Introduction
Chemotherapy is one of the treatment options for
malignant tumors, and many of the recently developed molecular
targeted drugs have been adopted as standard treatments, leading to
an improved patient prognosis (1).
The improvement in prognosis of adolescent and young adult female
cancer patients aged 15 to 39 years has led to an increase in their
desire to conceive and bear a child after cancer treatment
(2).
Primary liver cancer is one of six major types of
cancer and the third leading cause of cancer death (3). Hepatocellular carcinoma (HCC) is the
most common type of primary liver cancer. Patients with hepatitis C
virus (HCV) develop chronic liver cirrhosis, with up to 20% of
these patients developing cirrhosis and approximately 2% developing
HCC (4). Up to 80% of the causes of
HCC are due to HCV and hepatitis B virus (HBV) (5,6), HCC
caused by HBV is common in younger patients in East Asia (7), but non-B and non-C HCC negative for
these two viruses has been increasing in Japan in recent years.
Alcoholic liver disease or non-alcoholic fatty liver disease is
often the cause of these disorders, but in half of the cases, the
cause remains unknown (8).
Surgery, drug therapy, and catheter ablation are
performed for HCC, but the recurrence rate is high and the survival
rate remains low (9). As evidence
for neoadjuvant chemotherapy, there are reports of resectable cases
in which the molecular targeted drug sorafenib has been used
(10), and lenvatinib has shown
non-inferiority to sorafenib for unresectable HCC in a phase 3
trial (11).
Lenvatinib exhibits antitumor effects by inhibiting
the growth of vascular endothelial cells and the formation of
vessel-like luminal structures (12). However, ovarian insufficiency is not
a recognized complication of treatment with lenvatinib. We report a
case of primary ovarian insufficiency during administration of
lenvatinib for non-B, non-C HCC in a young woman.
Case report
A 25-year-old woman with epigastralgia visited her
previous doctor, who diagnosed her as having a liver tumor. She
underwent percutaneous liver biopsy resulting in a pathological
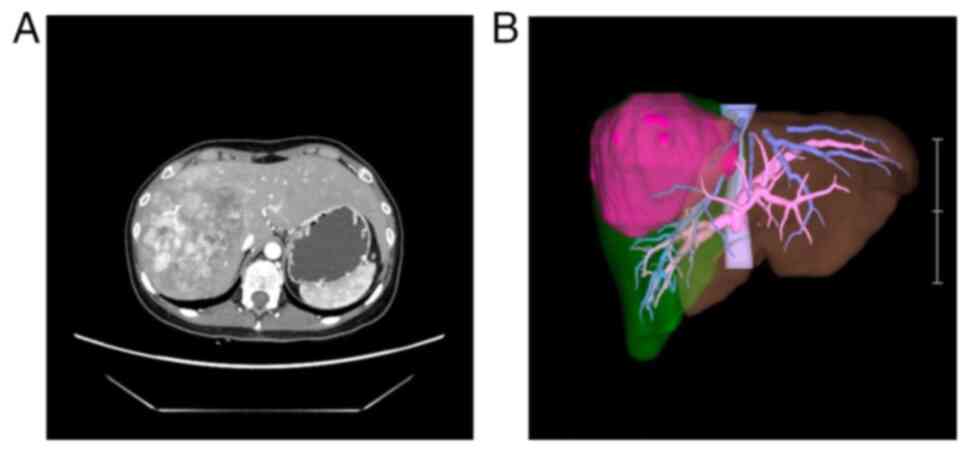
diagnosis of HCC. Computed tomography revealed a 103×98×100-mm
tumor in the right lobe of the liver (Fig. 1). Her Child-Pugh classification was
A. 99mTc-labelled diethylene triamine pentaacetate-galactosyl-human
serum albumin scintigraphy was performed before surgery (13). The radioactivity of the liver
regions of interest (ROI) divided by that of the liver-plus-heart
ROI at 15 min (LHL15) was 0.969, and radioactivity of the heart ROI
at 15 min divided by that at 3 min (HH15) was 0.753, thus revealing
no reduction in hepatic blood flow due to the tumor. Inferior
mediastinal lymphadenopathy was noted as an extrahepatic
metastasis. She was ultimately diagnosed as having stage IVB HCC
and was considered for lenvatinib treatment.
She was 155 cm tall, weighed 49 kg, and had a body
mass index of 20.4 kg/m2, HbA1C of 5.4%, and no
diabetes. HBV and HCV antibody tests were negative. She had a
thyroid-stimulating hormone level of 2.48 µU/ml, levels of free
thyroid hormones 4 and 3 of 1.25 ng/dl and 2.91 pg/ml,
respectively, and normal thyroid function tests. Diagnostic imaging
of the pelvic area showed no abnormal findings such as tumors in
her uterus or ovaries. She had no history of pregnancy, and her
menstrual cycle was regular. Imaging studies revealed left
pulmonary thrombosis and left common iliac vein thrombosis for
which oral administration of rivaroxaban 15 mg/day was started.
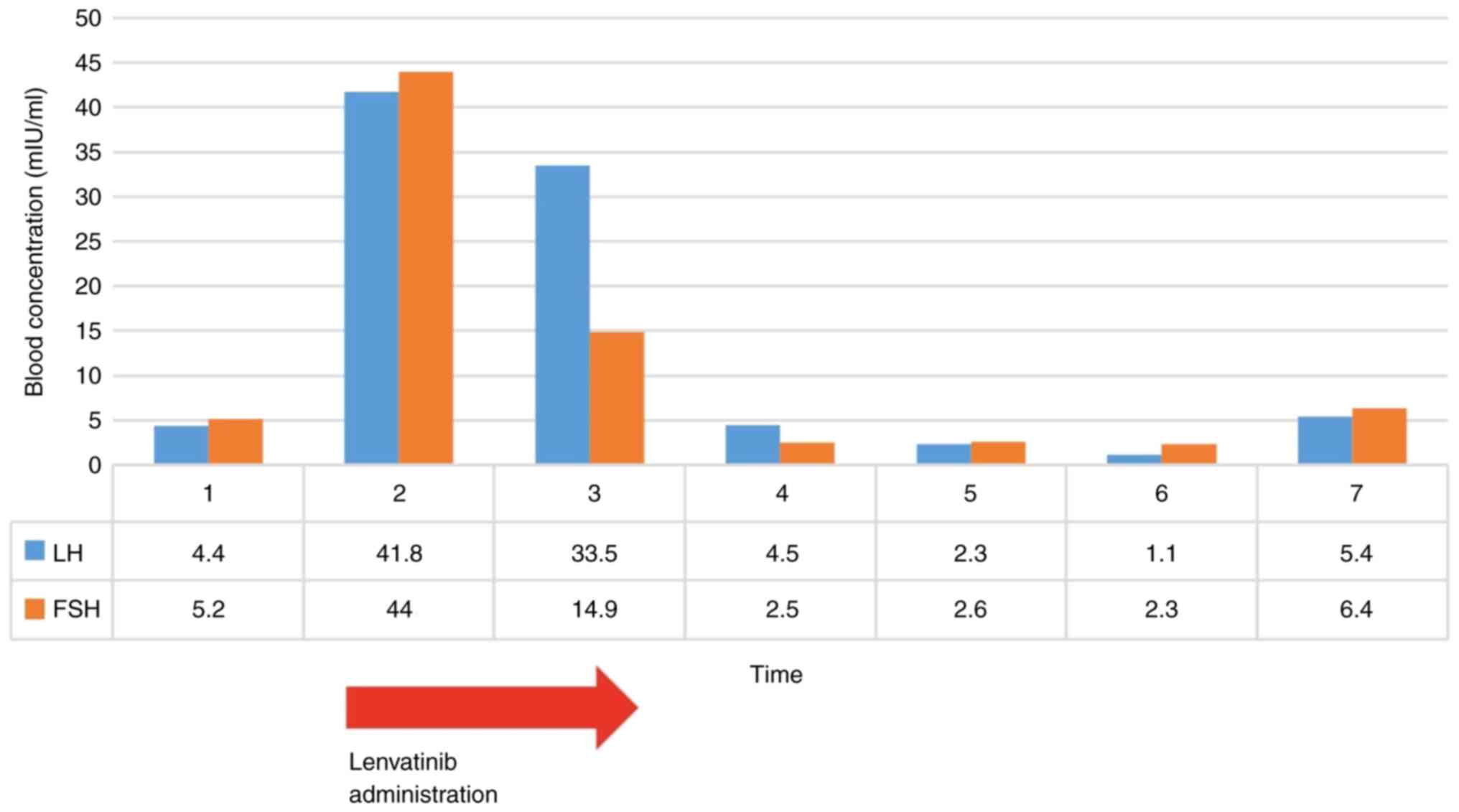
A blood examination on the day before lenvatinib
administration was started showed the following: luteinizing
hormone (LH) 4.40 mIU/ml, follicle-stimulating hormone (FSH) 5.2
mIU/ml, estradiol (E2) 57.4 pg/ml, and age-equivalent hormone
values. Oral administration of lenvatinib 8 mg/day was started as
chemotherapy for the extrahepatic lesions. However, she became
amenorrheic after the lenvatinib was started, and 48 days after the
start of administration, LH was 41.8 mIU/ml, FSH was 44 mIU/ml, and
E2 was 53.2 pg/ml, values indicating decreased ovarian function.
Lenvatinib administration at 8 mg/day was continued for 98 days.
Thirteen days after the end of lenvatinib administration, the
hormone values were LH 33.5 mIU/ml, FSH 14.9 mIU/ml, and E2 474
pg/ml, indicating slight improvement. Menstruation then resumed
(Fig. 2). Before oral
administration of lenvatinib, tumor marker alpha-fetoprotein (AFP)
was 10.4 ng/ml, and protein induced by vitamin K absence or
antagonist-II (PIVKA-II), which reflects Des-gamma
carboxyprothrombin, was 440 mAU/ml. PIVKA-II improved to 177 mAU/ml
after administration of lenvatinib. After the hepatectomy, AFP
decreased to 2.6 ng/ml and PIVKA-II was 38 mAU/ml.
The only adverse event (AE) other than amenorrhea
was mild general fatigue at 3 months after administration, but no
major lenvatinib-related AEs such as hand-foot skin reaction,
hypertension, proteinuria, and diarrhea were observed. Her weight
did not change by more than 5% during the observation period.
Imaging studies showed shrinkage of the liver tumor.
An extended right hepatectomy, cholecystectomy, and dissection of
liver perihilar lymph node #111 were then performed. The
pathological findings showed a tumor macroscopically classified as
a confluent multinodular type and histologically classified as HCC
with an expansive pattern. The ratio of lymph node metastasis was
1/15. Thirty days later, the mediastinal lymph nodes were resected
thoracoscopically, and no lymph node metastasis was observed. At
the time of liver resection, her LH was 4.5 mIU/ml, FSH was 2.5
mIU/ml, and E2 was 235 pg/ml. Transvaginal ultrasound showed
endometrial thickening, and she began to have regular menses. One
year after receiving lenvatinib, she had a LH of 1.10 mIU/ml, FSH
of 2.3 mIU/ml, and E2 of 159 pg/ml, and her menstrual cycles were
regular. At 2 years after starting treatment, she had no metastasis
or recurrence of liver cancer, and her menstruation remained
regular without amenorrhea.
The patient consented to the publication of this
case report, and ethical committee approval was not required.
Discussion
There have been no reports of primary ovarian
insufficiency (POI) caused by lenvatinib. Our young female patient
with liver cancer showed amenorrhea and high FSH levels during the
administration of lenvatinib as preoperative chemotherapy for 3
months and then recovered to normal menstruation.
Lenvatinib is an oral inhibitor of multiple receptor
tyrosine kinases that suppresses stem cell factor (SCF)-producing
tumors via vascular endothelial growth factor (VEGF), fibroblast
growth factor (FGF), SCF, inhibition of tyrosine-protein kinase
(KIT), and VEGF signaling. Lenvatinib inhibits the kinases VEGF
receptor (VEGFR) 1–3, fibroblast growth factor receptor (FGFR) 1–4,
platelet-derived growth factor receptor (PDGFR), rearranged during
transfection (RET), and KIT (14,15)
(Fig. 3). An in vitro study
also reported that it suppresses FGFR and PDGFR signaling (16).
Lenvatinib was found to inhibit kinase-insert
domain-containing receptor and KIT kinases more strongly than did
imatinib (12). The antiangiogenic
activity of lenvatinib in an in vivo experiment was similar to that
of lenvatinib 10 mg/kg and sorafenib 100 mg/kg in an experiment
using human pancreatic cancer VEGF121 (17).
The effect of lenvatinib on malignant tumors has
been reported in HCC (8), thyroid
carcinoma (18), and advanced
endometrial carcinoma (19).
Currently, treatment of advanced HCC with lenvatinib significantly
prolongs overall survival compared to sorafenib, and lenvatinib has
become one of the first-line treatment drugs (11).
Typical AEs of lenvatinib include hand-foot skin
reaction, general fatigue, appetite loss, hypertension, diarrhea,
and proteinuria, among others. However, our patient experienced
only amenorrhea and mild general fatigue (20–23).
The toxicities of lenvatinib are hypertension and proteinuria,
which are likely to occur with drugs that target the VEGF pathway
(24). Bevacizumab, sorafenib, and
sunitinib, which also act on the VEGF pathway, have similar
mechanisms of hypertension risk (25–27).
VEGF has several isoforms: VEGF145 is expressed in
carcinoma cells, whereas VEGF121 and 165 are expressed in the ovary
(28). Follicle development
requires angiogenesis, for which the action of VEGF is important
(29,30). When VEGF Trap was administered to
macaques during the early luteal phase in in vivo experiments on
the corpus luteum, attenuation of E2 and elevation of LH and FSH
occurred. These findings suggested that VEGF plays an important
role in ovarian function and fertility (31). The anti-angiogenic drug bevacizumab
is thought to affect the process of ovulation by this mechanism
(32). In the present patient, it
is most likely that the inhibitory effect of lenvatinib on VEGF
impaired follicle angiogenesis, inhibited follicle development, and
caused temporary ovarian insufficiency (Fig. 2).
The definition of POI is ovarian insufficiency in
women under 40 years of age that causes infertility (33), the criterion of which is a FSH ≥40
mIU/ml with amenorrhea (34).
According to a prospective cohort study by Coulam et al
(35), premature ovarian failure
was reported to occur in 0.1% of women by age 30 years and in 1% by
age 40 years, and it increased with age. The present patient met
the criterion of FSH ≥40 associated with amenorrhea during
lenvatinib administration, and ovarian insufficiency could be
diagnosed. There have been no reports of ovarian insufficiency due
to administration of the Xa inhibitor rivaroxaban, which was taken
during the same period (36).
Antiphospholipid antibody syndrome has been reported to decrease
ovarian reserve in blood coagulation disorders. In our patient,
although lower extremity venous thrombosis was observed before
starting lenvatinib, her menstrual cycle was normal, and no
elevation of FSH was observed. While it can be difficult to
accurately evaluate the impact of blood coagulation abnormalities
associated with tumor growth on ovarian function, the timing of the
administration of lenvatinib and the onset of amenorrhea coincided,
suggesting that the trigger for amenorrhea was the administration
of lenvatinib (37–39).
There are few reports of ovarian insufficiency
caused by molecular targeted drugs. An 18-year-old female patient
with breast angiosarcoma was reported to have POI due to the
effects of pazopanib (40), which
is also an inhibitor of multiple receptor tyrosine kinase.
Pazopanib also inhibits VEGFR, PDGFR, and c-Kit (41). c-Kit and PDGFR are thought to be
involved in primary follicle formation (42), but three regimens (doxorubicin,
ifosfamide, and gemcitabine, among others) were administered in
this case, and there was a delay before the administration of
pazopanib.
Ifosfamide-containing regimens have also been
reported to cause ovarian insufficiency (43) that may result in some ovarian
damage. Multitargeted tyrosine kinase inhibitors that impair VEGFR
include sorafenib and sunitinib, and these drugs may also have side
effects that impair ovarian function (26,27).
The administration of pazopanib, sorafenib, and sunitinib doses in
in vivo rat experiments did not cause a meaningful change in the
number of ovarian follicles (44).
In conclusion, a young woman with HCC experienced
transient ovarian hypofunction as a possible side effect of
lenvatinib use. Although this side effect has not been reported so
far, to our knowledge, when treating such patients, it may be
necessary to consider the risk of POI caused by molecular targeted
drugs that impair the VEGF pathway.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YA, YI, NS, MO, KI, AK, TT, HK and MW made
substantial contributions to the conception and design of the
study, acquired data and revised the manuscript critically for
important intellectual content. YA, YI and MO analyzed and
interpreted the data. YA and HK drafted the manuscript. KI made the
pathological diagnosis. YA and YI confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Written informed consent for participation in this
study was obtained from the patient using our institutional consent
form. All identifying information has been removed or anonymized to
ensure confidentiality. Approval from our institutional ethical
committee was not required.
Patient consent for publication
Written informed consent for publication was
obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Santos R, Ursu O, Gaulton A, Bento AP,
Donadi RS, Bologa CG, Karlsson A, Al-Lazikani B, Hersey A, Oprea TI
and Overington JP: A comprehensive map of molecular drug targets.
Nat Rev Drug Discov. 16:19–34. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kao WH, Kuo CF, Chiou MJ, Liu YC, Wang CC,
Hong JH, Hsu JT, Chiang YJ and Chuang YF: Adverse birth outcomes in
adolescent and young adult female cancer survivors: A nationwide
population-based study. Br J Cancer. 122:918–924. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Bisceglie AM: Natural history of
hepatitis C: Its impact on clinical management. Hepatology.
31:1014–1018. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
McGlynn KA, Petrick JL and El-Serag HB:
Epidemiology of hepatocellular carcinoma. Hepatology. 73 (Suppl
1):S4–S13. 2021. View Article : Google Scholar
|
|
7
|
Lam CM, Chan AO, Ho P, Ng IO, Lo CM, Liu
CL, Poon RT and Fan ST: Different presentation of hepatitis
B-related hepatocellular carcinoma in a cohort of 1863 young and
old patients-implications for screening. Aliment Pharmacol Ther.
19:771–777. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tateishi R, Okanoue T, Fujiwara N, Okita
K, Kiyosawa K, Omata M, Kumada H, Hayashi N and Koike K: Clinical
characteristics, treatment, and prognosis of non-B, non-C
hepatocellular carcinoma: A large retrospective multicenter cohort
study. J Gastroenterol. 50:350–360. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhu J, Tang B, Lv X, Meng M, Weng Q, Zhang
N, Li J, Fan K, Zheng L, Fang S, et al: Identifying
apoptosis-related transcriptomic aberrations and revealing clinical
relevance as diagnostic and prognostic biomarker in hepatocellular
carcinoma. Front Oncol. 10:5191802021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye SL, Chen X, Yang J, Bie P, Zhang S, Liu
F, Liu L, Zhou J, Dou K, Yip CS and Yang X: Evaluation of sorafenib
in Chinese unresectable hepatocellular carcinoma patients with
prior surgery and portal vein tumor thrombosis: A subset analysis
of GIDEON study data. Tumour Biol. 39:10104283176950302017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kudo M, Finn RS, Qin S, Han KH, Ikeda K,
Piscaglia F, Baron A, Park JW, Han G, Jassem J, et al: Lenvatinib
versus sorafenib in first-line treatment of patients with
unresectable hepatocellular carcinoma: A randomised phase 3
non-inferiority trial. Lancet. 391:1163–1173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsui J, Yamamoto Y, Funahashi Y,
Tsuruoka A, Watanabe T, Wakabayashi T, Uenaka T and Asada M: E7080,
a novel inhibitor that targets multiple kinases, has potent
antitumor activities against stem cell factor producing human small
cell lung cancer H146, based on angiogenesis inhibition. Int J
Cancer. 122:664–671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Harada K, Mizuguchi T, Katagiri Y,
Kawamoto M, Nakamura Y, Meguro M, Ota S, Sasaki S, Miyanishi K,
Sonoda T, et al: Area between the hepatic and heart curves of
(99m)Tc-galactosyl-human serum albumin scintigraphy represents
liver function and disease progression for preoperative evaluation
in hepatocellular carcinoma patients. J Hepatobiliary Pancreat Sci.
19:667–673. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tohyama O, Matsui J, Kodama K, Hata-Sugi
N, Kimura T, Okamoto K, Minoshima Y, Iwata M and Funahashi Y:
Antitumor activity of lenvatinib (e7080): An angiogenesis inhibitor
that targets multiple receptor tyrosine kinases in preclinical
human thyroid cancer models. J Thyroid Res. 2014:6387472014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iesato A, Li S, Roti G, Hacker MR, Fischer
AH and Nucera C: Lenvatinib targets PDGFR-β pericytes and inhibits
synergy with thyroid carcinoma cells: Novel translational insights.
J Clin Endocrinol Metab. 106:3569–3590. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Glen H, Mason S, Patel H, Macleod K and
Brunton VG: E7080, a multi-targeted tyrosine kinase inhibitor
suppresses tumor cell migration and invasion. BMC Cancer.
11:3092011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamamoto Y, Matsui J, Matsushima T,
Obaishi H, Miyazaki K, Nakamura K, Tohyama O, Semba T, Yamaguchi A,
Hoshi SS, et al: Lenvatinib, an angiogenesis inhibitor targeting
VEGFR/FGFR, shows broad antitumor activity in human tumor xenograft
models associated with microvessel density and pericyte coverage.
Vasc Cell. 6:182014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gild ML, Bullock M, Robinson BG and
Clifton-Bligh R: Multikinase inhibitors: A new option for the
treatment of thyroid cancer. Nat Rev Endocrinol. 7:617–624. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Makker V, Colombo N, Herráez AC, Santin
AD, Colomba E, Miller DS, Fujiwara K, Pignata S, Baron-Hay S,
Ray-Coquard I, et al: Lenvatinib plus pembrolizumab for advanced
endometrial cancer. N Engl J Med. 386:437–448. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rimel BJ, Crane EK, Hou J, Nakayama J,
MacDonald J, Lutz K, Makker V and O'Cearbhaill RE: Tyrosine kinase
inhibitor toxicities: A society of gynecologic oncology review and
recommendations. Gynecol Oncol. 174:148–156. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hiraoka A, Kumada T, Kariyama K, Takaguchi
K, Atsukawa M, Itobayashi E, Tsuji K, Tajiri K, Hirooka M, Shimada
N, et al: Clinical features of lenvatinib for unresectable
hepatocellular carcinoma in real-world conditions: Multicenter
analysis. Cancer Med. 8:137–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Calo CA, Levine MD, Brown MD, O'Malley DM
and Backes FJ: Combination lenvatinib plus pembrolizumab in the
treatment of ovarian clear cell carcinoma: A case series. Gynecol
Oncol Rep. 46:1011712023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Backes FJ, Wei L, Chen M, Hill K,
Dzwigalski K, Poi M, Phelps M, Salani R, Copeland LJ, Fowler JM, et
al: Phase I evaluation of lenvatinib and weekly paclitaxel in
patients with recurrent endometrial, ovarian, fallopian tube, or
primary peritoneal cancer. Gynecol Oncol. 162:619–625. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Keizer RJ, Gupta A, Gillavry MR, Jansen M,
Wanders J, Beijnen JH, Schellens JH, Karlsson MO and Huitema AD: A
model of hypertension and proteinuria in cancer patients treated
with the anti-angiogenic drug E7080. J Pharmacokinet Pharmacodyn.
37:347–363. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu X, Wu S, Dahut WL and Parikh CR: Risks
of proteinuria and hypertension with bevacizumab, an antibody
against vascular endothelial growth factor: Systematic review and
meta-analysis. Am J Kidney Dis. 49:186–193. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu S, Chen JJ, Kudelka A, Lu J and Zhu X:
Incidence and risk of hypertension with sorafenib in patients with
cancer: A systematic review and meta-analysis. Lancet Oncol.
9:117–123. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu X, Stergiopoulos K and Wu S: Risk of
hypertension and renal dysfunction with an angiogenesis inhibitor
sunitinib: Systematic review and meta-analysis. Acta Oncol.
48:9–17. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Geva E and Jaffe RB: Role of vascular
endothelial growth factor in ovarian physiology and pathology.
Fertil Steril. 74:429–438. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Stouffer RL, Martínez-Chequer JC,
Molskness TA, Xu F and Hazzard TM: Regulation and action of
angiogenic factors in the primate ovary. Arch Med Res. 32:567–575.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tamanini C and De Ambrogi M: Angiogenesis
in developing follicle and corpus luteum. Reprod Domest Anim.
39:206–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fraser HM, Wilson H, Morris KD, Swanston I
and Wiegand SJ: Vascular endothelial growth factor Trap suppresses
ovarian function at all stages of the luteal phase in the macaque.
J Clin Endocrinol Metab. 90:5811–5818. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Imai A, Ichigo S, Matsunami K, Takagi H
and Kawabata I: Ovarian function following targeted anti-angiogenic
therapy with bevacizumab. Mol Clin Oncol. 6:807–810. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ebrahimi M and Asbagh FA: Pathogenesis and
causes of premature ovarian failure: An update. Int J Fertil
Steril. 5:54–65. 2011.PubMed/NCBI
|
|
34
|
Rebar RW: Premature ovarian failure.
Obstet Gynecol. 113:1355–1363. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Coulam CB, Adamson SC and Annegers JF:
Incidence of premature ovarian failure. Obstet Gynecol. 67:604–606.
1986.PubMed/NCBI
|
|
36
|
Burness CB and Perry CM: Rivaroxaban: A
review of its use in the treatment of deep vein thrombosis or
pulmonary embolism and the prevention of recurrent venous
thromboembolism. Drugs. 74:243–262. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rodrigues VO, Soligo AGES and Pannain GD:
Antiphospholipid antibody syndrome and infertility. Rev Bras
Ginecol Obstet. 41:621–627. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yamakami LY, Serafini PC, de Araujo DB,
Bonfá E, Leon EP, Baracat EC and Silva CA: Ovarian reserve in women
with primary antiphospholipid syndrome. Lupus. 23:862–867. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vega M, Barad DH, Yu Y, Darmon SK,
Weghofer A, Kushnir VA and Gleicher N: Anti-mullerian hormone
levels decline with the presence of antiphospholipid antibodies. Am
J Reprod Immunol. 76:333–337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De Sanctis R, Lorenzi E, Agostinetto E,
D'Amico T, Simonelli M and Santoro A: Primary ovarian insufficiency
associated with pazopanib therapy in a breast angiosarcoma patient:
A CARE-compliant case report. Medicine (Baltimore). 98:e180892019.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Verweij J and Sleijfer S: Pazopanib, a new
therapy for metastatic soft tissue sarcoma. Expert Opin
Pharmacother. 14:929–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nilsson EE, Detzel C and Skinner MK:
Platelet-derived growth factor modulates the primordial to primary
follicle transition. Reproduction. 131:1007–1015. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Long JP, Wan F, Zhang F, Zhou J and Don
LF: DTC chemotherapy regimen is associated with higher incidence of
premature ovarian failure in women of reproductive age with breast
cancer. Eur Rev Med Pharmacol Sci. 20:1087–1092. 2016.PubMed/NCBI
|
|
44
|
Yildiz C, Kacan T, Akkar OB, Karakus S,
Kacan SB, Ozer H and Cetin A: Effects of pazopanib, sunitinib, and
sorafenib, anti-VEGF agents, on the growth of experimental
endometriosis in rats. Reprod Sci. 22:1445–1451. 2015. View Article : Google Scholar : PubMed/NCBI
|