Introduction
Non-small cell lung cancer (NSCLC) accounts for
80–85% of lung cancers and >15% of patients with NSCLC are
diagnosed with locally advanced stage IIIA/IIIB disease (1). The second most prevalent histological
subtype of NSCLC is squamous non-small cell lung cancer (SqCLC),
characterized by poor prognosis and lack of specific target agents
(2). Patients with stage IIIA/IIIB
SqCLC are particularly challenging to treat and the 5-year survival
rate is only 36–15% (3).
For patients with operable and potentially
resectable NSCLC, neoadjuvant therapy is a viable treatment option.
For patients with stage IIIA/IIIB NSCLC, neoadjuvant therapy may
downstage the cancers and make them more operable, potentially
enhancing the rate of complete resection (4). In the era of chemotherapy,
meta-analyses of randomized trials of neoadjuvant chemotherapy
showed a significant survival advantage over surgery alone with a
5% increase in the 5-year overall survival (OS) rate (5).
In stage IV NSCLC, immune checkpoint inhibitors
(ICIs) have been the new standard of care as first-line therapy,
including anti-programmed cell death 1 (PD-1) and PD-1 ligand 1
antibodies alone, or combined with chemotherapy, in accordance with
the survival advantages over chemotherapy alone (6). Recently, clinical trials exploring
neoadjuvant immunotherapy in resectable NSCLC have shown promising
results, with the major pathologic response ranging from 21 to 45%
(7,8). However, new therapeutic approaches are
required for individuals with driver gene mutations and those who
have contraindications to immunotherapy, as ICIs are not
appropriate for everyone.
Angiogenesis is one of the most important features
in cancers and is associated with more aggressive disease (9). Malignant cells secrete angiogenic
cytokines to induce endothelial cell migration and angiogenesis to
accelerate the growth of new vessels (10). Antiangiogenic therapy aims to
disrupt those processes by normalizing the abnormal vasculature in
tumors and improving delivery of drugs, enhancing its anti-tumor
effect. Bevacizumab and recombinant human endostatin (Endostar)
combined with cytotoxic drugs have been approved in advanced NSCLC
by the Chinese National Medical Products Administration (NMPA).
Recombinant human endostatin with chemotherapy had a better
therapeutic effect than chemotherapy alone in advanced SqCLC,
according to a meta-analysis. In addition, combination therapy did
not increase the incidence of adverse reactions (11).
The present study aimed to explore the efficacy and
safety of Endostar combined with chemotherapy as the neoadjuvant
treatment in patients with stage IIIA/IIIB SqCLC.
Patients and methods
Subjects
This study was performed in line with the principles
of the Declaration of Helsinki. All methods were performed in
accordance with the relevant guidelines and regulations. Patients
with locally advanced SqCLC (TNM stage: IIIA/IIIB) treated with
Endostar combined with chemotherapy as neoadjuvant therapy from
January 1, 2017 to December 31, 2019 at Zhejiang Cancer Hospital
(Hangzhou, China) were included. Exclusion criteria in this study
were as follows: i) Patients with other types of malignancy; ii)
patients who were lost to follow-up. Patient data were collected,
including age, gender, histological subtype, clinical TNM stage
(8th edition of the American Joint Committee on Cancer
Tumor-Node-Metastasis staging system) (12), date of start of treatment, regimen
of chemotherapy, cycles of chemotherapy, cycles of chemotherapy
before surgery/radiotherapy, percentage of target lesions change,
the best response [evaluated using computed tomography (CT)
according to the Response Evaluation Criteria in Solid Tumors
version 1.1 (RECIST) (13)],
surgical resection, date of surgery, pathological stage,
radiotherapy, date of disease progression, date of death, adverse
events.
Treatment schedule
Endostar
Endostar was administered at a dose of 7.5
mg/m2 by intravenous (iv) infusion daily on day
(D)1-D14; repeated every 3 weeks.
Chemotherapy
Participants received one of the platinum-based
chemotherapies as follows: i) DP regimen: Docetaxel 60
mg/m2 by iv infusion on D1; nedaplatin 100
mg/m2 by iv infusion on D1; repeated every 3 weeks; ii)
TP regimen: Paclitaxel 175 mg/m2 or albumin paclitaxel
260 mg/m2 by iv infusion on D1; nedaplatin 100
mg/m2 or cisplatin 75 mg/m2 by iv infusion on
D1; repeated every 3 weeks; iii) GP regimen: Gemcitabine 1,000
mg/m2 by iv infusion on D1; nedaplatin 100
mg/m2 or cisplatin 75 mg/m2 by iv infusion on
D1; repeated every 3 weeks.
Follow-up procedures
Patients receiving Endostar and chemotherapy were
evaluated for response every two treatment cycles during treatment
and then every 2 months after treatment. The response evaluation of
the tumor to therapy was based on computed tomography or magnetic
resonance imaging scanning. The short-term efficacy was defined
based on version 1.1 of the RECIST guidelines (13). The objective response rate (ORR) was
defined as the percentage of patients who had a tumor response
[complete response (CR) and/or partial response (PR)]. The
long-term efficacy was evaluated according to progression-free
survival (PFS) and OS. PFS was defined as the time from the
initiation of treatment to radiological evidence of progressive
disease (PD). OS was calculated from the initiation of treatment to
mortality. Adverse events (AEs) were graded according to the
National Cancer Institute Common Terminology Criteria for Adverse
Events version 5.0 (14).
Statistical analysis
Statistical analysis was performed using the
software SPSS 23.0 (IBM Corp.). Continuous variables were expressed
as the median (minimum and maximum), and qualitative data were
expressed as frequencies and percentages. Survival analysis was
conducted using the Kaplan-Meier method and subgroups were compared
using the log-rank test. All P-values were two-sided and P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient cohort and clinical
characteristics
In total, 27 patients with locally advanced SqCLC
(TNM stage: IIIA/IIIB) treated with Endostar combined with
chemotherapy as neoadjuvant therapy from January 1, 2017 to
December 31, 2019 were included. After efficacy assessment, 10
patients (10/27; 37%) underwent radical surgical resection. Of the
17 patients who did not receive surgery, 7 patients underwent
sequential radiotherapy (Fig.
1).
Baseline characteristics, treatment details and
efficacy of the 27 patients are presented in Table I. All of the 27 patients were male
and the median age was 64 (49–74) years. A total of 24 (88.9%)
patients exhibited a performance status of 1, while 3 patients
(11.1%) displayed a performance status of 2. All 27 patients had
squamous cell carcinoma on histopathology of their biopsy specimen.
Among them, there were 6 (22.3%) cases of moderately differentiated
carcinoma, 4 (14.8%) cases of moderate-low differentiated
carcinoma, 4 (14.8%) cases of poorly differentiated carcinoma and
13 (48.1) cases of squamous carcinoma that could not be staged. A
total of 13 (48.1%) patients were diagnosed with stage IIIA and 14
(51.9%) patients with stage IIIB. 26 (96.3%) patients reported a
smoking history, while only 1 patient (3.7%) had no smoking
history. None of the 27 patients exhibited any protein expression,
including anaplastic lymphoma kinase, ROS proto-oncogene 1 and
hepatocyte growth factor receptor (c-Met) protein expression, among
others. The 27 patients received a median of 3 (range, 2–6) cycles
of combination of Endostar and chemotherapy. Among them, 9 patients
received Endostar plus docetaxel and nedaplatin, 9 received
Endostar plus paclitaxel and nedaplatin/cisplatin, 3 received
Endostar plus gemcitabine and nedaplatin/cisplatin and 6 received
Endostar plus albumin-paclitaxel and nedaplatin.
 | Table I.Patients' clinical characteristics and
treatment information. |
Table I.
Patients' clinical characteristics and
treatment information.
| Item | Value |
|---|
| Male sex | 100 (100.0) |
| Age, years | 64 (49–74) |
| Performance
status |
|
| 1 | 24 (88.9) |
| 2 | 3 (11.1) |
| Histology of squamous
cell carcinoma | 100 (100.0) |
| Differentiation |
|
| G2 | 6 (22.3) |
| G2-3 | 4 (14.8) |
| G3 | 4 (14.8) |
| NA | 13 (48.1) |
| Stage |
|
| IIIA | 13 (48.1) |
| IIIB | 14 (51.9) |
| Smoking history |
|
| Yes | 26 (96.3) |
| No | 1 (3.7) |
| ALK/ROS-1/c-MET
protein expression |
|
|
Negative | 5 (18.5) |
| NA | 22 (81.5) |
| Regimen of
chemotherapy |
|
| Docetaxel
+ Nedaplatin | 9 (33.3) |
|
Paclitaxel +
Nedaplatin/Cisplatin | 9 (33.3) |
|
Gemcitabine +
Nedaplatin/Cisplatin | 3 (11.1) |
|
Albumin-paclitaxel +
Nedaplatin | 6 (22.3) |
| Cycles of
chemotherapy and Endostar | 3 (2–6) |
| Local
treatment |
|
|
Surgical resection | 10 (37.0) |
|
Radiotherapy | 7 (25.9) |
|
None | 10 (37.0) |
Efficacy of Endostar combined with
chemotherapy
After treatment by Endostar combined with
chemotherapy, 37% of the patients (10/27) underwent surgery and the
radical resection rate was 90% (9/10). The ORR was 63% (17/27) for
the total population and 80% (8/10) for patients who received
surgery. Of note, 100% (27/27) of the patients achieved disease
control after treatment with Endostar combined with chemotherapy
(Fig. 2; Table II). In the patients who underwent
surgical resection, postoperative pathology showed that 100%
(10/10) achieved pathological downstaging. Among them, 90% (9/10)
achieved downstaging of the T stage and 80% (8/10) achieved
downstaging of the N stage. Furthermore, 1 (10%) patient achieved a
pathological CR after surgery (Table
III).
 | Table II.Response in the total population and
patients who received surgery. |
Table II.
Response in the total population and
patients who received surgery.
| Response | The total
population (n=27) | Patients who
received surgery (n=10) |
|---|
| Radical resection
rate | 9 (33.3) | 9 (90.0) |
| Pathological
downstaging | 10 (37.0) | 10 (100.0) |
| Partial
response | 17 (63.0) | 8 (80.0) |
| Stable disease | 10 (37.0) | 2 (20.0) |
| Objective
response | 17 (63.0) | 8 (80.0) |
| Disease
control | 27 (100.0) | 2 (20.0) |
 | Table III.TNM stage and tumor size of patients
who underwent surgical resection. |
Table III.
TNM stage and tumor size of patients
who underwent surgical resection.
|
| Before
treatment | After surgery |
|
|
|---|
|
|
|
|
|
|
|---|
| Patient no. | TNM stage | Tumor size, cm | TNM stage | Tumor size, cm | Downgrade N | Downgrade T |
|---|
| 1 | cT2bN2M0, IIIA | 4.3×3.7 | ypT2aN2M0,
IIIA | 3.5×2.8 | No | Yes |
| 2 | cT2aN2M0, IIIA | 3.6 | ypT1bN2M0,
IIIA | 2.0×1.8 | No | Yes |
| 3 | cT3N2M0, IIIB | 5.4×3.8 | ypT1aN0M0, IA | 0.8×0.7 | Yes | Yes |
| 4 | cT3N2M0, IIIB | 5.1×4.6 | ypT0N0M0, pCR | No nodules | Yes | Yes |
| 5 | cT2aN2M0, IIIA | 3.5×3.2 | ypT1cN1M0, IIB | 3 .0×3.0 | Yes | Yes |
| 6 | cT3N2M0, IIIB | 6.4×4.9 | ypT1cN0M0, IA | 2.9×3.0 | Yes | Yes |
| 7 | cT3N1M0, IIIA | 6.0×3.8 | ypT1cN1M0, IIB | 2.5×1.5 | Yes | No |
| 8 | cT4N1M0, IIIA | NA | ypT1cN0M0, IA | 2.1×1.2 | Yes | Yes |
| 9 | cT4N2M0, IIIB | 9.7×4.3 | ypT1bN1M0, IIB | 2.0×2.0 | Yes | Yes |
| 10 | cT2aN2M0, IIIA | 2.3×3.1 | ypT1aN0M0, IA | No nodules | Yes | Yes |
Survival analysis
The last follow-up date was December 26, 2021 and
the median follow-up time was 38.0 months (range, 27.8–57.7
months). Until the last follow-up, 20 patients had disease
progression; 17 patients died of lung cancer progression and none
died of other diseases or unknown causes. The median PFS was 13.5
months (95% CI: 9.6–17.4 months) and the median OS was 27.9 months
(95% CI: 18.8–37.0 months) for the total cohort. Figs. 3 and 4 show two representative examples.
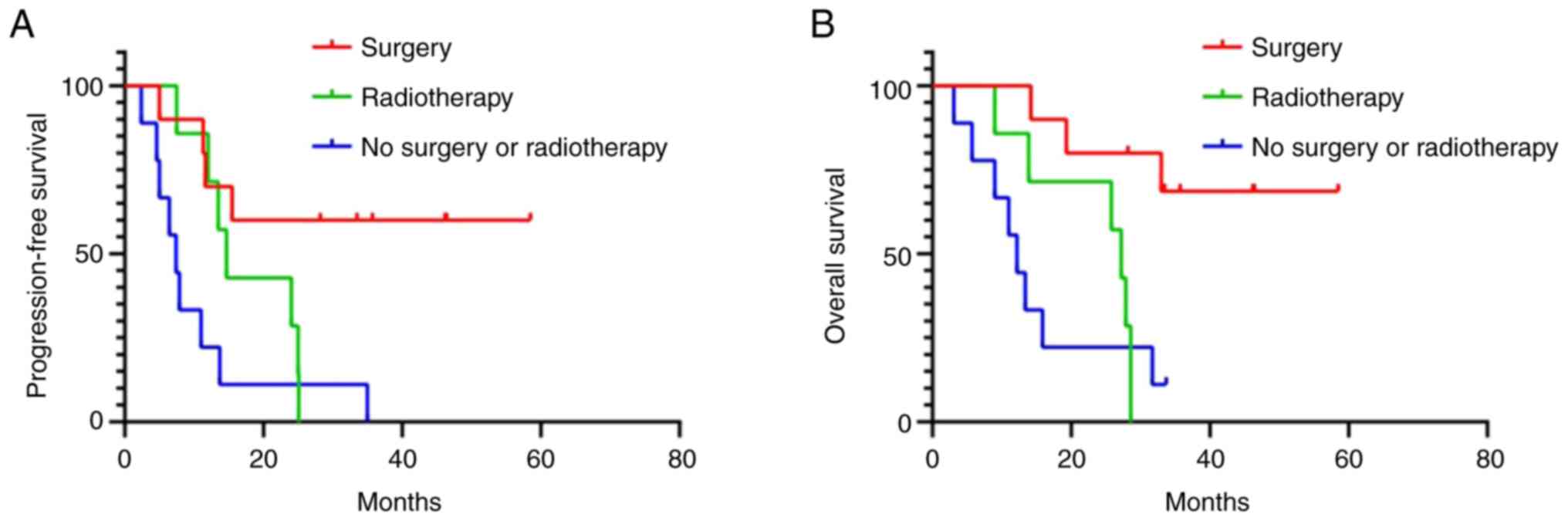
In the subgroup analysis, the median PFS of patients
who underwent surgical resection (not reached; NR) was
significantly longer than that of patients who did not undergo
surgery (11.0 months; 95%CI: 3.0–19.0 months; P=0.003). The median
OS of patients underwent surgical resection (NR) was significantly
longer than that of patients who did not undergo surgery (15.9
months; 95%CI: 0–32.6 months; P=0.004). Patients without surgery or
radiotherapy had the worst median PFS (7.4 months; 95%CI: 4.5–10.3
months; P=0.002) and OS (12.2 months; 95%CI: 8.5–15.9 months;
P=0.009; Fig. 5).
The association of short-term efficacy and long-term
survival in the total cohort was further analyzed. The results
indicated that patients who achieved PR had better PFS [24 (95%CI:
5.2–42.8) vs. 6.4 (95%CI: 2.5–10.3) months; P<0.001] and OS
[37.2 (95%CI: ∞-∞) vs. 13.4 (95%CI: 7.7–19.1) months; P=0.001] than
patients who did not achieve a PR (Fig.
6).
AEs
Endostar combined with chemotherapy was well
tolerated in the total cohort. The most common AEs were anemia,
which occurred in 69.4% (19/27) of patients, followed by
hypertension in 29.6% (8/27) of patients. Most of the AEs were
grade 1–2 and only 4 (14.8%) patients showed grade 3–4 AEs
(Table IV).
 | Table IV.AEs during Endostar combined with
chemotherapy. |
Table IV.
AEs during Endostar combined with
chemotherapy.
| AE | CTCAE grade 1 | CTCAE grade 2 | CTCAE grade 3 | CTCAE grade 4 |
|---|
| Leukocytopenia | 2 (7.4) | 2 (7.4) | 1 (3.7) | 2 (7.4) |
| Anemia | 17 (63.0) | 2 (7.4) | 0 (0.0) | 0 (0.0) |
|
Thrombocytopenia | 3 (11.1) | 3 (11.1) | 1 (3.7) | 0 (0.0) |
| Hypertension | 8 (29.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
Discussion
Previously reported phase III clinical trials
evaluating neoadjuvant chemotherapy in stage IIIA/IIIB NSCLC showed
unsatisfactory and inconsistent results. Roth et al
(15) explored the efficacy of
cyclophosphamide+etoposide+cisplatin as neoadjuvant therapy in
stage IIIA NSCLC and 60 patients were included with 37% of SqCLC
cases. The ORR was 35% in the total cohort and the complete
resection rate was 39% in patients receiving induction
chemotherapy. The median OS was 64 and 11 months in patients
receiving induction chemotherapy and surgery alone, respectively
(15). In another study,
mitomycin+ifosfamide+cisplatin were evaluated in stage IIIA NSCLC;
60 patients were included with 70% of SqCLC cases. The ORR was 60%
and the complete resection rate was 85% for the total population.
However, the median OS was only 22 months in patients receiving
induction chemotherapy (16). Nagai
et al (17) explored the
efficacy of cisplatin+vindesine in stage IIIA NSCLC and 62 patients
were included with 24% of SqCLC cases. The ORR was 28% in the total
cohort and the complete-resection rate was 65% in patients
receiving induction chemotherapy. The median OS was 17 months in
patients receiving induction chemotherapy (17). Mattson et al (18) explored the efficacy of docetaxel in
stage IIIA-IIIB NSCLC and 274 patients were included with 62% of
SqCLC cases. The ORR was 28% in the total cohort and the complete
resection rate was 77% in patients receiving induction
chemotherapy. The median OS was 14.8 months in patients receiving
induction chemotherapy (18).
Given the success of targeted therapy and
immunotherapy for patients with stage III/IV lung cancer, there is
increasing interest in exploring these agents as neoadjuvant
therapy in earlier disease settings (19). The neoadjuvant targeted therapy
tended to have a higher ORR than chemotherapy. However, whether
tumor shrinkage of neoadjuvant-targeted therapy could translate
into an improvement of OS remains to be determined. In the CTONG
1103 trial, the erlotinib group achieved a higher ORR compared with
the group treated with gemcitabine plus cisplatin as neoadjuvant
therapy (54.1 vs. 34.3%), but the OS was not significantly
different between the two groups (45.8 vs. 39.2 months) (20). In the past years, the advent of
neoadjuvant immunotherapy has revolutionized the treatment
landscape of NSCLC. Early findings from various ongoing clinical
trials suggest that neoadjuvant ICIs alone or combined with
chemotherapy may significantly reduce systemic recurrence. However,
the median OS data are not yet mature. In addition, ICIs are not
suitable for all patients, including those with immunological
contraindications and those with driver gene alterations (21).
In the present study, the efficacy and safety of
Endostar combined with chemotherapy as the neoadjuvant treatment in
patients with stage IIIA/IIIB SqCLC were explored. The results were
better than the previous results of neoadjuvant platinum-based
two-drug chemotherapy (15–17). Endostar, as a novel recombinant
human endostatin, was approved by the NMPA in 2005 to treat
advanced NSCLC. Targeting the growth of vessels in tumors, Endostar
was found to exert its antiangiogenic effects through the
VEGF-triggered signaling pathway (22). In the HELPER study, the addition of
Endostar to concurrent etoposide, cisplatin and radiotherapy in
patients with unresectable stage III NSCLC achieved a median PFS
and OS of 13.3 and 34.7 months, respectively (23). A meta-analysis of 15 clinical
studies indicated that Endostar combined with vinorelbine plus
cisplatin improved the ORR and one-year OS rate of advanced NSCLC
(24). In the cohort of the present
study, the most common AEs were anemia (69.4% of patients),
followed by hypertension (29.6% of patients). Most of the AEs were
grade 1–2 and only 14.8% of patients showed grade 3–4 AEs. In a
clinical trial by Bao et al (25), the addition of Endostar to
concurrent chemoradiotherapy did not increase toxicity. A
meta-analysis also demonstrated that the combination of Endostar
and platinum-based doublet chemotherapy (PBDC) was not associated
with a higher incidence of leukopenia, thrombocytopenia and anemia
compared with PBDC alone (26).
Of note, the present study had certain limitations.
First, constrained by the retrospective and single-arm design, the
results of the current study lacked comparison with patients
receiving chemotherapy alone, which weakened the reliability of the
study. Therefore, prospective studies are needed to further
validate the present results. Furthermore, the sample size of the
present study was relatively small. Consequently, a multi-center
study is needed to address the issue and provide a more robust
result based on a larger sample size.
In conclusion, the combination of Endostar and
chemotherapy demonstrated promising efficacy and was well tolerated
among patients diagnosed with stage IIIA/IIIB SqCLC. Due to the
limited cohort size, further prospective studies are necessary to
investigate the potential efficacy of this neoadjuvant therapy.
Acknowledgements
Not applicable.
Funding
This work was supported by the Natural Science Foundation of
Zhejiang Provincial (grant no. LY23H160003) and Zhejiang Medical
technology program (grant no. 2023KY069).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WH and YJ designed the study. FC, SD, CG, YZ, YJ and
WH contributed to the data collection and investigation. FC and SD
wrote the original draft of the manuscript. CG and YZ confirm the
authenticity of the raw data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Zhejiang Cancer Hospital
(Zhejiang, China) approved the present study (approval no.
IRB-2023-410).
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nicholson AG, Tsao MS, Beasley MB, Borczuk
AC, Brambilla E, Cooper WA, Dacic S, Jain D, Kerr KM, Lantuejoul S,
et al: The 2021 WHO classification of lung tumors: Impact of
advances since 2015. J Thorac Oncol. 17:362–387. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng SH and Yang ST: The new 8th TNM
staging system of lung cancer and its potential imaging
interpretation pitfalls and limitations with CT image
demonstrations. Diagn Interv Radiol. 25:270–279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blumenthal GM, Bunn PA Jr, Chaft JE,
McCoach CE, Perez EA, Scagliotti GV, Carbone DP, Aerts HJWL, Aisner
DL, Bergh J, et al: Current status and future perspectives on
neoadjuvant therapy in lung cancer. J Thorac Oncol. 13:1818–1831.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burdett S, Stewart L, Auperin A and Pignon
JP: Chemotherapy in non-small-cell lung cancer: An update of an
individual patient data meta-analysis. J Clin Oncol. 23:924–925;
author reply 925–926. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Duma N, Santana-Davila R and Molina JR:
Non-Small cell lung cancer: Epidemiology, screening, diagnosis, and
treatment. Mayo Clin Proc. 94:1623–1640. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Pembrolizumab versus Chemotherapy for PD-L1-positive
non-small-cell lung cancer. N Engl J Med. 375:1823–1833. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Forde PM, Chaft JE, Smith KN, Anagnostou
V, Cottrell TR, Hellmann MD, Zahurak M, Yang SC, Jones DR,
Broderick S, et al: Neoadjuvant PD-1 blockade in resectable lung
cancer. N Engl J Med. 378:1976–1986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Alshangiti A, Chandhoke G and Ellis PM:
Antiangiogenic therapies in non-small-cell lung cancer. Curr Oncol.
25 (Suppl 1):S45–S58. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rapisarda A and Melillo G: Role of the
VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res.
114:237–267. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng L, Wang Z, Jing L, Zhou Z, Shi S,
Deng R, Liu Y and Meng Q: Recombinant human endostatin combined
with chemotherapy for advanced squamous cell lung cancer: A
meta-analysis. World J Surg Oncol. 19:642021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Detterbeck FC, Boffa DJ, Kim AW and Tanoue
LT: The eighth edition lung cancer stage classification. Chest.
151:193–203. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schwartz LH, Litiere S, de Vries E, Ford
R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J,
et al: RECIST 1.1-Update and clarification: From the RECIST
committee. Eur J Cancer. 62:132–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-Version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Roth JA, Fossella F, Komaki R, Ryan MB,
Putnam JB Jr, Lee JS, Dhingra H, De Caro L, Chasen M, McGavran M,
et al: A randomized trial comparing perioperative chemotherapy and
surgery with surgery alone in resectable stage IIIA non-small-cell
lung cancer. J Natl Cancer Inst. 86:673–680. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosell R, Gómez-Codina J, Camps C, Javier
Sánchez J, Maestre J, Padilla J, Cantó A, Abad A and Roig J:
Preresectional chemotherapy in stage IIIA non-small-cell lung
cancer: A 7-year assessment of a randomized controlled trial. Lung
Cancer. 26:7–14. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nagai K, Tsuchiya R, Mori T, Tada H,
Ichinose Y, Koike T and Kato H; Lung Cancer Surgical Study Group of
the Japan Clinical Oncology Group, : A randomized trial comparing
induction chemotherapy followed by surgery with surgery alone for
patients with stage IIIA N2 non-small cell lung cancer (JCOG 9209).
J Thorac Cardiovasc Surg. 125:254–260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mattson KV, Abratt RP, ten Velde G and
Krofta K: Docetaxel as neoadjuvant therapy for radically treatable
stage III non-small-cell lung cancer: A multinational randomised
phase III study. Ann Oncol. 14:116–122. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Uprety D, Mandrekar SJ, Wigle D, Roden AC
and Adjei AA: Neoadjuvant immunotherapy for NSCLC: Current concepts
and future approaches. J Thorac Oncol. 15:1281–1297. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong WZ, Chen KN, Chen C, Gu CD, Wang J,
Yang XN, Mao WM, Wang Q, Qiao GB, Cheng Y, et al: Erlotinib versus
gemcitabine plus cisplatin as neoadjuvant treatment of stage
IIIA-N2 EGFR-Mutant non-small-cell lung cancer (EMERGING-CTONG
1103): A Randomized phase II study. J Clin Oncol. 37:2235–2245.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kang J, Zhang C and Zhong WZ: Neoadjuvant
immunotherapy for non-small cell lung cancer: State of the art.
Cancer Commun (Lond). 41:287–302. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ling Y, Yang Y, Lu N, You QD, Wang S, Gao
Y, Chen Y and Guo QL: Endostar, a novel recombinant human
endostatin, exerts antiangiogenic effect via blocking VEGF-induced
tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem
Biophys Res Commun. 361:79–84. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhai Y, Ma H, Hui Z, Zhao L, Li D, Liang
J, Wang X, Xu L, Chen B, Tang Y, et al: HELPER study: A phase II
trial of continuous infusion of endostar combined with concurrent
etoposide plus cisplatin and radiotherapy for treatment of
unresectable stage III non-small-cell lung cancer. Radiother Oncol.
131:27–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
An J and Lv W: Endostar (rh-endostatin)
versus placebo in combination with vinorelbine plus cisplatin
chemotherapy regimen in treatment of advanced non-small cell lung
cancer: A meta-analysis. Thoracic Cancer. 9:606–612. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bao Y, Peng F, Zhou QC, Yu ZH, Li JC,
Cheng ZB, Chen L, Hu X, Chen YY, Wang J, et al: Phase II trial of
recombinant human endostatin in combination with concurrent
chemoradiotherapy in patients with stage III non-small-cell lung
cancer. Radiother Oncol. 114:161–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rong B, Yang S, Li W, Zhang W and Ming Z:
Systematic review and meta-analysis of Endostar (rh-endostatin)
combined with chemotherapy versus chemotherapy alone for treating
advanced non-small cell lung cancer. World J Surg Oncol.
10:1702012. View Article : Google Scholar : PubMed/NCBI
|