Introduction
Liver cancer is the second leading cause of
cancer-related mortality worldwide, among which hepatocellular
carcinoma (HCC) accounts for ~90% of new cases, featuring a male
preponderance and rising mortality rate over the past decade
(1–3). The major etiologies of HCC include
hepatitis B and/or C virus infections, superfluous alcohol intake
and fatty liver disease (4,5). Curative treatment modalities,
including liver resection or transplantation, and microwave
ablation, provide a survival benefit for patients with early-stage
HCC; however, as the majority of patients with HCC are diagnosed at
an intermediate or advanced stage, these patients are not
considered candidates for these curative treatments (6,7).
Currently, diverse interventional treatment approaches, such as
bridging therapies, are administered in order make patients
eligible for curative resection treatments, thus prolonging the
survival profiles of patients with intermediate- to advanced-stage
HCC (8,9). However, there is still a lack of
consensus on the optimal bridging strategy for these patients.
Thus, further investigations are required into bridging
strategies.
Transarterial chemoembolization (TACE) is an
established first-line palliative treatment for patients with
unresectable intermediate-stage HCC. According to recent studies,
TACE is effective in promoting a reduction in tumor size and a
decrease in tumor burden; it is therefore recognized as a promising
bridging therapy for the management of HCC (10–13).
For example, a previous study indicated that TACE was an effective
bridging strategy for subsequent curative radical treatment in
patients with HCC, with a successful downstaging rate of 59.4%
(14).
Immunotherapy with strategies targeting immune
checkpoints [especially programmed-death 1 (PD-1) inhibitor] have
been developed for antitumor treatment and the combination of these
with chemotherapy has been shown to exhibit an enhanced clinical
benefit in patients with diverse malignancies (15–17).
Camrelizumab is a PD-1 inhibitor developed in China, which has
received considerable attention, since it exerts satisfactory
antitumor efficacy and it may serve as a promising strategy for the
downstaging of HCC followed by liver transplant or surgical
resection of the tumor (18–20).
Furthermore, there is evidence to indicate that the combination of
TACE and programmed cell death protein 1 (PD-1) inhibitor is
associated with a prolonged progression-free survival (PFS)
compared with TACE monotherapy for patients with intermediate- to
advanced-stage HCC (21,22). However, these previous studies do
not evaluate the effect of TACE plus camrelizumab as a downstaging
strategy for HCC.
Therefore, the present pilot study aimed to explore
the potential of TACE plus camrelizumab as a bridging therapy prior
to surgery in patients with intermediate-stage HCC.
Patients and methods
Patients
In the present prospective pilot study, 11 patients
with HCC with intermediate-stage disease [classified by China Liver
Cancer (CNLC) staging] who received TACE plus camrelizumab as
bridging therapy prior to surgery were enrolled between June 2019
and July 2020 at Handan Central Hospital (Handan, China). The
enrollment criteria included: i) A diagnosis of primary HCC in
accordance with the guidelines for liver cancer (23); ii) age ≥18 years; iii) CNLC IIa or
IIb stage disease (23); iv)
Eastern Cooperative Oncology Group performance status (ECOG PS)
score of 0 to 1 (24); v) patients
had to be ineligible for direct surgery due to one or more of the
following reasons: Large and multiple tumors, insufficient residual
liver, poor hepatic function, severe complications relating to a
high surgical risk, and a potential risk of severe post-excision
cirrhosis and post-excision liver failure; and vi) scheduled for
TACE combined with camrelizumab as a bridging therapy prior to
surgery. Patients were excluded if the following applied: i)
Vascular invasion, bile duct invasion or distant metastasis; ii)
contraindications to TACE or camrelizumab; iii) an uncontrolled
infection or moderate to severe myelosuppression; i) other
malignant diseases; or v) pregnant or lactating female patients.
The Institutional Review Board of Handan Central Hospital (Handan,
China) approved the present study and all patients provided written
informed consent.
Assessment prior to treatment
Clinical examinations prior to treatment were
performed for all patients and the main clinical data were
documented, including age, sex, hepatitis B (HBV) status, liver
cirrhosis, ECOG PS score, Child-Pugh stage (25), nodule number, tumor capsule, tumor
size, pathological differentiation and CNLC stage. The CNLC stage
was evaluated in line with the Guidelines for Diagnosis and
Treatment of Primary Liver Cancer in China (2017 Edition) (23).
Treatment process
The total treatment process was as follows: First,
all patients received one cycle of TACE; at 2 weeks following TACE,
patients began to receive camrelizumab treatment for 2–6 cycles;
following camrelizumab treatment, according to the surgical
indications, tumor resection was performed. The detailed TACE
procedures were conducted as reported in a previous study (26). In brief, following the
superselective catheterization of feeding arteries for tumors, a
mixture of ethiodized poppyseed oil (Jiangsu Hengrui Pharmaceutical
Co., Ltd) and oxaliplatin (200 mg) (Jiangsu Hengrui Pharmaceutical
Co., Ltd) was infused into the feeding arteries of the tumor and
polyvinyl alcohol particles (Jiangsu Hengrui Pharmaceutical Co.,
Ltd, China) were then injected as embolization material. The
embolization endpoint was that the blood flow reached complete or
near stasis in the feeding arteries for the tumor. Camrelizumab was
administered intravenously at a dose of 3 mg/kg, with a treatment
cycle of every 3 weeks.
Assessments
For the evaluation of the treatment response, an
abdominal contrast-enhanced CT examination was performed for the
patients at 2 weeks following TACE treatment and following
camrelizumab treatment. The clinical response was assessed based on
the modified Response Evaluation Criteria in Solid Tumors (27), including the complete response (CR),
partial response (PR), stable disease (SD) and progressive disease
(PD). Furthermore, the objective response rate (ORR) and disease
control rate (DCR) were calculated as CR + PR and CR + PR + SD,
respectively. When the patients had completed the whole
camrelizumab treatment protocol, the CNLC stage of the patients was
assessed again. Furthermore, the downstaging success was assessed,
which was defined as a change in CNLC stage from stage II to I
following TACE combined with camrelizumab bridging therapy
(14). To monitor the change in
tumor markers, the α-fetoprotein (AFP) level was detected prior to
TACE, following TACE, following camrelizumab treatment and
following surgical resection. In addition, adverse events were
documented for safety assessment.
Follow-up
Following surgery, the patients were followed up
every 3 to 6 months, during which the date of disease relapse or
patient mortality were recorded in detail for survival evaluation.
Relapse-free survival (RFS) and overall survival (OS) were
calculated for survival analysis.
Statistical analysis
Variables are presented using numbers with
percentages or the mean with standard deviation. Paired comparisons
for CNLC stage and AFP at different time-points were performed
using McNemar's test or the Wilcoxon signed-rank test, as
appropriate. The associations of HBV history and anti-HBV treatment
with downstaging success were determined using Fisher's exact test.
The RFS and OS were examined using Kaplan-Meier curves and the
comparison of the RFS and OS between two groups was performed using
the log-rank test. Statistical analysis and graph plotting were
completed using SPSS 22.0 software (IBM Corp.) and 7.00 software
(GraphPad Software; Dotmatics), respectively. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics of the
patients with HCC
In the present study, the 11 patients with HCC had a
mean age of 60.4±9.6 years (Table
I). The cohort comprised 8 (72.7%) males and 3 (27.3%) females.
Regarding the Child-Pugh stage, there were 8 (72.7%) and 3 (27.3%)
patients with Child-Pugh stages A and B, respectively. In terms of
the CNLC stage, there were 5 (45.5%) and 6 (54.5%) patients with
CNLC stage IIa and CNLC stage IIb, respectively. Detailed
information on the patients with HCC is provided in Table I.
 | Table I.Characteristics of patients with
hepatocellular carcinoma (n=11). |
Table I.
Characteristics of patients with
hepatocellular carcinoma (n=11).
| Item | Value |
|---|
| Age, years | 60.4±9.6 |
| Sex |
|
|
Male | 8 (72.7) |
|
Female | 3 (27.3) |
| HBV history | 7 (63.6) |
| Liver
cirrhosis | 3 (27.3) |
| ECOG PS score |
|
| 0 | 8 (72.7) |
| 1 | 3 (27.3) |
| Child-Pugh
stage |
|
| A | 8 (72.7) |
| B | 3 (27.3) |
| Nodule number | 3.4±1.0 |
| Tumor size >3
cm | 11 (100.0) |
| Tumor capsular | 8 (72.7) |
| Pathological
differentiation |
|
|
Well | 6 (54.5) |
|
Moderate | 3 (27.3) |
|
Poor | 2 (18.2) |
| CNLC stage |
|
|
IIa | 5 (45.5) |
|
IIb | 6 (54.5) |
Treatment response following
TACE/camrelizumab treatment in patients with HCC
Following TACE therapy, the CR, PR, SD, PD, ORR and
DCR were 9.1, 63.6, 27.3, 0.0, 72.7 and 100.0%, respectively
(Fig. 1A). Furthermore, following
camrelizumab treatment, the CR, PR, SD, PD, ORR and DCR were 18.2,
81.8, 0.0, 0.0, 100.0 and 100.0%, respectively (Fig. 1B).
 | Figure 1.Assessment of the treatment response
of patients with HCC. CR, PR, SD, PD, ORR and DCR following (A)
TACE and (B) following camrelizumab treatment in patients with
intermediate-stage HCC. HCC, hepatocellular carcinoma; TACE,
transarterial chemoembolization; CR, complete response; PR, partial
response; SD, stable disease; PD, progressive disease; ORR,
objective response rate; DCR, disease control rate. |
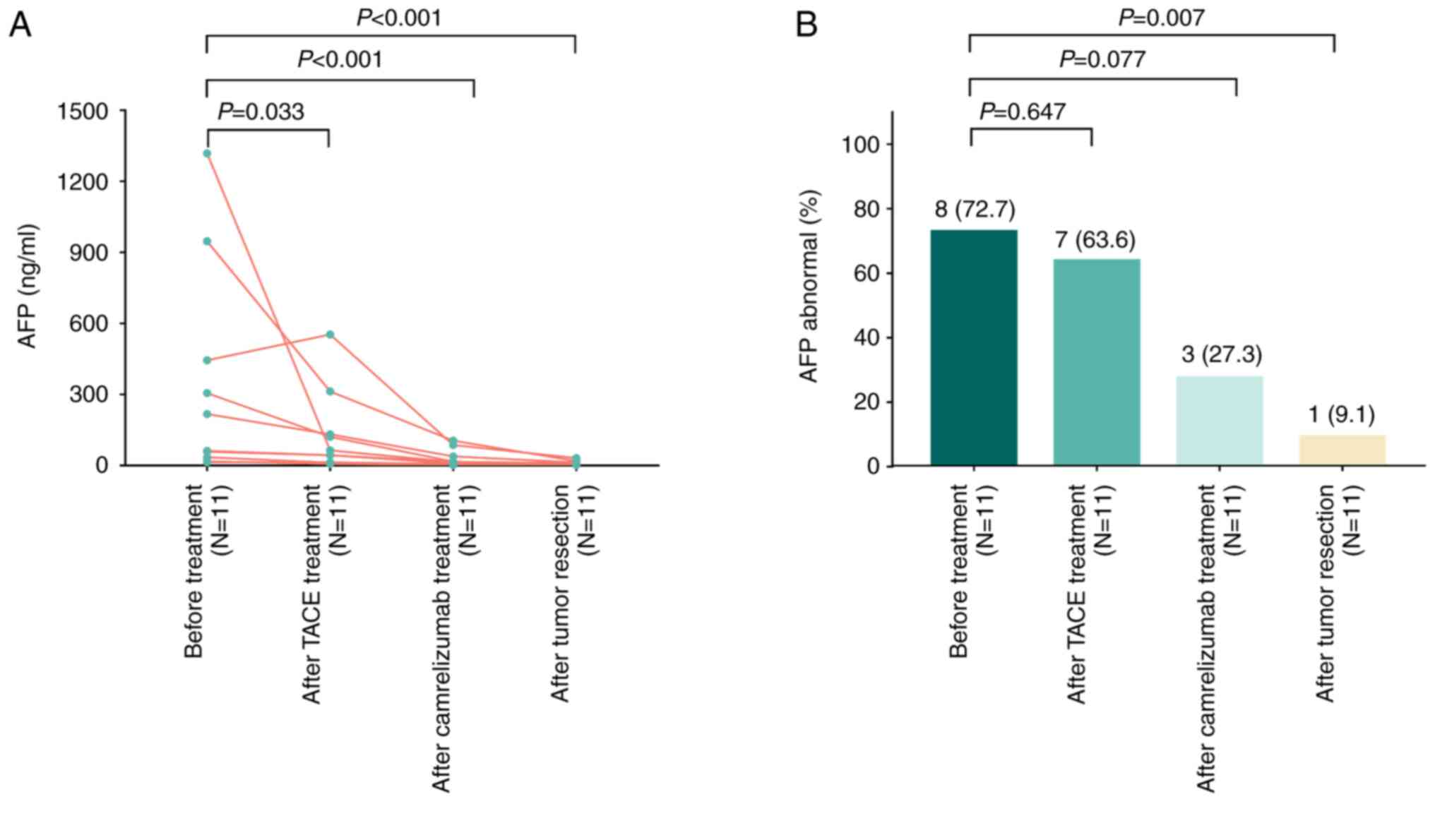
Longitudinal change in AFP during the
treatment of patients with HCC
The AFP levels were documented at four time-points
(including prior to TACE, following TACE, following camrelizumab
treatment and following surgical resection). The results revealed
that the AFP levels exhibited a marked decrease over these four
time-points (all P<0.05; Fig.
2A). Furthermore, the proportion of patients with abnormal AFP
levels was 72.7, 63.6, 27.3 and 9.1% at these four time-points,
respectively; this proportion significantly decreased following
tumor resection when compared with that prior to treatment
(P=0.007; Fig. 2B).
Downstaging success in patients with
HCC
Prior to treatment, there were 0 (0.0%), 0 (0.0%), 5
(45.5%) and 6 (64.5%) patients with CNLC stages Ia, Ib, IIa and
IIb, respectively; following treatment, there were 1 (9.1%), 6
(54.5%), 4 (36.4%) and 0 (0.0%) patients with CNLC stages Ia, Ib,
IIa and IIb, respectively (Table
II). By comparison, the CNLC stage was decreased following
treatment (P=0.007). Furthermore, the downstaging success was
63.6%. In the current study, 2 patients with an HBV history had HBV
reactivation and they received anti-HBV treatment, while the other
5 patients with an HBV history did not have any HBV reactivation
and thus, they did not receive anti-HBV treatment. Statistically,
neither HBV history nor anti-HBV treatment affected the downstaging
success (Table SI).
 | Table II.Down-staging success rate in the
patients (n=11). |
Table II.
Down-staging success rate in the
patients (n=11).
| Item | Before
treatment | After
treatment | P-value |
|---|
| CNLC stage |
|
| 0.007 |
| Ia | 0 (0.0) | 1 (9.1) |
|
| Ib | 0 (0.0) | 6 (54.5) |
|
|
IIa | 5 (45.5) | 4 (36.4) |
|
|
IIb | 6 (54.5) | 0 (0.0) |
|
| Down-staging
success |
| 7 (63.6) |
|
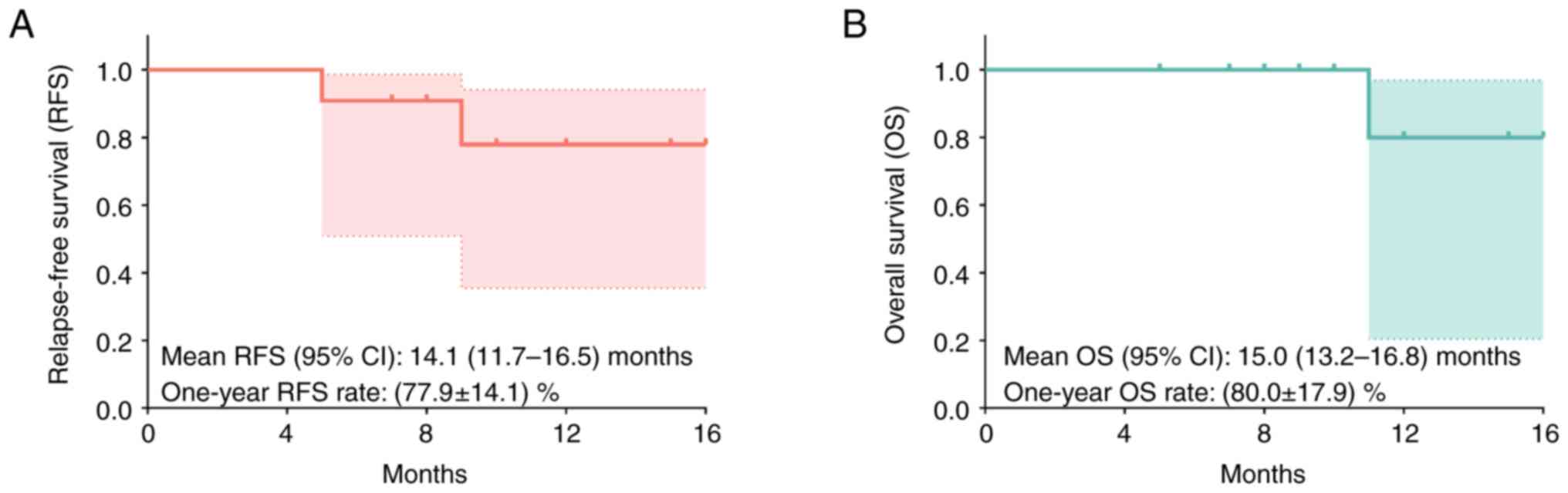
RFS and OS of patients with HCCs
According to the follow-up records following
surgery, the mean RFS (95% CI) was 14.1 (11.7–16.5) months and the
1-year RFS rate was 77.9±14.1% (Fig.
3A). Furthermore, the mean OS (95% CI) was 15.0 (13.2–16.8)
months and the 1-year OS rate was 80.0±17.9% (Fig. 3B).
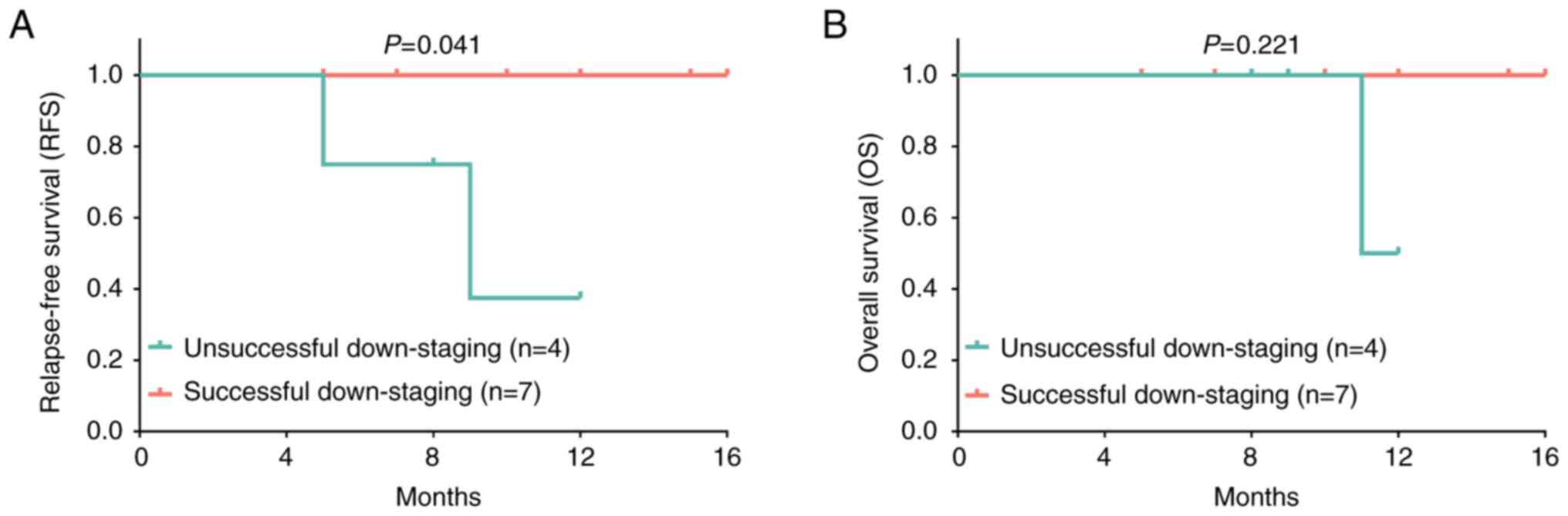
Association of downstaging success
with the survival profiles of patients with HCC
According to the downstaging success, all patients
were divided into the unsuccessful downstaging group (n=4) and the
successful downstaging group (n=7). Further comparative analysis
revealed that the RFS was increased in the successful downstaging
group compared with that in the unsuccessful downstaging group
(P=0.041; Fig. 4A); however, the OS
was similar between the successful and unsuccessful downstaging
groups (P=0.221; Fig. 4B). Detailed
information (including clinical features, treatment response,
disease relapse, survival status, etc.) of each patient with HCC is
presented in Table III.
 | Table III.Detailed data of each patient with
hepatocellular carcinoma. |
Table III.
Detailed data of each patient with
hepatocellular carcinoma.
|
|
|
|
|
| CNLC stage | Treatment
response |
|
|
|
|
|
|---|
|
|
|
|
|
|
|
|
|
|
|
|
|
|---|
| No. | Age, years | Gender | Child-Pugh
stage | Pathological
differentiation | Before | After | Down-staging | TACE | Camrelizumab | Tumor
resection | Disease
relapse | RFS, months | Death | OS, months |
|---|
| 1 | 54 | Male | A | Well | IIa | Ia | Yes | CR | CR | Yes | No | 5 | No | 5 |
| 2 | 48 | Male | A | Well | IIb | Ib | Yes | PR | PR | Yes | No | 12 | No | 12 |
| 3 | 65 | Female | B | Poor | IIb | Ib | Yes | SD | PR | Yes | No | 10 | No | 10 |
| 4 | 41 | Male | B | Moderate | IIb | IIa | No | PR | PR | Yes | No | 12 | No | 12 |
| 5 | 68 | Male | A | Well | IIa | Ib | Yes | PR | CR | Yes | No | 7 | No | 7 |
| 6 | 70 | Female | A | Poor | IIb | IIa | No | SD | PR | Yes | Yes | 5 | No | 9 |
| 7 | 56 | Male | A | Moderate | IIa | Ib | Yes | PR | PR | Yes | No | 10 | No | 10 |
| 8 | 59 | Male | A | Well | IIa | IIa | No | PR | PR | Yes | No | 8 | No | 8 |
| 9 | 69 | Female | B | Well | IIa | IIa | No | SD | PR | Yes | Yes | 9 | Yes | 11 |
| 10 | 68 | Male | A | Moderate | IIb | Ib | Yes | PR | PR | Yes | No | 15 | No | 15 |
| 11 | 66 | Male | A | Well | IIb | Ib | Yes | PR | PR | Yes | No | 16 | No | 16 |
Common adverse events in patients with
HCC
The adverse events that occurred during the
treatment period were mild and well-tolerated in the patients with
HCC, as indicated in Table IV. In
detail, there were 6 (54.5%) patients with reactive cutaneous
capillary endothelial proliferation (RCCEP), 5 (45.5%) patients
with pain and 4 (36.4%) patients with fever.
 | Table IV.Common adverse events in the patients
(n=11). |
Table IV.
Common adverse events in the patients
(n=11).
| Adverse event | n (%) |
|---|
| RCCEP | 6 (54.5) |
| Pain | 5 (45.5) |
| Fever | 4 (36.4) |
Discussion
Camrelizumab is a humanized high-affinity IgG4-κ
anti-PD-1 monoclonal antibody that was first administered for the
treatment of relapsed/refractory classical Hodgkin's lymphoma
(17); recent studies have
demonstrated its efficacy in the treatment of multiple
malignancies, including HCC (17,22,28).
For instance, a previous randomized, open-label, multicenter study
indicated that the ORR was 14.7% and the 6-month OS rate was 74.4%
in patients with advanced-stage HCC who received camrelizumab
treatment (29). Furthermore, a
non-randomized, open-label study reported that camrelizumab
combined with apatinib led to an ORR of 34.3%, a median PFS rate of
5.7 months and a 12-month OS rate of 74.7% in patients with
advanced-stage HCC (28). In
addition, other studies have demonstrated that the combination of
TACE and PD-1 inhibitors (such as nivolumab, sintilimab or
pembrolizumab) has potential for use as an effective treatment
strategy for patients with intermediate- and advanced-stage HCC
(30,31). Other studies have also suggested the
role of TACE as an effective bridging therapy for patients with
initially unresectable HCC (32,33).
Hence, according to the aforementioned evidence, it was
hypothesized that the combination of TACE and camrelizumab may also
have potential value as a novel bridging strategy for patients with
intermediate-stage HCC.
In the present study, it was observed that the ORR
and DCR were 72.7 and 100.0% following TACE therapy, and 100.0 and
100.0% following camrelizumab treatment, respectively. Of note, the
treatment response of TACE plus camrelizumab in the present study
was markedly higher than that achieved with camrelizumab alone
(ORR, 36.4%; DCR, 81.8%), as previously reported (34), suggesting an elevated treatment
response of TACE plus camrelizumab compared with that of
camrelizumab alone in patients with intermediate-stage HCC. A
possible reason for this may be that TACE therapy has the
advantages of minimal trauma and high targeting, thereby
effectively suppressing the progression of HCC and leading to a
markedly increased treatment efficacy in patients with HCC
(30,32). In addition, in the present study, it
was found that the AFP level was the highest prior to TACE,
followed by after TACE and camrelizumab treatment, and was the
lowest following surgical resection. This evidence reflected the
good treatment response of TACE plus camrelizumab in the treatment
of patients with intermediate-stage HCC. The use of AFP to reflect
treatment efficacy in patients with HCC is in accordance with
previous studies (35,36). Furthermore, in the present study,
the downstaging success rate following TACE plus camrelizumab was
63.6%, which was relatively increased compared with the successful
downstaging rate achieved with TACE alone (ranging from 23.7 to
55.0%) in patients with HCC who were not suitable for curative
treatments reported in previous studies (37–39).
This phenomenon may be attributed to the extra application of
camrelizumab, which enhanced the antitumor activities and inhibited
HCC growth in patients with HCC (40). In addition, the procedure of TACE
may be different in the current study compared with previous
studies (37–39). Therefore, the downstaging
superiority of TACE plus camrelizumab compared with TACE alone
should be further validated in comparative studies.
In addition, in terms of survival profiles, it was
observed that successful downstaging was associated with RFS;
however, successful downstaging was also associated with OS,
although without a statistically significant difference in patients
with intermediate-stage HCC. This may have been due to the
relatively small sample size in the present study. The
aforementioned results were consistent with those of previous
studies demonstrating that patients with successful downstaging
exhibit improved survival compared to patients with unsuccessful
downstaging (10,14,37). A
possible reason for this may be that the combination of TACE plus
camrelizumab as a bridging therapy helps patients with
intermediate-stage HCC achieve downstaging, and the decreased HCC
stage was associated with favorable survival.
Regarding the safety profiles, in detail, 6 patients
experienced RCCEP, 5 patients reported pain and 4 patients
exhibited fever; however, there were no severe adverse events,
suggesting mild and manageable safety profiles in patients treated
with TACE plus camrelizumab as a bridging therapy. The explanations
for these adverse events are as follows: i) According to the
existing evidence, RCCEP, as a reflection of the activated immune
response, is considered a common skin reaction related to the use
of camrelizumab (41). In addition,
the presence of RCCEP did not require the discontinuation of
treatment or dose reduction, and it often spontaneously regressed
(41). ii) Furthermore, based on
previous research (19), pain and
fever are part of post-embolization syndrome and appear frequently
following the application of TACE, which explained the occurrence
of fever and pain in the present study.
Of note, there are certain limitations to the
present study: i) The sample size of the present study was
relatively small; therefore, the results require further validation
using a larger sample size; ii) considering the relatively short
follow-up period, the efficacy and safety of the combined use of
TACE plus camrelizumab as a bridging therapy require to be further
assessed in patients with intermediate-stage HCC in studies with
longer follow-up times; iii) the present study suggested efficacy
of TACE plus camrelizumab as a bridging therapy prior to surgery;
however, its superior efficacy over monotherapy requires a control
group for further validation; iv) the current study used AFP to
reflect the treatment efficacy of TACE plus camrelizumab, while
other possible markers, such as AFP-L3, as well as markers of liver
function, should be examined to further reflect the efficacy and
safety of TACE plus camrelizumab.
In conclusion, the present study demonstrated that
TACE plus camrelizumab may be a potential effective and safe
strategy as a bridging therapy prior to surgery in patients with
intermediate-stage HCC. However, further randomized, controlled
trials should be conducted for validation.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PF and SH conceptualized and supervised the study.
HH, XW, SX and XN performed analysis and interpretation of data. SX
and XN provided resources and performed visual-ization. LC and ZY
conducted the investigation, prepared the original draft and wrote
the manuscript. PF and SH confirm the authenticity of all the raw
data. All authors contributed critically to the manuscript
revision. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The Institutional Review Board of Handan Central
Hospital (Handan, China) approved the present study and all
patients provided written informed consent to participate.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vogel A, Meyer T, Sapisochin G, Salem R
and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lubel JS, Roberts SK, Strasser SI,
Thompson AJ, Philip J, Goodwin M, Clarke S, Crawford DH, Levy MT
and Shackel N: Australian recommendations for the management of
hepatocellular carcinoma: A consensus statement. Med J Aust.
214:475–483. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ng CKY, Dazert E, Boldanova T,
Coto-Llerena M, Nuciforo S, Ercan C, Suslov A, Meier MA, Bock T,
Schmidt A, et al: Integrative proteogenomic characterization of
hepatocellular carcinoma across etiologies and stages. Nat Commun.
13:24362022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Younossi ZM and Henry L: Epidemiology of
non-alcoholic fatty liver disease and hepatocellular carcinoma.
JHEP Rep. 3:1003052021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mocan L: Multimodal therapy for
hepatocellular carcinoma: The role of surgery. Eur Rev Med
Pharmacol Sci. 25:4470–4477. 2021.PubMed/NCBI
|
|
7
|
Wen N, Cai Y, Li F, Ye H, Tang W, Song P
and Cheng N: The clinical management of hepatocellular carcinoma
worldwide: A concise review and comparison of current guidelines:
2022 update. Biosci Trends. 16:20–30. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Arita J, Ichida A, Nagata R, Mihara Y,
Kawaguchi Y, Ishizawa T, Akamatsu N, Kaneko J and Hasegawa K:
Conversion surgery after preoperative therapy for advanced
hepatocellular carcinoma in the era of molecular targeted therapy
and immune checkpoint inhibitors. J Hepatobiliary Pancreat Sci.
29:732–740. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin Z, Chen D, Liang S and Li X:
Neoadjuvant therapy for hepatocellular carcinoma. J Hepatocell
Carcinoma. 9:929–946. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Makary MS, Khandpur U, Cloyd JM, Mumtaz K
and Dowell JD: Locoregional therapy approaches for hepatocellular
carcinoma: Recent advances and management strategies. Cancers
(Basel). 12:19142020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Affonso BB, Galastri FL, da Motta Leal
Filho JM, Nasser F, Falsarella PM, Cavalcante RN, de Almeida MD,
Felga GEG, Valle LGM and Wolosker N: Long-term outcomes of
hepatocellular carcinoma that underwent chemoembolization for
bridging or downstaging. World J Gastroenterol. 25:5687–5701. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Parikh ND, Waljee AK and Singal AG:
Downstaging hepatocellular carcinoma: A systematic review and
pooled analysis. Liver Transpl. 21:1142–1152. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Melchiorre F, Patella F, Pescatori L,
Pesapane F, Fumarola E, Biondetti P, Brambillasca P, Monaco C,
Ierardi AM and Franceschelli Gand Carrafiello G: DEB-TACE: A
standard review. Future Oncol. 14:2969–2984. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai L, Li H, Guo J, Zhao W, Duan Y, Hou X,
Cheng L, Du H, Shao X, Diao Z and Li C: Drug-eluting bead
transarterial chemoembolization is an effective downstaging option
for subsequent radical treatments in patients with hepatocellular
carcinoma: A cohort study. Clin Res Hepatol Gastroenterol.
45:1015352021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gandhi L, Rodriguez-Abreu D, Gadgeel S,
Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ,
Powell SF, et al: Pembrolizumab plus chemotherapy in metastatic
non-small-cell lung cancer. N Engl J Med. 378:2078–2092. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fang W, Yang Y, Ma Y, Hong S, Lin L, He X,
Xiong J, Li P, Zhao H, Huang Y, et al: Camrelizumab (SHR-1210)
alone or in combination with gemcitabine plus cisplatin for
nasopharyngeal carcinoma: Results from two single-arm, phase 1
trials. Lancet Oncol. 19:1338–1350. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Markham A and Keam SJ: Camrelizumab: First
global approval. Drugs. 79:1355–1361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jin ZC, Zhong BY, Chen JJ, Zhu HD, Sun JH,
Yin GW, Ge NJ, Luo B, Ding WB, Li WH, et al: Real-world efficacy
and safety of TACE plus camrelizumab and apatinib in patients with
HCC (CHANCE2211): A propensity score matching study. Eur Radiol.
Jun 27–2023.(Epub ahead of print). View Article : Google Scholar
|
|
19
|
Zhu C, Dai B, Zhan H and Deng R:
Neoadjuvant transarterial chemoembolization (TACE) plus PD-1
inhibitor bridging to tumor resection in intermediate-stage
hepatocellular carcinoma patients. Ir J Med Sci. 192:1065–1071.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Y, Zhang Z, Liao W, Hu K and Wang Z:
Combination of sorafenib, camrelizumab, transcatheter arterial
chemoembolization, and stereotactic body radiation therapy as a
novel downstaging strategy in advanced hepatocellular carcinoma
with portal vein tumor thrombus: A case series study. Front Oncol.
11:6503942021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kudo M, Ueshima K, Ikeda M, Torimura T,
Tanabe N, Aikata H, Izumi N, Yamasaki T, Nojiri S, Hino K, et al:
Randomized, open label, multicenter, phase II trial comparing
transarterial chemoembolization (TACE) plus sorafenib with TACE
alone in patients with hepatocellular carcinoma (HCC): TACTICS
trial. J Clin Oncol. 36:2062018. View Article : Google Scholar
|
|
22
|
Zhu XD, Tang ZY and Sun HC: Targeting
angiogenesis for liver cancer: Past, present, and future. Genes
Dis. 7:328–335. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhou J, Sun HC, Wang Z, Cong WM, Wang JH,
Zeng MS, Yang JM, Bie P, Liu LX, Wen TF, et al: Guidelines for
diagnosis and treatment of primary liver cancer in China (2017
Edition). Liver Cancer. 7:235–260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shvarts O, Lam JS, Kim HL, Han KR, Figlin
R and Belldegrun A: Eastern Cooperative Oncology Group performance
status predicts bone metastasis in patients presenting with renal
cell carcinoma: Implication for preoperative bone scans. J Urol.
172:867–870. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Palmieri C and Macpherson IR: A review of
the evidence base for utilizing Child-Pugh criteria for guiding
dosing of anticancer drugs in patients with cancer and liver
impairment. ESMO Open. 6:1001622021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma Y, Zhao C, Zhao H, Li H, Chen C, Xiang
H, Zheng C, Ma C, Luo C, Qiu H, et al: Comparison of treatment
efficacy and safety between drug-eluting bead transarterial
chemoembolization with CalliSpheres® microspheres and
conventional transarterial chemoembolization as first-line
treatment in hepatocellular carcinoma patients. Am J Transl Res.
11:7456–7470. 2019.PubMed/NCBI
|
|
27
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu J, Shen J, Gu S, Zhang Y, Wu L, Wu J,
Shao G, Zhang Y, Xu L, Yin T, et al: Camrelizumab in combination
with apatinib in patients with advanced hepatocellular carcinoma
(RESCUE): A nonrandomized, open-label, phase II Trial. Clin Cancer
Res. 27:1003–1011. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin S, Ren Z, Meng Z, Chen Z, Chai X,
Xiong J, Bai Y, Yang L, Zhu H, Fang W, et al: Camrelizumab in
patients with previously treated advanced hepatocellular carcinoma:
A multicentre, open-label, parallel-group, randomised, phase 2
trial. Lancet Oncol. 21:571–580. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Montasser A, Beaufrere A, Cauchy F,
Bouattour M, Soubrane O, Albuquerque M and Paradis V: Transarterial
chemoembolization enhances programmed death 1 and programmed
death-ligand 1 expression in hepatocellular carcinoma.
Histopathology. 79:36–46. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Waidmann O: Recent developments with
immunotherapy for hepatocellular carcinoma. Expert Opin Biol Ther.
18:905–910. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Han K and Kim JH: Transarterial
chemoembolization in hepatocellular carcinoma treatment: Barcelona
clinic liver cancer staging system. World J Gastroenterol.
21:10327–10335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gyori GP, Felsenreich DM, Silberhumer GR,
Soliman T and Berlakovich GA: Multimodality locoregional treatment
strategies for bridging HCC patients before liver transplantation.
Eur Surg. 49:236–243. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen J, Hu X, Li Q, Dai W, Cheng X, Huang
W, Yu W, Chen M, Guo Y and Yuan G: Effectiveness and safety of
toripalimab, camrelizumab, and sintilimab in a real-world cohort of
hepatitis B virus associated hepatocellular carcinoma patients. Ann
Transl Med. 8:11872020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mehta N, Frenette C, Tabrizian P, Hoteit
M, Guy J, Parikh N, Ghaziani TT, Dhanasekaran R, Dodge JL,
Natarajan B, et al: Downstaging outcomes for hepatocellular
carcinoma: Results from the multicenter evaluation of reduction in
tumor size before liver transplantation (MERITS-LT) consortium.
Gastroenterology. 161:1502–1512. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Degroote H, Pinero F, Costentin C,
Notarpaolo A, Boin IF, Boudjema K, Baccaro C, Chagas AL, Bachellier
P, Ettorre GM, et al: International study on the outcome of
locoregional therapy for liver transplant in hepatocellular
carcinoma beyond Milan criteria. JHEP Rep. 3:1003312021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jang JW, You CR, Kim CW, Bae SH, Yoon SK,
Yoo YK, Kim DG and Choi JY: Benefit of downsizing hepatocellular
carcinoma in a liver transplant population. Aliment Pharmacol Ther.
31:415–423. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chapman WC, Majella Doyle MB, Stuart JE,
Vachharajani N, Crippin JS, Anderson CD, Lowell JA, Shenoy S, Darcy
MD and Brown DB: Outcomes of neoadjuvant transarterial
chemoembolization to downstage hepatocellular carcinoma before
liver transplantation. Ann Surg. 248:617–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Otto G, Herber S, Heise M, Lohse AW, Monch
C, Bittinger F, Hoppe-Lotichius M, Schuchmann M, Victor A and
Pitton M: Response to transarterial chemoembolization as a
biological selection criterion for liver transplantation in
hepatocellular carcinoma. Liver Transpl. 12:1260–1267. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L
and Liu X: Application of PD-1 blockade in cancer immunotherapy.
Comput Struct Biotechnol J. 17:661–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang F, Qin S, Sun X, Ren Z, Meng Z, Chen
Z, Chai X, Xiong J, Bai Y, Yang L, et al: Reactive cutaneous
capillary endothelial proliferation in advanced hepatocellular
carcinoma patients treated with camrelizumab: Data derived from a
multicenter phase 2 trial. J Hematol Oncol. 13:472020. View Article : Google Scholar : PubMed/NCBI
|