Introduction
Synovial sarcoma (SS) is a relatively common
high-grade sarcoma, accounting for 5–10% of all soft tissue
sarcomas, most commonly occurring in the juxta-articular location
(1). SS located in the pleura are
rare and rarely reported in the literature, with the majority of
cases reported (2). Nearly 40 cases
of primary SS of the pleura (PSSP) have been reported since
Gaertner et al (3) published
the first case in 1996. It has been reported in all age groups.
Since most of the published papers are case reports, there is no
clear prognosis or recurrence rate reported in the literature. It
is frequently misdiagnosed as lung cancer or other pleural tumors
before surgery due to the lack of clinical and imaging specificity.
SS has a comparatively poorer prognosis and higher recurrence rate.
To date, <50 cases have been published in the English language.
The present study reported a rare case of two simultaneous PSSP in
an adolescent.
Case report
An 18-year-old male patient presented with a 1-month
history of repeated sporadic dry cough and wheezing admitted to the
The Second People's Hospital of Liaocheng (Linqing, China). The
patient had no history of smoking or asbestos exposure. The cough
worsened after exercise and in the lateral decubitus position
accompanied by general fatigue, no fever, no blood in sputum and no
hemoptysis. The patient's laboratory results were as follows:
Erythrocyte sedimentation rate, 52 mm/h ↑ (normal range, ≤20 mm/h);
platelets, 383×109/l ↑ (normal range,
100–300×109/l); C-reactive protein, 98.46 mg/l ↑ (normal
range, 0–10 mg/l); prothrombin time, 15.40 sec ↑ (normal range,
9.4–12.5 sec); fibrinogen, 5.8 g/l ↑ (normal range, 2–4 g/l),
D-dimer determination, 5.57 mg/l ↑ (normal range, 0–0.5 mg/l).
Hydropleural biochemistry was as follows: Hydropleural protein,
51.8 g/l ↑ (normal range, 0–30 g/l); hydropleural lactate
dehydrogenase, 1,022 U/l ↑ (normal range, 0–200 U/l); hydropleural
cholesterol, 1.62 mmol/l ↑ (normal range, 0–1.60 mmol/l). Computed
tomography of the chest revealed two tumors in the left parietal
pleura (7.8×2.8 cm; 6.5×5.8 cm), with unclear boundaries with the
adjacent chest wall, localized thickening of soft tissue in the
left anterior intercostal space at 8 and 9, left pleural effusion
(considered hemorrhagic), incomplete expansion of the left upper
lobe of the lung and shadow consolidation of the left lower lobe of
the lung (Fig. 1). Abdominal and
pelvic CT and bone scans were normal without evidence of
metastasis. Three pleural effusion cytological examinations (one
per day for three consecutive days) showed no tumor cells. The
patient refused to undergo preoperative MRI, positron emission
tomography-CT and biopsy for financial reasons. The patient
underwent a left intrathoracic tumor resection. During the
operation, a fifth intercostal incision was performed on the left
lateral chest. The exploration revealed pleural adhesion,
separation adhesion, blood clots in the chest, the formation of
pleural fiberboard in the left lower lobe of the lung, and two
lesions in the chest; the larger one was located near the spine at
the level of the lower lung ligament, closely related to the
descending aorta, and the other one was located in the costophrenic
Angle. The hemoaccumulation in the chest was cleared, two lesions
were completely resected. During the operation, rapid freeze
pathology examination, performed according to standard procedures,
was used to confirm that the surgical margin was negative, and the
final specimen pathology, performed according to standard
procedures, also confirmed that the margin was negative. The
operation was deemed successful.
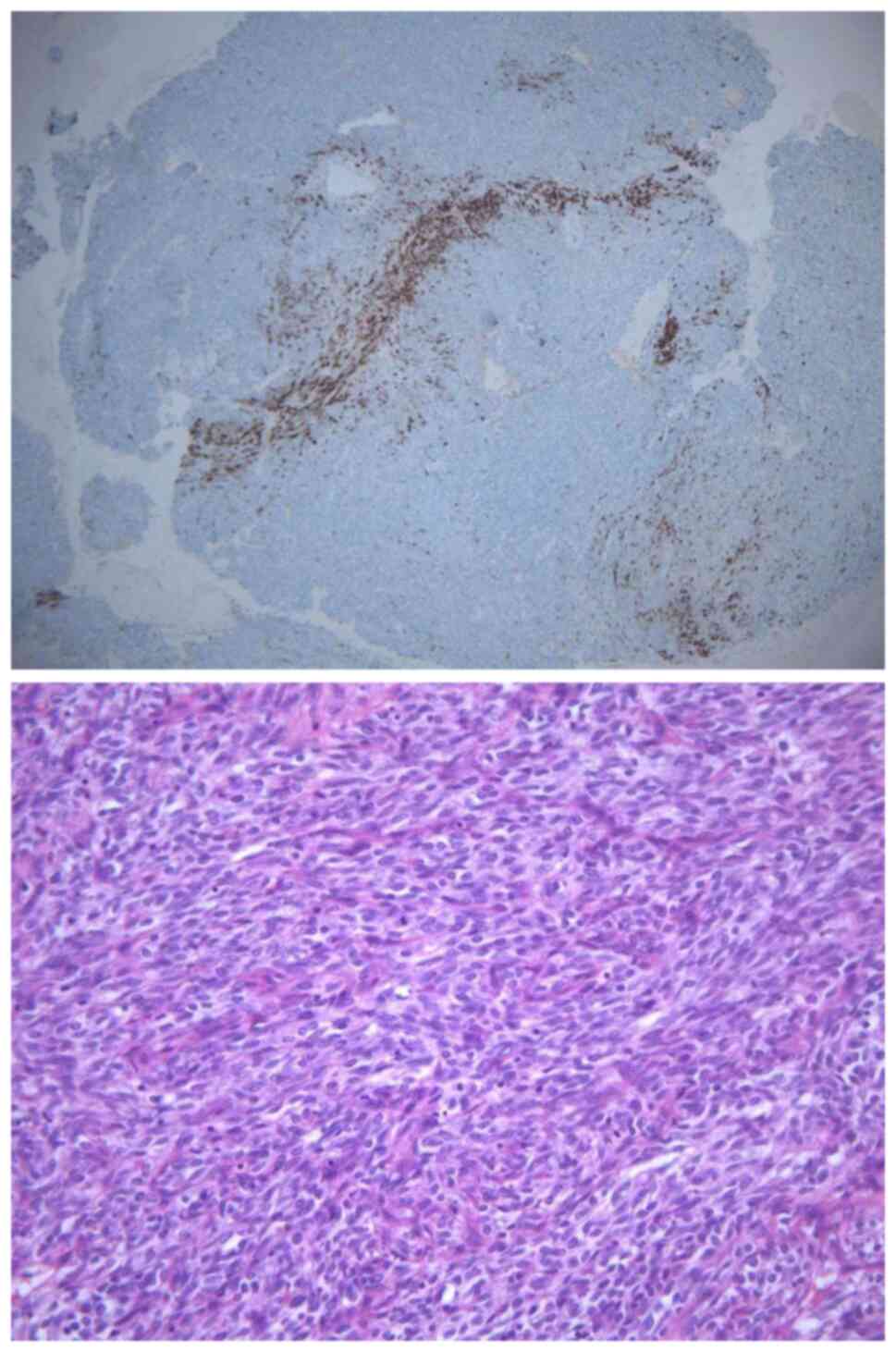
By histopathology with H&E staining (performed
according to standard procedures; Fig.
2, bottom), the tumor was confirmed to be a monophasic synovial
sarcoma (consisting of spindle-like cells in a perivascularomatous
vascular morphology, with mitotic or interwoven bundles of
spindle-like cells). Immunohistochemistry performed according to
routine procedures was used revealed the following: Cytokeratin
(CK) (−), epithelial membrane antigen (EMA) (−), CD99 molecule
(CD99/MIC-2) (−), signal transducer and activator of transcription
6 (STAT6) (−), smooth muscle actin (SMA) (−), Desmin (−), receptor
tyrosine kinase (CD117) (−), WT1 transcription factor (WT1) (−),
RING finger-like protein Ini1 (INI-1) (−), premelanosome protein
(HMB45) (−), tumor protein p63 (p63) (small amount +), BCL2
apoptosis regulator (Bcl-2) (part +), antigen identified by
monoclonal antibody Ki 67 (Ki67) (+ 40%), methylation of histone 3
lysine 27 (H3K27ME3) (+), cyclin D1 (+),CD34 molecule (CD34) (+),
calretinin (CR) (+) (Fig. 2, top),
podoplanin (D2-40) (part +), transducer-like enhancer split 1
(TLE-1) (+) and SS18 subunit of BAF chromatin remodeling complex
SSX family member 2 (SS18-SSX) (+). In brief, consecutive parallel
sections were stained with the following antibodies (the dilution
was according to the manufacturers' recommendations for
immunohistochemistry for each antibody): CK [rabbit anti-human
monoclonal antibody (mAb); cat. no. RAB-0050], EMA (rabbit
anti-human mAb; cat. no. kit-0011), CD99 (mouse anti-human mAb;
cat. no. MAB-0059), STAT6 (rabbit anti-human mAb; cat. no.
RMA-0845), SMA (mouse anti-human mAb; cat. no. kit-0006), Desmin
(mouse anti-human mAb; cat. no. MAB-0766), CD117 (rabbit anti-human
mAb; cat. no. kit-0029), WT1 (rabbit anti-human mAb; cat. no.
MAB-0678), INI-1 (mouse anti-human mAb; cat. no. MAB-0696), HMB45
(rabbit anti-human mAb; cat. no. MAB-0098), p63 (mouse anti-human
mAb; cat. no. MAB-0694), Bcl-2 (mouse anti-human mAb; cat. no.
MAB-0711), Ki67 (mouse anti-human mAb; cat. no. MAB-0672), cyclin
D1 (rabbit anti-human mAb; cat. no. RMA-0541), CD34 (mouse
anti-human mAb; cat. no. kit-0004), CR (mouse anti-human mAb; cat.
no. MAB-0716), D2-40 (mouse anti-human mAb; cat. no. MAB-0567),
TLE-1 (mouse anti-human mAb; cat. no. MAB-0686), SS18-SSX (rabbit
anti-human mAb; cat. no. RMA-1049; all from Maixin Fuzhou) and
H3K27ME3 (mouse anti-human mAb; cat. no. P68431; Absin). The
secondary antibodies used were goat anti-mouse IgG-FITC antibody
(cat. no. abs20140; Absin) and Elivision™ plus polymer HRP
(mouse/rabbit) IHC Kit (cat. no. KIT-9903; Maixin Fuzhou). Genetic
testing (fluorescence in situ hybridization), performed
according to routine procedures (4), indicated SS-18 (+) (Fig. 3).
After surgery, the patient received ifosfamide and
doxorubicin combined chemotherapy. He underwent four cycles of
chemotherapy (cyclophosphamide 9 g, once a day for 5 days;
doxorubicin 150 mg, once a day for 3 days; 21 days as a cycle) and
has been well followed up. After six months of follow-up, the
symptoms of dry cough and wheezing disappeared. All of the
laboratory results were within normal limits and no post-operative
complications, tumor recurrence or metastasis occurred.
Discussion
PSSP is a highly malignant and rare tumor type that
is common in adolescents and is not associated with smoking.
Typical symptoms include acute chest pain, dyspnea, hemoptysis and
hemorrhagic effusion in the ipsopleural cavity (5). Diagnosis of SSP is often difficult
owing to its rarity and its similarity (clinically and
histologically) to other types of pleural tumor, particularly
sarcomatous mesothelioma. The most common presentation is a
well-defined mass with effusion on CT (2). On CT of the chest, a synovial sarcoma
of the pleura is commonly characterized as a heterogeneously
enhanced mass with well-defined margins, cortical bone destruction,
tumor calcifications and tumor infiltration of the chest wall
musculature (6). Duran-Mendicuti
et al (7) reported 5 cases
of primary pleural synovial sarcoma, showing radiologically uneven
enhancement and well-defined masses without calcification. The
pathologic types may be divided into monophasic, biphasic and
poorly differentiated types. In monophasic synovial sarcoma,
spindle cells may be seen interwoven into bundles.
Immunohistochemical examination of synovial sarcoma is
characterized by positive staining of cytokeratin and epithelial
cell membrane antigen, negative staining of nerve (S100) and smooth
muscle markers and uniform staining of epithelial cell marker
BerEp4, which facilitates the differentiation from malignant
mesothelioma. Most synovial sarcomas exhibit at least an exocentric
immune response to cytokeratin and epithelial membrane antigens,
which are usually more prominent in epithelial components. CD99 and
Bcl-2 were also detected in most cases.
Therefore, the diagnosis of PSSP requires clinical,
radiological, pathological and immunohistochemical examination to
exclude other primary tumors and metastatic sarcomas.
Although there is no standardized treatment for
PSSP, based on the generally effective treatment for soft tissue
sarcomas, a multidisciplinary treatment regimen that includes
radical excision as the primary means of treatment, combined with
chemotherapy (doxorubicin and ifosfamide) and radiotherapy, may be
recommended (8). Prior to radical
resection, neoadjuvant chemotherapy may be beneficial because it
causes tumor volume reduction and has the potential to treat
micrometastases, but there is no experience in PSSP. A previous
study reported that the disease-free survival of 5 patients after
surgical resection of PSSP was 2–14 months (7). Despite aggressive combination therapy,
the prognosis is uncertain and long-term follow-up is
warranted.
The present study described a case of primary
pleural synovial sarcoma, the first published case to date of two
simultaneous intrapleural tumors, treated by radical resection plus
chemotherapy. Postoperative pathology, immunohistochemistry,
genetic testing and radiological examination confirmed malignant
tumors. At six months after surgery, the patient is currently in
good health with no recurrence or metastasis.
In conclusion, PSSP is a rare and aggressive
neoplasm in adolescents; it is difficult to diagnose with imaging
alone, especially in the case of two masses in the pleura at the
same time and hemorrhagic pleural effusion. Genetic testing may
help confirm the diagnosis. Radical surgery is the main treatment
in combination, followed by adjuvant chemotherapy. The long-term
outcome remains to be seen, as PSSP has a poor prognosis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ and CM designed the study. YZ was the principal
person responsible for the study and wrote the original manuscript.
XX provided the surgical details described in the manuscript. YZ,
CM and HL performed histological analysis of the specimens and
provided all pathological details described in the study. YZ and CM
performed analysis and interpretation of CT imaging data. CM, XX
and HL performed a critical literature review, contributed to the
acquisition, analysis and interpretation of data and contributed to
the drafting of the Introduction and Discussion sections. YZ and XX
confirm the authenticity of all the raw data. All authors have read
and approved the final version of the study.
Ethics approval and consent to
participate
The study was conducted in accordance with the
ethical standards from the 1964 Declaration of Helsinki and its
later amendments; local ethical approval was obtained from the
Ethics Committee of the Second People's Hospital of Liaocheng
(Linqing, China).
Patient consent for publication
Written informed consent was obtained from the
patient for the case information and images to be published in this
case report.
Competing interests
All authors declare they have no competing
interests.
References
|
1
|
Attanoos RL and Pugh MR: The diagnosis of
pleural tumors other than mesothelioma. Arch Pathol Lab Med.
142:902–913. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sandeepa HS, Kate AH, Chaudhari P, Chavan
V, Patole K, Lokeshwar N and Chhajed PN: Primary pleural synovial
sarcoma: A rare cause of hemorrhagic pleural effusion in a young
adult. J Cancer Res Ther. 9:517–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaertner E, Zeren EH, Fleming MV, Colby TV
and Travis WD: Biphasic synovial sarcomas arising in the pleural
cavity. A clinicopathologic study of five cases. Am J Surg Pathol.
20:36–45. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Amary MF, Berisha F, Bernardi Fdel C,
Herbert A, James M, Reis-Filho JS, Fisher C, Nicholson AG,
Tirabosco R, Diss TC and Flanagan AM: Detection of SS18-SSX fusion
transcripts in formalin-fixed paraffin-embedded neoplasms: Analysis
of conventional RT-PCR, qRT-PCR and dual color FISH as diagnostic
tools for synovial sarcoma. Mod Pathol. 20:482–496. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu Y, Lin J, Sun H and Xie S: Primary
pleural synovial sarcoma in an adolescent: A case report. Transl
Cancer Res. 9:3771–3775. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kang MK, Cho KH, Lee YH, Han IY, Yoon YC,
Park KT, Kang DK and Kim BM: Primary synovial sarcoma of the
parietal pleura: A case report. Korean J Thorac Cardiovasc Surg.
46:159–161. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Duran-Mendicuti A, Costello P and Vargas
SO: Primary synovial sarcoma of the chest: Radiographic and
clinicopathologic correlation. J Thorac Imaging. 18:87–93. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamaki M, Yonehara S and Noriyuki T: Large
primary pleural synovial sarcoma with severe dyspnea: A case
report. Surg Case Rep. 3:292017. View Article : Google Scholar : PubMed/NCBI
|