Introduction
Malignant neoplasms of the nasal cavity and
paranasal sinuses represent 0.2% of all human primary malignant
tumors, and their incidence is about 0.1–1.4 new cases/year/100.000
inhabitants (1,2). Among primary malignant neoplasms of
the sinonasal tract nasal, sinonasal adenocarcinomas account for
10–20% (3); the majority of them
show a salivary gland origin, while others show histological
features resembling those of colon adenocarcinoma. One particular
subtype of sinonasal adenocarcinoma was named intestinal-type
adenocarcinoma (ITAC), which is responsible for less than 4% of
total malignancies in this region (4), and can occur sporadically or
associated with specific workers' categories that are exposed to
hardwood and leather dust (5): in
fact, these high-risk individuals show an approximately 500-fold
higher incidence (6,7), and ITACs arising in subjects with
occupational dust exposure are more often diagnosed in men, and
show a significant propensity to develop in the ethmoid sinuses
(8–10). In addition, the ITACs connected with
occupational exposure (hardwood dust, leather dust) are preceded by
the intestinal metaplasia of the respiratory mucosal tract.
ITAC represent a clinically aggressive entity that
is typically associated with a tendency to recur locally, a low
incidence of distant metastases, and an overall mortality of
approximately 53% (10). Several
studies reported how the most important prognosticators in patients
with ITAC are the histopathological grading, and the pT
classification (9–12). Surgery combined with postoperative
radiotherapy (RT) and, in some cases, with adjuvant chemotherapy
(CH) is the best treatment option (13).
Although the classic prognostic factors maintain a
great utility in predicting the clinical behavior of ITAC (13,14),
nevertheless it is not clear why some ITACs present a more
aggressive behavior in comparison to others with the same type of
features in terms of histological subtype and clinical stage
(15–25). In the last ten years, information
about the molecular mechanisms involved in the pathogenesis of head
and neck squamous-cell carcinomas (HNSCC) are rapidly increasing
(26–36); recently, some authors showed that
epigenetic alterations have a critical role in HNSCC carcinogenesis
(37–39). MicroRNAs (miRNAs), a type of small
non-protein-coding RNA molecules that modulate the expression of
target genes, operating as gene expression repressors at
post-transcriptional level, and affecting the translation or
causing the degradation of mRNA targets, are now considered as
crucial components of the epigenome, orchestrating events ranging
from organogenesis to immunity, and they are known to be important
in influencing the origin of many diseases, including malignant
tumors (40–54).
To achieve further knowledge about the phenotype and
possible mechanisms of ethmoidal ITAC development, for the first
time we investigated the role and the prognostic value of
miR-let-7a, a head and neck squamous-cell carcinoma (HNSCC) related
microRNA (53–55) in a well-characterized and
homogeneous cohort of patients affected by primary ethmoidal ITAC,
associated with occupational exposure and treated by primary
surgery (56,57).
Materials and methods
Patients and specimen selection
This retrospective cohort study analyzed
consecutively the medical charts of all patients with primary ITAC,
treated by surgery with curative intent at the Department of
Otorhinolaryngology, Umberto I University General Hospital (Marche
Polytechnic University, Ancona, Italy) between January 2000 and
January 2010. From the medical records, the following data were
collected: age at the diagnosis, gender, occupational history, site
of the tumor, postoperative staging (pTNM classification),
histological findings, disease-free survival (DFS), and overall
survival (OS).
Inclusion criteria: complete clinical data, 5
follow-up years at least, uniformity of histological
differentiation throughout the tumor sample, and the availability
of normal formalin-fixed paraffin-embedded (FFPE) tissue
samples.
Exclusion criteria: patients with previous or
synchronous second malignancies, or patients who underwent previous
radiation therapy or chemotherapy, or patients who died of
postoperative complications. As additional, specifical exclusion
criteria were applied regarding the surgical approach for removing
the tumors as previously described and suggested in the literature
(13,58,59).
Absolute contraindications for an endoscopic approach were erosion
of nasal bones or floor of the nasal cavity, extensive involvement
of the nasal pathway (except the nasolacrimal duct), infiltration
of the walls of the maxillary sinus (except the medial one), and
invasion of the orbital content.
Length of survival was calculated from the date of
surgery to the date of the latest clinical follow-up, or to the
date of death by disease or other causes. Representative tissue
blocks were collected from the archives of the section of Pathology
Department of Marche Polytechnic University (Ancona, Italy). The
paired samples from tumor tissues and adjacent normal tissues were
obtained from patients with ITAC who had undergone surgical
resection. The diagnosis and assessment of the histological grading
(G, from G1 to G3) of tumor differentiation were made on 4–6
µm-thick paraffin tissue sections stained with conventional
hematoxylin and eosin according to WHO Classification of Head and
Neck Tumors (56).
In all the cases, ITAC tumor diagnosis was
confirmed, as described by Barnes (10).
The identification of the anatomical site of the
tumor (T, from T1 to T4), nodal involvement (N0, N1, N2), and
clinical-pathologic stage were determined according to AJCC/UICC
TNM classification (7th edition) (57).
Written informed consent was obtained at the time of
surgery from each patient included in the study, and samples were
processed after approval of the Ethical Committee of the Marche
Regional Hospital, Ancona, Italy, Rec. no. 501 of November 29,
2011.
Patient cohort and workup
The clinical data were collected prospectively from
patients, then updated retrospectively after the follow-up review.
All patients underwent complete clinical examination and were
staged by multiplanar CT and by contrast-enhanced MRI (or
contrast-enhanced CT whenever an MRI could not be obtained). After
imaging evaluation, a biopsy with the patient under local
anesthesia was performed. Treatment planning was discussed by the
local multidisciplinary team.
Surgery
All patients were treated by surgery that was,
depending on the position and the extension of the tumor or a
craniofacial and cranioendoscopic resection or a transnasal
endoscopic approach with or without transnasal craniectomy. In all
cases we used surgical techniques already described in the
literature (13,58,59).
Patients who had absolute contraindication to
endoscopic approach as describe in the exclusion criteria performed
traditional open surgical approach (13).
Histological evaluation
Tissue blocks were collected, and the histological
slides were examined by a senior pathologist (C.R.) to confirm the
diagnosis of ITAC and to assess the grade of histopathological
differentiation of each tumor, according to WHO criteria [56], as follows: G1=well-differentiated,
G2=moderately differentiated, and G3=poorly-differentiated.
Surgical and histological reports were analyzed, and
all the lesions were retrospectively staged according to the TNM
classification (57).
Adjuvant therapy
Although advanced stage, poor differentiation, and
presence of positive surgical margins were the main considered
factors, the indication for adjuvant RT and /or CHT was discussed
for each patient by the multidisciplinary team, also considering
age, comorbidities, previous treatment and, especially for
low-stage ITAC, the availability of the patient for adequate
follow-up.
Follow-up
All patients were followed according to our
institutional protocols by endoscopic evaluation and MRI every 2
and 4 months, respectively, during the first year, both endoscopic
evaluation and MRI every 6 months until the fifth year, and
clinical evaluation and MRI yearly thereafter; this follow-up
protocol was the same applied in literature on large sample of
patients (59).
miRNA detection by reverse
transcription-quantitative PCR (RT-qPCR)
Expression levels of miR-let-7a were measured in 23
FFPE samples of ethmoidal ITAC and in the corresponding adjacent
healthy normal tissues considered as control (CTR).
The expression of miR-let-7a was also evaluated
according to: tumor grade of differentiation G (G1 or G2 vs. G3),
tumor extension (T1 or T2 vs. T 3 or T4), tumor stage (I or II vs.
III or IV), and presence or absence of recurrence.
Total RNA was extracted from FFPE samples using FFPE
RNA/DNA purification Kit (Norgen, Canada). MiRNAs were quantified
by RT-qPCR using TaqMan miRNA assays (Applied Biosystems, Foster
City, CA, USA) according to the Manufacturer's protocol. Data were
analyzed with the iCycler (Bio-Rad Laboratories, Segrate, Milan,
Italy) with an automatic setting for assigning the baseline.
RT-qPCR data were standardized to RNU48.
The 2−∆∆Cq method was used for
quantification (60). Relative miR
expression obtained from RT-qPCR was calculated using Ct (cycle
threshold), i.e., the fractional cycle number where a fluorescent
signal reaches the detection threshold. Levels of miRs expressed
with reference to RNU48 were turned into linear form using the
formula 2-DCt, DCt=Ct miR-X-Ct RNU), and reported as arbitrary
units (a.u.). Ct values from RT-qPCR assays >35 were considered
as not expressed. The intra- and inter-assay variability of miR
measurements were <5% and <10%, respectively.
Statistical analysis
Results are expressed as mean ± standard deviation
(SD) or as median, quartile and confidence interval (CI).
Comparisons between and among groups were performed using
two-tailed Student's paired t-test (two groups) and analysis of
variances (one-way ANOVA), followed by post-hoc Tukey analysis,
respectively. P<0.05 was considered statistically significant.
All statistical analyses were performed using the SPSS statistical
package (SPPS Inc. Chicago, IL).
Results
Patient data
Overall, twenty-three patients met the inclusion
criteria. Patient population consisted of twenty-one (91.3%) males
and two (8.7%) females, with a mean age of 66.3 years (range 54–77
yr). All the patients had a known history of occupational exposure
to hardwood dust and the ITAC was in the ethmoid region in all
cases, as confirmed by endoscopic and imaging (enhanced TC and/or
MRI) evaluation. No patients presented clinical and radiological
(cN) positive lymph nodes at diagnosis. The main
clinicopathological features of the patients included in the study
are summarized in Table I. No
patients underwent selective neck dissection (ND).
 | Table I.Overview of the clinical and
pathological characteristics of patients with primary ethmoidal
intestinal-type sinonasal adenocarcinoma. |
Table I.
Overview of the clinical and
pathological characteristics of patients with primary ethmoidal
intestinal-type sinonasal adenocarcinoma.
| Patient | Age, years | Sex | TNM stage | Grade | CH | CHT | RT |
|---|
| 1 | 70 | M | T2N0M0 | 3 | Yes | No | No |
| 2 | 55 | M | T3N0M0 | 2 | Yes | No | Yes |
| 3 | 67 | M | T3N0M0 | 2 | Yes | No | Yes |
| 4 | 60 | F | T1N0M0 | 1 | Yes | No | No |
| 5 | 62 | M | T3 N0M0 | 3 | Yes | No | Yes |
| 6 | 54 | M | T3N0M0 | 2 | Yes | No | Yes |
| 7 | 54 | M | T3N0M0 | 3 | Yes | No | Yes |
| 8 | 74 | M | T3N0M0 | 1 | Yes | No | Yes |
| 9 | 74 | M | T2N0M0 | 2 | Yes | No | Yes |
| 10 | 58 | M | T1N0M0 | 1 | Yes | No | No |
| 11 | 74 | M | T2N0M0 | 3 | Yes | No | Yes |
| 12 | 70 | M | T2N0M0 | 2 | Yes | No | Yes |
| 13 | 72 | M | T3N0M0 | 1 | Yes | No | Yes |
| 14 | 68 | M | T3N0M0 | 3 | Yes | No | Yes |
| 15 | 77 | M | T3N0M0 | 2 | Yes | No | Yes |
| 16 | 67 | M | T3N0M0 | 3 | Yes | No | Yes |
| 17 | 77 | M | T2N0M0 | 2 | Yes | No | No |
| 18 | 65 | M | T3N0M0 | 3 | Yes | No | Yes |
| 19 | 63 | M | T2N0M0 | 1 | Yes | No | Yes |
| 20 | 61 | F | T1N0M0 | 1 | Yes | No | No |
| 21 | 65 | M | T2N0M0 | 2 | Yes | No | No |
| 22 | 61 | M | T4aN0M0 | 3 | Yes | No | Yes |
| 23 | 76 | M | T4bN0M0 | 3 | Yes | No | Yes |
Histological findings, post-operative
staging (pTNM), and adjuvant therapy
The patients following pTNM classification were
distributed as follows: three (13%) in stage I, seven (30.4%) in
stage II, eleven (47.8%) in stage III, and two (8.7%) in stage
IV.
Looking at the severity of the tumor (histological
grade), the patients were distributed as follows: six patients
affected by Grade 1(well-differentiated) (Fig. 1A), eight by Grade 2 (moderately
differentiated) (Fig. 1B), and nine
suffering from Grade 3 tumor (poorly differentiated) (Fig. 1C).
The immunohistochemistry analysis on the tissue
showed that cases classified as ITAC had variable cellular
appearance, and consisted of a mixture of tall columnar cells,
atypical stratified cylindrical cells like the cells seen in
conventional colorectal adenocarcinoma, goblet cells, and large
round to polygonal non-descriptive epithelial cells.
None of the patients had a tumor ‘within’ (R1) or
‘close to’ (<1 mm, Rclose) the surgical margins. Nineteen (83%)
of the patients underwent post-operative conventional RT on the
primary site; nobody was treated by adjuvant CH.
miR-let-7a expression
miR-let-7a expression levels in ethmoidal ITAC
tissues were significantly lower than in adjacent normal tissues
(P<0.05) (Fig. 2). Moreover,
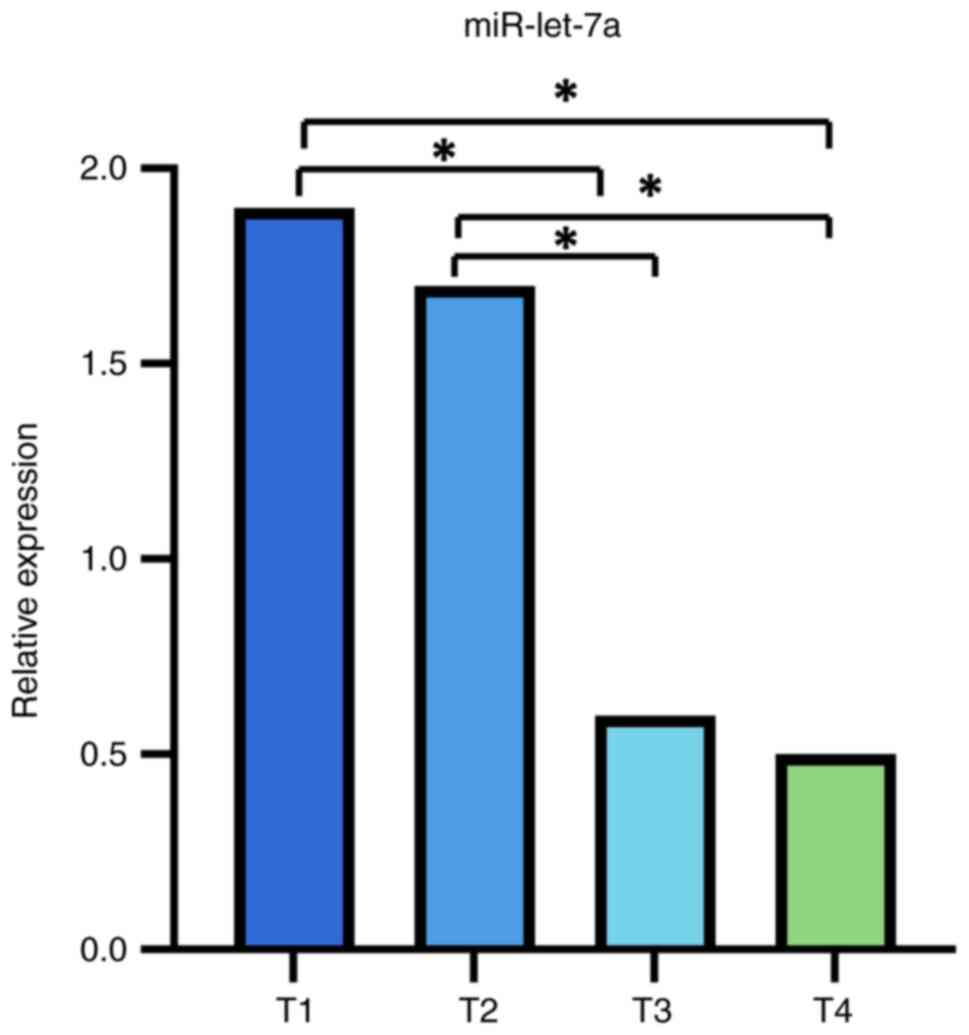
miR-let-7a varies with the pT stage of the tumor, being
lower-expressed in the more advanced stages (pT3-pT4) compared to
earlier stages (pT1-pT2) (mean expression level ± SD;
1.452707±1.4367189 vs. 4.094017±2.7465375; P<0.05) (Fig. 3). Moreover, there was a
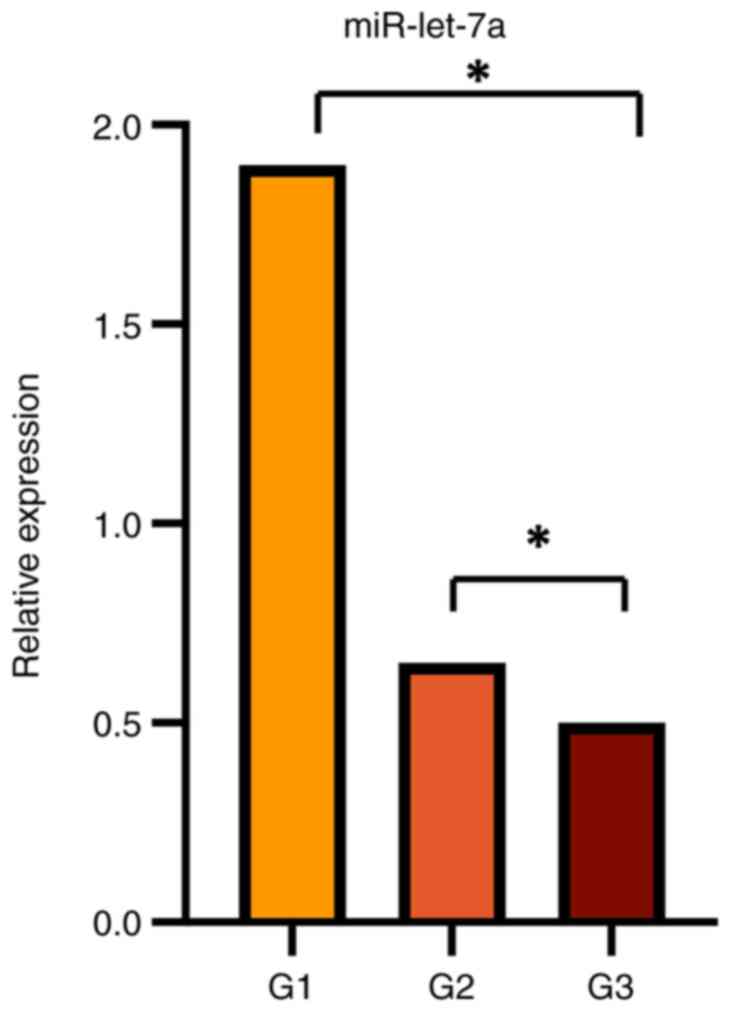
statistically significant relationship between miR-let-7a
down-expression and G3 histological grading (P<0.05) (Fig. 4). No other significant findings were
found between miR-let-7a expression and the other
clinicopathological parameters, including DFS.
Discussion
The results of our study showed, for the first time,
that the overall expression levels of miR-let-7a were significantly
down-regulated in tumor tissues as compared with the adjacent
non-pathologic tissues (approximately three times lower in tumor
samples than in normal tissue) (P<0.05). The down-regulation of
miR-let-7a was associated with the pT stage, reaching the minimum
levels of expression in pT3 and pT4 samples (P<0.05).
Furthermore, we found a down-regulation of
miR-let-7a expression (P<0.05) in poorly-differentiated tumors
(G3) compared with moderately- and well-differentiated tumors
(G1-G2), indicating a potential role of miR-let-7a in the cell
differentiation of ITACs. No other statistically significant
variances were found looking at the up/down regulation of
miR-let-7a related to other clinicopathological parameters,
including DFS.
Despite their histological similarity to colorectal
carcinomas, there is a scarce amount of data about the molecular
events that are involved in ITAC pathogenesis. A wide number of
tumorigenesis pathways have been identified in colorectal
adenocarcinomas, and these pathways are related to mutation and
inactivation of various oncogenes, tumor suppressor genes, and DNA
mismatch repair genes, including K-ras, APC, p53, MLH1, and MSH2
(16,17,61).
Assuming that the morphological similarities to
colorectal adenocarcinomas might reflect equivalent genetic
alterations, the presence of activating mutations of Ras oncogenes
and TP53 mutations in ITAC were investigated in many studies. TP53
mutations were found in 18–44% of mostly occupational ITACs,
whereas K-Ras mutations were observed in 10–15% of ITACs. The
results of these studies suggested that mutations of K-Ras and
other Ras genes are relatively uncommon in ITAC and, similarly,
TP53 mutations in ITACs have not been widely demonstrated (18,19,20,62,63).
Other authors found that K-Ras mutation and C-erb-2 expression
might be associated with a more aggressive behavior and poorer
outcome (21).
Licitra et al (64) described two genetic ITACs subgroups,
characterized by differences in TP53 mutational status or protein
functionality, that significantly influence the pathological
response to primary CH and, ultimately, the prognosis.
Perez-Ordonez and colleagues investigated the role
of DNA mismatch repair (MMR) gene defects or disruptions of
E-cadherin/β-catenin complex in ITAC by testing the
immunohistochemical expression of the MMR gene products, E-cadherin
and β-catenin, in a cohort of patients with sporadic ITACs, and
they found that the nuclear expression of MLH1, MSH2, MSH3 and MSH6
were preserved in these tumors, suggesting that mutations or
promoter methylation of MMR genes do not play a role in ITAC
pathogenesis (65).
An interesting finding was performed by Kennedy and
al., who found that sinonasal ITACs have a distinctive phenotype,
with all the cases expressing CK20, CDX-2, villin, and most ITACs
also expressing CK7. So the expression pattern of CK7, CK20, CDX-2,
and villin positive may be used to distinguish these tumors from
other non-ITACs of the sinonasal tract (66).
Furthermore, published data showed how miRNAs may
have an important role in the carcinogenesis process because more
than 50% of miRNAs were found in cancer-associated genomic regions
or fragile sites (67,68). Some miRNAs seem to promote the
cancer onset, while others inhibit the cell proliferation and
survival. Basically, both miRNA classes are showing important
connection with cancer development, being able to act like novel
oncogenes or tumor suppressors, respectively (69,70).
Measuring miRNAs expression as a biomarker may bring an important
advantage in cancer diagnosis, prognosis and therapy. Also,
concerning head and neck tumors there are many studies that have
reported significant associations among miRNA profiles and patient
survival (23,27-30,33-36).
At present time, due to the rarity of this type of
cancer, there is still a lack of literature evaluating the
expression of miRNAs in ITAC of the paranasal sinuses (25). Our research team previously
investigated the status of MiR-126 and we found that it was reduced
in ITACs compared with benign tumors, suggesting the potential role
of this miRNA acting as a circulating biomarker for the detection
of malignant transformation (25).
On the basis of these findings, to explore other
pathways involved in the molecular pathogenesis of ITACs, in the
current study we tried to investigate the expression of miR-let-7a,
which is an HNSCC related microRNA (53,55).
To analyze the prognostic role of this miRNA, its expression levels
were then retrospectively correlated with clinicopathological
characteristics of the tumor itself and the patient's outcome to
evaluate its independent prognostic relevance.
The let-7 gene family consists of 11 very closely
related genes and miR-let-7a is currently the best characterized
member. Recently the miR-let-7a expression was found to be reduced
in different tumor model tissues, compared to adjacent healthy
tissue and, therefore, miR-let-7a could probably act as a tumor
suppressor miRNA (55,71–75).
miR-let-7a was found poorly expressed in many types
of malignancies such as lung, colon, thyroid, and renal cancer
(70–74). Furthermore, data from recent
published works suggested that the down-regulation of let-7 miRNA
family gene, targeting RAS oncogene, may be related with poor
survival and relapse in surgical treated non-small cell lung cancer
(53,71–72).
The expression of miR-let-7a is also reduced in gastric cancer and
relates to tumor cells differentiation degree. A xenograft model of
mice showed that miR-let-7a acts as suppressor for the growth of
gastric cancer in vivo and in vitro (76).
Long et al (55) evaluated the expression of miR-let-7a
in a sample of 48 patients surgically treated for laryngeal primary
carcinoma, and compared miR-let-7a levels among tumor tissue and
adjacent healthy tissue. Results showed that in 37 out of 48
patients a statistically significant reduction in miR-let-7a
expression level was present in all tumors with different clinical
stages, compared with normal larynx tissues.
miR-let-7a could be a tumor suppressor in laryngeal
cancer by inhibiting cell growth and inducing cell apoptosis. This
action would be possible by down-regulating the protein expression
of oncogenes such as RAS and c-MYC (target genes). Ras proteins are
membrane associated GTPase, signaling proteins that regulate
cellular growth and differentiation, while MYC is an evolutionarily
conserved nuclear protein also involved in the control of cell
proliferation and differentiation (55). An inverse correlation was observed
among RAS/c-MYC protein and miR-let-7a expression in laryngeal
tumor, suggesting that increased RAS and c-MYC protein expressions
may be caused by the loss of miR-let-7a expression (55).
A down-regulation of tumor miR-let-7a suppressor
family exists also in tumors of the Ewing's sarcoma family (ESFT).
The mechanism by which miR-let-7a expression modulates the growth
of ESFT has been shown to be mediated by its target gene HMGA2. An
overexpression of miR-let-7a and the consequent repression of HMGA2
inhibit the tumorigenicity of ESFT cells (77).
The present study shows some limitations. In this
work we did not consider the prognostic value of the expression of
miR-let-7a, which will be the object of a second retrospective
analysis. Second, although we tried to evaluate a homogeneous
cohort of patients in terms of pTNM stage and treatment, our data
were achieved from a retrospective cohort study and the patients
cohort remains heterogeneous in some crucial clinical aspects
(different pTNM classification, non-uniform surgical approach,
mode, and effectiveness of complementary protocol treatment,
follow-up). Moreover, the study did not conduct a sensitivity
analysis due to the small sample size (n=43). Finally,
understanding the connections between miRNAs deregulated in cancer
and cellular signaling pathways involved in cancer was hindered by
our limited knowledge of miRNA target recognition.
Despite these limitations, we presented a pilot
study through highly standardized retrospective analysis of a
single head and neck cancer institution.
In conclusion, this study provides the first
evidence that a down-regulation of miR-let-7a in ethmoidal ITAC is
associated with advanced stage (pT3 and pT4) disease, and with
poorly differentiated tumors (G3). Our data suggest that the
specific mutation of this gene, in combination with additional
genetic events, could play a role in ITAC pathogenesis.
The analysis performed on a small sample of patients
will necessarily be extended to a larger cohorts, and our
single-institution results would require validation through a
broader prospective and multicenter analysis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FMG and MR conceived the study. FMG wrote the first
draft. ADS and PDL analyzed the data, defined the conclusions,
revised the manuscript and helped write the manuscript. ADS and MS
were involved in image curation by selecting the images and drawing
the graphs. AC, AP, GI, AS and MS collected clinical data, and were
involved in the analyses of data and definition of the results. CR,
MT, MS and FO were involved in collection and analysis of
histologic findings. FMG, FO and MR analyzed the data and
participated in the definition of the conclusions. FMG and MR
confirm the authenticity of all the raw data. All authors were
involved in critical review of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethical Committee of
the Marche Regional Hospital, Ancona, Italy, Rec. no. 501 of
November 29, 2011. All patients provided written informed consent
to participate in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Robin PD, Powell DJ and Stansbie JM:
Carcinoma of the nasal cavity and paranasal sinuses: Incidence and
presentation of different histological types. Clin Otolaryngol
Allied Sci. 4:431–456. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Batsakis JG, Rice DH and Solomon AR: The
pathology of head and neck tumors: Squamous and mucous-gland
carcinomas of the nasal cavity, paranasal sinuses, and larynx, part
6. Head Neck Surg. 2:497–508. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Weber AL and Stanton AC: Malignant tumors
of the paranasal sinuses: Radiologic, clinical, and histopathologic
evaluation of 200 cases. Head Neck Surg. 6:761–776. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lopez JI, Nevado M, Eizaguirre B and Perez
A: Intestinal-type adenocarcinoma of the nasal cavity and paranasal
sinuses. A clinicopathologic study of 6 cases. Tumori. 76:250–254.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kleinsasser O and Schroeder HG:
Adenocarcinomas of the inner nose after exposure to wood dust.
Morphological findings and relationships between histopathology and
clinical behavior in 79 cases. Arch Otorhinolaryngol. 245:1–15.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Leclerc A, Cortes MM, Gerin M, Luce D and
Brugere J: Sinonasal cancer and wood dust exposure: Results from a
case-control study. Am J Epidemiol. 140:340–349. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stellman SD, Demers PA, Colin D and
Boffetta P: Cancer mortality and wood dust exposure among
participants in the American cancer society cancer prevention
study-II (CPS-II). Am J Ind Med. 34:229–237. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hadfield EH: A study of adenocarcinoma of
the paranasal sinuses in woodworkers in the furniture industry. Ann
R Coll Surg Engl. 46:301–319. 1970.PubMed/NCBI
|
|
9
|
Klintenberg C, Olofsson J, Hellquist H and
Sökjer H: Adenocarcinoma of the ethmoid sinuses. A review of 28
cases with special reference to wood dust exposure. Cancer.
54:482–488. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barnes L: Intestinal-type adenocarcinoma
of the nasal cavity and paranasal sinuses. Am J Surg Pathol.
10:192–202. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Franchi A, Gallo O and Santucci M:
Clinical relevance of the histological classification of sinonasal
intestinal-type adenocarcinomas. Hum Pathol. 30:1140–1145. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fiaux-Camous D, Chevret S, Oker N,
Turri-Zanoni M, Lombardi D, Choussy O, Frederic D, Jorissen M, de
Gabory L, Malard O, et al: Prognostic value of the seventh
AJCC/UICC TNM classification of intestinal-type ethmoid
adenocarcinoma: Systematic review and risk prediction model. Head
Neck. 39:668–678. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Maccariello G, Deganello A, Choussy O,
Gallo O, Vitali D, De Raucourt D and Georgalas C: Endoscopic nasal
versus open approach for the management of sinonasal
adenocarcinoma: A pooled-analysis of 1826 patients. Head Neck. 38
(Suppl 1):E2267–E2274. 2016.PubMed/NCBI
|
|
14
|
Mills SE, Fechner RE and Cantrell RW:
Aggressive sinonasal lesion resembling normal intestinal mucosa. Am
J Surg Pathol. 6:803–809. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Batsakis JG, Mackay B and Ordonez NG:
Enteric-type adenocarcinoma of the nasal cavity. An electron
microscopic and immunocytochemical study. Cancer. 54:855–860. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McKinney CD, Mills SE and Franquemont DW:
Sinonasal intestinal-type adenocarcinoma: Immunohistochemical
profile and comparison with colonic adenocarcinoma. Mod Pathol.
8:421–426. 1995.PubMed/NCBI
|
|
17
|
Franchi A, Massi D, Baroni G and Santucci
M: CDX-2 homeobox gene expression. Am J Surg Pathol. 27:1390–1391.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saber AT, Nielsen LR, Dictor M, Hagmar L,
Mikoczy Z and Wallin H: K-ras mutations in sinonasal
adenocarcinomas in patients occupationally exposed to wood or
leather dust. Cancer Lett. 126:59–65. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perez P, Dominguez O, Gonzalez S, Trivino
A and Suarez C: Ras gene mutations in ethmoid sinus adenocarcinoma:
Prognostic implications. Cancer. 86:255–264. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu TT, Barnes L, Bakker A, Swalsky PA and
Finkelstein SD: K-ras-2 and p53 genotyping of intestinal-type
adenocarcinoma of the nasal cavity and paranasal sinuses. Mod
Pathol. 9:199–204. 1996.PubMed/NCBI
|
|
21
|
Perrone F, Oggionni M, Birindelli S,
Suardi S, Tabano S, Romano R, Moiraghi ML, Bimbi G, Quattrone P,
Cantu G, et al: TP53, p14ARF, p16INK4a and H-ras gene molecular
analysis in intestinal-type adenocarcinoma of the nasal cavity and
paranasal sinuses. Int J Cancer. 105:196–203. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Re M, Magliulo G, Tarchini P, Mallardi V,
Rubini C, Santarelli A and Lo Muzio L: p53 and BCL-2
over-expression inversely correlates with histological
differentiation in occupational ethmoidal intestinal-type sinonasal
adenocarcinoma. Int J Immunopathol Pharmacol. 24:603–609. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gallo O, Franchi A, Fini-Storchi I,
Cilento G, Boddi V, Boccuzzi S and Urso C: Prognostic significance
of c-erbB-2 oncoprotein expression in intestinal-type
adenocarcinoma of the sinonasal tract. Head Neck. 20:224–231. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Re M, Santarelli A, Mascitti M, Bambini F,
Lo Muzio L, Zizzi A and Rubini C: Trail overexpression inversely
correlates with histological differentation in intestinal-type
sinonasal adenocarcinoma. Int J Surg Oncol.
2013:2038732013.PubMed/NCBI
|
|
25
|
Tomassetti M, Re M, Monaco F, Gaetani S,
Rubini C, Bertini A, Pasquini E, Bersaglieri C, Bracci M,
Staffolani S, et al: MiR-126 in intestinal-type sinonasal
adenocarcinomas: Exosomal transfer of MiR-126 promotes anti-tumour
responses. BMC Cancer. 18:8962018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Re M, Magliulo G, Ferrante L, Zizzi A,
Santarelli A, Stramazzotti D, Lo Muzio L, Goteri G and Rubini C:
p63 expression in laryngeal squamous cell carcinoma is related to
tumor extension, histologic grade, lymph node involvement and
clinical stage. J Biol Regul Homeost Agents. 27:121–129.
2013.PubMed/NCBI
|
|
27
|
Re M, Zizzi A, Ferrante L, Stramazzotti D,
Goteri G, Gioacchini FM, Olivieri F, Magliulo G and Rubini C: p63
and Ki-67 immunostainings in laryngeal squamous cell carcinoma are
related to survival. Eur Arch Otorhinolaryngol. 271:1641–1651.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Re M, Ceka A, Rubini C, Ferrante L, Zizzi
A, Gioacchini FM, Tulli M, Spazzafumo L, Sellari-Franceschini S,
Procopio AD and Olivieri F: MiR-34c-5p is related to recurrence in
squamous cell carcinoma. Laryngoscope. 125:E306–E312. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lucarini G, Zizzi A, Re M, Sayeed MA, Di
Primio R and Rubini C: Prognostic implication of CEACAM1 expression
in squamous cell carcinoma of the larynx: Pilot study. Head Neck.
41:1615–1621. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Re M, Gioacchini FM, Scarpa A, Cassandro
C, Tulli M and Cassandro E: The prognostic significance of
E-cadherin expression in laryngeal squamous cell carcinoma: A
systematic review. Acta Otorhinolaryngologica Ital. 38:504–510.
2018. View Article : Google Scholar
|
|
31
|
Re M, Magliulo G, Gioacchini FM,
Bajraktari A, Bertini A, Ceka A, Rubini C, Ferrante L, Procopio AD
and Olivieri F: Expression levels and clinical significance of
miR-21-5p, miR-let-7a, and miR-34c-5p in laryngeal squamous cell
carcinoma. Biomed Res Int. 2017:39212582017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Re M, Gioacchini FM, Bajraktari A,
Tomassetti M, Kaleci S, Rubini C, Bertini A, Magliulo G and
Pasquini E: Malignant transformation of sinonasal inverted
papilloma and related genetic alterations: A systematic review. Eur
Arch Otorhinolaryngol. 274:2991–3000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gioacchini FM, Alicandri-Ciufelli M,
Rubini C, Magliluo G and Re M: Prognostic value of Bcl-2 expression
in squamous cell carcinoma of the larynx: A systematic review. Int
J Biol Markers. 30:e155–e160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gioacchini FM, Alicandri-Ciufelli M,
Kaleci S, Magliulo G, Presutti L and Re M: The prognostic value of
Cyclin D1 expression in head and neck sqamous cell carcinoma. Eur
Arch Otorhinolaryngol. 273:801–809. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gioacchini FM, Alicandri-Ciufelli M,
Magliulo G, Rubini C, Presutti L and Re M: The clinical relevance
of Ki-67 expression in laryngeal squamous cell carcinoma. Eur Arch
Otorhinolaryngol. 272:1569–1576. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Re M, Magliulo G, Salvolini E, Orciani M,
Gioacchini FM, Goteri G and Rubini C: Prognostic significance of
p53 and KAI-1 expression in patients with Laryngeal squamous cell
carcinoma. Anal Quant Cytol Histol. 32:247–253. 2010.PubMed/NCBI
|
|
37
|
Fan CY: Epigenetic alterations in head and
neck cancer: Prevalence, clinical significance, and implications.
Curr Oncol Rep. 6:152–161. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marsit CJ, Christensen BC, Houseman EA,
Karagas MR, Wrensch MR, Yeh RF, Nelson HH, Wiemels JL, Zheng S,
Posner MR, et al: Epigenetic profiling reveals etiologically
distinct patterns of DNA methylation in head and neck squamous cell
carcinoma. Carcinogenesis. 30:416–422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Worsham MJ, Chen KM, Meduri V, Nygren AO,
Errami A, Schouten JP and Benninger MS: Epigenetic events of
disease progression in head and neck squamous cell carcinoma. Arch
Otolaryngol Head Neck Surg. 132:668–677. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zamore PD and Haley B: Ribo-gnome: The big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schickel R, Boyerinas B, Park SM and Peter
ME: MicroRNAs: Key players in the immune system, differentiation,
tumorigenesis and cell death. Oncogene. 27:5959–5974. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cullen BR: Transcription and processing of
human microRNA precursors. Mol Cell. 16:861–865. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Avissar M, McClean MD, Kelsey KT and
Marsit CJ: MicroRNA expression in head and neck cancer associates
with alcohol consumption and survival. Carcinogenesis.
30:2059–2063. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen CZ: MicroRNAs as oncogenes and tumor
suppressors. N Engl J Med. 353:1768–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Iorio MV, Ferracin M, Liu CG, Veronese A,
Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M,
et al: MicroRNA gene expression deregulation in human breast
cancer. Cancer Res. 65:7065–7070. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Calin GA, Ferracin M, Cimmino A, Di Leva
G, Shimizu M, Wojcik SE, Iorio MV, Visone R, Sever NI, Fabbri M, et
al: A MicroRNA signature associated with prognosis and progression
in chronic lymphocytic leukemia. N Engl J Med. 353:1793–1801. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yan LX, Huang XF, Shao Q, Huang MY, Deng
L, Wu QL, Zeng YX and Shao JY: MicroRNA miR-21 overexpression in
human breast cancer is associated with advanced clinical stage,
lymph node metastasis and patient poor prognosis. RNA.
14:2348–2360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schetter AJ, Leung SY, Sohn JJ, Zanetti
KA, Bowman ED, Yanaihara N, Yuen ST, Chan TL, Kwong DL, Au GK, et
al: MicroRNA expression profiles associated with prognosis and
therapeutic outcome in colon adenocarcinoma. JAMA. 299:425–436.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen Y and Stallings RL: Differential
patterns of microRNA expression in neuroblastoma are correlated
with prognosis, differentiation, and apoptosis. Cancer Res.
67:976–983. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Avissar M, Christensen BC, Kelsey KT and
Marsit CJ: MicroRNA expression ratio is predictive of head and neck
squamous cell carcinoma. Clin Cancer Res. 15:2850–2855. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chang SS, Jiang WW, Smith I, Poeta LM,
Begum S, Glazer C, Shan S, Westra W, Sidransky D and Califano JA:
MicroRNA alterations in head and neck squamous cell carcinoma. Int
J Cancer. 123:2791–2797. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Childs G, Fazzari M, Kung G, Kawachi N,
Brandwein-Gensler M, McLemore M, Chen Q, Burk RD, Smith RV,
Prystowsky MB, et al: Low-level expression of microRNAs let-7d and
miR-205 are prognostic markers of head and neck squamous cell
carcinoma. Am J Pathol. 174:736–745. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ramdas L, Giri U, Ashorn CL, Coombes KR,
El-Naggar A, Ang KK and Story MD: miRNA expression profiles in head
and neck squamous cell carcinoma and adjacent normal tissue. Head
Neck. 31:642–654. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Long XB, Sun GB, Hu S, Liang GT, Wang N,
Zhang XH, Cao PP, Zhen HT, Cui YH and Liu Z: Let-7a microRNA
functions as a potential tumor suppressor in human laryngeal
cancer. Oncol Rep. 22:1189–1195. 2009.PubMed/NCBI
|
|
56
|
Cardesa A, Gale N, Nadal A and Zidar N:
Squamus cell carcinoma. Head and Neck tumors: Pathology &
Genetics. World Health Organization Classification on Tumors (WHO);
2005, pp. 118–119
|
|
57
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rampelli V, Ferrari M and Nicolai P:
Intestnal-type adenocarcinoma of the sinonasal tract: An update.
Curr Opin Otolaryngol Head Neck Surg. 26:115–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Nicolai P, Shreiber A, Villaret AB,
Lombardi D, Morassi L, Raffetti E, Donato F, Battaglia P,
Turri-Zanoni M, Bignami M and Castelnuovo P: Intestinal type
adenocarcinoma of the ethmoid: Outcomes of a treatment regimen
based on endoscopic surgery with or without radiotherapy. Head
Neck. 38:E996–E1003. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Chung DC: The genetic basis of colorectal
cancer: Insights into critical pathways of tumorigenesis.
Gastroenterology. 119:854–865. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Calvert PM and Frucht H: The genetics of
colorectal cancer. Ann Intern Med. 137:603–612. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Licitra L, Suardi S, Bossi P, Locati LD,
Mariani L, Quattrone P, Lo Vullo S, Oggionni M, Olmi P, Cantu G, et
al: Prediction of TP53 status for primary cisplatin, fluorouracil,
and leucovorin chemotherapy in ethmoid sinus intestinal-type
adenocarcinoma. J Clin Oncol. 22:4901–4906. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Perez-Ordonez B, Huynh NN, Berean KW and
Jordan RC: Expression of mismatch repair proteins, beta catenin,
and E cadherin in intestinal-type sinonasal adenocarcinoma. J Clin
Pathol. 57:1080–1083. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kennedy MT, Jordan RC, Berean KW and
Perez-Ordonez B: Expression pattern of CK7, CK20, CDX-2, and villin
in intestinal-type sinonasal adenocarcinoma. J Clin Pathol.
57:932–937. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Tu HF, Lin SC and Chang KW: MicroRNA
aberrances in head and neck cancer: Pathogenetic and clinical
significance. Curr Opin Otolaryngol Head Neck Surg. 21:104–111.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Lee YS and Dutta A: MicroRNAs: Small but
potent oncogenes or tumor suppressors. Curr Opin Investig Drugs.
7:560–564. 2006.PubMed/NCBI
|
|
70
|
Price C and Chen J: MicroRNAs in cancer
biology and therapy: Current status and perspectives. Genes Dis.
1:53–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Takamizawa J, Konishi H, Yanagisawa K,
Tomida S, Osada H, Endoh H, Harano T, Yatabe Y, Nagino M, Nimura Y,
et al: Reduced expression of the let-7 microTNAs in human lung
cancer in association with shortened postoperative survival. Cancer
Res. 64:3753–3756. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
He X, Duan C, Chen J, Ou-Yang X, Zhang Z,
Li C and Peng H: Let-7a elevates p21(WAF1) levels by targeting of
NIRF and suppresses the growth of A549 lung cancer cells. FEBS
Lett. 583:3501–3507. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Akao Y, Nakagawa Y and Naoe T: Let-7
microRNA functions as a potential growth suppressor in human colon
cancer cell. Biol Pharm Bull. 29:903–906. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Colamaio M, Calì G, Sarnataro D, Borbone
E, Pallante P, Decaussin-Petrucci M, Nitsch L, Croce CM, Battista S
and Fusco A: Let-7a down-regulation plays a role in thyroid
neoplasias of follicular histotype affecting cell adhesion and
migration through its ability to target the FXYD5 (Dysadherin)
gene. J Clin Endocrinol Matab. 97:E2168–E2178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Liu Y, Yin B, Zhang C, Zhou L and Fan J:
Hsa-let-7a functionsas a tumor suppressor in renal cell carcinoma
cell lines by targetingc-myc. Biochem Biophys Res Commun.
417:371–375. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Yang Q, Jie Z, Cao H, Greenlee AR, Yang C,
Zou F and Jiang Y: Low-level expression of let-7a in gastric cancer
and its involvement in tumorigenesis by targeting RAB40C.
Carcinogenesis. 32:713–722. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
De Vito C, Riggi N, Suvà ML, Janiszewska
M, Horlbeck J, Baumer K, Provero P and Stamenkovic I: Let-7a is a
direct EWS-FLI-1 target implicated in Ewing's sarcoma development.
PLoS One. 6:e235922011. View Article : Google Scholar : PubMed/NCBI
|