In addition,
thrombospondin-2, insulin-linked binding protein 2,
lysophosphatidic acid, autotaxin, inflammatory factors, coagulation
factors (61) and Dkkl (29) are possible simple protein biomarker
candidates. Mesothelin was identified by serial analysis of gene
expression as overexpressed in 80–90% of PC cells. However, limited
expression of mesothelin has been observed in normal tissues, being
thus a valuable diagnostic aid and a therapeutic target (137). Soluble mesothelin-related proteins
may potentially be part of a panel of markers for pancreatic
carcinoma, along with previously used markers, including CA19-9,
CEA, and tissue inhibitor of metalloproteinases-1 (138,139).
Several serum biomarkers, including protein-induced
by vitamin K absence or antagonist-II (PIVKA II), Duke pancreatic
monoclonal antigen type 2 (DUPAN-2), and s-pancreas-1 antigen
(Span-1) in conjunction with ‘classical’ non-specific tumor markers
(for example, CA19-9 or CEA) have been studied as risk factors for
the evolution of patients with PAC. PIVKA II was identified as
having higher serological levels in PC, thus being considered as a
possible biomarker for this pathology (140). A subsequent study by the same
group of authors revealed that PIVKA II is can also be used as a
predictive biomarker of postoperative evolution in small stages
(141). In addition, it has been
demonstrated that PIVKA II can function as an excellent marker in
rare cases of hepatoid pancreatic adenocarcinoma demonstrated,
while its increased levels have also been associated with a
positive diagnosis of hepatocellular carcinoma (142).
DUPAN-2, CEA and CA19-9 positivity, in conjunction
with certain levels for the first two and tumor sizes >30 mm,
are indications of resectable or non-resecable tumors (143). In addition, DUPAN-2 has been used
to monitor the response to chemotherapy (144). DUPAN-2 can potentially predict the
prolonged survival of patients with PC during initial systemic
therapy and may be useful in determining the optimal timing for
conversion surgery in initial systemic therapy (145). Thus, together with CA19-9, DUPAN
may aid in patient stratification and personalized treatment
decisions (146).
SPan-1 is a biomarker used in PC that plays a
predictive role in the evolution of the disease. Preoperative serum
SPan-1>37-41 U/ml (147,148)
levels are significantly associated with a higher early recurrence
risk following the curative resection of PC. Therefore, SPan-1 may
be useful for determining the best treatment option for patients
with resectable PC.
Span-1 is as useful as CA19-9 for monitoring the
success of gemcitabine chemotherapy (149). Moreover, SPan-1 and CA19-9 have
been identified as independent risk factors for early recurrence in
patients who underwent surgical resection for PC: Patients with
both biomarkers presented with a higher rate of lymph node
metastasis than patients with one increased biomarker or none
altered (150), whereas higher
levels of CA19-9, SPAN-1 together with low mitochondrial OGG1
expression are indicators of perineural invasion (151).
Recently, the former two biomarkers have been
included in various scores, e.g., a preoperative tumor marker index
whose high values have been associated with larger tumors, lymph
node metastases, and worse prognostic outcomes in terms of both
relapse-free survival and overall survival (152). Another example of a predictive
score is the early recurrence prediction score, which identifies
patients with poor prognoses and avoids unnecessary surgery
(153). However, elevated
post-operative CA19-9 instead of either Span-1 or DUPAN-2 (154), was identified as the strongest
predictive marker of poor survival in the pre- and post-operative
period, being thus a biomarker of choice for post-operative
evolution. However, complementing the preoperative serum levels of
CEA, CA19-9 with values of Span-1 and DUPAN-II would have been
necessary to further support surgical indication.
Liquid biopsies and next-generation sequencing of
circulating tumor cells enable novel PC diagnostics and
therapeutics, allowing prognosis (57,155)
by detecting circulating nucleic acid-based biomarkers (42,156),
including non-coding miRNAs (157). Genetic testing for germline BRCA1
and 2 pathogenic variants is crucial for all newly diagnosed
patients with mPDA, considering the solid hereditary component of
the disease (31,37,39).
Additionally, identifying active oncogenic pathways and gene-gene
interactions has been suggested to reveal oncogene addiction and
synthetic lethality, which can provide a basis for developing
personalized treatments (155).
Additionally, identifying active oncogenic pathways and gene-gene
interactions can reveal oncogene addiction and synthetic lethality,
which can provide a basis for developing personalized treatments
(155). However, challenges
including sensitivity and analytical limitations still exist, which
require further research (156).
Identifying biomarkers that accurately predict
disease recurrence or response to chemotherapy would substantially
aid individual risk assessment and treatment selection, possibly
also leading to novel therapies by becoming targets for molecular
intervention in specific subsets of patients.
In conclusion, the positive and differential
diagnosis of metastatic adenocarcinoma with hepatoid morphology
revealed associations between standard histopathological
examination and the expression of immunohistochemical markers.
Furthermore, due to the highly aggressive clinical outcomes of this
malignancy, establishing the origin of peritoneal metastases is
crucial for the evaluation of the prognosis and response of the
tumor to surgical and oncological treatment. The establishment of a
neoadjuvant therapy for pancreatic neoplasms in general (118,158), and for tumors as uncharacteristic
and as aggressive as the hepatoid pancreatic adenocarcinoma
presented in the present study is rare; however,
immunohistochemical studies contribute to opening up new
perspectives for early diagnosis and improvement of neoadjuvant
treatments or curative strategies. Case report studies should not
be considered solely as descriptions of rarely occurring tumors but
an opportunity to gain further immunological insight. As has been
demonstrated in the present study, pancreatic adenocarcinoma is
very aggressive, regardless of its histopathological typology,
mainly associated with other aggravating factors including
multi-organ dissemination and risk factors (comorbidities, old
age).
Acknowledgement
Not applicable.
Funding
Publication of this paper was supported by the University of
Medicine and Pharmacy ‘Carol Davila’ through the institutional
program ‘Publish, not Perish’.
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
AI, RVT and LB participated in the
conceptualization of the study. LB performed the validation of the
manuscript plan according to available data. RC and NZ performed
the surgery, while AI and AMC conducted the medical investigation
of the pathology slides. AI, AMC, RC and NZ gathered all necessary
resources. AI and RVT conducted all data curation. AI, LB and RVT
prepared the original draft of the manuscript. AI, RVT, NZ, RC, and
AMC reviewed and edited the manuscript. LB and RVT realized the
Figures. AI, LB and RVT supervised the present study. AI and LB
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient's informed consent was obtained to use
the biological material for ancillary studies, including also the
consent for the publication of personal information as presented in
imaging body scans.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AFP
|
α-fetoprotein
|
|
CDH1
|
cadherin-1
|
|
CDH2
|
cadherin-2
|
|
CDKN2A
|
cyclin-dependent kinase inhibitor
2
|
|
CDX2
|
caudal-type homeobox transcription
factor 2
|
|
CK
|
cytokeratin
|
|
CRS
|
cytoreductive surgery
|
|
Dkkl
|
Dickkopf-1
|
|
DUPAN-2
|
duke pancreatic monoclonal antigen
type 2
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
FOLFIRINOX
|
folinic acid, 5-fluorouracil,
irinotecan and oxaliplatin
|
|
HC
|
hepatoid carcinoma
|
|
HIPEC
|
hyperthermic intraperitoneal
chemotherapy
|
|
mPDA
|
metastatic pancreatic
adenocarcinoma
|
|
MUC 5AC
|
mucin 5AC
|
|
nab-paclitaxel
|
nanoparticle albumin-bound
paclitaxel
|
|
PAC
|
pancreatic adenocarcinoma
|
|
PAX8
|
paired box gene 8
|
|
PARP
|
poly ADP-ribose polymerase
|
|
PC
|
pancreatic cancer
|
|
PD
|
pancreaticoduodenectomy
|
|
PIVKA II
|
protein-induced by vitamin K absence
or antagonist-II
|
|
SNAI1
|
Snail family transcriptional
repressor 1
|
|
SPan-1
|
s-pancreas-1 antigen
|
|
Six1
|
sineoculis homeobox homolog 1
|
|
US
|
ultrasound
|
|
VIM
|
vimentin
|
|
VR
|
vascular resection
|
|
WT1
|
Wilms tumor 1
|
|
ZEB1
|
Zinc finger E-box-binding homeobox
1
|
References
|
1
|
Choi WK, Cho DH, Yim CY and Lee NR:
Primary hepatoid carcinoma of the ovary: A case report and review
of the literature. Medicine (Baltimore). 99:e200512020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stamatova D, Theilmann L and Spiegelberg
C: A hepatoid carcinoma of the pancreatic head. Sur Case Rep.
2:782016. View Article : Google Scholar
|
|
3
|
Soofi Y, Kanehira K, Abbas A, Aranez J,
Bain A and Ylagan L: Pancreatic hepatoid carcinoma: A rare form of
pancreatic neoplasm. Diagn Cytopathol. 43:251–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen TC, Huang SC, Chang HC, Yeh TS, Ng KF
and Chen TC: Hepatoid microcarcinoma of the pancreas: A case report
and review of the literature. Chang Gung Med J. 35:285–291.
2012.PubMed/NCBI
|
|
5
|
Vanoli A, Argenti F, Vinci A, La Rosa S,
Viglio A, Riboni R, Necchi V, Pugliese L, Sessa F, Pietrabissa A
and Paulli M: Hepatoid carcinoma of the pancreas with lymphoid
stroma: First description of the clinical, morphological,
immunohistochemical, and molecular characteristics of an unusual
pancreatic carcinoma. Virchows Arch. 467:237–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zeng SX, Tan SW, Fong CJTH, Liang Q, Zhao
BL, Liu K, Guo J and Tao J: Hepatoid carcinoma of the pancreas: A
case report and review of the literature. World J Clin Cases.
8:1116–1128. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Uribe Rivera AK, Alvarez Larraondo M, Taxa
Rojas L, Bravo Taxa M and Zevallos Cardenas A: Hepatoid carcinoma
of the ovary-A case report and literature review. Gynecol Oncol
Rep. 32:1005642020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Acosta AM and Pins MR: Hepatoid carcinoma
of the ovary: Clinical, histopathologic, and immunophenotypic
features. Arch Pathol Lab Med. 143:883–889. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Karakas Y, Lacin S and Yalcin S: Recent
advances in the management of pancreatic adenocarcinoma. Expert Rev
Anticancer Ther. 18:51–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tesfaye AA, Kamgar M, Azmi A and Philip
PA: The evolution into personalized therapies in pancreatic ductal
adenocarcinoma: Challenges and opportunities. Expert Rev Anticancer
Ther. 18:131–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tijeras-Raballand A, Hilmi M,
Astorgues-Xerri L, Nicolle R, Bièche I and Neuzillet C: Microbiome
and pancreatic ductal adenocarcinoma. Clin Res Hepatol
Gastroenterol. 45:1015892021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Attiyeh MA, Amini A, Chung V and Melstrom
LG: Multidisciplinary management of locally advanced pancreatic
adenocarcinoma: Biology is King. J Surg Oncol. 123:1395–1404. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ogden J, Xie H, Ma W and Hubbard J: The
management of older adults with pancreatic adenocarcinoma.
Geriatrics. 3:852018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ushio J, Kanno A, Ikeda E, Ando K, Nagai
H, Miwata T, Kawasaki Y, Tada Y, Yokoyama K, Numao N, et al:
Pancreatic ductal adenocarcinoma: Epidemiology and risk factors.
Diagnostics (Basel). 11:5622021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Panchal K, Sahoo RK, Gupta U and
Chaurasiya A: Role of targeted immunotherapy for pancreatic ductal
adenocarcinoma (PDAC) treatment: An overview. Int Immunopharmacol.
95:1075082021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pang Y, Holmes MV, Chen Z and Kartsonaki
C: A review of lifestyle, metabolic risk factors, and blood-based
biomarkers for early diagnosis of pancreatic ductal adenocarcinoma.
J Gastroenterol Hepatol. 34:330–345. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sikdar N, Saha G, Dutta A, Ghosh S,
Shrikhande SV and Banerjee S: Genetic alterations of periampullary
and pancreatic ductal adenocarcinoma: An overview. Curr Genomics.
19:444–463. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Garcia-Carbonero N, Li W, Cabeza-Morales
M, Martinez-Useros J and Garcia-Foncillas J: New Hope for
pancreatic ductal adenocarcinoma treatment targeting endoplasmic
reticulum stress response: A systematic review. Int J Mol Sci.
19:24682018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chappuis PO, Ghadirian P and Foulkes WD:
The role of genetic factors in the etiology of pancreatic
adenocarcinoma: An update. Cancer Inve. 19:65–75. 2001. View Article : Google Scholar
|
|
20
|
Duvillié B, Kourdoughli R, Druillennec S,
Eychène A and Pouponnot C: Interplay between diabetes and
pancreatic ductal adenocarcinoma and insulinoma: The role of aging,
genetic factors, and obesity. Front Endocrinol (Lausanne).
11:5632672020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Eskelinen MJ and Haglund UH: Prognosis of
human pancreatic adenocarcinoma: Review of clinical and
histopathological variables and possible uses of new molecular
methods. Eur J Surg. 165:292–306. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diaz KE and Lucas AL: Familial pancreatic
ductal adenocarcinoma. Am J Pathol. 189:36–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bellotti R, Speth C, Adolph TE, Lass-Flörl
C, Effenberger M, Öfner D and Maglione M: Micro- and mycobiota
dysbiosis in pancreatic ductal adenocarcinoma development. Cancers
(Basel). 13:34312021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bou Zerdan M, Shatila M, Sarwal D,
Bouferraa Y, Bou Zerdan M, Allam S, Ramovic M and Graziano S:
Single cell RNA sequencing: A new frontier in pancreatic ductal
adenocarcinoma. Cancers (Basel). 14:45892022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kothari A and Flick MJ: Coagulation
signaling through PAR1 as a therapeutic target in pancreatic ductal
adenocarcinoma. Int J Mol Sci. 22:51382021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin T, Dai C and Xu F: Surgical and local
treatment of hepatic metastasis in pancreatic ductal
adenocarcinoma: Recent advances and future prospects. Ther Adv Med
Oncol. 12:1758835920933032020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu X, Gu J, Fu D and Jin C: Dose surgical
resection of hepatic metastases bring benefits to pancreatic ductal
adenocarcinoma? A systematic review and meta-analysis. Int J Surg.
48:149–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Parte S, Nimmakayala RK, Batra SK and
Ponnusamy MP: Acinar to ductal cell trans-differentiation: A
prelude to dysplasia and pancreatic ductal adenocarcinoma. Biochim
Biophys Acta Rev Cancer. 1877:1886692022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Igbinigie E, Guo F, Jiang SW, Kelley C and
Li J: Dkk1 involvement and its potential as a biomarker in
pancreatic ductal adenocarcinoma. Clin Chim Acta. 488:226–234.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mere Del Aguila E, Tang XH and Gudas LJ:
Pancreatic ductal adenocarcinoma: New insights into the actions of
vitamin A. Oncol Res Treat. 45:291–298. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Das S and Cardin D: Targeting DNA damage
repair pathways in pancreatic adenocarcinoma. Curr Treat Options
Oncol. 21:622020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schmitt A, Feldmann G, Zander T and
Reinhardt HC: Targeting defects in the cellular DNA damage response
for the treatment of pancreatic ductal adenocarcinoma. Oncol Res
Treat. 41:619–625. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Qian Y, Gong Y, Fan Z, Luo G, Huang Q,
Deng S, Cheng H, Jin K, Ni Q, Yu X and Liu C: Molecular alterations
and targeted therapy in pancreatic ductal adenocarcinoma. J Hematol
Oncol. 13:1302020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hayashi H, Higashi T, Miyata T, Yamashita
Y and Baba H: Recent advances in precision medicine for pancreatic
ductal adenocarcinoma. Ann Gastroenterol Surg. 5:457–466. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rainone M, Singh I, Salo-Mullen EE,
Stadler ZK and O'Reilly EM: An emerging paradigm for germline
testing in pancreatic ductal adenocarcinoma and immediate
implications for clinical practice: A review. JAMA Oncol.
6:7642020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu C, Yang P, Liu B and Tang Y: Is there a
CDKN2A-centric network in pancreatic ductal adenocarcinoma? Onco
Targets Ther. 13:2551–2562. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Macchini M, Centonze F, Peretti U, Orsi G,
Militello AM, Valente MM, Cascinu S and Reni M: Epidemiology and
geographic distribution of BRCA1-2 and DNA Damage response genes
pathogenic variants in pancreatic ductal adenocarcinoma patients.
Cancer Treat Rev. 104:1023572022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pimenta JR, Ueda SKN and Peixoto RD:
Excellent response to olaparib in a patient with metastatic
pancreatic adenocarcinoma with germline BRCA1 mutation after
progression on FOLFIRINOX: Case report and literature review. Case
Rep Oncol. 13:904–910. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rebelatto TF, Falavigna M, Pozzari M,
Spada F, Cella CA, Laffi A, Pellicori S and Fazio N: Should
platinum-based chemotherapy be preferred for germline BReast CAncer
genes (BRCA) 1 and 2-mutated pancreatic ductal adenocarcinoma
(PDAC) patients? A systematic review and meta-analysis. Cancer
Treat Rev. 80:1018952019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Reinacher-Schick A, Arnold D, Venerito M,
Goekkurt E, Kraeft AL and Seufferlein T: Platinum-Based
chemotherapy in locally advanced or metastatic pancreatic ductal
adenocarcinoma: Summary of evidence and application in clinical
practice. Oncol Res Treat. 45:752–763. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Singh RR and O'Reilly EM: New treatment
strategies for metastatic pancreatic ductal adenocarcinoma. Drugs.
80:647–669. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zemanek T, Melichar B, Lovecek M, Soucek P
and Mohelnikova-Duchonova B: Biomarkers and pathways of
chemoresistance and chemosensitivity for personalized treatment of
pancreatic adenocarcinoma. Pharmacogenomics. 20:113–127. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dardare J, Witz A, Merlin JL, Gilson P and
Harlé A: SMAD4 and the TGFβ pathway in patients with pancreatic
ductal adenocarcinoma. Int J Mol Sci. 21:35342020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jian S, Kong D and Tian J: Expression of
miR-425-5p in pancreatic carcinoma and its correlation with tumor
immune microenvironment. J Invest Surg. 36:22167562023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nan P, Dong X, Bai X, Lu H, Liu F, Sun Y
and Zhao X: Tumor-stroma TGF-β1-THBS2 feedback circuit drives
pancreatic ductal adenocarcinoma progression via integrin
αvβ3/CD36-mediated activation of the MAPK pathway. Cancer Lett.
528:59–75. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rajagopal MU, Bansal S, Kaur P, Jain SK,
Altadil T, Hinzman CP, Li Y, Moulton J, Singh B, Bansal S, et al:
TGFβ drives metabolic perturbations during epithelial mesenchymal
transition in pancreatic cancer: TGFβ induced EMT in PDAC. Cancers
(Basel). 13:62042021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Murthy D, Attri KS and Singh PK:
Phosphoinositide 3-Kinase signaling pathway in pancreatic ductal
adenocarcinoma progression, pathogenesis, and therapeutics. Front
Physiol. 9:3352018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dolay K, Malya FU and Akbulut S:
Management of pancreatic head adenocarcinoma: From where to where?
World J Gastrointest Surg. 11:143–154. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Vaish U, Jain T, Are AC and Dudeja V:
Cancer-Associated fibroblasts in pancreatic ductal adenocarcinoma:
An update on heterogeneity and therapeutic targeting. Int J Mol
Sci. 22:134082021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Khokhar AS, Sher AF and Schattner M:
Interventional endoscopy in the diagnosis and management of
pancreatic adenocarcinoma. Chin Clin Oncol. 6:63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rémond MS, Pellat A, Brezault C, Dhooge M
and Coriat R: Are targeted therapies or immunotherapies effective
in metastatic pancreatic adenocarcinoma? ESMO Open. 7:1006382022.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Seo YD and Katz MHG: Preoperative therapy
for pancreatic adenocarcinoma-precision beyond anatomy. Cancer.
128:3041–3056. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lafond M, Lambin T, Drainville RA, Dupré
A, Pioche M, Melodelima D and Lafon C: Pancreatic ductal
adenocarcinoma: Current and emerging therapeutic uses of focused
ultrasound. Cancers (Basel). 14:25772022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ladd AM and Diehl DL: Artificial
intelligence for early detection of pancreatic adenocarcinoma: The
future is promising. World J Gastroenterol. 27:1283–1295. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Constantin AL, Cazacu IM, Stroescu C,
Copăescu C and Săftoiu A: Prognostic biomarkers related to tumoral
microenvironment in pancreatic ductal adenocarcinoma: A systematic
review. Rom J Morphol Embryol. 62:671–678. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kulkarni NM, Mannelli L, Zins M, Bhosale
PR, Arif-Tiwari H, Brook OR, Hecht EM, Kastrinos F, Wang ZJ, Soloff
EV, et al: White paper on pancreatic ductal adenocarcinoma from
society of abdominal radiology's disease-focused panel for
pancreatic ductal adenocarcinoma: Part II, update on imaging
techniques and screening of pancreatic cancer in high-risk
individuals. Abdom Radiol (NY). 45:729–742. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Buscail E, Maulat C, Muscari F, Chiche L,
Cordelier P, Dabernat S, Alix-Panabières C and Buscail L: Liquid
biopsy approach for pancreatic ductal adenocarcinoma. Cancers
(Basel). 11:8522019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Barhli A, Cros J, Bartholin L and
Neuzillet C: Prognostic stratification of resected pancreatic
ductal adenocarcinoma: Past, present, and future. Dig Liver Dis.
50:979–990. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lupinacci RM, Bachet J-B, André T, Duval A
and Svrcek M: Pancreatic ductal adenocarcinoma harboring
microsatellite instability/DNA mismatch repair deficiency. Towards
personalized medicine. Surg Oncol. 28:121–127. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Leal AD, Messersmith WA and Lieu CH:
Neoadjuvant treatment of localized pancreatic adenocarcinoma. J
Gastrointest Oncol. 12:2461–2474. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kapszewicz M and Małecka-Wojciesko E:
Simple serum pancreatic ductal adenocarcinoma (PDAC) Protein
Biomarkers-Is There Anything in Sight? J Clin Med. 10:54632021.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Iyengar S, Nevala-Plagemann C and
Garrido-Laguna I: Updates on adjuvant and neoadjuvant treatment
strategies for surgically resectable and borderline resectable
pancreatic ductal adenocarcinoma. Ther Adv Med Oncol.
13:1758835921104582021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Lawlor R, Veronese N, Nottegar A, Malleo
G, Smith L, Demurtas J, Cheng L, Wood LD, Silvestris N, Salvia R,
et al: Prognostic Role of High-Grade tumor budding in pancreatic
ductal adenocarcinoma: A systematic review and meta-analysis with a
focus on epithelial to mesenchymal transition. Cancers. 11:1132019.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
O'Connor D, Brown M, Eatock M, Turkington
RC and Prue G: Exercise efficacy and prescription during treatment
for pancreatic ductal adenocarcinoma: A systematic review. BMC
Cancer. 21:432021. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Thobie A, Mulliri A, Bouvier V, Launoy G,
Alves A and Dejardin O: Same chance of accessing resection? impact
of socioeconomic status on resection rates among patients with
pancreatic adenocarcinoma-A systematic review. Health Equity.
5:143–150. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
He J, Zhao Q, Liu Q, Li F, He L, Liu M and
Yan X: Surgical resection of pancreatic hepatoid carcinoma followed
by combined transarterial chemoembolization and immunotherapy: A
case report. Onco Targets Ther. 14:4575–4578. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tomino T, Ninomiya M, Matono R, Narutomi
F, Oshiro Y, Watanabe K, Taniguchi D, Nishimura S, Zaitsu Y,
Kajiwara Y, et al: Pure pancreatic hepatoid carcinoma: A surgical
case report and literature review. Surg Case Rep. 5:1862019.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zeeshan MS and Ramzan Z: Current
controversies and advances in the management of pancreatic
adenocarcinoma. World J Gastrointest Oncol. 13:472–494. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Gholap NN, Davies MJ, Mostafa SA and
Khunti K: Diagnosing type 2 diabetes and identifying high-risk
individuals using the new glycated haemoglobin (HbA1c) criteria. Br
J Gen Pract. 63:e165–e167. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Trinh HS, Luong TH, Lai TT and Nguyen TK:
Mixed pancreatic hepatoid carcinoma: A surgical case report and
literature review. Int J Surg Case Rep. 83:1059512021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Cardona D, Grobmyer S, Crawford JM and Liu
C: Hepatocellular carcinoma arising from ectopic liver tissue in
the pancreas. Virchows Arch. 450:225–229. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kubota K, Kita J, Rokkaku K, Iwasaki Y,
Sawada T, Imura J and Fujimori T: Ectopic hepatocellular carcinoma
arising from pancreas: A case report and review of the literature.
World J Gastroenterol. 13:4270–4273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Veerankutty FH, Yeldho V, Tu SA, Venugopal
B, Manoj KS and Vidhya C: Hepatoid carcinoma of the pancreas
combined with serous cystadenoma: A case report and review of the
literature. Hepatobiliary Surg Nutr. 4:35462–35362. 2015.PubMed/NCBI
|
|
74
|
Yahaya A, Wa Kammal WS, Abd Shukor N and
Osman SS: Oesophageal hepatoid carcinoma with liver metastasis, a
diagnostic dilemma. Malays J Pathol. 41:59–63. 2019.PubMed/NCBI
|
|
75
|
Schneitler S: Metastasized pancreatic
carcinoma with neoadjuvant FOLFIRINOX therapy and R0 resection.
World J Gastroenterol. 21:6384–6390. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Orth M, Metzger P, Gerum S, Mayerle J,
Schneider G, Belka C, Schnurr M and Lauber K: Pancreatic ductal
adenocarcinoma: Biological hallmarks, current status, and future
perspectives of combined modality treatment approaches. Radiat
Oncol. 14:1412019. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Sohal DPS, Kennedy EB, Cinar P, Conroy T,
Copur MS, Crane CH, Garrido-Laguna I, Lau MW, Johnson T,
Krishnamurthi S, et al: Metastatic pancreatic cancer: ASCO
guideline update. J Clin Oncol. JCO20013642020.PubMed/NCBI
|
|
78
|
Brown DB, Gonsalves CF, Yeo CJ, Witkiewicz
AK and Carr BI: One Year survival with poorly differentiated
metastatic pancreatic carcinoma following chemoembolization with
gemcitabine and cisplatin. Oncol Rep. 24:767–769. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Kuo PC, Chen SC, Shyr YM, Kuo YJ, Lee RC
and Wang SE: Hepatoid carcinoma of the pancreas. World J Surg
Oncol. 13:1852015. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Schawkat K, Manning MA, Glickman JN and
Mortele KJ: Pancreatic ductal adenocarcinoma and its variants:
Pearls and perils. Radiographics. 40:1219–1239. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yang C, Sun L, Lai JZ, Zhou L, Liu Z, Xi
Y, Tao Y, Dooley E and Cao D: Primary hepatoid carcinoma of the
pancreas: A Clinicopathological study of 3 cases with review of
additional 31 cases in the literature. Int J Surg Pathol. 27:28–42.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Al-Obaidy KI, Williamson SR, Shelman N,
Idrees MT and Ulbright TM: Hepatoid teratoma, hepatoid yolk sac
tumor, and hepatocellular carcinoma: A morphologic and
immunohistochemical study of 30 cases. Am J Surg Pathol.
45:127–136. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Su JS, Chen YT, Wang RC, Wu CY, Lee SW and
Lee TY: Clinicopathological characteristics in the differential
diagnosis of hepatoid adenocarcinoma: A literature review. World J
Gastroenterol. 19:321–327. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lai YCC, Cheng CC, Lai YS and Liu YH:
Cytokeratin 18-associated Histone 3 modulation in hepatocellular
carcinoma: A mini review. Cancer Genomics Proteomics. 14:219–223.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Shimonishi T, Miyazaki K and Nakanuma Y:
Cytokeratin profile relates to histological subtypes and
intrahepatic location of intrahepatic cholangiocarcinoma and
primary sites of metastatic adenocarcinoma of liver.
Histopathology. 37:55–63. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Gurzu S and Jung I: Aberrant pattern of
the cytokeratin 7/cytokeratin 20 immunophenotype in colorectal
adenocarcinomas with BRAF mutations. Pathol Res Pract. 208:163–166.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yokoyama S, Hamada T, Higashi M, Matsuo K,
Maemura K, Kurahara H, Horinouchi M, Hiraki T, Sugimoto T, Akahane
T, et al: Predicted prognosis of patients with pancreatic cancer by
machine learning. Clin Cancer Res. 26:2411–2421. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Al-Arashi MYH and Byers HR: Cutaneous
clear cell squamous cell carcinoma in situ: Clinical, histological
and immunohistochemical characterization. J Cutan Pathol.
34:226–233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Miettinen M and Fetsch JF: Distribution of
keratins in normal endothelial cells and a spectrum of vascular
tumors: Implications in tumor diagnosis. Hum Pathol. 31:1062–1067.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Austgulen R, Chedwick L, Vogt Isaksen C,
Vatten L and Craven C: Trophoblast apoptosis in human placenta at
term as detected by expression of a cytokeratin 18 degradation
product of caspase. Arch Pathol Lab Med. 126:1480–1486. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Kuan SF, Montag AG, Hart J, Krausz T and
Recant W: Differential expression of mucin genes in mammary and
extramammary Paget's disease. Am J Surg Pathol. 25:1469–1477. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Kirtonia A, Pandey AK, Ramachandran B,
Mishra DP, Dawson DW, Sethi G, Ganesan TS, Koeffler HP and Garg M:
Overexpression of laminin-5 gamma-2 promotes tumorigenesis of
pancreatic ductal adenocarcinoma through EGFR/ERK1/2/AKT/mTOR
cascade. Cell Mol Life Sci. 79:3622022. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Qazi AM, Gruzdyn O, Semaan A, Seward S,
Chamala S, Dhulipala V, Sethi S, Ali-Fehmi R, Philip PA, Bouwman
DL, et al: Restoration of E-cadherin expression in pancreatic
ductal adenocarcinoma treated with microRNA-101. Surgery.
152:704–713. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Çoban EA, Tecimil D, Kașikci E, Bayrak ÖF
and Şahin F: E-cadherin might be a stage-dependent modulator in
aggressiveness in pancreatic cancer cells. Turk J Biol. 44:230–237.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Hong SM, Li A, Olino K, Wolfgang CL,
Herman JM, Schulick RD, Iacobuzio-Donahue C, Hruban RH and Goggins
M: Loss of E-cadherin expression and outcome among patients with
resectable pancreatic adenocarcinomas. Mod Pathol. 24:1237–1247.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Jiao F, Hu H, Han T, Zhuo M, Yuan C, Yang
H and Wang L and Wang L: Aberrant expression of nuclear HDAC3 and
cytoplasmic CDH1 predict a poor prognosis for patients with
pancreatic cancer. Oncotarget. 7:16505–16516. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Zhao L, Wang YX, Xi M, Liu SL, Zhang P,
Luo LL and Liu MZ: Association between E-cadherin (CDH1)
polymorphisms and pancreatic cancer risk in Han Chinese population.
Int J Clin Exp Pathol. 8:57532015.PubMed/NCBI
|
|
98
|
Saito T, Mizukami H, Umetsu S, Uchida C,
Inaba W, Abe M, Takahashi K, Kudo K, Itabashi C, Yagihashi S and
Hakamada K: Worsened outcome in patients with pancreatic ductal
carcinoma on long-term diabetes: Association with E-cadherin1
(CDH1) promoter methylation. Sci Rep |. 7:180562017. View Article : Google Scholar
|
|
99
|
Dhayat SA, Traeger MM, Rehkaemper J,
Stroese AJ, Steinestel K, Wardelmann E, Kabar I and Senninger N:
Clinical impact of Epithelial-to-Mesenchymal transition regulating
MicroRNAs in pancreatic ductal adenocarcinoma. Cancers (Basel).
10:3282018. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Kang Y, Ling J, Suzuki R, Roife D,
Chopin-Laly X, Truty MJ, Chatterjee D, Wang H, Thomas RM, Katz MH,
et al: SMAD4 regulates cell motility through transcription of
N-Cadherin in human pancreatic ductal epithelium. PLoS One.
9:1079482014. View Article : Google Scholar
|
|
101
|
Ottenhof NA, De Wilde RF, Morsink FH, de
Leng WW, Ausems MG, Morreau H, van Hillegersberg R, Offerhaus GJ
and Milne AN: Pancreatic ductal adenocarcinoma in hereditary
diffuse gastric cancer. A case report. Hum Pathol. 43:457–461.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Zhou L, Sheng WW, Jia C, Shi X, Cao R,
Wang G, Lin Y, Zhu F, Dong Q and Dong M: Musashi2 promotes the
progression of pancreatic cancer through a novel ISYNA1-p21/ZEB-1
pathway. J Cell Mol Med. 24:10560–10572. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Hu D, Ansari D, Pawlowski K, Zhou Q, Sasor
A, Welinder C, Kristl T, Bauden M, Rezeli M, Jiang Y, et al:
Proteomic analyses identify prognostic biomarkers for pancreatic
ductal adenocarcinoma. Oncotarget. 9:9789–9807. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Kurahara H, Takao S, Maemura K, Mataki Y,
Kuwahata T, Maeda K, Ding Q, Sakoda M, Iino S, Ishigami S, et al:
Epithelial-mesenchymal transition and mesenchymal-epithelial
transition via regulation of ZEB-1 and ZEB-2 expression in
pancreatic cancer. J Surg Oncol. 105:655–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Vannier C, Mock K, Brabletz T and Driever
W: Zeb1 regulates E-cadherin and Epcam (epithelial cell adhesion
molecule) expression to control cell behavior in early zebrafish
development. J Biol Chem. 288:18643–18659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yu C, Wang M, Chen M, Huang Y and Jiang J:
Upregulation of microRNA-138-5p inhibits pancreatic cancer cell
migration and increases chemotherapy sensitivity. Mol Med Rep.
12:5135–5140. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Lerbs T, Bisht S, Schölch S, Pecqueux M,
Kristiansen G, Schneider M, Hofmann BT, Welsch T, Reissfelder C,
Rahbari NN, et al: Inhibition of Six1 affects tumour invasion and
the expression of cancer stem cell markers in pancreatic cancer.
BMC Cancer. 17:2492017. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Lahat G, Lubezky N, Loewenstein S, Nizri
E, Gan S, Pasmanik-Chor M, Hayman L, Barazowsky E, Ben-Haim M and
Klausner JM: Epithelial-to-Mesenchymal transition (EMT) in
intraductal papillary mucinous neoplasm (IPMN) is associated with
high tumor grade and adverse outcomes. Ann Surg Oncol. 21 (Suppl
4):S750–S757. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Blevins MA, Towers CG, Patrick AN, Zhao R
and Ford HL: The SIX1-EYA transcriptional complex as a therapeutic
target in cancer. Expert Opin Ther Targets. 19:213–225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Nomura A, Majumder K, Giri B, Dauer P,
Dudeja V, Roy S, Banerjee S and Saluja AK: Inhibition of NF-kappa B
pathway leads to deregulation of epithelial-mesenchymal transition
and neural invasion in pancreatic cancer. Lab Invest. 96:1268–1278.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Zaheer A, Wadhwa V, Oh J and Fishman EK:
Pearls and pitfalls of imaging metastatic disease from pancreatic
adenocarcinoma: A systematic review. Clin Imaging. 39:750–758.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Larentzakis A, Anagnostou E, Georgiou K,
Vrakopoulou GZ, Zografos CG, Zografos GC and Toutouzas KG: Place of
hyperthermic intraperitoneal chemotherapy in the armament against
pancreatic adenocarcinoma: A survival, mortality and morbidity
systematic review. Oncol Lett. 21:2462021. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Tao L, Yuan C, Ma Z, Jiang B and Xiu D:
Surgical resection of a primary tumor improves survival of
metastatic pancreatic cancer: A population-based study. Cancer
Manag Res. 9:471–479. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Larentzakis A, Anagnostou E, Georgiou K,
Vrakopoulou GZ, Zografos C, Zografos G and Toutouzas K: Place of
hyperthermic intraperitoneal chemotherapy in the armament against
pancreatic adenocarcinoma: A survival, mortality and morbidity
systematic review. Oncol Lett. 21:2462021. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Brind'Amour A, Webb M, Parapini M, Sidéris
L, Segedi M, Chung SW, Chartier-Plante S, Dubé P, Scudamore CH and
Kim PTW: The role of intraperitoneal chemotherapy in the surgical
management of pancreatic ductal adenocarcinoma: A systematic
review. Clin Exp Metastasis. 38:187–196. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Moletta L, Serafini S, Valmasoni M,
Pierobon ES, Ponzoni A and Sperti C: Surgery for recurrent
pancreatic cancer: Is it effective? Cancers (Basel). 11:9912019.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
La Salvia A, Persano I, Parlagreco E,
Audisio A, Cani M and Brizzi MP: Pancreatic adenocarcinoma and
pancreatic high-grade neuroendocrine carcinoma: Two sides of the
moon. Med Oncol. 39:1682022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Winner M, Goff SL and Chabot JA:
Neoadjuvant therapy for Non-metastatic pancreatic ductal
adenocarcinoma. Semin Oncol. 42:86–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Zhou Y, Liao S, You J and Wu H: Conversion
surgery for initially unresectable pancreatic ductal adenocarcinoma
following induction therapy: A systematic review of the published
literature. Updates Surg. 74:43–53. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Liberko M, Kolostova K, Szabo A, Gurlich
R, Oliverius M and Soumarova R: Circulating tumor cells,
circulating tumor DNA and other blood-based prognostic scores in
pancreatic ductal adenocarcinoma-mini-review. In Vivo. 35:31–39.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Attard JA, Farrugia A, Pathanki A, Roberts
KJ, Dasari B, Isaac J, Ma YT and Chatzizacharias NA: Treatment
strategies for the optimal management of locally advanced
pancreatic adenocarcinoma with curative intent: A systematic
review. Pancreas. 49:1264–1275. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Moorcraft SY, Khan K, Peckitt C, Watkins
D, Rao S, Cunningham D and Chau I: FOLFIRINOX for locally advanced
or metastatic pancreatic ductal adenocarcinoma: The royal marsden
experience. Clin Colorectal Cancer. 13:232–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Korrel M, Lof S, Van Hilst J, Alseidi A,
Boggi U, Busch OR, van Dieren S, Edwin B, Fuks D, Hackert T, et al:
Predictors for survival in an international cohort of patients
undergoing distal pancreatectomy for pancreatic ductal
adenocarcinoma. Ann Surg Oncol. 28:1079–1087. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Fancellu A, Petrucciani N, Porcu A, Deiana
G, Sanna V, Ninniri C, Perra T, Celoria V and Nigri G: The impact
on survival and morbidity of Portal-Mesenteric resection during
pancreaticoduodenectomy for pancreatic head adenocarcinoma: A
systematic review and meta-analysis of comparative studies. Cancers
(Basel). 12:19762020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chen K, Pan Y, Zhang B, Maher H and Cai X:
Laparoscopic versus open pancreatectomy for pancreatic ductal
adenocarcinoma: A systematic review and meta-analysis. Int J Surg.
53:243–256. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
de Liguori Carino N, O'Reilly DA,
Siriwardena AK, Valle JW, Radhakrishna G, Pihlak R and McNamara MG:
Irreversible Electroporation in pancreatic ductal adenocarcinoma:
Is there a role in conjunction with conventional treatment? Eur J
Surg Oncol. 44:1486–1493. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Yang DJ, Xiong JJ, Lu HM, Wei Y, Zhang L,
Lu S and Hu WM: The oncological safety in minimally invasive versus
open distal pancreatectomy for pancreatic ductal adenocarcinoma: A
systematic review and meta-analysis. Sci Rep. 9:11592019.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ferreira BP, Vasquez J and Carilli A:
Metastatic hepatoid carcinoma of the pancreas: First description of
treatment with capecitabine and temozolomide. Am J Med Sci.
353:610–612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Brunner M, Wu Z, Krautz C, Pilarsky C,
Grützmann R and Weber GF: Current clinical strategies of pancreatic
cancer treatment and open molecular questions. Int J Mol Sci.
20:45432019. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Oba A, Ho F, Bao QR, Al-Musawi MH,
Schulick RD and Del Chiaro M: Neoadjuvant treatment in pancreatic
cancer. Front Oncol. 10:2452020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Varghese AM, Lowery MA, Yu KH and O'Reilly
EM: Current management and future directions in metastatic
pancreatic adenocarcinoma: Review of metastatic pancreatic
adenocarcinoma. Cancer. 122:3765–3775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Duffy A and Kummar S: Metastatic
pancreatic adenocarcinoma: Current standards, future directions. Am
J Ther. 17:79–85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Akhuba L, Tigai Z and Shek D: Where do we
stand with immunotherapy for advanced pancreatic ductal
adenocarcinoma: A synopsis of clinical outcomes. Biomedicines.
10:31962022. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Sendur M: Current treatment options for
metastatic pancreatic adenocarcinoma. UHOD. 25:263–274. 2015.
View Article : Google Scholar
|
|
135
|
Conroy T and Mitry E: Chemotherapy of
metastatic pancreatic adenocarcinoma: Challenges and encouraging
results. Bull Cancer. 98:1439–1446. 2011.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ballehaninna UK and Chamberlain RS: The
clinical utility of serum CA 19-9 in the diagnosis, prognosis and
management of pancreatic adenocarcinoma: An evidence based
appraisal. J Gastrointest Oncol. 3:105–119. 2012.PubMed/NCBI
|
|
137
|
Kriz D, Ansari D and Andersson R:
Potential biomarkers for early detection of pancreatic ductal
adenocarcinoma. Clin Transl Oncol. 22:2170–2174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Wu H, Ou S, Zhang H, Huang R, Yu S, Zhao M
and Tai S: Advances in biomarkers and techniques for pancreatic
cancer diagnosis. Cancer Cell Int. 22:2202022. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Brand RE, Nolen BM, Zeh HJ, Allen PJ,
Eloubeidi MA, Goldberg M, Elton E, Arnoletti JP, Christein JD,
Vickers SM, et al: Serum biomarker panels for the detection of
pancreatic cancer. Clin Cancer Res. 17:805–816. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Tartaglione S, Pecorella I, Zarrillo SR,
Granato T, Viggiani V, Manganaro L, Marchese C, Angeloni A and
Anastasi E: Protein Induced by Vitamin K Absence II (PIVKA-II) as a
potential serological biomarker in pancreatic cancer: A pilot
study. Biochem Med (Zagreb). 29:0207072019. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Tartaglione S, Mancini P, Viggiani V,
Chirletti P, Angeloni A and Anastasi E: PIVKA-II: A biomarker for
diagnosing and monitoring patients with pancreatic adenocarcinoma.
PLoS One. 16:e02516562021. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Chan HLY, Vogel A, Berg T, De Toni EN,
Kudo M, Trojan J, Eiblmaier A, Klein HG, Hegel JK, Sharma A, et al:
Performance evaluation of the Elecsys PIVKA-II and Elecsys AFP
assays for hepatocellular carcinoma diagnosis. JGH Open. 6:292–300.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Okada KI, Kawai M, Tani M, Hirono S,
Miyazawa M, Shimizu A, Kitahata Y and Yamaue H: Predicting factors
for unresectability in patients with pancreatic ductal
adenocarcinoma. J Hepatobiliary Pancreat Sci. 21:648–653. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Ozaki K, Hayashi H, Ikuta Y, Masuda T,
Akaboshi S, Ogata K, Matumoto K, Ogawa K, Kamio T, Baba H and
Takamori H: Conversion surgery for initially unresectable
pancreatic ductal adenocarcinoma with synchronous liver metastasis
after treatment with FOLFIRINOX. Clin J Gastroenterol. 12:603–608.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Sunagawa Y, Yamada S, Sato Y, Morimoto D,
Sonohara F, Takami H, Inokawa Y, Hayashi M, Kanda M, Tanaka C, et
al: Novel prognostic implications of DUPAN-2 in the era of initial
systemic therapy for pancreatic cancer. Ann Surg Oncol.
27:2081–2089. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Takagi T, Nagai M, Nishiwada S, Terai T,
Yasuda S, Matsuo Y, Doi S, Kohara Y and Sho M: Importance of triple
tumor markers as biomarkers in patients with pancreatic ductal
adenocarcinoma. Ann Gastroenterol Surg. 7:326–335. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Hosokawa Y, Nagakawa Y, Sahara Y,
Takishita C, Katsumata K and Tsuchida A: Serum SPan-1 is a
significant risk factor for early recurrence of pancreatic cancer
after curative resection. Dig Surg. 34:125–132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Nishio K, Kimura K, Amano R, Yamazoe S,
Ohrira G, Nakata B, Hirakawa K and Ohira M: Preoperative predictors
for early recurrence of resectable pancreatic cancer. World J Surg
Oncol. 15:162017. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Tsutsumi K, Kawamoto H, Hirao K,
Sakakihara I, Yamamoto N, Noma Y, Fujii M, Kato H, Ogawa T, Ishida
E, et al: Monitoring of CA19-9 and SPan-1 can facilitate the
earlier confirmation of progressing pancreatic cancer during
chemotherapy. Pancreatology. 12:409–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Shimizu T, Asakuma M, Tomioka A, Inoue Y,
Hirokawa F, Hayashi M and Uchiyama K: Span-1 and CA19-9 as
predictors of early recurrence and lymph node metastasis for
patients with invasive pancreatic cancer after pancreatectomy. Am
Surg. 84:109–113. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Inokuchi S, Itoh S, Yoshizumi T, Yugawa K,
Yoshiya S, Toshima T, Takeishi K, Iguchi T, Sanefuji K, Harada N,
et al: Mitochondrial expression of the DNA repair enzyme OGG1
improves the prognosis of pancreatic ductal adenocarcinoma.
Pancreatology. 20:1175–1182. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Miyata T, Hayashi H, Yamashita YI,
Matsumura K, Nakao Y, Itoyama R, Yamao T, Tsukamoto M, Okabe H,
Imai K, et al: Prognostic value of the preoperative tumor marker
index in resected pancreatic ductal adenocarcinoma: A retrospective
single-institution study. Ann Surg Oncol. 28:1572–1580. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Ishido K, Kimura N, Wakiya T, Nagase H,
Hara Y, Kanda T, Fujita H and Hakamada K: Development of a
biomarker-based scoring system predicting early recurrence of
resectable pancreatic duct adenocarcinoma. Ann Surg Oncol.
29:1281–1293. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Kondo N, Murakami Y, Uemura K, Nakagawa N,
Takahashi S, Ohge H and Sueda T: Comparison of the prognostic
impact of pre- and post-operative CA19-9, SPan-1, and DUPAN-II
levels in patients with pancreatic carcinoma. Pancreatology.
17:95–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Ho J, Li X, Zhang L, Liang Y, Hu W, Yau
JCW, Chan H, Gin T, Chan MTV, Tse G and Wu WKK: Translational
genomics in pancreatic ductal adenocarcinoma: A review with
re-analysis of TCGA dataset. Semin Cancer Biol. 55:70–77. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Pietrasz D, Sereni E, Lancelotti F, Pea A,
Luchini C, Innamorati G, Salvia R and Bassi C: Circulating tumour
DNA: A challenging innovation to develop ‘precision onco-surgery’
in pancreatic adenocarcinoma. Br J Cancer. 126:1676–1683. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
McGowan R, Sally Á, McCabe A, Moran BM and
Finn K: Circulating nucleic acids as novel biomarkers for
pancreatic ductal adenocarcinoma. Cancers (Basel). 14:20272022.
View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Araujo RLC, Silva RO, de Pádua Souza C,
Milani JM, Huguet F, Rezende AC and Gaujoux S: Does neoadjuvant
therapy for pancreatic head adenocarcinoma increase postoperative
morbidity? A systematic review of the literature with
meta-analysis. J Surg Oncol. 121:881–892. 2020.PubMed/NCBI
|
|
159
|
Taniere P, Borghi-Scoazec G, Saurin JC,
Lombard-Bohas C, Boulez J, Berger F, Hainaut P and Scoazec JY:
Cytokeratin expression in adenocarcinomas of the esophagogastric
junction: A comparative study of adenocarcinomas of the distal
esophagus and of the proximal stomach. Am J Surg Pathol.
26:1213–1221. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Kim MA, Lee HS, Yang H-K and Kim WH:
Cytokeratin expression profile in gastric carcinomas. Hum Pathol.
35:576–581. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Xu W, Zhang MW, Huang J, Wang X, Xu SF, Li
Y and Wang SJ: Correlation between CK18 gene and gastric carcinoma
micrometastasis. World J Gastroenterol. 11:6530–6534. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Chen ZM and Wang HL: Alteration of
cytokeratin 7 and cytokeratin 20 expression profile is uniquely
associated with tumorigenesis of primary adenocarcinoma of the
small intestine. Am J Surg Pathol. 28:1352–1359. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Kelder W, van den Berg A, van der Leij J,
Bleeker W, Tiebosch AT, Grond JK, Baas PC and Plukker JT: RT-PCR
and immunohistochemical evaluation of sentinel lymph nodes after in
vivo mapping with Patent Blue V in colon cancer patients. Scand J
Gastroenterol. 41:1073–1078. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Athanassiadou P, Psyhoyiou H, Grapsa D,
Gonidi M, Ketikoglou I and Patsouris E: Cytokeratin 8 and 18
expression in imprint smears of chronic viral hepatitis, autoimmune
hepatitis and hepatocellular carcinoma. Acta Cytologica. 51:61–65.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Terracciano LM, Glatz K, Mhawech P, Vasei
M, Lehmann FS, Vecchione R and Tornillo L: Hepatoid adenocarcinoma
with liver metastasis mimicking hepatocellular carcinoma: An
immunohistochemical and molecular study of eight cases. Am J Surg
Pathol. 27:1302–1312. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Cabibi D, Licata A, Barresi E, Craxì A and
Aragona F: Expression of cytokeratin 7 and 20 in pathological
conditions of the bile tract. Pathol Res Pract. 199:65–70. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Castrillon DH, Lee KR and Nucci MR:
Distinction between endometrial and endocervical adenocarcinoma: An
immunohistochemical study. Int J Gynecol Pathol. 21:4–10. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Chu P, Wu E and Weiss LM: Cytokeratin 7
and cytokeratin 20 expression in epithelial neoplasms: A survey of
435 cases. Mod Pathol. 13:962–972. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Vang R, Gown AM, Barry TS, Wheeler DT,
Yemelyanova A, Seidman JD and Ronnett BM: Cytokeratins 7 and 20 in
primary and secondary mucinous tumors of the ovary: Analysis of
coordinate immunohistochemical expression profiles and staining
distribution in 179 cases. Am J Surg Pathol. 30:1130–1139. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Harnden P, Kennedy W, Andrew AC and
Southgate J: Immunophenotype of transitional metaplasia of the
uterine cervix. Int J Gynecol Pathol. 18:125–129. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Tot T: Patterns of distribution of
cytokeratins 20 and 7 in special types of invasive breast
carcinoma: a study of 123 cases. Ann Diagn Pathol. 3:350–356. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Cruz I, Ciudad J, Cruz JJ, Ramos M,
Gómez-Alonso A, Adansa JC, Rodríguez C and Orfao A: Evaluation of
multiparameter flow cytometry for the detection of breast cancer
tumor cells in blood samples. Am J Clin Pathol. 123:66–74. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Leivo I: Sinonasal adenocarcinoma: Update
on classification, immunophenotype and molecular features. Head
Neck Pathol. 10:68–74. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Bejarano PA, Nikiforov YE, Swenson ES and
Biddinger PW: Thyroid transcription factor-1, thyroglobulin,
cytokeratin 7, and cytokeratin 20 in thyroid neoplasms. Appl
Immunohistochem Mol Morphol. 8:189–194. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Ikeda S, Fujimori M, Shibata S, Okajima M,
Ishizaki Y, Kurihara T, Miyata Y, Iseki M, Shimizu Y, Tokumoto N,
et al: Combined immunohistochemistry of beta-catenin, cytokeratin
7, and cytokeratin 20 is useful in discriminating primary lung
adenocarcinomas from metastatic colorectal cancer. BMC Cancer.
6:312006. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Mhawech P, Uchida T and Pelte MF:
Immunohistochemical profile of high-grade urothelial bladder
carcinoma and prostate adenocarcinoma. Hum Pathol. 33:1136–1140.
2002. View Article : Google Scholar : PubMed/NCBI
|