Introduction
Primary small bowel cancer refers to a
gastrointestinal malignant tumor originating from the duodenum,
jejunum, or ileum. The most common histological types of small
bowel cancer are adenocarcinomas, neuroendocrine tumors,
gastrointestinal stromal tumors and lymphomas (1). The clinical manifestations of small
bowel adenocarcinoma (SBA) are atypical. Of patients with SBA,
~35-36.4% have distant metastases (2–4); among
whom, ~1.6% develop ovarian metastases (5). Due to their similar clinical symptoms,
the differential diagnosis between metastatic ovarian cancer and
primary ovarian cancer primarily relies on histopathology and
immunohistochemistry. Metastasectomy can prolong the median overall
survival (OS) of patients with advanced SBA to 28.6 months
(6). The present study reported the
case of a 45-year-old woman with jejunal adenocarcinoma who
developed tumor metastasis to the right and left ovaries as well as
the abdominopelvic cavity successively after surgical resection of
the primary site. As of February 2023, the patient has survived for
73 months and has a high quality of life. In this case, surgery
after multidisciplinary team (MDT) evaluation in advanced SBA
prolonged the patient's survival. Immunohistochemistry has also
been reported as a method to identify primary ovarian cancers from
secondary ovarian cancers. This case is presented following the
CARE reporting checklist (available at http://www.care-statement.org/checklist).
Case report
In February 2017, a 45-year-old woman who presented
with a change in bowel habits and abdominal pain was suspected of
SBA and consequently underwent a small bowel tumorectomy (R0
resection) at Zhangzhou Hospital (Fujian, China). No history of
familial syndromes such as familial adenomatous polyposis or Lynch
syndrome and no medical-surgical history of interest were reported.
The patient had no history of allergies and had never smoked or
drank alcohol. The cancer was located intraoperatively at the upper
end of the jejunum, ~80 cm from Treitz's ligament, with a size of
~4.0×5.0 cm. The postoperative pathology finding showed that the
mass was a moderately to poorly differentiated SBA, which was of
the ulcerated type and ~2.5×2.5×1.0 cm in size. The SBA mass
invaded the adipose tissue of the serous layer of the small
intestine and nerve fibers, but not the regional lymph nodes (0/13
next to the mass and 0/30 next to the intestine) or the upper or
lower surgical margins. According to the AJCC 8th Edition (7), the tumor diagnosis was SBA (T4N0M0
stage IIB) and the patient was treated with four cycles of
5-fluorouracil hyperthermic intraperitoneal perfusion chemotherapy
and eight cycles of SOX chemotherapy after surgery.
In February 2018, during a regular review at the
Affiliated Hospital of Xiamen University (Fujian, China), the
result of the positron emission tomography-computed tomography
(PET-CT) examination suggested an irregular mixed image on the
right side of the pelvic cavity, which was ~9.36×7.47 cm in size,
partly hypermetabolic and poorly demarcated from the right
appendage. A hypermetabolic small nodule was observed above the
lesion, which was ~1.66×1.12 cm in size. The patient was then
transferred to the Sino-German Gynecology Department of the
Affiliated Hospital of Southwest Medical University (Luzhou, China)
due to health insurance reimbursement policies. After the MDT
evaluated the condition, the patient underwent a right adnexectomy
and resection of small nodules of the jejunal serosa. The left
ovary was explored intraoperatively and found to be clear of
metastases. As the patient was not menopausal and requested to keep
the left ovary, the left ovary was not removed. The specimens were
fixed in 4% formaldehyde solution for 1 h at room temperature, and
then subjected to gradient ethanol dehydration, paraffin embedding
and sectioning (thickness, 3–5 µm) to make paraffin sections. After
heating at 60°C, paraffin sections were dewaxed with xylene and
rehydrated in a descending alcohol series. Sections were
successively stained with hematoxylin stain (cat. no. BA4021;
Zhuhai Baso Biotechnology Co., Ltd.) for 5–10 min and eosin stain
(cat. no. BA4022; Zhuhai Baso Biotechnology Co., Ltd.) for 3–5 min
at room temperature. Finally, the sections were sealed with neutral
gum resin. Immunohistochemistry was performed using the MaxVision
two-step method. After sections underwent dewaxing, hydration and
antigen retrieval, they were added with primary antibodies and
incubated at 37°C for 2 h. The primary antibodies used were mouse
anti-human CK20 monoclonal antibody reagent (cat. no. MAB-0834;
Fuzhou Maixin Biotech Co., Ltd.) and rabbit anti-human CDX2
monoclonal antibody reagent (cat. no. PA207; Suzhou Abcarta Medical
Technology Co., Ltd.). They did not need to be diluted.
Subsequently, sections were added with secondary antibodies and
incubated at 37°C for 30 min. The secondary antibody used was
MaxVision™ HRP-polymer anti-mouse/rabbit IHC kit (cat. no.
KIT-5020; Fuzhou Maixin Biotech Co., Ltd.), which had a peroxidase
conjugate. It did not need to be diluted. Finally, the specimens
were stained with MaxVision III UItra DAB (cat. no. KIT-0038;
Fuzhou Maixin Biotech Co., Ltd.) at 25°C for 3–5 min, re-stained
with hematoxylin (cat. no. BA4021; Zhuhai Baso Biotechnology Co.,
Ltd.) at 37°C for 3–5 min, dehydrated at 25°C for 20 sec, clearing
with xylene and sealed with neutral gum resin. Postoperative
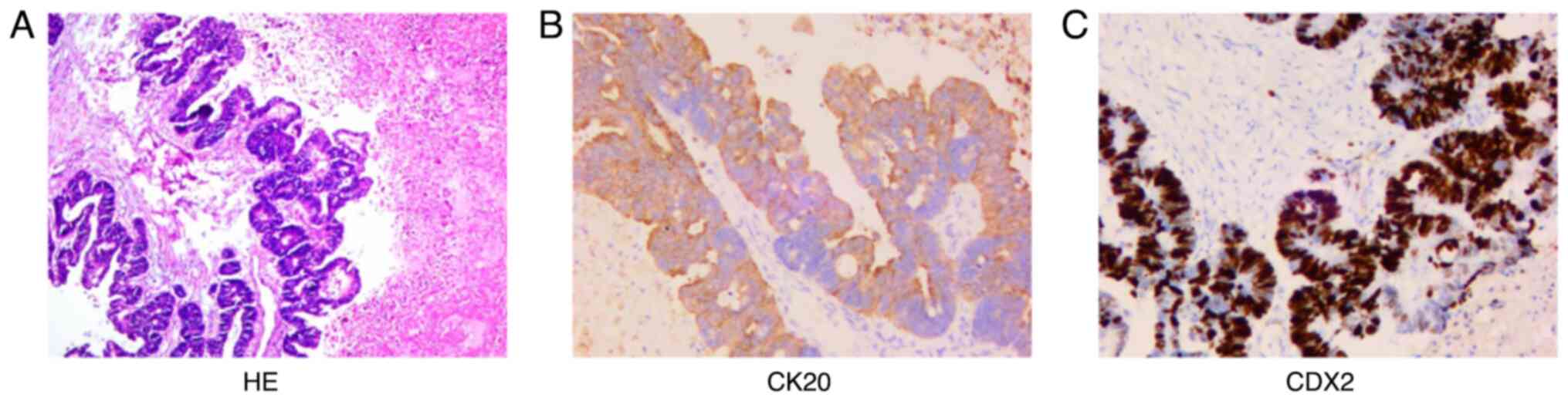
pathological findings (Fig. 1)
showed that the jejunum nodule was granulation and scar tissue. The
right ovarian adenocarcinoma was ~8.5×6.0×5 cm in size and the
capsule was not involved. The most significant immunophenotypic
results (Fig. 1) were CK20 (+) and
CDX2 (+). Combined with histomorphological analyses,
immunophenotyping and the history of the disease, the tumor was
diagnosed as metastatic adenocarcinoma of the right ovary
originating from SBA. Postoperative chemotherapy and targeted
therapy were not administered.
In July 2018, during a regular review at the
Affiliated Hospital of Xiamen University (Fujian, China), the
result of ultrasonography showed a mixed echogenic mass (~5.3×3.9
cm in size) in the left adnexa uteri, while CDFI showed that it was
visible on the Doppler blood velocity signal in the solid region.
Therefore, the patient returned to the Sino-German Gynecology
Department of the Affiliated Hospital of Southwest Medical
University (Fujian, China). After evaluation of the condition by
the MDT, the patient underwent a left adnexectomy and a total
hysterectomy. The method used for histology was the same as
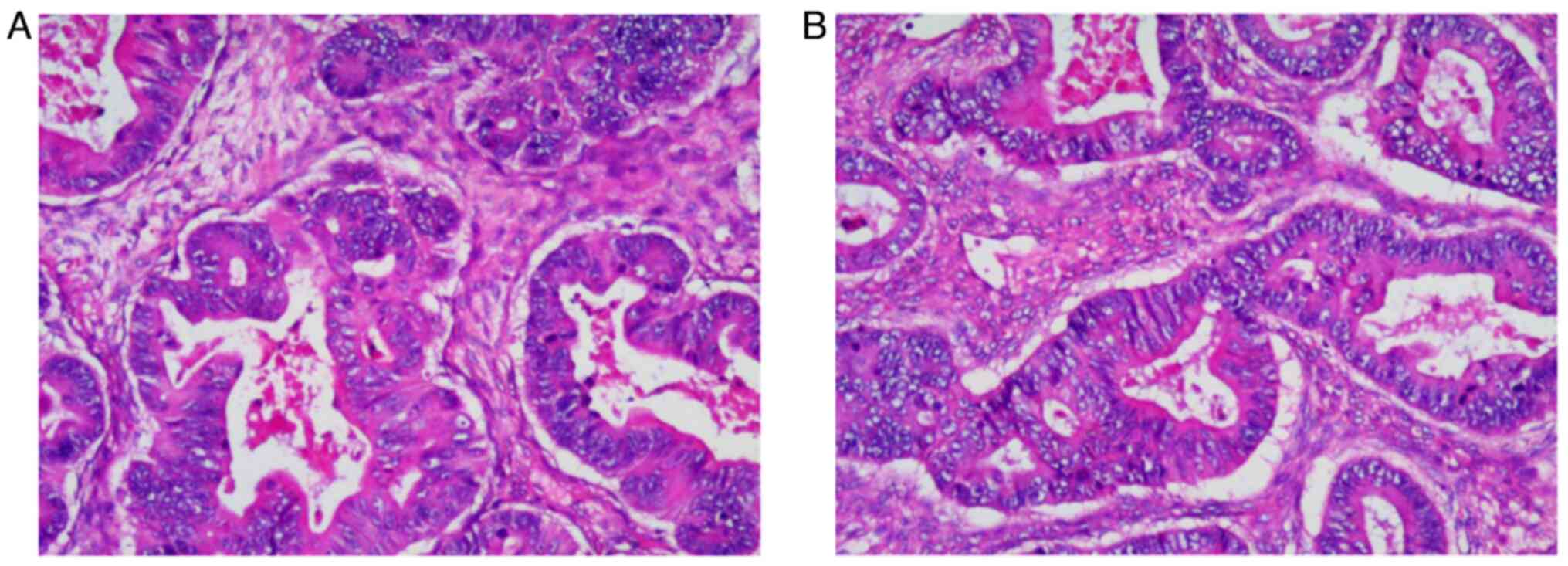
aforementioned. The postoperative pathological findings (Fig. 2) showed that the left ovarian mass
was an intestinal-type adenocarcinoma with necrosis, which was
~6.0×5.0×3.0 cm in size, without intravascular cancer embolus or
neural invasion. No immunohistochemistry examination was performed
due to the left and right metastatic ovarian adenocarcinomas
sharing the same histomorphology. The tumor was diagnosed as
metastatic adenocarcinoma of the left ovary originating from SBA.
Postoperative chemotherapy and targeted therapy were not
administered.
In June 2019, because of a change in bowel habits
with stomach pains, the patient returned to the gastrointestinal
surgery department of the Affiliated Hospital of Southwest Medical
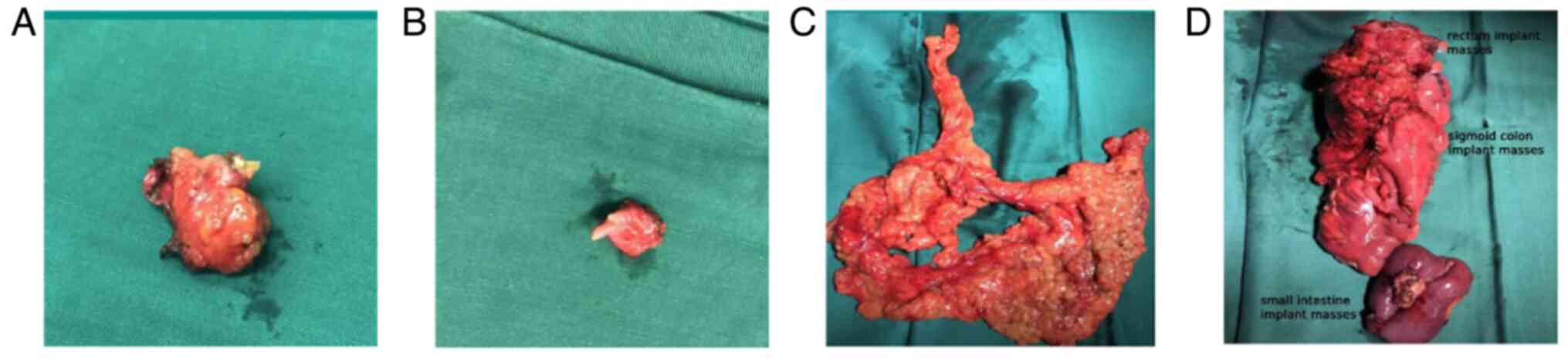
University (Fujian, China). PET-CT (Fig. 3) showed local bowel wall thickening
of the upper rectum and sigmoid colon and splenic flexure of the
colon with increased glucose metabolism (SUVmax: ~4.3), which
suggested the possibility of tumor lesions. Following evaluation by
the MDT, a left colectomy with partial ileectomy, large
omentectomy, abdominal wall implant node resection, vaginal residue
resection and bilateral bladder angle implant node resection (R0
resection) was performed (Fig. 4).
The method used for histology and immunohistochemistry was the same
as aforementioned. The primary antibodies used were mouse
anti-human CK20 monoclonal antibody reagent (cat. no. MAB-0834;
Fuzhou Maixin Biotech Co. Ltd.) and mouse anti-human villin
monoclonal antibody reagent (cat. no. MAB-0540; Fuzhou Maixin
Biotech Co., Ltd.). They did not need to be diluted. The
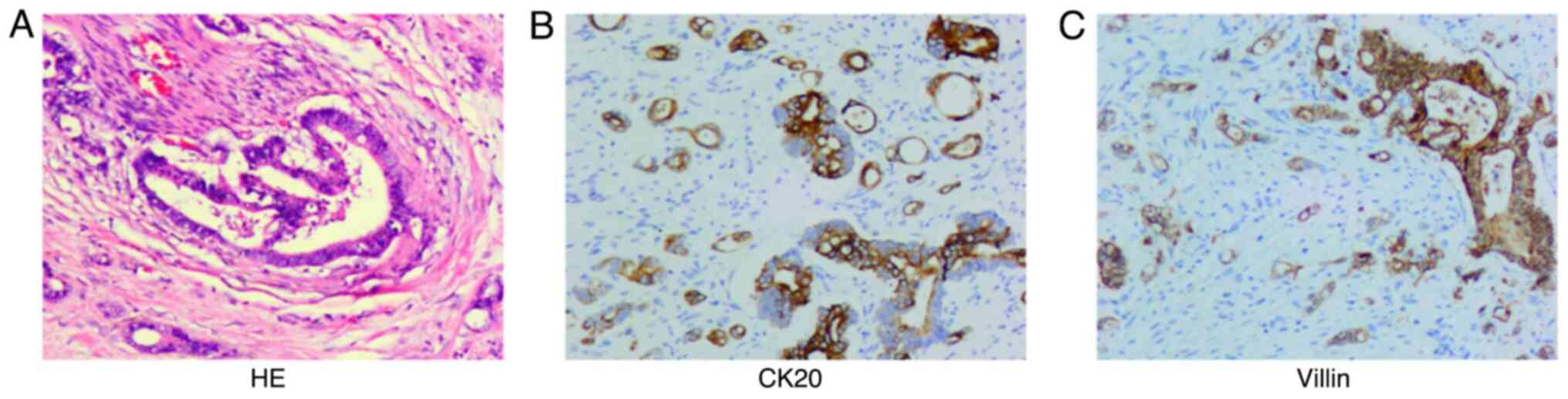
postoperative pathological findings (Fig. 5) showed a low differentiated
adenocarcinoma of the left colon, with a size of ~6.0×6.0×2 cm and
of the terminal ileum, with a size of ~3.0×3.0×2.0 cm. The most
significant immunophenotypic results (Fig. 5) were CK20 (+) and villin (+). Taken
together, these findings suggested that the tumor was diagnosed as
metastatic adenocarcinoma of the small intestine. The patient's
samples were sent to the Guangzhou Clinical Laboratory Center for
high-throughput sequencing of 21 colorectal tumor genes (Table I). Postoperatively, the patient was
treated with five cycles of lobaplatin hyperthermic intraperitoneal
perfusion chemotherapy, 12 cycles of cetuximab with mFOLFOX6, q14d
and capecitabine 1,250 mg/m2 d1-14 as maintenance
therapy for 6 months. No recurrence or metastasis of SBA was found
during regular follow-ups.
 | Table I.High-throughput sequencing results of
21 colorectal tumor genes. |
Table I.
High-throughput sequencing results of
21 colorectal tumor genes.
| Type |
| Content | Result | Medication
suggestions | Treatment |
|---|
| Genetic testing for
targeted drugs | - | Genes | KRAS, NRAS, BRAF,
PIK3CA, EGFR, PTEN, MET, HER2 and HRAS | No mutations | - | Recommended drugs:
cetuximab, panitumumab or bevacizumab |
| Genetic testing for
immunosuppressants | MSI testing | Microsatellite
loci | BAT-25, BAT-26,
D2S123, D5S346 and D17S250 | MSS | Pembrolizumab and
nivolumab with low sensitivity |
|
| Genetic testing for
chemotherapeutic drugs | Chemotherapeutic
drugs | Platinum-based
drugs | XPC, MTHFR, GSTP1 and
XRCC1 | - | Moderate risk of
toxicity and poor efficacy | Optional drugs:
fluorouracil, capecitabine or irinotecan |
|
|
| Paclitaxel | ABCB1 |
| High risk of
toxicity |
|
|
|
| Etoposide | DYNC2H1 |
| Good effective |
|
|
|
| Gemcitabine | NT5C2 |
| Fast drug
clearance |
|
|
|
| Capecitabine and
flurouracil | DPYD, TYMS, UMPS and
TP53 |
| Low risk of toxicity
and moderate efficacy |
|
|
|
| Cyclophosphamide | MTHFR, GSTP1 and
SOD2 |
| Low risk of toxicity
and good efficacy |
|
|
|
| Methotrexate | MTHFR, ABCB1, SLCO1B1
and MTRR |
| High risk of
toxicity |
|
|
|
| Irinotecan | UGT1A1, SEMA3C and
C8orf34 |
| Low risk of toxicity
and moderate efficacy |
|
|
|
| Pemetrexed | MTHFR |
| Good effective |
|
|
|
| Anthracycline-based
drugs | NQO1 and CBR3 |
| High risk of
toxicity |
|
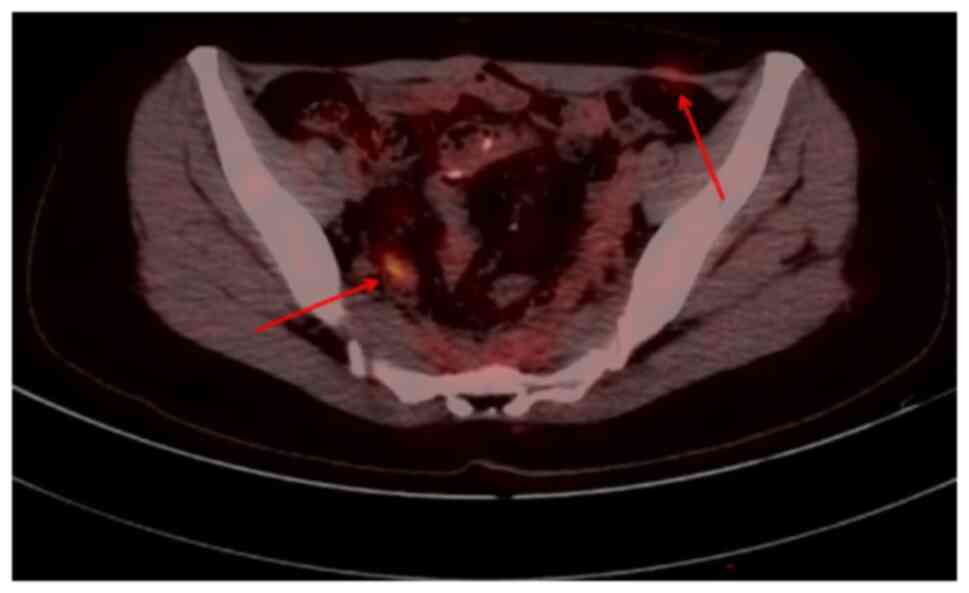
In January 2022, when the patient was reviewed at
our hospital, PET-CT (Fig. 6)
showed local bowel wall thickening, increased glucose metabolism on
the right side of the presacral space and soft tissue nodules with
slightly increased glucose metabolism on the left side of the
aponeurosis area of the musculus obliquus externus abdominis; this
suggested the possibility of tumor recurrence or metastasis
(SUVmax: ~3.2). Following a comprehensive evaluation by the MDT,
the lesion could not be precisely removed by surgery and therefore
the patient was treated with eight cycles of bevacizumab plus
mFOLFOX6, q14d. When the tumor was determined to show a partial
response by PET-CT, the patient was given a regimen of bevacizumab
400 mg plus capecitabine 1.5 g q14d for four cycles of maintenance
treatment (August 8, 2022). Then, the patient refused to continue
medication maintenance. As of February 2023, the patient has
survived for 73 months and has a high quality of life. The
treatments were well tolerated by the patient. Serious or potential
adverse reactions were not reported. Additionally, a timeline has
been created to make it easier to follow the progress of the case
(Fig. 7).
Discussion
SBA is a type of gastrointestinal cancer with a low
incidence, accounting for only 3% of all gastrointestinal cancers
(8), often occurring in the
duodenum (52–57.9%), jejunum (15.6–29%), ileum (10–13%), or other
locations in the small intestine (4–15.7%) (2–4). The
onset of SBA is relatively insidious and some patients already have
distant metastasis when diagnosed with SBA. Among them, ~1.6% of
patients with SBA have ovarian metastasis, including left ovarian
(16.7%), right ovarian (27.8%) and bilateral ovarian metastases
(55.6%) (5). Therefore, the jejunal
adenocarcinoma with ovarian metastasis reported here is rare.
The PubMed database was searched for literature on
ovarian metastasis from small bowel cancer from January 1990 to
September 2023, using the following search terms: (small bowel
cancer) OR (small intestine cancer) OR (jejunum cancer) OR
(duodenum cancer) OR (ileum cancer) AND (metastatic ovarian
cancer). Only English-language literature were selected for
documented case reports of ovarian metastases from SBA and there
were 10 cases (Table II) (9–18).
There are some differences between this case and cases in Table II. Of the 10 patients, 40% had
bilateral ovarian metastases and 50% had right ovarian metastases.
By contrast, the patient in this case developed right ovarian
metastases, followed by left ovarian metastases. The patient has
survived for 73 months after the primary cancer resection and 30
months without recurrence after the third metastasectomy. The
patient's survival time is much longer than that of 10 patients in
Table II. In the opinion of the
authors, when the patient in this case presented with right
ovarian, left ovarian and abdominopelvic implant successively, the
three metastasectomies performed after MDT evaluations may have
prolonged the survival time of the patient. There are also some
similarities between this case and 10 cases in Table II. In this case, the patient also
presented with SBA. The patient also developed ovarian metastases
and underwent operations and adjuvant chemotherapy. Meanwhile,
doctors used histopathology and immunohistochemistry to diagnose
metastatic ovarian cancer.
 | Table II.Reported cases of ovarian metastasis
from small bowel adenocarcinoma. |
Table II.
Reported cases of ovarian metastasis
from small bowel adenocarcinoma.
| First author,
year | Case no. | Age (years) | Primary tumor
site | Side | Size (cm) | Pathology | Surgery | Adjuvant
chemotherapy | Result | (Refs.) |
|---|
| Iijima et al,
2020 | 1 | 34 | Jejunum | Both | 3.3 (right) 1.3
(left) | Yes | ND | ND | Died 9.8 months after
the initial diagnosis | (9) |
| Liu et al,
2018 | 1 | 53 | Jejunum | Both | 7 (right) 15
(left) | Yes | ATH + BSO + OMT +
jejunectomy | ND | ND | (10) |
| Dunsmore and Lovell,
1998 | 1 | 12 | Jejunum | Both | 9×6.5×4 (right)
7×4×3.5 (left) | Yes | (1st) Jejunectomy
(2nd) BSO | 5-FU + leucovorin +
α-interferon | Died 23 months after
the initial diagnosis | (11) |
| Kilic and Abadi,
2000 | 1 | 53 | Jejunum | Right | 20×18×15 | Yes | RSO +
jejunectomy | No | Died 6 days after
the surgery | (12) |
| Maekawa et
al, 2010 | 1 | 50 | Jejunum | Both | 16×12×13 (right)
5×4×4 (left) | Yes | (1st) ATH + BSO +
OMT + PLA (2nd) Jejunectomy | S-1 | No recurrence for
24 months | (13) |
| Mitsushita et
al, 2017 | 1 | 34 | Jejunum | Right then
Left | 26×23×13
(right) | Yes | (1st) RSO (2nd) ATH
+ LSO + PAN + OMT + jejunectomy | Capecitabine +
oxaliplatin + bevacizumab | Recurrence 26
months after the 2nd surgery | (14) |
| Tsuruchi et
al, 1995 | 1 | 49 | Jejunum | Right | 25×18×12 | Yes | ATH + BSO + OMT +
PLA + PAN + jejunectomy | 5-FU +
cisplatin | No recurrence for 8
months | (15) |
| Iwata et al,
2020 | 1 | 59 | Ileum | Right | 8.5 | Yes | ATH + BSO + OMT +
ileectomy | Capecitabine +
oxaliplatin | No recurrence for
24 months | (16) |
| Andresen et
al, 2001 | 1 | 65 | Ileum | Right | ND | Yes | Rightsided
hemicolectomy + ileostomy | ND | Died 6 weeks after
the surgery | (17) |
| Loke et al,
1997 | 1 | 44 | Duodenojejunal
flexure | Both | 10.5 (right) 11
(left) | Yes | (1st) Small bowel
resection (2nd) ATH + BSO + appendicectomy | ND | ND | (18) |
| Total | 10 | 45.3a | Jejunum 7 | Both 5 | Maximum 1.3–26 | 10/10 | Surgery 9 ND 1 | Performed 5 |
|
|
|
|
|
| Ileum 2 | Right 4 | diameter | (100%) |
| Not performed |
|
|
|
|
|
| Duodenojejunal | Right then |
|
|
| 1 ND 4 |
|
|
|
|
|
| flexure 1 | Left 1 |
|
|
|
|
|
|
The differential diagnosis of metastatic ovarian
cancer and primary ovarian cancer is challenging. Imaging
examinations such as ultrasound and PET-CT can only clarify the
site of the lesion, but not the origin of the lesion.
Histomorphologically, metastatic ovarian cancer may present with
characteristic intraluminal necrotic debris (‘dirty necrosis’)
(19); however, the use of
immunohistochemistry is still needed to definitively diagnose
cancer.
The immunophenotype and molecular mechanism of SBA
are still unclear and the diagnosis and differential diagnosis of
SBA primarily refer to the immunophenotype of colorectal neoplasms.
Positive expression of CK20, CDX2 and SATB2 is found in colorectal
metastatic ovarian cancer, all of which are considered sensitive
markers for colorectal tumors. Primary ovarian cancer often shows
the positive expression of CK7 and MUC2/5AC, while β-catenin, CA125
and CEA also have some significance in the differential diagnosis
(9). In this case, when the patient
developed non-synchronized bilateral ovarian metastasis, the MDT
relied mainly on the histopathology and immunohistochemistry of the
lesion to diagnose the disease.
Surgical resection is the primary treatment for SBA.
Version 2.2022 of the NCCN guidelines for SBA (20) suggested that metastasectomy may be
an option if the advanced tumor lesion is considered resectable
following evaluation by an experienced MDT. Of patients with SBA,
~13% have synchronous peritoneal metastasis and a poor prognosis,
with a median OS of 5.8 and 11 months for patients after primary
cancer resection (21). Meanwhile,
Rompteaux et al (6) reported
a median OS of 28.6 months for patients with metastasectomy and a
median recurrence-free survival (RFS) of 18.7 months. By contrast,
the patient in the present study has survived for 73 months after
the primary cancer resection, 61 months after the first
metastasectomy, 45 months after developing abdominal implant
metastases and 30 months without recurrence after the third
metastasectomy. The survival time of the patient has far exceeded
the median OS and RFS reported in the retrospective analysis above.
This case demonstrates that appropriate surgery could prolong
survival in patients with advanced SBA and that a comprehensive
evaluation by the MDT is essential.
In conclusion, the jejunal adenocarcinoma with
ovarian metastasis reported in the present report is rare. The
differential diagnosis between metastatic ovarian cancer and
primary ovarian cancer mainly relies on histopathology and
immunohistochemistry. After a comprehensive evaluation by an
experienced MDT, surgery can be of great benefit to terminal cancer
patients with SBA. The present study also has some shortcomings.
The MDT should consider the need for a hysterectomy plus bilateral
adnexectomy when a patient presents with a metastatic lesion in the
right ovary and chemotherapy and targeted therapy should be
actively recommended after surgery. Moreover, the patient was
treated with cetuximab after the resection of abdominopelvic
implant metastases, which lacked a recommendation by SBA
guidelines. Only a few cases of SBA have been reported in China and
abroad; there is currently a lack of large prospective clinical
trials and the efficacy of cetuximab is debatable.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The patient declined to allow the authors to upload
the high-throughput sequencing data to a public database to protect
privacy. The published article includes other data generated or
analyzed during the study.
Authors' contributions
XH contributed substantially to data acquisition and
analysis as well as writing the manuscript; YZ, AT, SD, JF and YJ
contributed to the treatment of the diseases and the collection of
case information; XX, DZ and LC contributed to the diagnosis of the
diseases and the collection of case information; SC and XD
contributed to data acquisition and analysis in addition to
revising the study critically for important intellectual content;
HY contributed substantially to the conception of the case study
and agreed to be accountable for all of the aspects of the work in
ensuring that questions related to the accuracy or integrity of any
part of the work were appropriately investigated and resolved; XH
and HY confirmed the authenticity of all of the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this manuscript and all of the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aparicio T, Zaanan A, Svrcek M,
Laurent-Puig P, Carrere N, Manfredi S, Locher C and Afchain P:
Small bowel adenocarcinoma: Epidemiology, risk factors, diagnosis
and treatment. Dig Liver Dis. 46:97–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dabaja BS, Suki D, Pro B, Bonnen M and
Ajani J: Adenocarcinoma of the small bowel: Presentation,
prognostic factors, and outcome of 217 patients. Cancer.
101:518–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Halfdanarson TR, McWilliams RR, Donohue JH
and Quevedo JF: A single-institution experience with 491 cases of
small bowel adenocarcinoma. Am J Surg. 199:797–803. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Akce M, Jiang R, Zakka K, Wu C, Alese OB,
Shaib WL, Behera M and El-Rayes BF: Clinical outcomes of small
bowel adenocarcinoma. Clin Colorectal Cancer. 18:257–268. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruls J, Simons M, Overbeek LI, Bulten J,
Massuger LF and Nagtegaal ID: A national population-based study
provides insight in the origin of malignancies metastatic to the
ovary. Virchows Arch. 467:79–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rompteaux P, Gagnière J, Gornet JM, Coriat
R, Baumgaertner I, Lecomte T, Afchain P, Zaanan A, Pocard M, Bachet
JB, et al: Resection of small bowel adenocarcinoma metastases:
Results of the ARCAD-NADEGE cohort study. Eur J Surg Oncol.
45:331–335. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. 8th edition.
Springer; New York: 2017, View Article : Google Scholar
|
|
8
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iijima K, Oozeki M, Ikeda K, Honda H,
Ishibashi H, Yamaoka M, Fujieda S, Saitoh H, Goto M, Araki M and
Amagai K: A case of small bowel adenocarcinoma wherein nivolumab
conferred temporary benefit in disease control. Clin J
Gastroenterol. 13:372–376. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
An-Chieh Liu A, Chen CH, Liu WM and Chang
CW: A rare Krukenberg tumor arising from a primary adenocarcinoma
of the small intestine. Taiwan J Obstet Gynecol. 57:319–322. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Dunsmore KP and Lovell MA: Small bowel
adenocarcinoma metastatic to the ovaries in 12-year-old girl. J
Pediatr Hematol Oncol. 20:498–501. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kilic G and Abadi M: Jejunal
adenocarcinoma presenting as a primary ovarian carcinoma. Gynecol
Oncol. 78:255–258. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maekawa H, Sato K, Komatsu Y, Orita H and
Sakurada M: Jejunal cancer detected after a resection of bilateral
ovarian metastasis: Report of a case. Surg Today. 40:1084–1087.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mitsushita J, Netsu S, Suzuki K, Nokubi M
and Tanaka A: Metastatic ovarian tumors originating from a small
bowel adenocarcinoma-a case report and brief literature review. Int
J Gynecol Pathol. 36:253–260. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tsuruchi N, Kubota H, Tsukamoto N and
Kurano A: Primary jejunal adenocarcinoma masquerading as a primary
ovarian malignancy. Gynecol Oncol. 58:129–132. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iwata N, Shikama A, Takao W, Hosokawa Y,
Itagaki H, Tasaka N, Akiyama A, Ochi H, Minaguchi T, Arita M, et
al: Ovarian metastases from ileum cancer in a patient with germline
EPCAM gene deletion successfully treated with surgical resection
and CAPOX chemotherapy: A case report. BMC Med Genet. 21:762020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Andresen DM, Pedersen FH and Rasmussen KL:
Adenocarcinoma of the small intestine mistaken as a primary ovarian
cancer. Arch Gynecol Obstet. 265:214–215. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Loke TKL, Lo SS and Chan CS: Case report:
Krukenberg tumours arising from a primary duodenojejunal
adenocarcinoma. Clin Radiol. 52:154–155. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kir G, Gurbuz A, Karateke A and Kir M:
Clinicopathologic and immunohistochemical profile of ovarian
metastases from colorectal carcinoma. World J Gastrointest Surg.
2:109–116. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Benson AB, Venook AP, Al-Hawary MM, Arain
MA, Chen YJ, Ciombor KK, Cohen SA, Cooper HS, Deming DA,
Garrido-Laguna I, et al: Small bowel adenocarcinoma, version
1.2020, NCCN clinical practice guidelines in oncology. J Natl Compr
Canc Netw. 17:1109–1133. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Legué LM, Simkens GA, Creemers GJM,
Lemmens VEPP and de Hingh IHJT: Synchronous peritoneal metastases
of small bowel adenocarcinoma: Insights into an underexposed
clinical phenomenon. Eur J Cancer. 87:84–91. 2017. View Article : Google Scholar : PubMed/NCBI
|