Introduction
Urothelial carcinoma (UC) is a common tumor found in
clinical settings. The majority of UC (90–95%), resides in the
bladder and is known as urinary bladder urothelial carcinoma (UBUC)
(1). In 2020, UBUC ranked as the
10th most commonly diagnosed malignancy, with approximately 573,000
new cases and 213,000 deaths around the world (2). Although most UC cases involve the
urinary bladder, ~5% originate in the upper urinary tract (3). Notably, 15–25% of invasive UC cases
exhibit morphological variations, such as squamous, glandular,
trophoblastic or small cell/high-grade neuroendocrine
differentiation, either individually or in combination (4). Patients with early stage UC are often
asymptomatic or present only with hematuria, and once distant
metastases develop, the prognosis becomes poor (5,6). UC
typically metastasizes to distant organs at an advanced stage after
the initial diagnosis (6). In
addition, occult cancer is rare, and its primary lesion is
difficult to detect using standard clinical methods. To date, most
reported cases of occult cancer occur in breast cancer, with few
cases in thyroid and gynecological cancer types (6). However, to the best of our knowledge,
occult UC with mediastinal metastasis has not previously been
reported. The current study presents an unusual case of occult
urothelial cancer manifesting as a mediastinal metastasis at an
early stage.
Case report
A 70-year-old man was initially diagnosed with
coronary artery disease at the Department of Cardiology at Weifang
People's Hospital (Weifang, China) in September 2022. The patient
presented with precordial pain, characterized by stabbing
sensations radiating to the back of the shoulder, which were
particularly intense during the nighttime. The patient was treated
conservatively with oral isosorbide mononitrate [20 mg, twice a day
(bid)] for two weeks. During this period, a computed tomography
(CT) scan revealed a mediastinal mass in the upper mediastinum
posterior to the esophagus, which was considered benign and was not
treated further. The patient had a history of smoking for >40
years, with 20 cigarettes per day, and had no notable family
medical history.
Over the subsequent 5 months, the patient
experienced worsening chest pain with a numerical rating scale
(7) score of 2, and reported
additional symptoms, including blood in the sputum, chest tightness
and shortness of breath after physical activity. In late January
2023, a follow-up chest CT conducted at the Department of
Cardiology of the Affiliated Hospital of Weifang Medical University
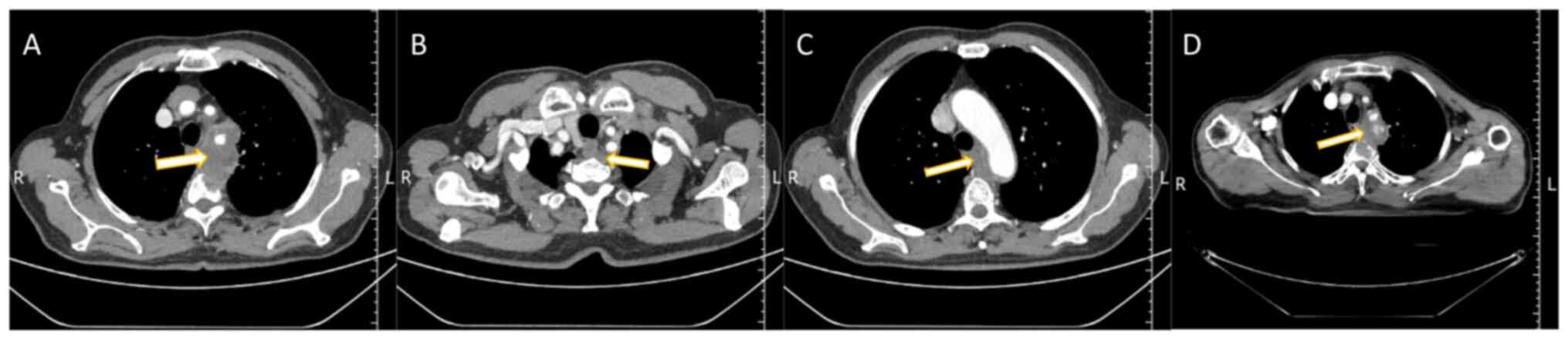
(Weifang, China) revealed a soft-tissue density mass in the upper
mediastinum posterior to the esophagus. The mass, with unclear
boundaries, was ~68.5×45.9×60.0 mm in size. Additionally, there was
evidence of bone destruction in the T2 and T3 vertebrae (Fig. 1A-C), suggesting the possibility of a
malignant tumor. Subsequent examinations included a CT-guided
biopsy of the mediastinal mass, and the postoperative pathology
results identified the mass as a metastatic migratory cell
carcinoma with a urothelial epithelial origin, characterized as a
poorly differentiated carcinoma (tumor biopsy tissue was fixed with
10% neutral buffered formalin at 37°C for 6–8 h. Following gradient
ethanol dehydration, xylene transparency and wax dipping, and
embedding, the 3-micron paraffin sections were subjected to
hematoxylin-eosin staining, where hematoxylin staining was
performed for 6–8 min at 22°C and eosin staining was performed for
20–30 sec. Then gradient ethanol dehydration, xylene transparency,
sealing, air drying, and finally the sections were successively
observed and diagnosed under light microscope at 40 magnification)
(Fig. 2A). Immunohistochemical
(IHC) analysis yielded positive findings for p40 (cat. no.
RMA-1006), cytokeratin 5/6 (CK5/6; cat. no. MAB-0744), p63 (cat.
no. MAB-0694), GATA binding factor 3 (GATA-3; cat. no. MAB-0695),
CK7 (cat. no. MAB-0828) and Ki-67 labeling index (40%) (cat. no.
MAB-0672) (Fig. 2B-G) and negative
findings for CD117 (cat. no. Kit-0029), CD5 (cat. no. MAB-0827),
CD56 (cat. no. MAB-0743), synaptophysin (Syn) (cat. no. MAB-0742)
and thyroid transcription factor-1 (TTF-1) (cat. no. MAB-0599). The
paraffin-embedded tissue sections were stained with
immunohistochemistry. The tissues were all fixed in 10% neutral
buffered formalin at 37°C for 6–8 h. The paraffin sections with the
thickness of 3 microns were rinsed with PBS liquid was washed three
times, 5 min each time, then 3% methanol hydrogen peroxide liquid
was dropwise added on the slides to block endogenous peroxidase
(22°C, 10 min), PBS was washed once, and then the primary
antibodies were dropwise added (all primary antibodies were
purchased from ready-to-use antibodies of China Fuzhou Maixin
Biotech, Co., Ltd.), Then incubated for 1 h in a wet box at the
constant temperature of 37°C, and rinsed with PBS solution for 3
times, 5 min each time after incubation, then the secondary
antibody (purchased from China Fuzhou Maixin Biotech, Co., Ltd.,
ready-to-use antibody, cat. no. KIT-5030) was added dropwise, and
then incubated for 30 min at the constant temperature of 37°C in a
wet box, rinsed with PBS solution for 3 times, 5 min each time,
then DAB was added dropwise for 3–5 min, and the staining was
stopped by washing with distilled water. The sections were observed
under light microscope (magnification, ×40). All the analyses were
conducted according to standard procedures. Brain magnetic
resonance imaging (MRI) showed no metastases, and abdominal MRI
revealed no primary lesions in the urinary tract. In February 2023,
the patient's family sought a second opinion by taking the
pathology slides to a referral hospital. After reviewing the
initial puncture pathology findings and the consultation opinion
from the referral hospital, the mediastinal mass was considered to
be of urological origin. Consequently, the patient was diagnosed
with occult UC with mediastinal metastases, without primary lesions
in the urinary tract. After pre-treatment preparations, the patient
started chemotherapy in February 2023. Two cycles of gemcitabine
[1,000 mg/m2 on day 1, day 8, every 21 days (q21d)] and
cisplatin (70 mg/m2 on days 1–2, q21d) chemotherapy were
administered to stabilize the patient's condition, and
osteoprotective treatment with inkadronate disodium (5 mg, q28d)
was administered to address the vertebral destruction. Local
radiotherapy for the mediastinal tumor and thoracic spine
metastases was started 1 month later in April 2023, with a
radiation dosage of 2 Gy/30 fractions. In May 2023, a follow-up CT
scan was performed, revealing a reduction in the size of the
mediastinal mass after radiotherapy (Fig. 1D). This finding led to an adjustment
in the radiotherapy regimen; while the radiation dosage remained
unchanged, the radiation field was reduced. The patient finished
radiotherapy after 43 days. The patient experienced
radiotherapy-induced esophagitis, manifesting as a feeling of
obstruction during eating, for which a month of symptomatic
treatment with continuous intravenous infusion of sodium riboflavin
phosphate, intermittent intravenous infusion of sodium
methylprednisolone succinate, and oral administration of Kangfuxin
solution had been provided. Notably, the patient maintained good
overall health and did not experience myelosuppression following
the radiation therapy up to the time of writing this study. The
patient had no obvious discomfort and had a good prognosis. The
patient was asked to have a follow up every 2 months
thereafter.
 | Figure 2.Histological analyses of biopsied
tissue samples. (A) Hematoxylin and eosin staining revealing
polygonal tumor cells with eosinophilic cytoplasm and nuclear
atypia (magnification, ×400; scale bar, 55 µm). Immunohistochemical
staining showing positive expression of (B) p63, (C) CK7, (D) GATA
binding factor 3, (E) p40, (F) CK5/6 and (G) Ki-67 (40%) in tumor
cells. Immunohistochemical staining showing negative expression of
(H) CD5, (I) CD56, (J) CD117, (K) synaptophysin, (L) thyroid
transcription factor-1 in tumor cells. (Magnification, ×400; scale
bar, 55 µm). CK, cytokeratin. |
Discussion
Primary mediastinal tumors commonly include germ
cell tumors, thymomas and lymphomas. All cases of UC demonstrate
strong diffuse expression of p63 and the majority of cases (94%)
demonstrate strong expression of CK7 (8). GATA-3, a zinc finger transcription
factor, has now been demonstrated as a valuable, sensitive and
relatively specific marker for conventional urothelial carcinoma
(9–12). TTF-1 or napsin A and p40 or p63 are
the best markers for lung adenocarcinoma and the squamous cell
carcinoma (SCC) spectrum, respectively (13–15).
Thymic carcinoma is positive for CK5 and CD117 immunoreactivity
(16). Established IHC markers for
diagnosing neuroendocrine tumor neoplasms include Syn, CD56 and
TTF-1, which demonstrate notable sensitivity and specificity in
their diagnostic utility (17–19).
IHC staining for GATA3 in mediastinal masses is not routinely
performed by the Affiliated Hospital of Weifang Medical University
(Weifang, China). In the present case, the tumor cells detected by
hematoxylin and eosin staining were arranged in nested and solid
sheets under the microscope, and the possibility of urothelial and
SCC was considered; therefore, IHC staining for GATA3 was
performed. In this case, the IHC analysis of the mediastinal tumor
revealed significant findings. Positive results for p63, GATA-3 and
CK7 supported the diagnosis of UC, with GATA-3 positivity being
specific for this type of carcinoma. The presence of p40(+) and
CK5/6(+) cells indicated squamous cell differentiation within the
tumor, while the negative result for CD117 suggested no tumors of
germ cell origin or stromal tumor metastasis, as well as thymic
carcinoma. Additionally, the absence of CD5 did not support a
diagnosis of thymoma, the negative findings for CD56 and Syn
effectively excluded the possibility of a neuroendocrine carcinoma,
and the negative TTF-1 status made a pulmonary origin less likely.
The pathological morphology of the tumor, in conjunction with these
IHC results, supported a diagnosis of metastatic UC. This diagnosis
was further confirmed by a pathological consultation at a
higher-level hospital. Moreover, a comprehensive abdominal MRI scan
revealed no primary lesions in the urinary tract, thereby
solidifying the diagnosis of metastatic UC without identifiable
primary urinary tract lesions.
UC arises from the uroepithelium, affecting the
proximal urethra, urinary bladder and upper urinary tract, with
bladder cancer accounting for 90% of UC cases (20). Globally, UC is a leading cause of
cancer-related deaths and stands as the most common malignant tumor
in the urinary system, with approximately half a million patients
diagnosed each year (6,21). UC usually invades nearby tissues and
organs, but a small percentage of UC cases can metastasize to
distant organs, including the lymph nodes, liver, lungs, bones and
adrenal glands (22). The pelvis
(68%), spine (12% cervical, 38% thoracic and 34% lumbar), ribs
(24%) and femurs (22%) are the most common sites of bone metastasis
(23). In the present study, the
patient presented with bone destruction in the T2 and T3 spinal
areas. This condition was not a result of metastasis; instead, it
was caused by the invasion of the vertebral body by a mediastinal
mass. Notably, cases of mediastinal metastases are quite rare.
Thoracic metastases from UC are frequently noted,
predominantly as solid parenchymal lesions, which are
characteristic of hematogenous seeding in the lung. Hiensch et
al (24) highlighted several
atypical thoracic metastases from UC, including mediastinal
lymphadenopathy. Notably, all these cases showed clear evidence of
primary tumors. Occult cancers are characterized by metastases that
manifest before the primary site is detected, with the primary
lesions often being challenging to identify. This phenomenon is
observed in breast, thyroid and genitourinary cancers.
Specifically, occult breast cancer accounts for 0.3–1% of all
breast cancer cases, with axillary nodal metastasis as the first
presentation (25). Occult thyroid
cancer primarily leads to regional lymph node metastasis and is
rarely associated with distant metastasis (26). Clinical evidence suggests that
>30% of men without a history of prostate cancer have occult
prostate cancer (27), and ~50% of
patients with UC have occult regional or distant metastases at the
muscle-invasive bladder cancer stage (28). To the best of our knowledge, the
present study documents only the second case of occult UC. Bu et
al (6) previously reported a
case of occult UC with widespread multiorgan metastases. However,
the present study uniquely focused on the mediastinal metastasis of
occult UC.
In the realm of imaging examinations, CT is
effective in identifying metastatic diseases, although it is
suboptimal for local staging up to T3a (29). A review by Mirmomen et al
(30) highlighted that the accuracy
of CT in detecting perivesical infiltration for tumors at stage ≥T3
ranged between 49 and 93%. MRI is more accurate for the early
diagnosis of local lesions and has become the preferred imaging
method for tumor staging, with a reported accuracy rate of >90%
(31). Therefore, MRI of the
abdomen and pelvis was selected to identify the primary site in the
present case. Unexpectedly, no lesions were detected in the urinary
tract. Fluoro-2-deoxy-D-glucose positron emission tomography-CT
(FDG PET-CT) has demonstrated high specificity for lymph node
staging in patients with UC (32).
A prospective study by Kibel et al (33) showed that out of 42 bladder UC cases
initially assessed as non-metastatic, 7 cases of occult metastasis
were identified by FDG PET/CT (33). This finding underscores the value of
FDG PET-CT in the comprehensive assessment of UC, especially in
detecting covert metastatic spread. However, FDG PET-CT is not the
first choice for diagnosing local lesions due to its high cost.
Cystoscopy and ureteroscopy are considered the gold standards for
evaluating urinary tract conditions, and a multipoint biopsy can be
used to identify carcinoma in situ (34–37).
Nevertheless, in clinical practice, cystoscopy and ureteroscopy are
rarely supported in patients with negative imaging results and no
urinary symptoms. A biopsy of the area where the mass occurs is
usually performed to determine the origin of the primary lesion to
diagnose occult UC. In the present study, the patient's
pathological IHC results showed a urinary epithelial origin. A
urologist was consulted who reported that occult UC could be
diagnosed combining the aforementioned even without endoscopy, as
long as the imaging did not reveal a space-occupying lesion.
However, the patient refused to undergo endoscopy. Therefore, IHC
analysis was the foundational method for the pathological diagnosis
of mediastinal masses in this case. IHC staining for p63 and CK7
helps distinguish adenocarcinoma of the prostate from UC of the
bladder (38). Notably, the
expression of CK7 is found in 100% of transitional cell carcinomas
(39). Furthermore, IHC staining
for GATA3 helps distinguish UCs from SCCs of the penile urethra
(40). CK5/6, typically present in
the normal keratinizing epidermis, is also expressed in the basal
cells of both low- and high-grade UCs (41). These IHC markers play a significant
role in accurately diagnosing and differentiating UC, particularly
when other diagnostic procedures are not an option.
Current imaging techniques do not support the
visualization of UC in situ (42). Therefore, a rare case of metastasis
of carcinoma in situ cannot be ruled out. Metastasis without
an invasive component is extremely rare, with only three other
documented cases in the medical literature (43–45).
Kim et al (43) reported a
case of inguinal and pelvic lymph node metastases from carcinoma
in situ in the penis. Avrach and Christensen (44) described a case of erythroplasia of
Queyrat with associated lymph node involvement. Additionally, Eng
et al (45) detailed a case
of lymph node metastasis from carcinoma in situ in the
penis. Several relevant theories have attempted to explain this
unusual phenomenon of metastasis from carcinoma in situ.
Tumor cells can disseminate even from earliest epithelial
alterations, and that carcinoma in situ may have the ability
to transfer tumor cells into the blood circulation (46). The finding of circulating tumour
cells in the peripheral blood (or bone marrow) of patients with
ductal carcinoma in situ supports the idea that cancer
dissemination may occur in the pre-infiltrative phase before tumour
progression, but it is unclear whether these cells are derived from
truly pre-invasive breast lesion or represent the earliest stage of
micro-infiltration (46).
Meanwhile, an article proposes a parallel progression model for
breast cancer, suggesting that early carcinogenesis and metastasis
are two separate processes rather than sequential events, and that
tiny cancers are capable of metastasis prior to invasion into the
breast; there is a small subset of cancer stem cells that have
metastatic potential from the outset and spread synchronously by
multiple pathways, and they are considered to be the founding cells
of metastatic lesions (47). That
is to say, primary (intramammary) cancer and metastases may appear
and grow simultaneously (48).
While these theories provide valuable insights, they remain
hypothetical and speculative. The occurrence of such events, though
acknowledged as true, is extremely rare in clinical practice.
Effective treatment plans should be developed based
on patient tumor status. For metastatic UC, gemcitabine combined
with cisplatin is one of the standard regimens of systemic
chemotherapy (49,50). In recent years, the use of
immunotherapy in UC treatment has gradually become a remedial
measure for first-line chemotherapy failure or as second-line
treatment (51–53). In addition, antibody-drug conjugates
have been approved for UC treatment (53,54).
Local radiotherapy may be beneficial for the survival of patients
with UC, especially those with oligometastases, as described in the
present case. A meta-analysis by Longo et al (55) revealed that local radiotherapy not
only promoted disease control but was also an effective and safe
treatment option for oligometastatic UC (55). Consistent with these findings, the
patient in the present study was treated with radiotherapy, and
subsequent radiological evaluation by CT showed a significant
overall response to the treatment, indicating a favorable
sensitivity of the disease to radiotherapy.
The present patient was diagnosed with occult UC
through a collaborative effort between pathologists and clinicians.
This case report highlights the diagnostic challenges posed by the
rarity of mediastinal metastatic occult UCs. In terms of symptoms,
the patient initially presented with chest pain and breathlessness;
these symptoms can be observed in UC and lung cancer, both of which
are smoking-related malignancies. This overlap necessitates a
careful differential diagnosis to avoid diagnostic errors. It is
hoped that this case report will prompt clinicians to consider
occult UC with mediastinal metastasis as a potential diagnosis in
similar clinical circumstances.
In summary, mediastinal metastasis originating from
UC is rare and may result in a poor prognosis. Therefore, when a
patient is diagnosed with a mediastinal tumor, clinicians should
consider the possibility of metastasis from UC. If the diagnosis of
a mediastinal tumor is confirmed and immunohistochemistry indicates
a uroepithelial origin, additional diagnostic procedures such as
retrograde urography and FDG PET-CT should be considered.
Subsequent individualized treatment in strict accordance with
oncology guidelines is effective in preventing complications. Early
interventions can effectively prolong survival time.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JFZ, XTP, XQL, YYC, XYL, LF, AL and ZL contributed
to the study conception and design. Material preparation, and data
collection and analysis were performed by ZL, XQL, YYC, XYL, LF and
AL. JFZ and XTP wrote the manuscript. JFZ, XTP, ZL and AL confirm
the authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki and approved by the Institutional Review
Board of the Affiliated Hospital of Weifang Medical University
(Weifang, China; approval no. wyfy-2023-96-026; 18 May 2023).
Patient consent for publication
Written informed consent has been obtained from
family members of the patient to publish this case report with the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CK
|
cytokeratin
|
|
GATA-3
|
GATA binding factor 3
|
|
CT
|
computed tomography
|
|
IHC
|
immunohistochemical
|
|
MRI
|
magnetic resonance imaging
|
|
UC
|
urothelial carcinoma
|
|
FDG PET-CT
|
fluoro-2-deoxy-D-glucose positron
emission tomography-CT
|
|
TTF-1
|
thyroid transcription factor-1
|
|
Syn
|
synaptophysin
|
References
|
1
|
Chan TC, Shiue YL and Li CF: The
biological impacts of CEBPD on urothelial carcinoma development and
progression. Front Oncol. 13:11237762023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
You X, Zhu C, Yu P, Wang X, Wang Y, Wang
J, Yu J and Wang K: Emerging strategy for the treatment of
urothelial carcinoma: Advances in antibody-drug conjugates
combination therapy. Biomed Pharmacother. 171:1161522024.(Epub
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Szarvas T, Módos O, Horváth A and Nyirády
P: Why are upper tract urothelial carcinoma two different diseases?
Transl Androl Urol. 5:636–647. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gandhi J, Chen JF and Al-Ahmadie H:
Urothelial carcinoma: Divergent differentiation and morphologic
subtypes. Surg Pathol Clin. 15:641–659. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rayn KN, Hale GR, Bloom JB, Gold SA,
Carvalho FLF, Mehralivand S, Czarniecki M, Wood BJ, Merino MJ,
Choyke P, et al: Incidental bladder cancers found on
multiparametric MRI of the prostate gland: A single center
experience. Diagn Interv Radiol. 24:316–320. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bu K, Shi Z, Lu Y, Zhao J and Li B: An
occult urothelial carcinoma with wide multiorgan metastases and its
genetic alteration profiling: Case report and literature review.
Medicine (Baltimore). 98:e152452019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alghadir AH, Anwer S, Iqbal A and Iqbal
ZA: Test-retest reliability, validity, and minimum detectable
change of visual analog, numerical rating, and verbal rating scales
for measurement of osteoarthritic knee pain. J Pain Res.
11:851–856. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carvalho JC, Thomas DG, McHugh JB, Shah RB
and Kunju LP: p63, CK7, PAX8 and INI-1: An optimal
immunohistochemical panel to distinguish poorly differentiated
urothelial cell carcinoma from high-grade tumours of the renal
collecting system. Histopathology. 60:597–608. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Higgins JP, Kaygusuz G, Wang L, Montgomery
K, Mason V, Zhu SX, Marinelli RJ, Presti JC Jr, van de Rijn M and
Brooks JD: Placental S100 (S100P) and GATA3: Markers for
transitional epithelium and urothelial carcinoma discovered by
complementary DNA microarray. Am J Surg Pathol. 31:673–680. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu H, Shi J, Wilkerson ML and Lin F:
Immunohistochemical evaluation of GATA3 expression in tumors and
normal tissues: A useful immunomarker for breast and urothelial
carcinomas. Am J Clin Pathol. 138:57–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ordóñez NG: Value of GATA3 immunostaining
in tumor diagnosis: A review. Adv Anat Pathol. 20:352–360. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rana C, Babu S, Agarwal H, Singhai A,
Kumar M, Singh V, Sinha RJ and Shankhwar SN: Diagnostic relevance
of GATA 3 expression in urinary bladder carcinoma of divergent
differentiation and other histological variants. Indian J Surg
Oncol. 12:678–685. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rossi G, Pelosi G, Barbareschi M, Graziano
P, Cavazza A and Papotti M: Subtyping non-small cell lung cancer:
Relevant issues and operative recommendations for the best
pathology practice. Int J Surg Pathol. 21:326–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pelosi G, Rossi G, Cavazza A, Righi L,
Maisonneuve P, Barbareschi M, Graziano P, Pastorino U, Garassino M,
de Braud F and Papotti M: ΔNp63 (p40) distribution inside lung
cancer: A driver biomarker approach to tumor characterization. Int
J Surg Pathol. 21:229–239. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lam KY, Dickens P and Chan AC: Tumors of
the heart. A 20-year experience with a review of 12,485 consecutive
autopsies. Arch Pathol Lab Med. 117:1027–1031. 1993.PubMed/NCBI
|
|
16
|
Nakagawa K, Matsuno Y, Kunitoh H, Maeshima
A, Asamura H and Tsuchiya R: Immunohistochemical KIT (CD117)
expression in thymic epithelial tumors. Chest. 128:140–144. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Parent AD, Bebin J and Smith RR:
Incidental pituitary adenomas. J Neurosurg. 54:228–231. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu L, Dong Y, Xue J, Xu S, Wang G, Kuang D
and Duan Y: SOX11 is a sensitive and specific marker for pulmonary
high-grade neuroendocrine tumors. Diagn Pathol. 17:22022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
He Y, Zhao L, Tang X, Jiang Q, Zhao X and
Cao Y: Prognostic implications of synaptophysin, CD56, thyroid
transcription factor-1, and Ki-67 in pulmonary high-grade
neuroendocrine carcinomas. Ann Diagn Pathol. 68:1522392024.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Giudici N, Bonne F, Blarer J, Minoli M,
Krentel F and Seiler R: Characteristics of upper urinary tract
urothelial carcinoma in the context of bladder cancer: A narrative
review. Transl Androl Urol. 10:4036–4050. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu YH, Chou MH, Meng E and Kao CC: A rare
case report of metastatic urothelial carcinoma to skull with
significant reossification after pembrolizumab. Medicina (Kaunas).
57:9872021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stellato M, Santini D, Cursano MC,
Foderaro S, Tonini G and Procopio G: Bone metastases from
urothelial carcinoma. The dark side of the moon. J Bone Oncol.
31:1004052021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hiensch R, Belete H, Rashidfarokhi M,
Galperin I, Shakil F and Epelbaum O: Unusual patterns of thoracic
metastasis of urinary bladder carcinoma. J Clin Imaging Sci.
7:232017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ofri A and Moore K: Occult breast cancer:
Where are we at? Breast. 54:211–215. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen WH, Wang YH, Lu YC, Huang CC and Wong
SL: Endobronchial metastasis from an occult papillary thyroid
carcinoma: A case report. Changgeng Yi Xue Za Zhi. 21:200–205.
1998.PubMed/NCBI
|
|
27
|
Iguchi T, Wang CY, Delongchamps NB,
Sunheimer R, Nakatani T, de la Roza G and Haas GP: Occult prostate
cancer effects the results of case-control studies due to
verification bias. Anticancer Res. 28:3007–3010. 2008.PubMed/NCBI
|
|
28
|
Merseburger AS, Matuschek I and Kuczyk MA:
Bladder preserving strategies for muscle-invasive bladder cancer.
Curr Opin Urol. 18:513–518. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Galgano SJ, Porter KK, Burgan C and
Rais-Bahrami S: The role of imaging in bladder cancer diagnosis and
staging. Diagnostics (Basel). 10:7032020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mirmomen SM, Shinagare AB, Williams KE,
Silverman SG and Malayeri AA: Preoperative imaging for locoregional
staging of bladder cancer. Abdom Radiol (NY). 44:3843–3857. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee CH, Tan CH, Faria SC and Kundra V:
Role of imaging in the local staging of urothelial carcinoma of the
bladder. AJR Am J Roentgenol. 208:1193–1205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aydh A, Abufaraj M, Mori K, Quhal F,
Pradere B, Motlagh RS, Mostafaei H, Karakiewicz PI and Shariat SF:
Performance of fluoro-2-deoxy-D-glucose positron emission
tomography-computed tomography imaging for lymph node staging in
bladder and upper tract urothelial carcinoma: A systematic review.
Arab J Urol. 19:59–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kibel AS, Dehdashti F, Katz MD, Klim AP,
Grubb RL, Humphrey PA, Siegel C, Cao D, Gao F and Siegel BA:
Prospective study of [18F]fluorodeoxyglucose positron emission
tomography/computed tomography for staging of muscle-invasive
bladder carcinoma. J Clin Oncol. 27:4314–4320. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Babjuk M, Böhle A, Burger M, Capoun O,
Cohen D, Compérat EM, Hernández V, Kaasinen E, Palou J, Rouprêt M,
et al: EAU Guidelines on non-muscle-invasive urothelial carcinoma
of the bladder: Update 2016. Eur Urol. 71:447–461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rafiemanesh H, Lotfi Z, Bakhtazad S,
Ghoncheh M and Salehiniya H: The epidemiological and histological
trend of bladder cancer in Iran. J Cancer Res Ther. 14:532–536.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bader MJ, Sroka R, Gratzke C, Seitz M,
Weidlich P, Staehler M, Becker A, Stief CG and Reich O: Laser
therapy for upper urinary tract transitional cell carcinoma:
Indications and management. Eur Urol. 56:65–71. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vicente-Rodriguez J, Chéchile G, Algaba F
and Amaral J Jr: Value of random endoscopic biopsy in the diagnosis
of bladder carcinoma in situ. Eur Urol. 13:150–152. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sakr SA, Abdel-Wahed MM and El-Sahra DG:
Immunohistochemical differential diagnosis between urothelial
carcinoma and prostate adenocarcinoma among Egyptian patients.
Biomed Pharmacother. 68:685–692. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim DH, Joo JE, Kim EK, Lee HJ and Lee WM:
The expressions of cytokeratin 7 and 20 in epithelial tumors: A
survey of 91 cases. Cancer Res Treat. 35:355–363. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chaux A, Han JS, Lee S, Gonzalez-Roibon N,
Sharma R, Burnett AL, Cubilla AL and Netto GJ: Immunohistochemical
profile of the penile urethra and differential expression of GATA3
in urothelial versus squamous cell carcinomas of the penile
urethra. Hum Pathol. 44:2760–2767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Akhtar M, Rashid S, Gashir MB, Taha NM and
Al Bozom I: CK20 and CK5/6 immunohistochemical staining of
urothelial neoplasms: A perspective. Adv Urol. 2020:49202362020.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kata SG and Aboumarzouk O: Are we closer
to seeing carcinoma in situ in the upper urinary tract? Cent
European J Urol. 69:157–161. 2016.PubMed/NCBI
|
|
43
|
Kim B, Garcia F, Touma N, Moussa M and
Izawa JI: A rare case of penile cancer in situ metastasizing to
lymph nodes. Can Urol Assoc J. 1:404–407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Avrach WW and Christensen HE:
Metastasizing erythroplasia Queyrat. Report of a case. Acta Derm
Venereol. 56:409–412. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Eng TY, Petersen JP, Stack RS and Judson
PH: Lymph node metastasis from carcinoma in situ of the penis: A
case report. J Urol. 153:432–434. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Banys M, Hahn M, Gruber I, Krawczyk N,
Wallwiener M, Hartkopf A, Taran FA, Röhm C, Kurth R, Becker S, et
al: Detection and clinical relevance of hematogenous tumor cell
dissemination in patients with ductal carcinoma in situ. Breast
Cancer Res Treat. 144:531–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Narod SA and Sopik V: Is invasion a
necessary step for metastases in breast cancer? Breast Cancer Res
Treat. 169:9–23. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Klein CA: Parallel progression of primary
tumours and metastases. Nat Rev Cancer. 9:302–312. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang HY, Wu CY, Chen JJ and Lee TH:
Treatment strategies and metabolic pathway regulation in urothelial
cell carcinoma: A comprehensive review. Int J Mol Sci. 21:89932020.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Yuasa T, Urakami S and Yonese J: Recent
advances in medical therapy for metastatic urothelial cancer. Int J
Clin Oncol. 23:599–607. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Rhea LP, Mendez-Marti S, Kim D and
Aragon-Ching JB: Role of immunotherapy in bladder cancer. Cancer
Treat Res Commun. 26:1002962021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ten Eyck JE, Kahlon N, Masih S, Hamouda DM
and Petros FG: Clinical evaluation of avelumab in the treatment of
advanced urothelial carcinoma: Focus on patient selection and
outcomes. Cancer Manag Res. 14:729–738. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Tassinari E, Mollica V, Nuvola G,
Marchetti A, Rosellini M and Massari F: Treatment options for
metastatic urothelial carcinoma after first-line chemotherapy.
Cancer Manag Res. 14:1945–1960. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Fenton SE and VanderWeele DJ:
Antibody-drug conjugates and predictive biomarkers in advanced
urothelial carcinoma. Front Oncol. 12:10693562023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Longo N, Celentano G, Napolitano L, La
Rocca R, Capece M, Califano G, Collà Ruvolo C, Mangiapia F, Fusco
F, Morra S, et al: Metastasis-directed radiation therapy with
consolidative intent for oligometastatic urothelial carcinoma: A
systematic review and meta-analysis. Cancers (Basel). 14:23732022.
View Article : Google Scholar : PubMed/NCBI
|