Introduction
Obesity has become a worldwide public health
problem. According to a report from the World Health Organization
(WHO), over 650 million people worldwide were diagnosed with
obesity in 2016 (1). With the
improvement of living standards, increased dietary intake and
decreased physical activity, the number of people with obesity in
China has significantly increased in recent years. The prevalence
of obesity in adults in China more than doubled between 2004 (3.1%)
and 2018 (8.1%). 85 million adults in China were obese in 2018,
which was three times as many as in 2004 (2). Obesity not only causes a number of
health, social and psychological problems but also significantly
increases the risk of various malignant tumors (3). Gastric cancer is a malignant tumor
with high morbidity and mortality worldwide, and its morbidity
(5.6%) and mortality (7.7%) are among the highest of all malignant
tumors (4). Standardized lymph node
dissection is crucial to improving the long-term prognosis of
patients with advanced gastric cancer (5). D2 lymph node dissection, as the
standard treatment for advanced gastric cancer, has been
unanimously recommended in Japanese Classification of Gastric
Carcinoma (6) and the US National
Comprehensive Cancer Network Gastric Cancer Guidelines (7), and a consensus has been reached
regarding its use. However, as yet there is no conformity of
opinion on the precise range of lymph node dissection for different
stages of gastric cancer. In theory, the prognosis of patients
should improve as more lymph nodes are dissected. Therefore,
reasonably expanding the scope of lymph node dissection by
performing D2+ lymph node dissection may improve the prognosis and
survival rate of patients. In addition, some studies have found
that D2+ lymph node dissection, which is the removal of lymph nodes
with an elevated risk of metastasis beyond the specified range of
D2, can improve the prognosis and survival rate of patients
(8,9). However, due to the difficulty in
exposing the surgical field of view in patients with obesity, it is
challenging to perform standardized D2 lymph node dissection, and
even more difficult to perform D2+ lymph node dissection in
patients with obesity. The safety and efficacy of D2+ compared with
D2 surgery requires further confirmation. The present study
retrospectively analyzed the clinical data of patients with obesity
with gastric cancer in a single hospital, with the aim of exploring
whether laparoscopic-assisted radical gastrectomy combined with D2+
lymph node dissection is feasible in patients with obesity and
gastric cancer and to provide a basis for clinical surgical
decision-making.
Materials and methods
Data collection
The clinical data of patients who underwent
laparoscopic radical gastrectomy in Henan Provincial People's
Hospital (Zhengzhou, China) from January 2016 to January 2018 were
collected and analyzed. The inclusion criteria were as follows: i)
Age between 18–75 years, either sex, body mass index (BMI) ≥25
kg/m2; ii) American Society of Anesthesiologists (ASA)
grade I–III; iii) postoperative conventional pathological diagnosis
of adenocarcinoma, where the tumor is located in the proximal,
middle or distal area; iv) pathological tumor-node-metastasis
(pTNM) stage after surgery of II–III, according to the TNM staging
criteria for gastric cancer in the 8th edition of the American
Joint Committee on Cancer (AJCC) in 2017 (10); and v) underwent laparoscopic radical
gastrectomy or conversion to laparotomy. The exclusion criteria
were as follows: i) Severe mental illness; ii) previous upper
abdominal surgery; iii) combined thoracoabdominal surgery; iv)
other malignant tumors in the past 5 years; v) severe
cardiopulmonary disease in preoperative evaluation, including
myocardial infarction, cerebral infarction, severe coronary heart
disease and chronic pulmonary disease within the past year; vi)
chemotherapy, radiotherapy or other targeted or immunotherapy
before surgery; and vii) distant metastasis of the tumor.
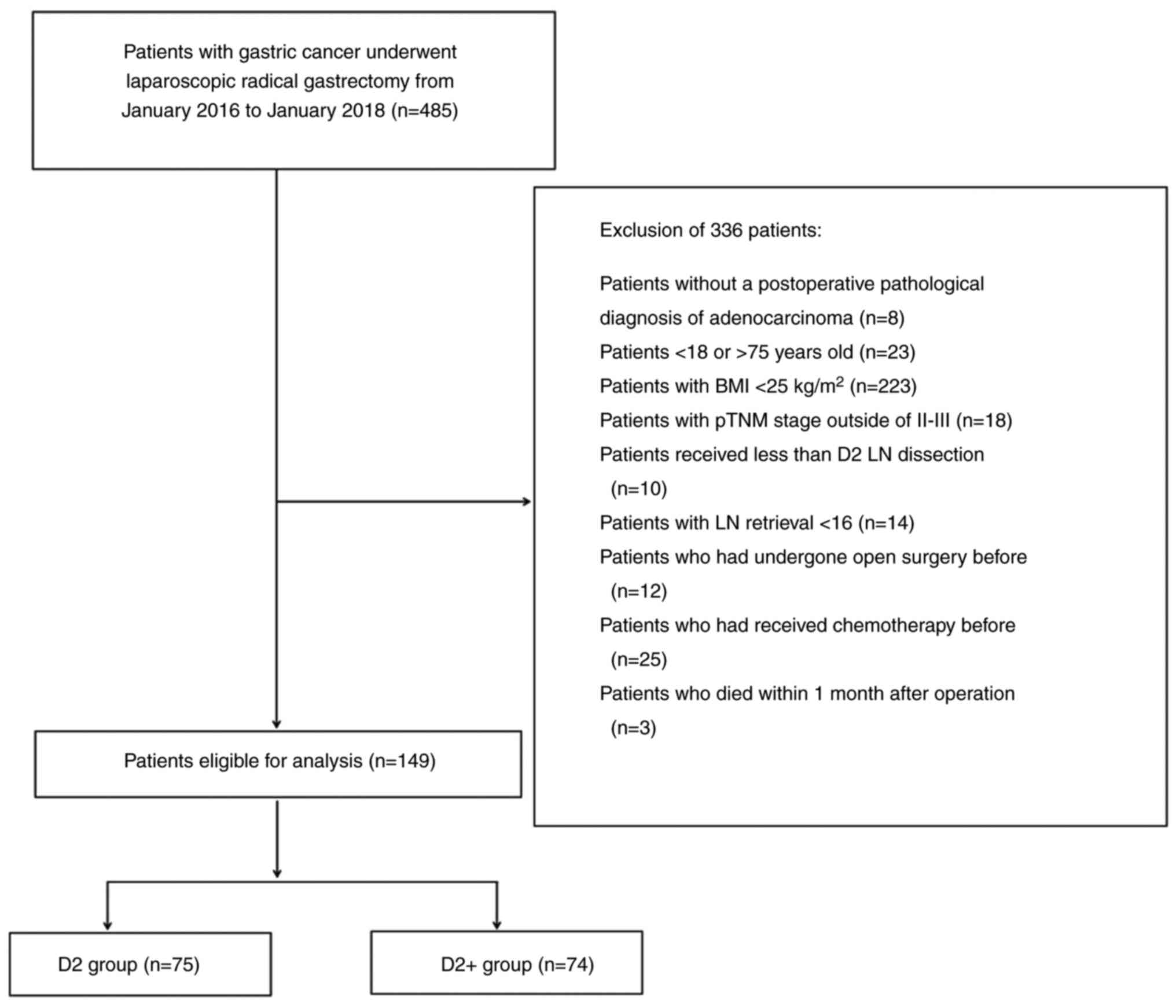
The 485 patients with gastric cancer who underwent
laparoscopic radical gastrectomy from January 2016 to January 2018
were screened for the study. The flow chart and reasons for
exclusion from the study are shown in Fig. 1. Following the exclusion of 336
patients, 149 patients were ultimately enrolled in the study.
Research methods
The standard scope of D2 lymph node dissection is
stipulated in the Chinese Society of Clinical Oncology (CSCO)
Guidelines for the Diagnosis and Treatment of Gastric Cancer
(11) as follows: In cases of
distal gastrectomy, node stations 1, 3, 4sb, 4d, 5, 6, 7, 8a, 9,
11p and 12a; for proximal gastrectomy, node stations 1, 2, 3, 4sa,
4sb, 7, 8a, 9, 10 and 11; during total gastrectomy, node stations
1–7, 8a, 9, 10, 11 and 12a. Patients who underwent laparoscopic
radical gastrectomy and standard D2 lymph node dissection were
included in the control group. Patients who underwent laparoscopic
radical gastrectomy and standard D2 lymph node dissection, as well
as the clearance of least one of lymph node stations 13, 14v, 8p,
12b, 12p and 16 had undergone D2+ lymph node dissection and were
included in the observation group. Postoperative complications were
classified according to the Clavien-Dindo complication
classification (12), with grades
I–II as minor complications and grades III–IV as severe
complications. Classification of macroscopic types of gastric
cancer according to Borrmann classification method, including four
types: I, II, III, and IV (13).
Follow-up
All patients were followed up by telephone. Patients
were followed up every 3 months for 2 years after surgery, every 6
months for the next 3–5 years, and once a year thereafter. The
deadline for final follow-up was February 2023.
Statistical analysis
SPSS 26.0 statistical software (IBM Corp.) was used
for data processing. The enumeration data are expressed as the mean
± standard deviation, and an independent sample t-test was used for
comparison. Categorical data are expressed as n (%), and the
two-sided χ2 test or Fisher's exact probability method
was used for comparison. Overall survival rates were plotted using
the Kaplan-Meier method and compared by the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
A total of 149 patients with gastric cancer were
included in the study, including 74 patients in the D2+ group and
75 patients in the D2 group. There was no difference in sex
composition between the two groups, with 52 males (70.3%) and 22
females (29.7%) in the D2+ group and 55 males (73.3%) and 20
females (26.7%) in the D2 group. There was also no difference in
age between the D2+ group (57.24±10.38 years) and the D2 group
(55.75±10.81 years). In addition, no difference in BMI was detected
between the D2+ group (27.35±1.03 kg/m2) and the D2
group (27.37±1.04 kg/m2). Furthermore, no difference in
ASA grade, preoperative complications, tumor location, tumor size,
Borrmann type, cancer-associated thrombus or pTNM staging was
identified between the two groups. The data are shown in Table I.
 | Table I.Comparison of clinical data and
pathological features of patients in the two groups. |
Table I.
Comparison of clinical data and
pathological features of patients in the two groups.
| Characteristic | D2+ group (n=74) | D2 group (n=75) | P-value |
|---|
| Sex |
|
| 0.678 |
| Male | 52 (70.3) | 55 (73.3) |
|
|
Female | 22 (29.7) | 20 (26.7) |
|
| Age, years | 57.24±10.38 | 55.75±10.81 | 0.390 |
| BMI,
kg/m2 | 27.35±1.03 | 27.37±1.04 | 0.903 |
| ASA grade |
|
| 0.828 |
| I | 12 (16.2) | 14 (18.7) |
|
| II | 48 (64.9) | 45 (60.0) |
|
|
III | 14 (18.9) | 16 (21.3) |
|
| Preoperative
complications |
|
|
|
|
Diabetes | 10 (13.5) | 8 (10.7) | 0.594 |
|
Anemia | 37 (50.0) | 46 (61.3) | 0.164 |
|
Hypoproteinemia | 23 (31.1) | 17 (22.7) | 0.247 |
| Tumor location |
|
| 0.323 |
|
Upper | 37 (50.0) | 35 (46.7) |
|
|
Central | 19 (25.7) | 14 (18.7) |
|
|
Lower | 18 (24.3) | 26 (34.7) |
|
| Tumor size, cm | 5.87±1.45 | 5.56±1.22 | 0.153 |
| Borrmann type |
|
| 0.179 |
| I | 0 (0) | 3 (4.0) |
|
| II | 9 (12.2) | 7 (9.3) |
|
|
III | 45 (60.8) | 52 (69.3) |
|
| IV | 20 (27.0) | 13 (17.3) |
|
| Cancer-associated
thrombus |
|
| 0.155 |
|
Yes | 47 (63.5) | 39 (52.0) |
|
| No | 27 (36.5) | 36 (48.0) |
|
| pTNM staging |
|
| 0.289 |
| II | 10 (13.5) | 15 (20) |
|
|
III | 64 (86.5) | 60 (80) |
|
Comparison of surgical conditions
between the two groups
The duration of surgery in the D2+ group
(282.55±23.02 min) was longer than that of the D2 group
(271.45±20.05 min), and the difference was statistically
significant (P<0.05). The number of lymph nodes dissected in the
D2+ group (28.57±7.19) was more than that in the D2 group
(25.29±6.41), with a statistically significant difference between
groups (P<0.05). The intraoperative blood loss in the D2+ group
(117.55±28.02 ml) was more than that in the D2 group (109.37±22.78
ml), albeit not significantly. The rate of conversion to open
laparotomy was 4/74 (5.4%) and 2/75 (2.7%) in the D2+ and D2
groups, respectively. The time to first flatus in the D2+ group
(4.49±1.32 days) was slightly earlier than that in the D2 group
(4.84±1.10 days), and the postoperative hospital stay in the D2+
group (10.46±2.39 days) was similar that in the D2 group
(10.69±2.27 days). The total cost of hospitalization in the D2+
group (8.64±1.25×104 yuan) was slightly more than that
in the D2 group (8.42±1.16×104 yuan), but the difference
was not significant. The data are shown in Table II.
 | Table II.Comparison of surgical treatment
between the two groups of patients. |
Table II.
Comparison of surgical treatment
between the two groups of patients.
| Surgical
factor | D2+ group
(n=74) | D2 group
(n=75) | P-value |
|---|
| Operation time,
mins | 282.55±23.02 | 271.45±20.05 | 0.002 |
| Intraoperative
blood loss, ml | 117.55±28.02 | 109.37±22.78 | 0.053 |
| Lymph nodes
dissected, n | 28.57±7.19 | 25.29±6.41 | 0.004 |
| Conversion to open
laparotomy | 4 (5.4) | 2 (2.7) | 0.665 |
| Surgical
method |
|
| 0.266 |
|
Proximal gastrectomy | 30 (40.5) | 32 (42.7) |
|
| Distal
gastrectomy | 16 (21.6) | 23 (30.7) |
|
| Total
gastrectomy | 28 (37.8) | 20 (26.7) |
|
| Time to first
flatus, days | 4.49±1.32 | 4.84±1.10 | 0.078 |
| Postoperative
hospital stay, days | 10.46±2.39 | 10.69±2.27 | 0.542 |
| Total
hospitalization expenses, ×104 yuan | 8.64±1.25 | 8.42±1.16 | 0.263 |
In a subgroup analysis according to the type of
gastrectomy, for patients with proximal gastrectomy, the duration
of surgery was the only factor that was found to be significantly
different, with the D2+ group having a longer operation time than
the D2 group. For patients undergoing distal gastrectomy, no
statistically significant difference was detected in any of the
surgery-associated data. For patients undergoing total gastrectomy,
the duration of surgery of the D2+ group was longer than that of
the D2 group and the number of lymph nodes dissected in the D2+
group was greater than that in the D2 group; these differences were
statistically significant (P<0.05). The data are presented in
Table III.
 | Table III.Subgroup analysis of the two groups
of patients. |
Table III.
Subgroup analysis of the two groups
of patients.
|
| Proximal
gastrectomy | Distal
gastrectomy | Total
gastrectomy |
|---|
|
|
|
|
|
|---|
| Surgical
factor | D2+ (n=30) | D2 (n=32) | P-value | D2+ (n=16) | D2 (n=23) | P-value | D2+ (n=28) | D2 (n=20) | P-value |
|---|
| Operation time,
mins | 282.87±14.80 | 272.28±16.74 | 0.011 | 275.81±25.59 | 273.22±21.65 | 0.734 | 286.07±28.17 | 268.10±23.45 | 0.024 |
| Intraoperative
blood loss, ml | 120.67±25.72 | 117.56±19.97 | 0.123 | 110.50±31.24 | 107.91±28.33 | 0.789 | 118.25±28.78 | 107.55±20.68 | 0.141 |
| Lymph nodes
dissected, n | 26.80±7.73 | 25.81±6.40 | 0.585 | 27.94±6.49 | 25.48±6.64 | 0.258 | 30.82±6.57 | 24.25±6.37 | 0.001 |
| Conversion to open
surgery | 2 (6.7) | 0 (0) | 0.230 | 0 (0) | 1 (4.3) | 1.000 | 2 (7.1) | 1 (5.0) | 1.000 |
| Exhaust time,
days | 4.43±1.36 | 4.97±1.00 | 0.084 | 4.44±1.37 | 4.57±1.12 | 0.751 | 4.57±1.29 | 4.95±1.23 | 0.313 |
| Postoperative
hospital stay, days | 10.63±2.31 | 10.69±1.96 | 0.921 | 10.06±1.81 | 11.13±2.85 | 0.194 | 10.50±2.80 | 10.20±1.99 | 0.683 |
| Total
hospitalization expenses, ×104 yuan | 8.82±1.34 | 8.69±1.34 | 0.698 | 8.49±1.20 | 8.17±0.85 | 0.322 | 8.52±1.20 | 8.27±1.12 | 0.455 |
| Complications | 10 (33.3) | 7 (21.9) | 0.312 | 2 (12.5) | 3 (13.0) | 1.000 | 7 (25.0) | 2 (10.0) | 0.348 |
| OS of 3 years | 17 (56.7) | 15 (46.9) | 0.441 | 9 (56.3) | 11 (47.8) | 0.748 | 14 (50.0) | 9 (45.0) | 0.732 |
| OS of 5 years | 12 (40.0) | 10 (31.3) | 0.472 | 6 (37.5) | 6 (26.1) | 0.498 | 10 (35.7) | 5 (25.0) | 0.430 |
Comparison of postoperative
complications
Among all the patients included in the study, 31 had
postoperative complications, accounting for 20.8% of cases. These
included 19 cases (25.7%) in the D2+ group and 12 cases (16%) in
the D2 group. In the D2+ group, there were 16 cases of minor
complications (21.6%) and 3 cases of severe complications (4.1%).
All minor complications were resolved by conservative treatment.
Two cases of severe complications comprised anastomotic fistula,
which were resolved by a second operation. There was also 1 case of
anastomotic bleeding, which was resolved by endoscopic hemostasis.
In the D2 group, there were 10 cases of minor complications (13.3%)
and 2 cases of severe complications (2.7%). All minor complications
were resolved by conservative treatment. One patient experienced
the severe complication of abdominal bleeding, and hemostasis was
achieved after a second operation. The patient with severe heart
failure was transferred to the intensive care unit for tracheal
intubation, cardiac intubation, diuretics and other treatments.
Following relief of the symptoms, the patient was transferred back
to the general ward, and the patient was finally cured and
discharged. Further analysis of the complications of the two groups
revealed that the postoperative complications comprised a
relatively high proportion of incision and pulmonary infections.
The incidence rates of total, minor and severe complications in the
D2+ group were slightly higher than those in the D2 group, but
analysis indicated that there was no significant difference. The
data are shown in Table IV. In the
subgroup analysis, no statistically significant difference in
postoperative complications was detected between the D2+ and D2
groups for any gastrectomy type (Table III).
 | Table IV.Comparison of postoperative
complications between the two groups of patients. |
Table IV.
Comparison of postoperative
complications between the two groups of patients.
| Postoperative
complications | D2+ group
(n=74) | D2 group
(n=75) | P-value |
|---|
| Total
complications | 19 (25.7) | 12 (16) | 0.146 |
| Clavien-Dindo
complication classification I–II | 16 (21.6) | 10 (13.3) | 0.183 |
| Delayed
gastric emptying | 3 | 2 |
|
|
Incision infection | 5 | 2 |
|
|
Lymphorrhagia | 1 | 0 |
|
|
Intra-abdominal infection | 2 | 2 |
|
|
Pancreatic leakage | 0 | 1 |
|
|
Intestinal obstruction | 2 | 0 |
|
|
Duodenal stump leakage | 0 | 1 |
|
|
Pulmonary infection | 3 | 1 |
|
| Deep
vein thrombosis | 0 | 1 |
|
| Clavien-Dindo
complication classification III–IV | 3 (4.1) | 2 (2.7) | 0.988 |
|
Abdominal bleeding | 0 | 1 |
|
|
Anastomotic fistula | 2 | 0 |
|
|
Anastomotic bleeding | 1 | 0 |
|
|
Pulmonary embolism | 0 | 0 |
|
| Heart
failure | 0 | 1 |
|
| Death within 30
days after surgery | 0 | 0 |
|
Clinical outcomes
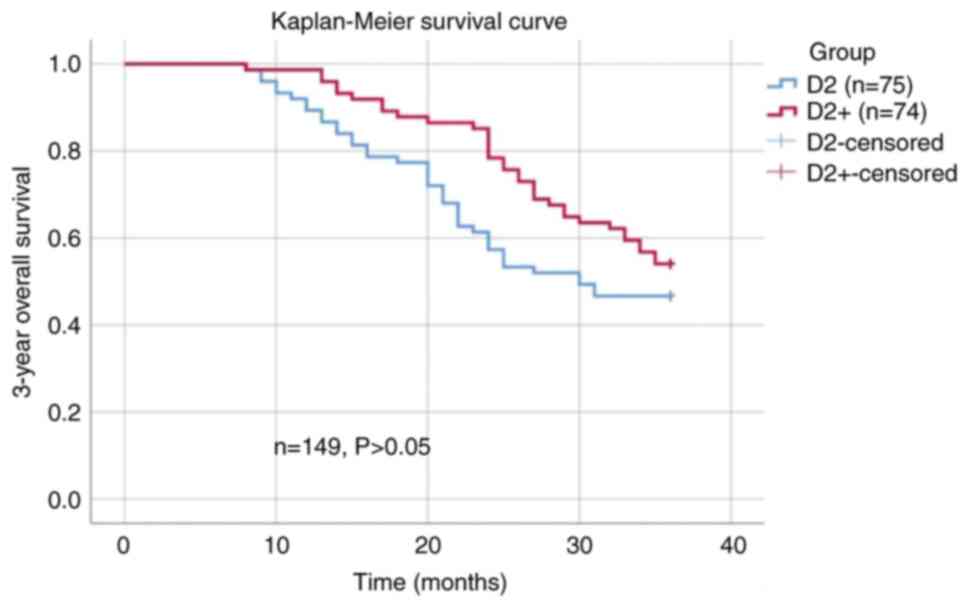
The 3-year overall survival rate of patients in the
D2+ group was 54.1%, and that of patients in the D2 group was
46.7%. The difference in 3-year overall survival rates between the
two groups was not found to be significant (Fig. 2). In addition, in the subgroup
analysis, no statistically significant differences were observed in
the 3-year overall survival between the D2+ and D2 groups (Table III).
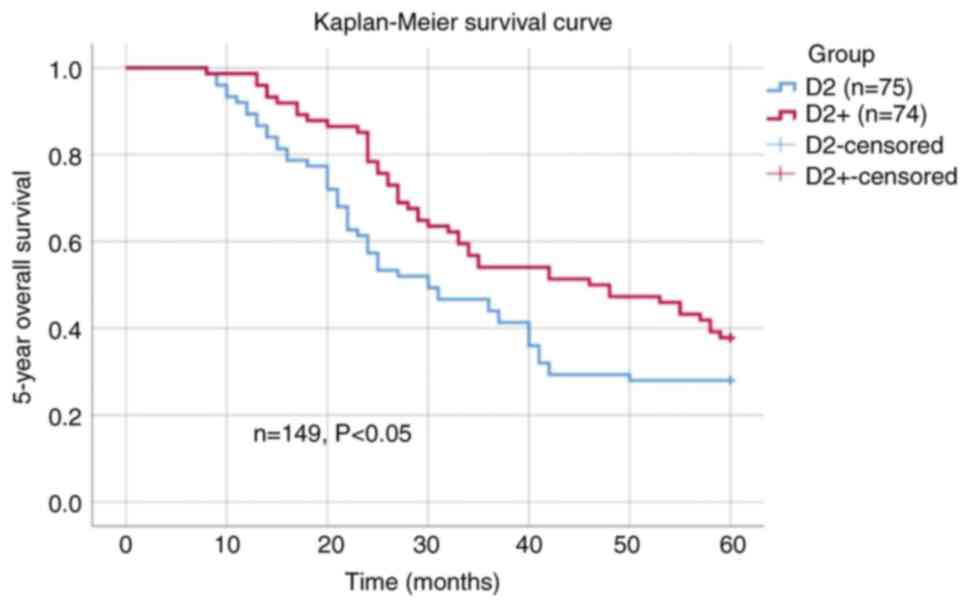
The 5-year overall survival rate of patients in the
D2+ group was 37.8%, and that of patients in the D2 group was
28.0%. The difference in 5-year overall survival rates between the
two groups was statistically significant (P<0.05; Fig. 3). However, in the subgroup analysis,
no statistically significant differences were found in 5-year
overall survival between the D2+ and D2 groups (Table III).
Discussion
The WHO defines adults with a BMI ≥30
kg/m2 as obese. However, the healthy BMI of the Asian
population is lower than that of the Western population, and the
body fat of Asian individuals is greater than that of Western
individuals with the same BMI. Therefore, adults of the Asian
population with a BMI ≥25 kg/m2 have an elevated risk of
weight-associated diseases. Based on the characteristics of the
Asia-Pacific population and of obesity-related diseases, the
obesity expert consultant of the WHO proposed that Asian
individuals with a BMI ≥25 kg/m2 should be considered
obese (14). Therefore, a BMI ≥25
kg/m2 was used as an obesity indicator in the present
study, and all 149 patients included had a BMI of this magnitude.
Obesity is a state involving the excessive accumulation or abnormal
distribution of fat in the body. It may cause a series of health,
social and psychological problems. A large-sample meta-analysis
found that obesity increases the risk of gastric cancer, and a
higher BMI is associated with a higher risk. In addition, a
subgroup analysis conducted on gastric cancer at different sites
found that obesity increases the risk of adenocarcinoma of the
esophagogastric junction but is not associated with the incidence
of distal gastric cancer (15). The
subjects included in the present study all had obesity and gastric
cancer. Proximal gastric cancer accounted for 50 and 46.7% of the
D2+ and D2 groups, respectively, which is a relatively high
proportion of patients, and is consistent with the findings of the
meta-analysis. Previous research has also found that a high BMI
significantly increases the risk of lymph node metastasis in
patients with gastric cancer (16).
As changes in the lifestyles of the Chinese
population have occurred, such as reductions in physical activity
and changes in diet, the proportion of the population that is obese
has been increasing. Therefore, the opportunities for
gastrointestinal surgeons to treat patients with obesity and
gastric cancer are also increasing. Laparoscopic technology is
widely used in clinical radical gastrectomy, but laparoscopic
surgery is more difficult in patients with obesity, and is
challenging for gastrointestinal surgeons. As well as having
increased quantities of intra-abdominal fat, obese patients have
fragile blood vessels, so they are more prone to bleeding during
surgery. In addition, large amounts of fat and omentum occupy the
surgical space, making it difficult to expose the visual field,
which may influence the effectiveness of surgery and postoperative
recovery. However, due to improvements achieved through increased
experience of laparoscopic surgery, the efficacy and feasibility of
applying this surgical technique to patients with obesity and
gastric cancer have been confirmed in a number of studies conducted
in various countries (17–19).
At present, the principal treatment for advanced
gastric cancer is surgery-based comprehensive treatment, and
sufficient gastrectomy and standardized lymph node dissection are
crucial to improving the long-term prognosis of patients with
advanced gastric cancer. After years of clinical research, a global
consensus has been reached on the use of D2 lymph node dissection
for patients with locally advanced gastric cancer (20). Appropriate lymph node dissection is
important for improving the prognosis of patients and reducing
surgical complications. In addition, for lymph nodes with a high
risk of metastasis outside the scope of D2 lymph node dissection,
whether it is advantageous to perform selective D2+ lymph node
dissection is a topic of debate for those researching gastric
cancer surgery. There has been a prolonged dispute about the
benefit of extended lymph node dissection for gastric cancer. In
theory, the removal of a larger area of lymph nodes by extended
lymphadenectomy should increase the likelihood of cure (21,22).
According to a previous report, the number of dissected lymph nodes
is an important indicator of the accuracy of pathological staging
and can predict the prognosis of patients; every additional 10
lymph node dissections can increase the 5-year survival rate by
5.7–10.9% (23). In addition, a
long-term multicenter study conducted in the USA showed that the
dissection of a sufficient number of lymph nodes can significantly
improve the long-term survival rate of patients (24). Furthermore, a study performed in
China by Liang et al (25)
found that standard D2+ para-aortic lymph nodal dissection) surgery
with the removal of ≥30 lymph nodes for N3-stage disease can
significantly improve the prognosis of patients (25). Some studies have also confirmed this
finding in Western populations. Based on their research results,
Ozmen et al (26) suggested
that D2+ lymph node dissection should be preferred for advanced
gastric cancer (IIIA-IIIB) as it increases the rate of survival,
and that D2+ lymph node dissection can be performed as safely as
standard D2 dissection by experienced surgeons without any increase
in postoperative morbidity and mortality. In addition, numerous
studies have confirmed the safety of D2+ lymph node dissection in
terms of surgical conditions and postoperative complications
(27–35). Based on these research results, it
is feasible to perform D2+ lymph node dissection in patients with
advanced gastric cancer. However, it is challenging for
gastrointestinal surgeons to perform laparoscopic surgery on
patients, and difficult to achieve standard D2 lymph node
dissection in patients with advanced stage gastric cancer who are
obese. However, there have been few studies on the curative effect
and safety of D2+ surgery for patients with obesity.
Since surgery in patients with obesity is onerous, a
number of studies have confirmed that obesity increases the
duration of laparoscopic surgery. A meta-analysis of >20,000
patients with gastric cancer showed that in patients with obesity
the duration of surgery was significantly longer than that of
patients with a normal BMI, and the occurrence of surgery-related
complications increased (36). In
addition, a Korean study showed that although patients with obesity
had prolonged surgery and fewer lymph nodes to be dissected, the
short- and long-term surgical effects were not significantly
different from those of patients with a normal BMI (37). In the present study, the BMI
baselines of the two groups of patients were very similar, which
excluded inconsistency of BMI as the cause of the difference in
surgical duration and simultaneously excluded the impact of BMI
differences on the research results. The present study showed that
the duration of surgery for D2+ lymph node dissection was longer
than that of D2 lymph node dissection for obese patients. Regarding
the reason for this, the lymph nodes around the stomach of patients
with obesity are often surrounded by larger quantities of fat. When
cleaning the lymph nodes, it is necessary to separate and clean
greater amounts of fat tissue from around the blood vessels. It is
challenging to obtain a clear surgical field of view and the space
for surgery in the abdominal cavity is limited. The surgical
assistant may not be able to apply sufficient force when pulling
the tissue, and tissues such as the greater omentum and the lesser
omentum may slide back and forth repeatedly to obscure the surgical
field of view. During surgery, the surgeon and assistants require
additional time to adjust the forceps. Also, to maintain sufficient
tension, assistants must apply greater force to resist the
gravitational pull of fat and gastric tissue. In addition, the
tissues of patients with obesity tend to be brittle and bleed
easily, particularly when stretched and exposed. Larger volumes of
intraoperative bleeding prolong the time required to achieve
hemostasis, requiring the use of gauze for compression and to clean
the accumulated blood, or the use of suction to remove the
accumulated blood. This prolongs the surgical duration even more.
Therefore, if lymph node groups in addition to those of D2 are
dissected, the operation will be more difficult and the duration of
surgery and volume of intraoperative blood loss will increase. In
the present study there was no significant difference in
postoperative pathological stages between the two groups because
the clinical baselines of the two groups were consistent when
screening the enrolled cases. Notably, the present study is a
retrospective analysis, so patients who decided to undergo D2+
dissection during surgery may have had a later intraoperative
clinical stage. For example, if the serosal surface of a patient
with distal gastric cancer is smooth and node station 6 is free
from metastasis and duodenal invasion, it would be decided during
the surgery to perform D2 dissection. However, if the serosa is
violated, node station 6 has enlarged lymph nodes or the duodenum
is invaded, it may be decided to perform D2 plus node station 13 or
14v excision during the surgery. Therefore, the study has certain
limitations due to its retrospective design. The indications for D2
or D2+ dissection during surgery are not clearly defined, so
patients in the D2+ group may generally have a higher clinical
stage. This will also increase the difficulty of surgery, leading
to an increase in the duration of surgery and intraoperative blood
loss in the D2+ group. Therefore, the results may be affected by
tumor stage. To avoid this, the pTNM staging of the groups was kept
consistent to prevent it from influencing the research results. In
addition, the D2+ group underwent the dissection of groups of lymph
nodes other than those of standard D2 surgery, so it is expected
that the number of lymph node dissections would be significantly
increased in the D2+ group compared with the D2 group. The present
study suggests that additional lymph node dissections can improve
the long-term survival of patients, which is confirmed by the
survival analysis. The 3-year overall survival rate of the D2+
group was slightly, but not significantly, higher than that of the
D2 group. However, the 5-year overall survival rate of the D2+
group was significantly higher than that of the D2 group. In the
subgroup analysis, no statistically significant difference in
5-year overall survival was found between the D2+ and D2 groups.
However, in all three subgroups, the 5-year overall survival of the
D2+ group was higher than that of the D2 group. The lack of
statistical significance may be due to the small sample size of the
three subgroups, particularly the distal gastrectomy subgroup with
only 39 cases. If a larger number of research cases are included in
future studies, statistically significant differences may be
obtained.
The present study found that the 3-year overall
survival rate of patients in the D2+ and D2 groups was 54.1 and
46.7%, respectively. In the JACCRO GC-07 clinical trial, the 3-year
relapse-free survival rate was 49.6–65.9% in patients with stage
III gastric cancer (38). The
present study mostly included patients with stage III gastric
cancer, with 86.5% of patients in the D2+ group and 80% in the D2
group having stage III disease. However, the 3-year overall
survival rate in the present study was markedly lower than that in
the JACCRO GC-07 study. It must be noted that education and
economic levels differ among provinces, and the compliance of
patients in Henan is poor. In addition, some patients have
insufficient understanding of gastric cancer, and consider that
surgery alone is sufficient treatment and that postoperative
adjuvant chemotherapy is unnecessary and expensive. Therefore, some
patients did not complete postoperative adjuvant chemotherapy,
while others completely refused it. However, in the JACCRO GC-07
study, patients all received postoperative adjuvant chemotherapy.
This may be the reason why the 3-year overall survival rate in the
present study is lower than that of the JACCRO GC-07 clinical
trial. In addition, poor compliance leads to a higher rate of
patients being lost to follow-up, which also reduces the 3-year
overall survival rate.
The results of the present study serve as a clinical
reference for D2+ lymph node dissection in patients with obesity.
The findings require confirmation in studies with a larger sample
size and randomized controlled study design. An increased number of
lymph node dissections requires a greater surgical scope and
increased surgical trauma. However, despite the D2+ group having a
prolonged surgical duration and increased intraoperative blood
loss, if there is no significant difference in postoperative
recovery and complications in comparison with D2 dissection, it is
feasible to reasonably expand the scope of lymph node dissection
for certain patients with obesity at stage II–III. The present
study found no significant difference in surgical methods, the rate
of conversion to open surgery, postoperative time to first flatus
postoperative hospital stay, total hospitalization costs,
postoperative complications or mortality within 30 days. Therefore,
D2+ lymph node dissection improves the prognosis of patients and
increases the long-term survival rate, without affecting
postoperative recovery or increasing postoperative
complications.
The present study has several limitations. First, as
already mentioned, it is a retrospective study, not a prospective
randomized controlled study. Second, the sample size of the study
is small, and the follow-up time is short. Third, although there
was no statistically significant difference in the characteristics
of the two groups of patients, this did not overcome all selection
bias. For example, factors associated with the selection of D2+ or
D2 lymph node resection remain. D2+ lymph node resection is more
likely to be performed in patients with a healthier physical
condition and younger average age, which may improve survival and
reduce the incidence of complications. Finally, the present study
was performed at a single center. The patients enrolled in the
study were all operated on by the same team of physicians in the
same hospital, including the same chief surgeon, first assistant
and mirror supporter. However, the hospital operates on a large
scale, performs a large number of surgeries and has a great amount
of experience in laparoscopic gastric cancer surgery. It is
undeniable that medical institutions with that perform a larger
number of surgeries and have more experienced gastrointestinal
surgeons can significantly reduce surgical complications and
patient mortality, and can significantly improve the rate of
survival (39,40). Although this eliminates the bias
caused by different surgeons, it also has certain limitations.
Since laparoscopic gastric cancer surgery is more complex than
colorectal surgery, the learning curve is longer, and surgery for
patients with obesity is particularly challenging. Therefore, it is
necessary to collaborate with multicenter medical institutions to
obtain a larger sample size and follow up the patients for a longer
time. The analysis of data from multiple centers will negate the
impact of differences in surgical experience on the research
results. In addition, larger-scale prospective randomized clinical
trials are necessary to obtain higher-level evidence of the
clinical efficacy and safety of this surgery. This should also
avoid the influence of selective bias on the research results.
Summarizing the results of the present study, it can
be concluded that among obese patients with gastric cancer,
although the D2+ group had a longer surgical duration and slightly
more intraoperative bleeding than the D2 group, there was no
difference in postoperative recovery or complications between the
two groups. Moreover, the D2+ group had a greater number of lymph
node dissections and a higher 5-year survival rate. Therefore, it
is suggested that D2+ lymph node dissection is safe to apply to
obese patients with gastric cancer who are treated in medical
institutions with a relatively high level of surgical
experience.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YCS and YG were responsible for the conception of
the present study. GTZ performed acquisition of data, statistical
analysis and revision of the tables and figures. XFS, YY and PZ
analyzed and interpreted the data. XDZ analyzed data. All authors
drafted the manuscript and revised it critically for important
intellectual content. YCS, YG and PZ confirm the authenticity of
all the raw data. All authors read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study
involving biological samples, medical record information and data
were approved by the Medical Ethics Committee of Henan Provincial
People's Hospital (Zhengzhou, China). Written informed consent was
obtained from all patients. All methods were performed in
accordance with the relevant guidelines and regulations
(Declaration of Helsinki).
Patient consent for publication
Written informed consent for the publication of
their data was obtained from all patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization, . Obesity and
overweight. Available from:. http://www.who.int/news-room/fact-sheets/detail/obesity-and-overweightJune
9–2021
|
|
2
|
Wang L, Zhou B, Zhao Z, Yang L, Zhang M,
Jiang Y, Li Y, Zhou M, Wang L, Huang Z, et al: Body-mass index and
obesity in urban and rural China: Findings from consecutive
nationally representative surveys during 2004-18. Lancet.
398:53–63. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sung H, Siegel RL, Torre LA,
Pearson-Stuttard J, Islami F, Fedewa SA, Goding Sauer A, Shuval K,
Gapstur SM, Jacobs EJ, et al: Global patterns in excess body weight
and the associated cancer burden. CA Cancer J Clin. 69:88–112.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen WQ, Li H, Sun KX, Zheng RS, Zhang SW,
Zeng HM, Zou XN, Gu XY and He J: Report of cancer incidence and
mortality in China, 2014. Zhonghua Zhong Liu Za Zhi. 40:5–13.
2018.(In Chinese). PubMed/NCBI
|
|
5
|
Lin GT, Chen QY, Zheng CH, Li P, Xie JW,
Wang JB, Lin JX, Lu J, Cao LL, Lin M, et al: Lymph node
noncompliance affects the long-term prognosis of patients with
gastric cancer after laparoscopic total gastrectomy. J Gastrointest
Surg. 24:540–550. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Japanese Gastric Cancer Association, .
Japanese classification of gastric carcinoma: 3rd English edition.
Gastric Cancer. 14:101–112. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network, .
NCCN clinical practice guidelines in oncology. Gastric cancer
(version 2.2023). https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdfAugust
29–2023
|
|
8
|
Liang Y, Cui J, Cai Y, Liu L, Zhou J, Li
Q, Wu J and He D: ‘D2 plus’ lymphadenectomy is associated with
improved survival in distal gastric cancer with clinical serosa
invasion: A propensity score analysis. Sci Rep. 9:191862019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xue L, Chen XL, Zhang WH, Yang K, Chen XZ,
Zhang B, Chen ZX, Chen JP, Zhou ZG and Hu JK: Risk factors and
prognostic significance of retropancreatic lymph nodes in gastric
adenocarcinoma. Gastroenterol Res Pract. 2015:3676792015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th edition.
Springer; New York, NY: 2017
|
|
11
|
Guidelines Working Committee of Chinese
Society of Clinical Oncology, . Chinese Society of Clinical
Oncology (CSCO) Guidelines for Diagnosis and Treatment of Gastric
Cancer (version 1.2023). People's Health Publishing House; Beijing:
2023
|
|
12
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Borrmann R: Geschwülste des Magens und
Duodenums. Verdauungsschlauch: Rachen und Tonsillen · Speiseröhre
Magen und Darm · Bauchfell. Borchardt H, Borrmann R, Christeller E,
Dietrich A, Fischer W, Von Gierke E, Hauser G, Kaiserling C, Koch
M, Koch W, et al: Springer Berlin Heidelberg; Berlin, Heidelberg:
pp. 812–1054. 1926, View Article : Google Scholar
|
|
14
|
Choo V: WHO reassesses appropriate
body-mass index for Asian populations. Lancet. 360:2352002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang P, Zhou Y, Chen B, Wan HW, Jia GQ,
Bai HL and Wu XT: Overweight, obesity and gastric cancer risk:
Results from a meta-analysis of cohort studies. Eur J Cancer.
45:2867–2873. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiao J, Shen K, Liu K, Wang Y, Fan H,
Cheng Q, Zhou X, Hu L, Wang G, Xu Z and Yang L: Obesity promotes
lipid accumulation in lymph node metastasis of gastric cancer: A
retrospective case-control study. Lipids Health Dis. 21:1232022.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kulig J, Sierzega M, Kolodziejczyk P,
Dadan J, Drews M, Fraczek M, Jeziorski A, Krawczyk M, Starzynska T
and Wallner G; Polish Gastric Cancer Study Group, : Implications of
overweight in gastric cancer: A multicenter study in a Western
patient population. Eur J Surg Oncol. 36:969–976. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Son SY, Jung DH, Lee CM, Ahn SH, Ahn HS,
Park DJ and Kim HH: Laparoscopic gastrectomy versus open
gastrectomy for gastric cancer in patients with body mass index of
30 kg/m2 or more. Surg Endosc. 29:2126–2132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sugimoto M, Kinoshita T, Shibasaki H, Kato
Y, Gotohda N, Takahashi S and Konishi M: Short-term outcome of
total laparoscopic distal gastrectomy for overweight and obese
patients with gastric cancer. Surg Endosc. 27:4291–4296. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Songun I, Putter H, Kranenbarg EM, Sasako
M and van de Velde CJH: Surgical treatment of gastric cancer:
15-Year follow-up results of the randomised nationwide Dutch D1D2
trial. Lancet Oncol. 11:439–449. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hartgrink HH, van de Velde CJ, Putter H,
Bonenkamp JJ, Klein Kranenbarg E, Songun I, Welvaart K, van Krieken
JH, Meijer S, Plukker JT, et al: Extended lymph node dissection for
gastric cancer: Who may benefit? Final results of the randomized
Dutch gastric cancer group trial. J Clin Oncol. 22:2069–2077. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ozmen MM, Zulfikaroglu B, Kucuk NO, Ozalp
N, Aras G, Koseoglu T and Koç M: Lymphoscintigraphy in detection of
the regional lymph node involvement in gastric cancer. Ann R Coll
Surg Engl. 88:632–638. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith DD, Schwarz RR and Schwarz RE:
Impact of total lymph node count on staging and survival after
gastrectomy for gastric cancer: Data from a large US-population
database. J Clin Oncol. 23:7114–7124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gholami S, Janson L, Worhunsky DJ, Tran
TB, Squires MH III, Jin LX, Spolverato G, Votanopoulos KI, Schmidt
C, Weber SM, et al: Number of lymph nodes removed and survival
after gastric cancer resection: An analysis from the US gastric
cancer collaborative. J Am Coll Surg. 221:291–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang YX, Liang H, Ding XW, Wang XN, Zhang
L, Wu LL, Liu HG and Jiao XG: The prognostic influence of D2
lymphadenectomy with para-aortic lymph nodal dissection for gastric
cancer in N3 stage. Zhonghua Wai Ke Za Zhi. 51:1071–1076. 2013.(In
Chinese). PubMed/NCBI
|
|
26
|
Ozmen MM, Zulfikaroglu B, Ozmen F, Moran
M, Ozalp N and Seckin S: D2 vs D2 plus para-aortic lymph node
dissection for advanced gastric cancer. Turk J Surg. 37:49–58.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sano T, Sasako M, Yamamoto S, Nashimoto A,
Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai K, Yamamura Y
and Okajima K: Gastric cancer surgery: Morbidity and mortality
results from a prospective randomized controlled trial comparing D2
and extended para-aortic lymphadenectomy-Japan clinical oncology
group study 9501. J Clin Oncol. 22:2767–2773. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marrelli D, Pedrazzani C, Neri A, Corso G,
DeStefano A, Pinto E and Roviello F: Complications after extended
(D2) and superextended (D3) lymphadenectomy for gastric cancer:
Analysis of potential risk factors. Ann Surg Oncol. 14:25–33. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Günther K, Horbach T, Merkel S, Meyer M,
Schnell U, Klein P and Hohenberger W: D3 lymph node dissection in
gastric cancer: Evaluation of postoperative mortality and
complications. Surg Today. 30:700–705. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bostanci EB, Kayaalp C, Ozogul Y, Aydin C,
Atalay F and Akoglu M: Comparison of complications after D2 and D3
dissection for gastric cancer. Eur J Surg Oncol. 30:20–25. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sasako M, Sano T, Yamamoto S, Kurokawa Y,
Nashimoto A, Kurita A, Hiratsuka M, Tsujinaka T, Kinoshita T, Arai
K, et al: D2 lymphadenectomy alone or with para-aortic nodal
dissection for gastric cancer. N Engl J Med. 359:453–462. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kulig J, Popiela T, Kolodziejczyk P,
Sierzega M and Szczepanik A; Polish Gastric Cancer Study Group, :
Standard D2 versus extended D2 (D2+) lymphadenectomy for gastric
cancer: An interim safety analysis of a multicenter, randomized,
clinical trial. Am J Surg. 193:10–15. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Danielson H, Kokkola A, Kiviluoto T, Sirén
J, Louhimo J, Kivilaakso E and Puolakkainen P: Clinical outcome
after D1 vs D2-3 gastrectomy for treatment of gastric cancer. Scand
J Surg. 96:35–40. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kunisaki C, Akiyama H, Nomura M, Matsuda
G, Otsuka Y, Ono H, Nagahori Y, Hosoi H, Takahashi M, Kito F and
Shimada H: Comparison of surgical results of D2 versus D3
gastrectomy (para-aortic lymph node dissection) for advanced
gastric carcinoma: A multi-institutional study. Ann Surg Oncol.
13:659–667. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu CW, Hsiung CA, Lo SS, Hsieh MC, Shia LT
and Whang-Peng J: Randomized clinical trial of morbidity after D1
and D3 surgery for gastric cancer. Br J Surg. 91:283–287. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu XS, Wu WG, Li ML, Yang JH, Ding QC,
Zhang L, Mu JS, Gu J, Dong P, Lu JH and Liu YB: Impact of being
overweight on the surgical outcomes of patients with gastric
cancer: A meta-analysis. World J Gastroenterol. 19:4596–4606. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kim JH, Chin HM, Hwang SS and Jun KH:
Impact of intra-abdominal fat on surgical outcome and overall
survival of patients with gastric cancer. Int J Surg. 12:346–352.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yoshida K, Kodera Y, Kochi M, Ichikawa W,
Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, et
al: Addition of docetaxel to oral fluoropyrimidine improves
efficacy in patients with stage III gastric cancer: Interim
analysis of JACCRO GC-07, a randomized controlled trial. J Clin
Oncol. 37:1296–1304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mahar AL, McLeod RS, Kiss A, Paszat L and
Coburn NG: A systematic review of the effect of institution and
surgeon factors on surgical outcomes for gastric cancer. J Am Coll
Surg. 214:860–868.e12. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Smith DL, Elting LS, Learn PA, Raut CP and
Mansfield PF: Factors influencing the volume-outcome relationship
in gastrectomies: A population-based study. Ann Surg Oncol.
14:1846–1852. 2007. View Article : Google Scholar : PubMed/NCBI
|