Introduction
Colorectal cancer is one of the most common types of
malignant tumors worldwide. According to GLOBOCAN 2020 estimates
(https://gco.iarc.fr/), colorectal cancer is the
third most-diagnosed malignancy and the second leading cause of
cancer-associated death. Regarding the pattern of metastasis in
colorectal cancer, any organ can be a site of metastasis, including
bone, the brain and distant lymph nodes, with the liver, lung and
peritoneum being the most common sites (1). The incidence of brain metastases from
colorectal carcinoma ranges from 0.06–4% and is more frequently
seen in cases of rectal cancer (1,2).
Even though the prognosis of colorectal cancer is
frequently poor with a median overall survival (OS) of 5–6 months,
the survival time may be extended with combination treatment
methods (3,4). The current therapeutic algorithm for
metastatic colorectal cancer (mCRC) management from the National
Comprehensive Cancer Network (5)
and European Society For Medical Oncology (6) may involve a combination of different
therapies which are tailored to the individual, based on the type
and timing of prior therapy and the toxicity profiles of essential
drugs. The main treatment approach includes systemic therapy and
radical resection, if possible, often in conjunction with the local
treatment of primary and metastatic sites. Regarding the increasing
importance of targeted therapy in the treatment of mCRC, the
mutational profile of the tumor is the focus of considerable
attention. Evaluation of the KRAS/NRAS and BRAF mutation status, as
well as HER2 amplifications and microsatellite instability/mismatch
repair status are recommended for patients with mCRC (5). In addition to systemic therapy,
locally ablative procedures or resection may be considered in cases
of liver or lung oligometastases (6). However, as brain metastases are rare,
there is a lack of data concerning the detection and management of
this type of metastasis. In the present article, a case of a
metachronous brain metastasis from rectal cancer treated using a
multidisciplinary approach is reported.
Case report
A 47-year-old male with no previous medical history
was admitted to Hanoi Medical University Hospital (Hanoi, Vietnam)
in May 2021 with increasing constipation and a palpable inguinal
lymph node. A colonoscopy showed a large serrated-ulcerative tumor
in the lower rectum and anal canal, protruding by more than half of
the bowel circumference. A biopsy of the mass revealed that it
comprised adenocarcinomatous tissue. A metastatic inguinal node was
confirmed via fine needle aspiration. On pelvic magnetic resonance
imaging (MRI), it was observed that the tumor invaded the internal
anal sphincter and levator ani muscle. In addition, malignant lymph
nodes were detected around the bilateral iliac vessels, pelvic area
and abdominal aorta, with irregular borders and enlarged sizes of
15×18 mm. The patient also received a cranial MRI and a computed
tomography scan of the thorax and abdomen, which did not show any
suspected metastasis sites (data not shown). Therefore, a diagnosis
of stage T4N2M1 lower rectal cancer was made.
As the genetic test result of the patient ruled out
RAS and BRAF mutations (test performed by Gene Solutions), the
patient was given a modified FOLFOXIRI regimen comprising 150
mg/m2 irinotecan, 85 mg/m2 oxaliplatin, 200
mg/m2 leucovorin and 2,400 mg/m2 fluorouracil
as a 48-h continuous infusion starting on day 1, every 2 weeks,
with cetuximab at an initial dose of 400 mg/m2, then 250
mg/m2 weekly, as an initial treatment for 12 cycles,
which ended in January 2022. The full sequencing data of the
patient are not publicly available due to patient privacy. Imaging
by cranial MRI, computed tomography of the thorax and abdomen, as
well as ultrasound for peripheral lymph nodes, showed a complete
clinical response, with lymph nodes measuring <10 mm on the
short axis and lesions in the rectum appearing as flat white scars.
At that time, the patient wished to discontinue the original
regimen due to the adverse effects of the systemic treatment, which
included anorexia and an intolerable cetuximab-induced rash, along
with the increasing financial burden of the therapy. Therefore, it
was decided to use capecitabine in a maintenance setting at a dose
of 1,250 mg/m2 twice a day from days 1–14, every 3
weeks. After two cycles of capecitabine, the patient developed a
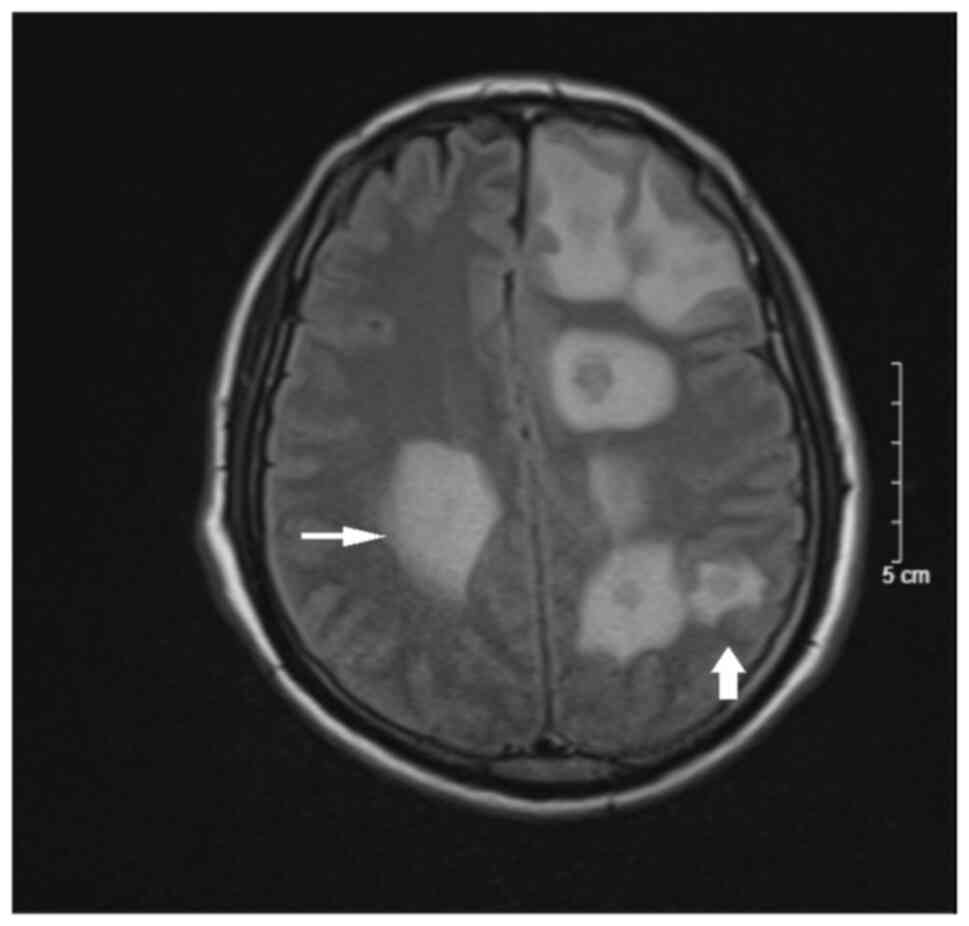
headache and mild paralysis. Cranial MRI revealed scattered nodules
in the bilateral brain parenchyma, mostly in the grey-white matter
border, hypo-intensity on T1-weighted imaging, hyper-intensity on
T2-weighted imaging, slightly restricted diffusion on
diffusion-weighted imaging, margin enhancement after contrast and
extensive cerebral edema (Figs. 1
and 2). Following the elimination
of all other possible causes, such as neurological infection or
stroke, a diagnosis of rectal cancer brain metastasis was made. The
patient was then given whole-brain radiation therapy (WBRT) with 30
Gy in 10 fractions.
After radiation therapy, the patient was reluctant
to restart infusional treatment, so was treated with the
aforementioned capecitabine regimen for three cycles. However,
after experiencing little to no improvement of the persistent
headache, the patient consented to a FOLFIRI and bevacizumab
regimen comprising 5 mg/kg bevacizumab, 400 mg/m2
fluorouracil intravenous bolus, 400 mg/m2 folinic acid,
180 mg/m2 irinotecan and 2,400 mg/m2
fluorouracil as a 46-h continuous infusion starting on day 1, every
2 weeks. This neurological symptom subsided after two cycles. The
cranial MRI of the patient performed in September 2022 after 6
cycles of the FOLFIRI bevacizumab regimen indicated a good response
of the brain metastasis with a decreased lesion size and no sign of
cerebral edema (Figs. 3 and
4). As of March 2023, the
administration of the FOLFIRI plus bevacizumab regimen to the
patient was continuing without adverse events. At this time, the
patient had been living with brain metastasis for >10 months and
maintained a stable neurological condition.
Discussion
Due to limited data on colorectal cancer metastases
to the brain, the management of this remains a challenge for
oncologists. Brain metastases are usually found in late-stage
advanced diseases, and the vast majority of patients also have
metastases at other sites (2). The
incidence of brain metastases in mCRC is 14.6%, of which 76% of
cases are asymptomatic (7). In the
present case, nervous system imaging was indicated only after the
patient presented with suspected neurological symptoms. Risk
factors associated with brain metastases in patients with
colorectal cancer have been noted in certain studies, which include
a RAS mutation and concomitant metastasis in the lung and liver
(3,8). However, none of these aspects was
observed in the present patient.
For initial management, the FOLFOXIRI triplet
regimen was selected because it has substantial efficacy in
metastatic disease (9). The
potential of adding cetuximab to the treatment plan was
demonstrated in the FIRE-3 trial, and the doses used in the trial
were applied to the present patient (10). However, exposure to all three active
cytotoxic agents (irinotecan, oxaliplatin and fluorouracil) might
be associated with a risk of increased toxicity. The original
FOLFOXIRI regimen comprising 165 mg/m2 irinotecan, 85
mg/m2 oxaliplatin, 200 mg/m2 leucovorin and
3,200 mg/m2 fluorouracil as a 48-h continuous infusion
starting on day 1 and administered every 2 weeks, has been reported
to cause a higher rate of grade 3 and 4 neutropenia compared with
the FOLFIRI combination (9). A
modified FOLFOXIRI regimen has been shown to have more favorable
efficacy and safety than FOLFOXIRI at the original doses (11). Despite receiving the modified
regimen, the present patient experienced persistent anorexia and a
cetuximab-induced rash during treatment.
The discontinuation of cetuximab may be a factor
leading to brain metastasis in the present patient. The efficacy
and safety of cetuximab as a maintenance therapy in patients
following the effective completion of chemotherapy have been
evaluated in the literature. For example, in one study cetuximab
maintenance therapy significantly improved the median progression
free survival (mPFS) of patients with mCRC to 11.6 months compared
with 6.1 months in the observation group (12). However, capecitabine is also a
standard treatment in mCRC. Despite the lack of head-to-head
comparisons between the two regimens as subsequent therapy,
capecitabine has been reported to lead to an mPFS of ~6.43 months,
which is lower than that of cetuximab (13). Nonetheless, there is no known
characteristic, feature or factor that is able to predict the
likelihood of brain metastases; therefore, clinicians are prompted
to take neurological signs into account at re-evaluation.
The standard approaches to brain metastases
treatment include surgery, stereotactic radiosurgery and WBRT,
either alone or in combination. In general, surgical resection is
performed for a single large brain metastasis with massive edema or
when the metastasis is in the eloquent brain area. Stereotactic
radiosurgery is usually indicated for oligometastases, whereas WBRT
is selected for patients who have multiple metastases or
large-sized oligometastases with uncontrolled extracranial
metastases, or who have a poor performance status (14). Patients with symptomatic brain
metastases are recommended to be offered local therapy, comprising
radiosurgery or radiation therapy plus surgery, according to the
American Society of Clinical Oncology-Society for
Neuro-Oncology-American Society for Radiation Oncology guidelines,
regardless of the systemic therapy used for the systemic disease
(14). For that reason, WBRT is a
suitable choice for the present patient. The effectiveness of WBRT
in the relief of symptoms and improvement of OS has been found in
previous studies, with a median OS of 2–9 months (2,15). Koo
et al (16) noted several
risk factors associated with a poor prognosis, including older age
(>65 years), multiple brain lesions (≥3), an elevated level of
carcinoembryonic antigen (>5 ng/ml) at brain metastasis
diagnosis, and extracranial metastases.
Evidence of the benefit of systemic therapy in
patients with brain metastases is limited, as its efficacy depends
on the ability of the treatment agent to cross the blood-brain
barrier. Bevacizumab is a humanized monoclonal antibody that
inhibits vascular endothelial growth factor (VEGF). Bevacizumab has
been shown to be beneficial to patients who have developed brain
metastasis from lung cancer and other solid tumors (17,18).
Additionally, bevacizumab therapy has been approved for the
treatment of recurrent glioblastoma and is currently being
evaluated for the treatment of newly diagnosed glioblastoma,
central nervous system lymphoma and secondary cerebral metastases
derived from glioblastoma (19).
Regarding its mechanism of action, it has been suggested that
bevacizumab may not have to cross the blood brain barrier to
function because of its ability to neutralize VEGF in the lumen of
the capillaries in the brain (19).
In one study, the administration of a bevacizumab-containing
chemotherapy regimen following neurosurgery, radiosurgery or WBRT
was found to result in an OS of 20.6 months after the diagnosis of
brain metastases (20). Another
study of 21 cases suggested that bevacizumab plays a role in the
treatment of brain metastasis from colorectal cancer; however, no
statistically significant result was observed due to the small
sample size (21). Chahine et
al (22) compared two groups of
patients with brain metastasis from colorectal cancer. When
patients with and without bevacizumab treatment were compared, it
was concluded that the antiangiogenic therapy significantly
improved median survival. However, the timing of bevacizumab
introduction, as well as the clinical response in terms of symptoms
and imaging, were not mentioned. In the present case, due to the
patient refusing immediate post-radiation infusional therapy,
capecitabine was used as an alternative treatment. Capecitabine has
been shown to be more beneficial than observation in patients with
mCRC (13). However, in the present
case, it was not until bevacizumab was administered that the
symptoms of the patient fully subsided. These observations suggest
that in antiangiogenic-naive patients with little symptomatic
improvement following radiation treatment, the addition of
bevacizumab to the treatment course later can bring benefits.
In conclusion, the brain is an uncommon site of
metastasis in colorectal cancer. Most cases of brain metastasis are
asymptomatic. For that reason, the early detection of brain
metastasis requires the close monitoring of neurological symptoms.
WBRT followed by bevacizumab and chemotherapy show satisfactory
results.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed in this study are
included in this published article.
Authors' contributions
HVN is the clinical oncologist who treated the
patient and revised the manuscript. DTP is the assistant doctor who
wrote the manuscript and made substantial contributions to the
conception of the study. TTN, KNTM, BTT and HLT assisted in the
patient treatment, collected clinical information and assisted with
the drafting of the manuscript. HVN and DTP confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent for the publication of this
study has been obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Riihimäki M, Hemminki A, Sundquist J and
Hemminki K: Patterns of metastasis in colon and rectal cancer. Sci
Rep. 6:297652016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walasek T, Reinfuss M, Kurzynski M, Pluta
E, Patla A, Mituś JW and Sas-Korczyńska B: Brain metastasis from
colorectal carcinoma. Clinical picture, treatment and prognosis.
Oncol Radiother. 2:11–16. 2018.
|
|
3
|
Müller S, Köhler F, Hendricks A, Kastner
C, Börner K, Diers J, Lock JF, Petritsch B, Germer CT and Wiegering
A: Brain metastases from colorectal cancer: A systematic review of
the literature and meta-analysis to establish a guideline for daily
treatment. Cancers (Basel). 13:9002021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Imaizumi J, Shida D, Narita Y, Miyakita Y,
Tanabe T, Takashima A, Boku N, Igaki H, Itami J and Kanemitsu Y:
Prognostic factors of brain metastases from colorectal cancer. BMC
Cancer. 19:7552019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Benson AB, Venook AP, Al-Hawary MM, Azad
N, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D,
Garrido-Laguna I, et al: Rectal cancer, version 2.2022, NCCN
clinical practice guidelines in oncology. J Natl Compr Canc Netw.
20:1139–1167. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshino T, Cervantes A, Bando H,
Martinelli E, Oki E, Xu RH, Mulansari NA, Govind Babu K, Lee MA,
Tan CK, et al: Pan-Asian adapted ESMO clinical practice guidelines
for the diagnosis, treatment and follow-up of patients with
metastatic colorectal cancer. ESMO Open. 8:1015582023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shindorf ML, Jafferji MS and Goff SL:
Incidence of asymptomatic brain metastases in metastatic colorectal
cancer. Clin Colorectal Cancer. 19:263–269. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ko FC, Liu JM, Chen WS, Chiang JK, Lin TC
and Lin JK: Risk and patterns of brain metastases in colorectal
cancer: 27-Year experience. Dis Colon Rectum. 42:1467–1471. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Falcone A, Ricci S, Brunetti I, Pfanner E,
Allegrini G, Barbara C, Crinò L, Benedetti G, Evangelista W,
Fanchini L, et al: Phase III trial of infusional fluorouracil,
leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with
infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as
first-line treatment for metastatic colorectal cancer: The gruppo
oncologico nord ovest. J Clin Oncol. 25:1670–1676. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Heinemann V, von Weikersthal LF, Decker T,
Kiani A, Kaiser F, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl
C, Seipelt G, et al: FOLFIRI plus cetuximab or bevacizumab for
advanced colorectal cancer: Final survival and per-protocol
analysis of FIRE-3, a randomised clinical trial. Br J Cancer.
124:587–594. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kazama K, Shiozawa M, Numata M, Sugano N,
Sato S, Uchiyama M, Sato M, Aoyama T, Tamagawa H, Oshima T, et al:
Comparison of safety and efficacy of fluorouracil + oxaliplatin +
irinotecan (FOLFOXIRI) and modified FOLFOXIRI with bevacizumab for
metastatic colorectal cancer: Data from clinical practice. Int J
Colorectal Dis. 37:337–348. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yuan M, Wang Z, Zhao Y, Feng T, Lv W and
Zhong H: Cetuximab can be an effective and low-toxicity maintenance
treatment drug in patients with metastatic colorectal cancer: A
real-world study of Zhejiang cancer hospital. Front Pharmacol.
12:6320762021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Luo HY, Li YH, Wang W, Wang ZQ, Yuan X, Ma
D, Wang FH, Zhang DS, Lin DR, Lin YC, et al: Single-agent
capecitabine as maintenance therapy after induction of XELOX (or
FOLFOX) in first-line treatment of metastatic colorectal cancer:
Randomized clinical trial of efficacy and safety. Ann Oncol.
27:1074–1081. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vogelbaum MA, Brown PD, Messersmith H,
Brastianos PK, Burri S, Cahill D, Dunn IF, Gaspar LE, Gatson NTN,
Gondi V, et al: Treatment for brain metastases: ASCO-SNO-ASTRO
guideline. J Clin Oncol. 40:492–516. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Damiens K, Ayoub JPM, Lemieux B, Aubin F,
Saliba W, Campeau MP and Tehfe M: Clinical features and course of
brain metastases in colorectal cancer: An experience from a single
institution. Curr Oncol. 19:254–258. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koo T, Kim K, Park HJ, Han SW, Kim TY,
Jeong SY, Park KJ and Chie EK: Prognostic factors for survival in
colorectal cancer patients with brain metastases undergoing whole
brain radiotherapy: Multicenter retrospective study. Sci Rep.
10:43402020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ascha MS, Wang JF, Kumthekar P, Sloan AE,
Kruchko C and Barnholtz-Sloan JS: Bevacizumab for the treatment of
non-small cell lung cancer patients with synchronous brain
metastases. Sci Rep. 9:177922019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Berghoff AS, Breckwoldt MO, Riedemann L,
Karimian-Jazi K, Loew S, Schlieter F, Furtner J, Cinci M, Thomas M,
Strowitzki MJ, et al: Bevacizumab-based treatment as salvage
therapy in patients with recurrent symptomatic brain metastases.
Neurooncol Adv 2. vdaa0382020.PubMed/NCBI
|
|
19
|
Chacko AM, Li C, Pryma DA, Brem S, Coukos
G and Muzykantov V: Targeted delivery of antibody-based therapeutic
and imaging agents to CNS tumors: Crossing the blood-brain barrier
divide. Expert Opin Drug Deliv. 10:907–926. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Finkelmeier F, You SJ, Waidmann O, Wolff
R, Zeuzem S, Bähr O and Trojan J: Bevacizumab in combination with
chemotherapy for colorectal brain metastasis. J Gastrointest
Cancer. 47:82–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li W, Wang T, Zhu Y, Yu H, Ma L, Ding Y,
Hong G and Lei D: Brain metastasis from colorectal cancer:
Treatment, survival, and prognosis. Medicine (Baltimore).
101:e302732022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chahine G, Ibrahim T, Felefly T,
El-Ahmadie A, Freiha P, El-Khoury L, Khalife-Saleh N and Saleh K:
Colorectal cancer and brain metastases: An aggressive disease with
a different response to treatment. Tumori J. 105:427–433. 2019.
View Article : Google Scholar : PubMed/NCBI
|