Introduction
Pulmonary cryptococcosis (PC) is a common
opportunistic fungal infection caused by Cryptococcus
neoformans or Cryptococcus gattii (1), which mainly invades the respiratory
system, followed by the central nervous system (CNS) (2). It usually occurs in immunocompromised
patients, such as patients with human immunodeficiency virus (HIV)
infection, solid organ transplantation or autoimmune diseases, as
well as patients who use corticosteroids and other
immunosuppressants (3,4). However, the incidence of PC has
recently increased rapidly in hosts with normal immune function
(5,6). Different immune statuses may affect
the pulmonary CT manifestations of cryptococcosis (7,8). The
diagnosis of PC is challenging due to its diverse and nonspecific
CT findings, which may mimic those of lung cancer, bacterial
pneumonia or tuberculosis (5).
Previous studies have indicated that advanced cancer may lead to
immunodeficiency and cause cryptococcosis (9,10).
Previous studies have reported on PC coexisting with lung carcinoma
(9,11–17).
Most of the reported cases presented with respiratory symptoms and
a small number of them were asymptomatic. Certain cases are
accompanied with other underlying diseases, such as diabetes, tumor
history or systemic lupus erythematosus (11,12,14,17).
In general, this coexistence relationship may be broadly divided
into two types; one is that cryptococcal infection occurs in lung
cancer nodules or masses (9,18); the
other is that cryptococcal infection and lung cancer nodules/masses
belong to two different lesions (15). It is radiologically nonspecific and
is usually found by surgical excision or percutaneous lung biopsy.
As with other early lung cancer, the treatment for lung cancer
nodules or masses is aggressive surgical resection, while PC
usually requires postoperative antifungal therapy. The prognosis of
lung cancer is related to the pathological and clinical stage of
lung cancer, and cryptococcal infection usually has a better
prognosis. The present study reported the case of a 65-year-old
patient with pulmonary adenocarcinoma complicated with PC
infection.
Case presentation
A 65-year-old Han Chinese woman who worked as a crop
farmer presented to Taihe Hospital (Shiyan, China) for a routine
physical examination in August 2018. The patient had no respiratory
symptoms and denied any other discomfort. Chest CT showed a
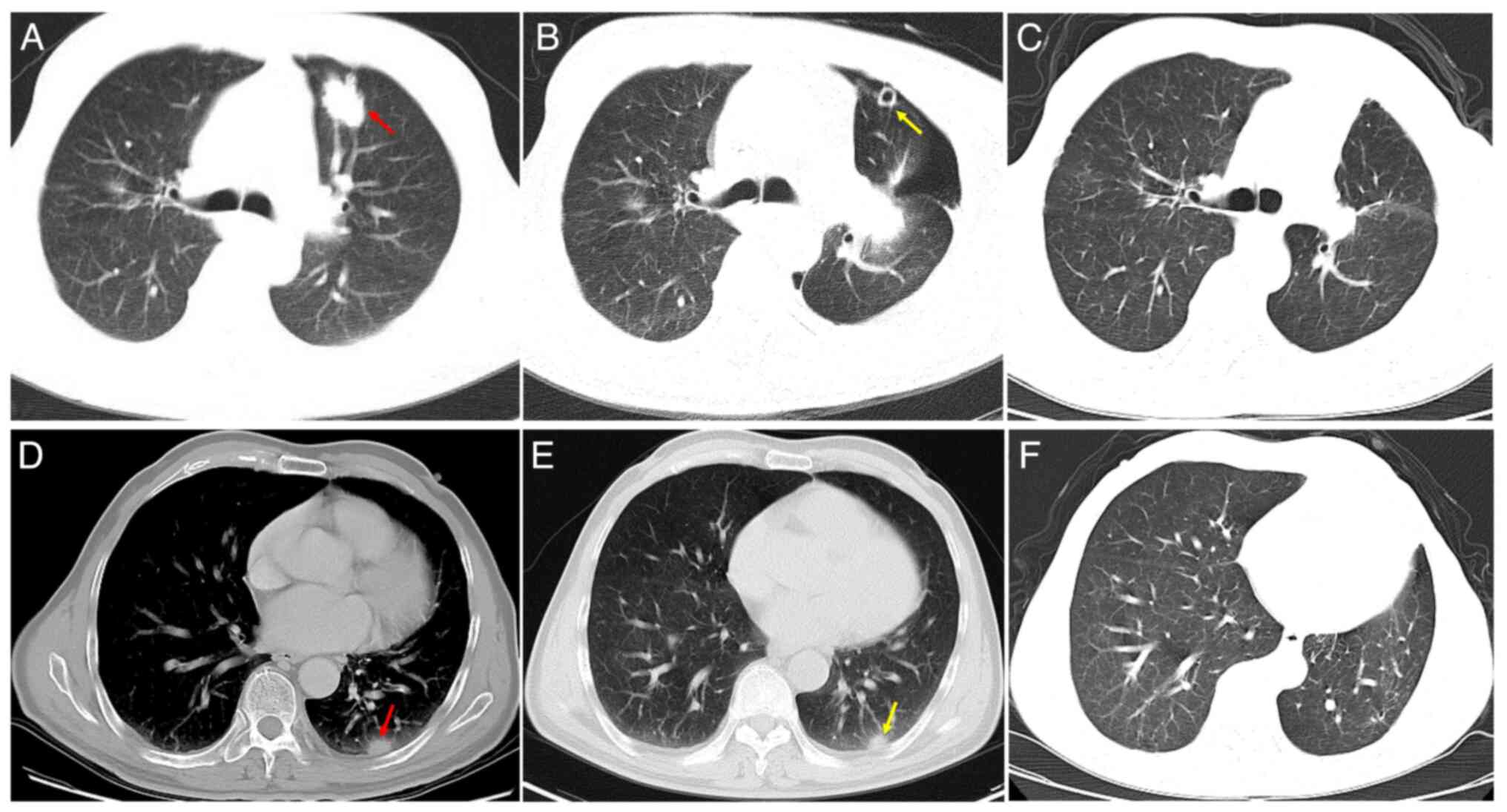
2.4×2.0-cm nodule in the anterior segment of the left superior lobe
(Fig. 1A), which was highly
suspected to be peripheral lung cancer. A 1.3×0.9-cm nodule was
detected in the posterior basal segment of the left lower lobe
(Fig. 1), which was suspected to be
intrapulmonary metastasis. Hospitalization was recommended for
further examination and treatment.
The patient denied any respiratory symptoms, such as
cough, sputum, fever, chest pain, wheezing or weight loss. Immune
function was normal, the patient had no history of travel or
exposure to pigeon feces or soil, no history of smoking or alcohol
consumption within the last month, and had not been extensively
treated with hormones and/or antibiotics before coming to the
hospital. The patient's medical history included surgery for
varicose veins in the left lower extremity 30 years earlier,
cataract surgery of the left eye 10 years ago and hemorrhoid
surgery 2 years previously. On admission, the vital signs etc. were
normal On physical examination, there were no skin lesions,
lymphadenopathy or splenomegaly.
Laboratory examination indicated the following:
Whole blood leukocytes, 4.82×109/l [neutrophils, 68.8%
(normal range, 50–70%); lymphocytes, 24.3% (normal range, 20–50%);
monocytes, 5.6% (normal range, 3–10%); eosinophils, 1.5% (normal
range, 0.4–8%); basophils, 0% (normal range, 0–1%)]; red blood
cells, 4.37×1012/l (normal range, 4.3 to
5.8×1012/l); hemoglobin, 127 g/l (normal range, 130–175
g/l); platelets, 175×109/l (normal range, 125 to
350×109/l); blood glucose, 4.45 mmol/l (normal range,
3.9–6.1 mmol/l); total bilirubin, 9.8 µmol/l (normal range,
3.42–20.5 µmol/l); aspartate aminotransferase, 17 U/l (normal
range, 0–40 U/l); alanine aminotransferase, 12 U/l (normal range,
0–50 U/l); lactate dehydrogenase, 99 IU/l (normal range, 100–240
IU/l); and highly sensitive C-reactive protein, 0.15 mg/l (normal
range, 0–5 mg/l). A urinalysis and microscopic examination were
normal. Tumor markers were as follows: Neuron-specific enolase,
10.1 ng/ml (normal range, 0–16.3 ng/ml); carcinoembryonic antigen,
1.77 µg/l (normal range, 0–5 µg/l); and ferritin, 357 ng/ml (normal
range, 30–400 ng/ml), all of which were at normal levels; however,
Cyfra21-1 was 3.75 µg/l higher than the upper limit of the normal
level (normal range, 0–3.3 µg/l). Sputum Gram staining (19) and bacterial culture showed no
microorganisms. Acid-fast staining (20) and sputum culture showed no acid-fast
bacteria. Bronchofiberscopy showed no lesions in the trachea and
bronchus, and bacterial, cytological and pathological examinations
from the bronchoscope provided negative results.
To obtain a definitive diagnosis, a CT-guided
percutaneous lung biopsy was performed on a nodule of
radiologically high suspicion of lung cancer in the anterior
segment of the left upper lobe, which was pathologically confirmed
to be adenocarcinoma. To evaluate the stage of lung cancer and
select appropriate treatment, a CT-guided percutaneous lung biopsy
was performed on the nodule in the posterior basal segment of the
left inferior lobe 1 week later, which was confirmed by
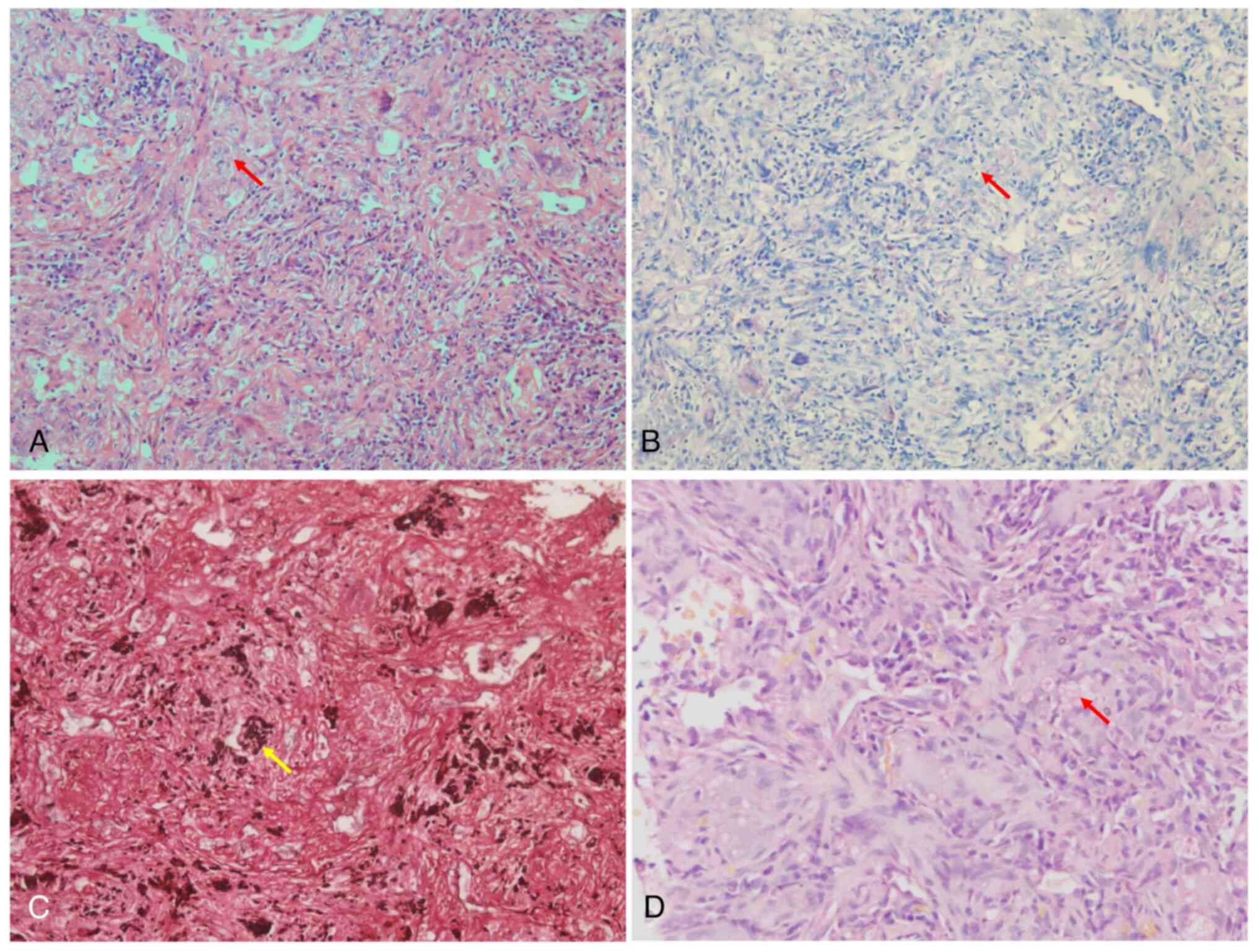
histopathology as PC infection. Histologically, hematoxylin &
eosin staining (21) revealed
granulomatous inflammation and yeast-form fungi in multinucleated
cells, and cryptococcus was identified by periodic acid Schiff
(PAS), Gomori methenamine silver (GMS) and mucicarmine (MC)
staining (Fig. 2A-D, respectively).
The above staining procedures were performed according to standard
protocols. Two weeks later, the patient underwent thoracoscopic
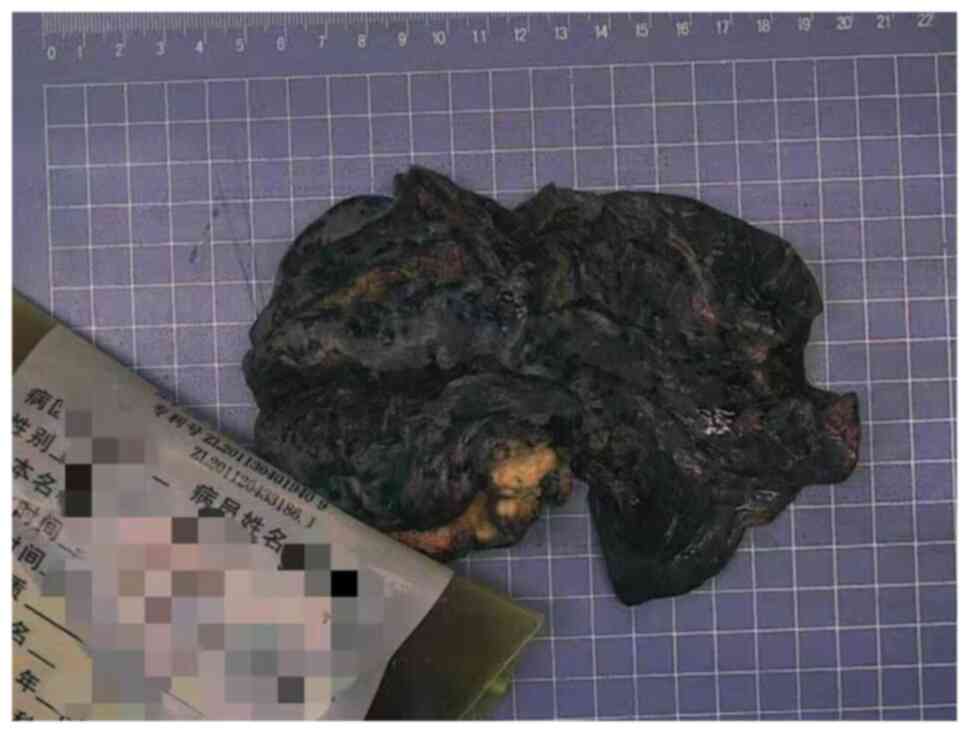
resection of the left lung cancer. Macroscopic examination revealed
that the excised 14×9.5×3.5-cm upper left lobe included a
3.1×2.5×2-cm mass with gray and grayish black sections, solid,
medium in texture, and indistinct from the surrounding boundary
(Fig. 3). The mass was adjacent to
the pleura and did not involve the bronchus. Histologically, the
tumor cells were moderately to poorly differentiated adenocarcinoma
of the acinar and micropapillary type (Fig. 4A-D), and no cryptococcal infection
was observed. No metastatic cancer was found in the lymph nodes
(0/11), the bronchial incisive margin was negative and the
pathological TNM stage was T2aN0Mx. In addition, the patient
received fluconazole (Pfizer Inc.) 200 mg/day antifungal therapy
for 6 months. Within five years after the resection, the patient
was admitted to the respiratory department of our hospital
regularly for further follow-up (every 6 months for the first 2
years and once a year for the last 3 years), and the latest
follow-up was in August 2023. The patient was in good condition and
contrast-enhanced CT showed no recurrence of either disease
(Fig. 1B, C, E and F).
Discussion
Cryptococcosis is a fatal fungal infection mainly
caused by Cryptococcus neoformans or Cryptococcus
gattii (1). Cryptococcosis
caused by Cryptococcus neoformans is common in China
(22). At present, PC is the third
most common pulmonary fungal infection in China and previous
studies have shown that the majority of cryptococcosis cases in
China were reported in HIV-uninfected patients (particularly
immunocompetent hosts) (22,23).
The case of the present study was a patient with normal immune
function, without any history of illness of the immune system,
underlying diseases such as diabetes or use of immunosuppressants
or glucocorticoids. PC can be confirmed by histopathology or tissue
culture (24). In the present case,
the histopathologic diagnosis of PC was obtained through biopsy,
surgery and special staining, such as PAS, GMS and MC. Of course,
in addition to invasive diagnostic methods, there are noninvasive
methods for PC, such as blood culture and Cryptococcus antigen
(CrAg) (25). However, culture
often yields negative results in immunocompetent hosts (26); occasionally, histopathologically
confirmed cases are culture-negative (27). The CrAg test is a sensitive and
specific test for the diagnosis of cryptococcosis in
immunocompromised patients (28).
However, the sensitivity is lower in patients with isolated PC. In
the present case, the nodule in the anterior segment of the left
upper lobe was highly suspected to be lung cancer on radiology, and
the nodule in the posterior basal segment of the left lower lobe
was suspected to be pulmonary metastasis. Therefore, initially, the
possibility of cryptococcus was not considered in advance, and
thus, no non-invasive tests, such as cryptococcal antigen testing,
were performed before percutaneous lung biopsy. PC symptoms are
nonspecific, presenting with cough, sputum, fever, dyspnea,
pleuritic chest pain, hemoptysis and malaise (3,29),
which are indistinguishable from other causes of pneumonia
(30). However, a subset of
patients are asymptomatic and the condition is usually detected
incidentally during chest radiological examination (23,31).
The patient of the present study had no clinical symptoms, even
with lung cancer of the upper lobe of the left lung. Different
immune statuses may affect the CT imaging features of patients with
PC (32). Based on previous
literature and clinical experience, pulmonary nodules/masses,
either solitary or multiple, were the most common CT findings in
PC, which usually occurs in the peripheral lung, adjacent to or
involving the pleura (29,32,33).
As reported in previous studies, when PC consists of solitary or
multiple nodules, these nodules may be confused with lung cancer on
chest CT and it is often difficult to distinguish PC from lung
carcinoma (5,16). Igai et al (34) tried to distinguish PC from lung
cancer by fluorodeoxyglucose positron emission tomography
(FDG-PET); however, their results showed that FDG-PET has
difficulty distinguishing PC from malignancies. In the patient of
the present study, the confirmed posterior basal pleural nodule of
the left lower lobe was consistent with this CT feature and the
final diagnosis was cryptococcal infection. In the present case,
multiple nodules were found in the left lung and based on chest CT,
it was highly suspected that the nodules in the anterior segment of
the left upper lobe were peripheral lung cancer, while the other
subpleural nodules in the posterior basal segment of the left lower
lobe were intrapulmonary metastases, which were later confirmed by
biopsy and surgery as adenocarcinoma and PC infection,
respectively. These imaging features of the present case were
consistent with those reported in the literature above. Huang et
al (12) suggested that
cryptococcosis lesions coexisted with lung cancer and resembled
primary or metastatic tumors. Harada et al (16) indicated that, since most patients
were in an immunocompetent state, the coexistence of cryptococcosis
and carcinoma was coincidental. However, Robinson et al
(9) thought that lung malignancy
may have resulted in a degree of immune suppression, predisposing
the patient to infection with cryptococcus. This issue is currently
controversial and further studies are needed to clarify the
possible relationship between lung cancer and cryptococcal
infection. It may be speculated that there is another possibility
that pulmonary cryptococcal infection can lead to the occurrence of
lung cancer. Similarly, The coexistence of pulmonary tuberculosis
and lung cancer is not an uncommon clinical observation (35), it has been proposed that chronic
inflammation in the lungs due to tuberculosis may cause clastogenic
activity in the DNA of bronchial epithelium. Another possibility is
lateral gene transfer; since Mycobacterium tuberculosis is
an intracellular organism, bacterial DNA may integrate into
bronchial epithelial cells to induce neoplastic transformation
(36). In addition, for cases
co-existing in the same nodule or mass, latent cryptococcus
infection may have a long-term chronic inflammatory stimulation,
and there is vast preclinical and clinical evidence suggesting that
strong and chronic inflammatory responses promote cancer
development and progression through different mechanisms (37,38).
The option that PC may cause lung cancer has not been reported, but
it is worthy of further research. Histopathology is still the most
important diagnostic method for PC and it is often necessary to
combine special staining to obtain a definitive diagnosis. It has
been reported that the detection rates of C. neoformans by
PAS, GMS, MC and Alcian blue staining were 100, 100, 87 and 67%,
respectively (39).
A comprehensive search of the PubMed, Google Scholar
and Web of Science databases was conducted and only 17 cases of PC
coexisting with pulmonary carcinoma have been reported in the
English language worldwide, which were from Japan, China, South
Korea and Australia (9,11–17).
The clinical characteristics of PC coexisting with pulmonary
carcinoma in the previous literature are summarized in Table I. The patient of the present case
study was asymptomatic; among the 17 patients reported in the
literature, 6 were asymptomatic. Furthermore, 12 patients were
immunocompetent and 5 patients had immunosuppressive and underlying
diseases, including diabetes mellitus, a history of gastric cancer
and thyroid adenoma resection, systemic lupus erythematosus,
chronic viral hepatitis B and a history of hormone use. Compared
with previous reports, the unique feature of the present case was
the relatively small size of the lung cancer and PC nodule, which
were 2.4×2.0 and 1.3×0.9 cm, respectively. The patient of the
present study had no underlying diseases and was immunocompetent.
Of the 17 patients reported in the literature, 7 were diagnosed
with coexisting cryptococcosis and carcinoma within the same lobe;
however, in the present case, the carcinoma nodule and cryptococcal
nodule were not in the same lobe. As reported in the literature,
the histological types of cancer in most cases were adenocarcinoma
(13 cases), 2 cases were squamous cell carcinoma and 2 cases were
alveolar cell carcinoma. The histological type of cancer in the
case of the present study was adenocarcinoma. In terms of
treatment, almost the same treatment method was adopted in the
present case and the previous literature, namely surgical excision
plus antifungal therapy. According to previous results, most of the
patients had a good prognosis and the patient of the present case
study was followed for 5 years with no recurrence of either
disease.
 | Table I.Features of previously reported cases
of PC coinciding with lung cancer. |
Table I.
Features of previously reported cases
of PC coinciding with lung cancer.
| Case no./age,
years/sex | Author, year | Country | Symptoms | Immuno-suppressive
underlying disease | Chest CT of lung
cancer/PC | Histologic subtypes
of cancer | Lung cancer TNM
staging | Therapy | Follow-up time
after discharge | Prognosis | (Refs.) |
|---|
| 1/73/M | Ahn, 2005 | South | Mild dyspnea | Diabetes | Anterior segment
of | Moderately | pT1aN0M0 | Surgical | 10 months | No | (11) |
|
|
| Korea | on exertion | mellitus and | the right upper
lobe | differentiated | (Stage IA) | excision + |
| recurrence |
|
|
|
|
| and cough | hypertension |
| squamous cell |
| AFT |
|
|
|
|
|
|
|
|
|
| carcinoma |
|
|
|
|
|
| 2/74/M | Robinson, | Australia | Right-sided | None | Left lower
lobe | Moderately | pT2N1 | Surgical | NA | NA | (9) |
|
| 1999 |
| pleuritic
chest |
| opacity | differentiated | (Stage IIB) | excision + |
|
|
|
|
|
|
| pain and mild |
|
| adenocarcinoma |
| AFT |
|
|
|
|
|
|
| dyspnea |
|
|
|
|
|
|
|
|
| 3/73/F | Kawasaki, | Japan | Asymptomatic | None | GGO, the left | Adenocarcinoma | pT1N0M0 | Surgical | 3 years | No | (13) |
|
| 2004 |
|
|
| anterior
superior |
| (Stage IA) | excision + |
| recurrence |
|
|
|
|
|
|
| subsegment/two |
|
| AFT |
|
|
|
|
|
|
|
|
| nodules, the
left |
|
|
|
|
|
|
|
|
|
|
|
| anterior basal |
|
|
|
|
|
|
|
|
|
|
|
| segment |
|
|
|
|
|
|
| 4/52/F | Li, 2018 | China | Cough | History of | Solitary
nodule, | Adenocarcinoma | pT1bN2M0 | Surgical | 3 years | No | (14) |
|
|
|
|
| thyroid | the right
posterior |
| (Stage IIIA) | excision + |
| recurrence |
|
|
|
|
|
| adenoma |
segment/multiple |
|
| ANCT + |
|
|
|
|
|
|
|
| resection | nodules, the
right |
|
| AFT |
|
|
|
|
|
|
|
|
| lateral basal |
|
|
|
|
|
|
|
|
|
|
|
| segment |
|
|
|
|
|
|
| 5/72/M | Yao, 2020 | China | Dry cough | None | Irregular mass,
left | Moderately- | NA | Surgical | 5 years | Cancer | (15) |
|
|
|
|
|
| hilum of the
lung/ | poorly |
| excision + |
| recurrence |
|
|
|
|
|
|
| multiple
nodules, | differentiated |
| AFT |
|
|
|
|
|
|
|
|
| dorsal segment
of | squamous cell |
|
|
|
|
|
|
|
|
|
|
| the right lower
lobe | carcinoma |
|
|
|
|
|
| 6/64/F | Huang., | China | Cough and | Diabetes | Solitary
nodule, | Invasive ADC | pT1aN0M0 | Surgical | 4 years | No | (12) |
|
| 2019 |
| sputum | mellitus | L-S3/solitary |
| (Stage IA) | excision + |
| recurrence |
|
|
|
|
| production |
| nodule, L-S7,8 |
|
| AFT |
|
|
|
| 7/55/M | Huang, | China | Asymptomatic | None | SNGGO, R-S6/ | Invasive ADC | pT2aN0M0 | Surgical | 7 years | No | (12) |
|
| 2019 |
|
|
| solitary
nodule, |
| (Stage IB) | excision + |
| recurrence |
|
|
|
|
|
|
| R-S3 |
|
| AFT |
|
|
|
| 8/69/F | Huang., | China | Asymptomatic | None | Solitary
nodule, | Non-mucinous | Tis | Surgical | 4 years | No | (12) |
|
| 2019 |
|
|
| R-S2/multiple | AIS |
| excision + |
| recurrence |
|
|
|
|
|
|
| nodules,
R-S2a |
|
| AFT |
|
|
|
| 9/57/F | Huang., | China | Cough and | Gastric | Solitary
nodule, | Invasive ADC | pT1aN0M0 | Surgical | 4 years | No | (12) |
|
| 2019 |
| sputum | cancer after | L-S1/solitary |
| (Stage IA) | excision + |
| recurrence |
|
|
|
|
| production | operation | nodule,
R-S1a |
|
| AFT |
|
|
|
| 10/43/F | Huang., | China | Cough, chest | None | Solitary
nodule, | Invasive | pT2aN0M0 | Surgical | 6 years | Cancer | (12) |
|
| 2019 |
| distress and |
| R-S3/solitary | mucinous | (Stage IB) | excision + |
| recurrence |
|
|
|
|
| chest pain |
| nodule,
R-S1a | ADC |
| AFT + |
|
|
|
|
|
|
|
|
|
|
|
| ANCT |
|
|
|
| 11/38/F | Huang., | China | Cough and | None | Solitary
nodule, | Invasive | pT1bN2M0 | Surgical | 8 years | Cancer | (12) |
|
| 2019 |
| phlegm with |
| R-S2/solitary | mucinous | (Stage IIIA) | excision + |
| recurrence |
|
|
|
|
| blood |
| nodule, R-S6 | ADC |
| AFT + |
|
|
|
|
|
|
|
|
|
|
|
| ANCT |
|
|
|
| 12/52/F | Huang., | China | Chest pain, | None | SNGGO, R-S2/ | Invasive ADC | pT1aN0M0 | Surgical | 4 years | No | (12) |
|
| 2019 |
| cough and |
| multiple
nodules, |
| (Stage IA) | excision + |
| recurrence |
|
|
|
|
| sputum |
| R-S6 |
|
| AFT |
|
|
|
|
|
|
| production |
|
|
|
|
|
|
|
|
| 13/67/F | Huang., | China | Fever? Cough | None | Air-space | Invasive | cT4N0Mib | AFT+ | 10 months | Deceased | (12) |
|
| 2019 |
| and sputum |
| consolidation, | mucinous ADC | (Stage IV) | ANCT |
|
|
|
|
|
|
| production |
| R-LL/air-space |
|
|
|
|
|
|
|
|
|
|
|
| consolidation,
R-LL |
|
|
|
|
|
|
| 14/69/M | Zheng, | China | Cough | None | Multiple
nodules, | Adenocarcinoma | NA | Surgical | 2 years | No | (17) |
|
| 2020 |
| and chest |
| the left upper
lobe/ |
|
| excision + |
| recurrence |
|
|
|
|
| discomfort |
| multiple
nodules, |
|
| AFT |
|
|
|
|
|
|
|
|
| the right lower
lobe |
|
|
|
|
|
|
| 15/54/M | Zheng, | China | Asymptomatic | None | GGO, the
posterior | Alveolar cell | NA | Surgical | 2 years | No | (17) |
|
| 2020 |
|
|
| segment of the
right | carcinoma |
| excision + |
| recurrence |
|
|
|
|
|
|
| upper lobe
apex/ |
|
| AFT |
|
|
|
|
|
|
|
|
| multiple
nodules, |
|
|
|
|
|
|
|
|
|
|
|
| the left upper
lobe |
|
|
|
|
|
|
| 16/46/F | Zheng, | China | Asymptomatic | Systemic lupus | Multiple
nodules, | Alveolar cell | NA | Surgical | 2 years | No | (17) |
|
| 2020 |
|
| erythematosus, | the dorsal
segment | carcinoma |
| excision + |
| recurrence |
|
|
|
|
|
| chronic viral | of the lower
lobe |
|
| AFT |
|
|
|
|
|
|
|
| hepatitis B
and | of the right
lung/ |
|
|
|
|
|
|
|
|
|
|
| use of methyl- | solitary nodule,
the |
|
|
|
|
|
|
|
|
|
|
| prednisolone | outer basal
segment |
|
|
|
|
|
|
|
|
|
|
| sodium | of the lower lobe
of |
|
|
|
|
|
|
|
|
|
|
| succinate | right lung |
|
|
|
|
|
|
| 17/71/M | Harada, | Japan | Asymptomatic | None | Solitary
thin-walled | Well- | T1N0M0 | NA | 1 year | No | (16) |
|
| 2006 |
|
|
| cavitary nodule,
the | differentiated | (Stage IA) |
|
| recurrence |
|
|
|
|
|
|
| apical segment
of | papillary |
|
|
|
|
|
|
|
|
|
|
| the right lung | adenocarcinoma |
|
|
|
|
|
The present case study reminds us of the possibility
of dualism in the diagnosis of multiple pulmonary nodules based on
CT examination, such as the coexistence of lung carcinoma and PC.
If medical conditions permit, lesion resection should be performed
to treat suspected malignant lung nodules, including cryptococcal
nodules that do not respond to antifungal therapy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated in the present study are not
publicly available to protect the patient's privacy but are
available from the corresponding author on reasonable request.
Authors' contributions
HW and MW were involved in the conception and design
of the study. HW and XC drafted the manuscript and performed the
acquisition, analysis and interpretation of data for the study. YT
and YW made contributions to the interpretation of the data for the
study and revised the manuscript critically for important
intellectual content. DY and YW acquired pathological and surgical
data of the patient/performed measurements. YZ and YL researched
the clinical case, participated in the treatment of the patient and
revised the manuscript. MW, HW and YT confirm the authenticity of
all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the ethics committee of
Taihe Hospital (Shiyan, China), and was performed in accordance
with the principles of Good Clinical Practice following the
Tri-Council guidelines.
Patient consent for publication
Written informed consent for anonymized information
and images to be published in this article was obtained from the
patient.
Competing interests
The authors declared that they have no competing
interests.
References
|
1
|
Perfect JR, Dismukes WE, Dromer F, Goldman
DL, Graybill JR, Hamill RJ, Harrison TS, Larsen RA, Lortholary O,
Nguyen MH, et al: Clinical practice guidelines for the management
of cryptococcal disease: 2010 update by the infectious diseases
society of america. Clin Infect Dis. 50:291–322. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yang R, Yan Y, Wang Y, Liu X and Su X:
Plain and contrast-enhanced chest computed tomography scan findings
of pulmonary cryptococcosis in immunocompetent patients. Exp Ther
Med. 14:4417–4424. 2017.PubMed/NCBI
|
|
3
|
Chang WC, Tzao C, Hsu HH, Lee SC, Huang
KL, Tung HJ and Chen CY: Pulmonary cryptococcosis: Comparison of
clinical and radiographic characteristics in immunocompetent and
immunocompromised patients. Chest. 129:333–340. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang RY, Chen YQ, Wu JQ, Wang X, Cao YH,
Zhao HZ and Zhu LP: Cryptococcosis in patients with hematological
diseases: A 14-year retrospective clinical analysis in a Chinese
tertiary hospital. BMC Infect Dis. 17:4632017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Setianingrum F, Rautemaa-Richardson R and
Denning DW: Pulmonary cryptococcosis: A review of pathobiology and
clinical aspects. Med Mycol. 57:133–150. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smith JA and Kauffman CA: Pulmonary fungal
infections. Respirology. 17:913–926. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qu J, Zhang X, Lu Y, Liu X and Lv X:
Clinical analysis in immunocompetent and immunocompromised patients
with pulmonary cryptococcosis in western China. Sci Rep.
10:93872020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cheng KB, Wu ZH, Liang S, Li HP and Xu JF:
Associations of serum cryptococcal antigen with different of
clinical characteristics: A comprehensive analysis of 378 pulmonary
cryptococcosis patients. Ann Palliat Med. 10:681–693. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robinson TD, Barnes DJ and Watson GF:
Coexistent cryptococcosis and carcinoma within a solitary pulmonary
nodule. Aust N Z J Med. 29:561–562. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Howard J, Thompson TZ, MacArthur RD,
Rojiani AM and White J: Widely disseminated cryptococcosis
manifesting in a previously undiagnosed human immunodeficiency
virus (HIV)-positive 18-year-old. Am J Case Rep. 21:e9244102020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahn IS, Kim HG, Ryu JS, Kim L, Kwak SM,
Lee HL, Yoon YH and Cho JH: A case of pulmonary cryptococcosis with
non-small cell lung cancer in idiopathic CD4+ T-lymphocytopenia.
Yonsei Med J. 46:173–176. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang J, Lan C, Li H, Chen S, Lin Q and
Weng H: Concomitant lung adenocarcinoma and pulmonary
cryptococcosis confirmed by pathologic examinations. Medicine
(Baltimore). 98:e183162019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kawasaki H, Ishikawa K, Kuniyoshi M, Ohta
M, Kawabata T and Hirayasu T: Lung adenocarcinoma with coexisting
pulmonary cryptococcoma. Jpn J Thorac Cardiovasc Surg. 52:21–25.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li L, Zhuang L, Zhou J and Shao C:
Pulmonary cryptococcosis coexisting with adenocarcinoma: A case
report and review of the literature. J Med Case Rep. 12:3272018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao K, Qiu X, Hu H, Han Y, Zhang W, Xia R,
Wang L and Fang J: Pulmonary cryptococcosis coexisting with central
type lung cancer in an immuocompetent patient: A case report and
literature review. BMC Pulm Med. 20:1612020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Harada T, Hakuma N, Kamimura A, Ito K and
Okamoto K: Pulmonary cryptococcosis within a pulmonary
carcinoma-review of reported cases. Intern Med. 45:369–372. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zheng GX, Tang HJ, Huang ZP, Pan HL, Wei
HY and Bai J: Clinical characteristics of pulmonary cryptococcosis
coexisting with lung adenocarcinoma: Three case reports. World J
Clin Cases. 8:6444–6449. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yuri T, Kimura A, Yoshizawa K, Emoto Y,
Kinoshita Y and Tsubura A: Pulmonary and meningeal cryptococcosis
after corticosteroid therapy for autoimmune hepatitis: Coexistence
of cryptococci within pulmonary cancer nodule. Case Rep Pathol.
2013:8071972013.PubMed/NCBI
|
|
19
|
Teixeira LM, Siqueira G, Shewmaker PL and
Facklam RR: Manual of Clinical Microbiology. (10th edition).
2011.
|
|
20
|
Diekema DJ, Pfaller MA and Murray PR:
Infection control epidemiology and clinical microbiology. 2006.
|
|
21
|
Murray GI: Laser Microdissection.
Molecular Biomethods Handbook; 2008, View Article : Google Scholar
|
|
22
|
Fang W, Fa Z and Liao W: Epidemiology of
cryptococcus and cryptococcosis in China. Fungal Genet Biol.
78:7–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu LP, Wu JQ, Xu B, Ou XT, Zhang QQ and
Weng XH: Cryptococcal meningitis in non-HIV-infected patients in a
Chinese tertiary care hospital, 1997–2007. Med Mycol. 48:570–579.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang H, Wang L, Luo Z, Li D, Luo G, Ren T,
You H, Liu Y, Tang Y and Wang M: Performance of rapid on-site
evaluation of touch imprints of lung tissue biopsies for the
diagnosis of pulmonary cryptococcosis in patients without HIV
infection. Mycoses. 65:635–642. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McFadden DC, Zaragoza O and Casadevall A:
Immunoreactivity of cryptococcal antigen is not stable under
prolonged incubations in human serum. J Clin Microbiol.
42:2786–2788. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fisher JF, Valencia-Rey PA and Davis WB:
Pulmonary cryptococcosis in the immunocompetent patient-many
questions, some answers. Open Forum Infect Dis. 3:ofw1672016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mukhopadhyay S, Farver CF, Vaszar LT,
Dempsey OJ, Popper HH, Mani H, Capelozzi VL, Fukuoka J, Kerr KM,
Zeren EH, et al: Causes of pulmonary granulomas: a retrospective
study of 500 cases from seven countries. J Clin Pathol. 65:51–57.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang MW, Clemons KV, Katzenstein DA and
Stevens DA: The cryptococcal antigen lateral flow assay: A
point-of-care diagnostic at an opportune time. Crit Rev Microbiol.
42:634–642. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ye F, Xie JX, Zeng QS, Chen GQ, Zhong SQ
and Zhong NS: Retrospective analysis of 76 immunocompetent patients
with primary pulmonary cryptococcosis. Lung. 190:339–346. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang CC, Sorrell TC and Chen SC:
Pulmonary cryptococcosis. Semin Respir Crit Care Med. 36:681–691.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kohno S, Kakeya H, Izumikawa K, Miyazaki
T, Yamamoto Y, Yanagihara K, Mitsutake K, Miyazaki Y, Maesaki S,
Yasuoka A, et al: Clinical features of pulmonary cryptococcosis in
non-HIV patients in Japan. J Infect Chemother. 21:23–30. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie LX, Chen YS, Liu SY and Shi YX:
Pulmonary cryptococcosis: Comparison of CT findings in
immunocompetent and immunocompromised patients. Acta Radiol.
56:447–453. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lacomis JM, Costello P, Vilchez R and
Kusne S: The radiology of pulmonary cryptococcosis in a tertiary
medical center. J Thorac Imaging. 16:139–148. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Igai H, Gotoh M and Yokomise H: Computed
tomography (CT) and positron emission tomography with
[18F]fluoro-2-deoxy-D-glucose (FDG-PET) images of pulmonary
cryptococcosis mimicking lung cancer. Eur J Cardiothorac Surg.
30:837–839. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liang HY, Li XL, Yu XS, Guan P, Yin ZH, He
QS and Zhou BS: Facts and fiction of the relationship between
preexisting tuberculosis and lung cancer risk: A systematic review.
Int J Cancer. 125:2936–2944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Molina-Romero C, Arrieta O and
Hernández-Pando R: Tuberculosis and lung cancer. Salud Publica Mex.
61:286–291. 2019. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Engels EA: Inflammation in the development
of lung cancer: Epidemiological evidence. Expert Rev Anticancer
Ther. 8:605–615. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yi XH, Kong J, Zhu MF, Zhang Y, Chen XF
and Zhong CS: Pathological diagnosis and ultrastructure features of
primary pulmonary cryptococcosis: A study of 27 cases. Zhonghua
Bing Li Xue Za Zhi. 33:424–428. 2004.(In Chinese). PubMed/NCBI
|