Introduction
Cervical cancer is the fourth most common malignant
tumor in the world and the most common malignant tumor of the
female reproductive system (1). It
is also the only malignant tumor with clear etiology that can be
prevented early and for which intervention is possible through
vaccination (2). In 2018, there
were ~570,000 new cases of cervical cancer worldwide, accounting
for 3.15% of all malignant tumors and ~310,000 deaths, accounting
for 3.26% of all malignant tumor deaths (3). Clinically, cervical cancer has no
obvious symptoms in the early stage, and the main symptoms in the
middle and advanced stages include increased leucorrhea and vaginal
contact bleeding (4). Early
cervical precancerous lesions have no visible symptoms, such as
watery vaginal secretions, postcoital bleeding and intermittent
drip bleeding (5). The early
symptoms of patients are often ignored due to the lack of
specificity; therefore, early cervical cancer screening is of value
for the prevention of cervical cancer (6). Persistent infection with HR-HPV has
been reported to be the main cause of cervical cancer (7). HR-HPV is a small double-stranded DNA
virus that can cause skin and mucous membrane lesions in humans.
Based on its carcinogenic risk, HPV is divided into low- and
high-risk HPV types (8,9). Persistent HR-HPV infection may
progress to CIN and eventually to invasive cervical cancer
(10). Clinical data have indicated
that persistent infection with high-risk human papillomavirus
(HR-HPV) is related to the incidence of cervical cancer (11). It has been reported that ≥70% of
women will have ≥1 HPV infection in their lifetime, but such
infections can be naturally cleared by the body's own immune system
in most cases, and only 1–4% of persistent HPV infections will
gradually develop into precancerous lesions or cervical cancer
(12). Therefore, HPV infection can
be regarded as being analogous to a ‘common cold’ of the cervix.
Clinical data also show that high-grade cervical intraepithelial
neoplasia (CIN) can potentially develop into invasive carcinoma
(13,14).
CIN is an important stage in the prevention and
treatment of cervical cancer. Early treatment of precancerous
lesions and the blocking of their further development into cancer
can effectively reduce the occurrence of cervical cancer (15). Colposcopy, an important method of
examination for cervical cancer screening, supports the early
detection and diagnosis of the disease. With digital colposcope
amplification technology, changes in the surface of the cervix can
be accurately and clearly observed, and the location of abnormal
cells can be clearly identified through biopsy. However,
false-negative phenomena may occur owing to the actions of the
examiner themselves (16). Previous
studies (12,17) have also reported that because of the
confusion of normal and abnormal transformation areas, the
difficulty in detecting endocervical lesions and the lack of
specificity in images and other factors, colposcopy can lead to
misdiagnosis. Therefore, it is necessary to explore a more accurate
examination method to improve the rate of cervical cancer detected
by early screening. In previous study (18), HR-HPV testing combined with
colposcopy was used to diagnose cervical cancer. In the present
study, the receiver operating characteristic (ROC) curve was used
to analyze the diagnostic value of HR-HPV testing combined with
colposcopy in differentiating cervical cancer from precancerous
lesions, and to provide a reference for the clinical diagnosis and
identification of this disease.
Materials and methods
Clinical data
A total of 397 patients with cervical cancer or
precancerous lesions, aged 26–71 years (average, 38.60±6.15 years
(mean ± SD), were diagnosed from August 2020 to December 2021 in
Jinan Licheng District Maternal and Child Health Care Family
Planning Service Center, China and were included in the present
study. The patients have 0–6 previous pregnancies with a mean of
(2.15±1.26) and the number of live births was 0–5 times with a mean
of (1.31±1.17 times. There were 136 cases with smooth cervix, 118
grade I cervical erosion, 100 grade II cervical erosion, and 43
grade III cervical erosion (19).
There were also 10 cases of menopause and 387 cases of
non-menopausal. Pathological diagnosis was divided into CIN I (mild
dysplasia; n=153 cases), CIN II (moderate dysplasia; n=101 cases),
CIN III (severe dysplasia and carcinoma in situ; n=86 cases)
and cervical cancer (n=57) (20).
The present study was reviewed and approved by the ethics committee
of Jinan Licheng District Maternal and Child Health Care Family
Planning Service Center, Jinan, Shandong, China. Samples are
collected and processed in February 2022.
Inclusion criteria
Patients who fulfilled the following criteria were
included in the present study: i) HR-HPV DNA detection and
colposcopy; ii) reported subjective symptoms, such as sexual
intercourse bleeding or increased leucorrhea; iii) complete
clinical and imaging data; iv) age ≥18 years and had sexual
experience; and v) patient consented to participate in the present
study.
Exclusion criteria
The following criteria were used to exclude patients
from the present study: i) Treatment with radiotherapy and/or
chemotherapy; ii) pregnancy or breastfeeding; iii) history of
cervical surgery; iv) other gynecological tumors; v) infections
with other viruses; vi) vaginal infectious lesions; vii) autoimmune
diseases; viii) history of hysterectomy; ix) <24 h since last
sexual intercourse; x) Within 48 h of vaginal medication; and xi)
active menstruation.
HR-HPV detection
The cervix was fully exposed with a vaginal
speculum, and a special HPV sampling brush rotated around the
cervix counterclockwise 3–5 times for ~10 sec. The sampling brush
was then removed and put in Digene sample storage solution. The
upper part of the sampling brush was discarded, and the reagent
bottle was covered with a cap. A total of 13 types of HR-HPV
including types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and
68 were assessed using the hybridization capture method using an
HCII-HPV-DNA genetic hybridization detection system (hybrid capture
II) (21,22) and an HCII reagent kit (Shanghai
Yaoyun Biotechnology Co., Ltd.), which was performed according to
manufacturer's protocols. All detection procedures and results were
strictly determined in accordance with the kit instructions. The
positive determination criteria were relative light unit (RLU)/cut
off (CO) ≥1.0 of the specimen.
Colposcopy
The domestic VIZ-GD optical and electronic
integrated colposcopy system (Beijing Siwei Saiyang Technology Co.,
Ltd.) was used to examine patients in the non-menstrual phase of
the menstrual cycle who had not had cervical intercourse and
neither drug administration, smear and irrigation, nor other
gynecological examinations were performed within 24 h before the
examination. Patients with acute inflammation were examined at a
later time, after recovery. Patients were placed in lithotomy
position, the bladder was drained and the cervix was exposed using
a vaginal speculum. The cervical surface secretions were wiped with
cotton balls for preliminary analysis, then 3% glacial acetic acid
was applied to the cervix for 1 min. Compound iodine solution was
applied to columnar and squamous epithelia, and their
transformation areas. The suspicious parts or iodine-free areas
were identified under colposcopy, and cervical canal scratching or
biopsy was performed. Biopsies were collected at the 3, 6, 9 and 12
o'clock positions on the cervix (23).
A total of 12 sagittal sections were taken at 12
o'clock in the cervical cone section, which were quickly frozen and
fixed with 10% formaldehyde. Then hematoxylin was nucleated for 3–5
min. After washing, 0.5% weak ammonia water returned to blue, eosin
was re-stained to cytoplasm for 1 min. After washing, gradient
ethanol was dehydrated, xylene transparent and neutral gum tablets
were sealed, and the sections were observed under a light
microscope. The pathological results were diagnosed by two senior
pathologists, and the results of the colposcopy were described and
diagnosed according to the new colposcopy terms of the 2011
International Federation for Cervical Pathology and Colposcopy
(24). The pathological diagnostics
were based on the International Cooperation on Cancer Reporting
(25); pathological diagnosis is
the gold standard for diagnosis of cervical cancer. Under
colposcopy, white acetic acid epithelium, with the severity of the
lesion positively associated with the whiteness of the epithelium,
white glands and rings, heterogenous vessels and punctured vessels
were observed. The Reid colposcopy index (RCI) score was proposed
by Reid in 1984; it can reduce the subjectivity of colposcopy
diagnosis and is currently the most widely accepted colposcopy
scoring system (26). Previous
studies (27,28) reported that colposcopy diagnosis
using RCI has a good consistency with histopathological diagnosis.
RCI was adopted for diagnosis and scored as follows: 1–2 points was
regarded as CIN I; 3–4 points was regarded as CIN II; and 5–6
points was regarded as CIN III.
Data comparison
Observation indicators were as follows: i)
HR-HPV-positive rate was compared among patients with different
lesion types, and the consistency of colposcopy and pathological
examination results was analyzed; ii) the diagnostic value and
efficacy of HR-HPV testing, colposcopy and combined examination for
cervical cancer and precancerous lesions were compared using
pathological examination results as the gold standard; and iii) the
detection rates were compared among patients with different types
of cervical lesions by colposcopy, and the association between
HR-HPV-positive rate and the severity of cervical lesions was
analyzed.
Statistical analysis
Data were processed using SPSS (version 22.0; IBM
Corp.). The positive rate of HR-HPV and sensitivity, specificity
were expressed as percentage, and the difference between groups was
compared using the χ2 test. The measurement data were
expressed as mean ± SD after Kolmogorov-Smirov normality testing.
The diagnostic value of HR-HPV testing, colposcopy and combined
examination for cervical cancer and precancerous lesions was
analyzed using the receiver operating characteristic (ROC) curve,
sensitivity and specificity were calculated according to the Jorden
index (29). MedCalc (version 19.4;
Beijing Huanzhong Ruichi Technology Co., Ltd.) software was used to
analyze z-score, and the diagnostic efficiency of combined
diagnosis and individual diagnosis of each index was compared.
Cohen's κ coefficient test was used to analyze the consistency
between the results of colposcopy and pathological examination.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HR-HPV testing results in patients
with cervical cancer and precancerous lesions
The positive rate of HR-HPV in patients with
cervical cancer was significantly higher than that in patients with
precancerous lesions, and the positive rate of HR-HPV in patients
with CIN I was significantly lower than that in patients with CIN
II and CIN III (both P<0.05; Table
I). There was no significant difference in the HR-HPV-positive
rates between patients with CIN II and CIN III (P>0.05). These
findings indicated that the detection rate of HR-HPV in patients
with cervical cancer increased with increasing degree of
lesion.
 | Table I.Comparison of HR-HPV testing results
in patients with cervical cancer, CIN I, CIN II and CIN III. |
Table I.
Comparison of HR-HPV testing results
in patients with cervical cancer, CIN I, CIN II and CIN III.
| HR-HPV type | CIN I (n=153) | CIN II (n=101) | CIN III (n=86) | Cervical cancer
(n=57) | χ2 | P-value |
|---|
| 16, n | 12 | 17 | 10 | 13 |
|
|
| 18, n | 3 | 3 | 5 | 2 |
|
|
| 31, n | 1 | 2 | 10 | 7 |
|
|
| 33, n | 3 | 7 | 5 | 8 |
|
|
| 35, n | 2 | 2 | 6 | 2 |
|
|
| 39, n | 0 | 2 | 3 | 1 |
|
|
| 45, n | 0 | 5 | 3 | 1 |
|
|
| 51, n | 0 | 6 | 2 | 0 |
|
|
| 52, n | 0 | 5 | 6 | 0 |
|
|
| 56, n | 1 | 2 | 2 | 4 |
|
|
| 58, n | 0 | 6 | 3 | 1 |
|
|
| 59, n | 1 | 2 | 2 | 0 |
|
|
| 68, n | 18 | 3 | 1 | 0 |
|
|
| Multiple
HR-HPV | 15 | 22 | 13 | 17 |
|
|
| Total, n (%) | 56 (36.60) | 84
(83.17)a | 71
(82.56)a,b | 56
(98.25)a,b | 110.9 | <0.001 |
Consistency of vaginal and
pathological examination results in patients with cervical cancer
and precancerous lesions
Cohen's κ coefficient of vaginal examination results
and pathological examination results in patients with cervical
cancer and precancerous lesions was 0.622, the diagnostic accuracy
was 90.43% (n=359/397), the positive predictive value was 65.57%
(n=40/61) and the negative predictive value was 94.94% (n=319/336)
(Table II). These findings
indicated that colposcopy had high consistency with pathological
examination of cervical cancer and precancerous lesions.
 | Table II.Consistency comparison between
vaginal examination results and pathological examination results in
patients with cervical cancer and precancerous lesions. |
Table II.
Consistency comparison between
vaginal examination results and pathological examination results in
patients with cervical cancer and precancerous lesions.
|
| Pathological
result |
|
|---|
| Examination
method |
|
|
|---|
| Positive | Negative | Total, n |
|---|
| Colposcopy |
|
|
|
|
Positive | 40 | 21 | 61 |
|
Negative | 17 | 319 | 336 |
| Total, n | 57 | 340 | 397 |
Differential value analysis of
colposcopy combined with HR-HPV testing for cervical cancer and
precancerous lesions
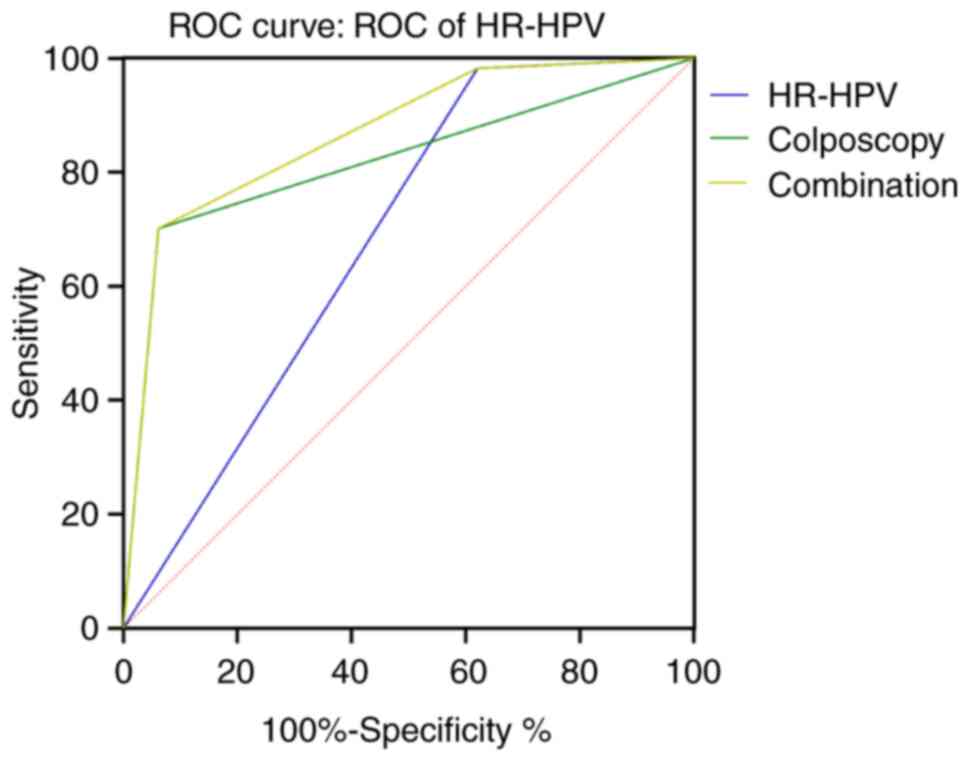
The area under the curve (AUC) of combined
colposcopy and HR-HPV testing for the identification of cervical
cancer and precancerous lesions was greater than that of colposcopy
or HR-HPV testing alone (Table
III; Fig. 1). This result
indicated that combined examination was better than either HR-HPV
testing and colposcopy alone in differentiating between cervical
cancer and precancerous lesions. Combined diagnosis is superior to
HR-HPV diagnosis alone (z=8.749, P<0.0001); Combined diagnosis
is better than colposcopic diagnosis alone (z=3.620; P=0.0003).
 | Table III.Differential value analysis of
colposcopy combined with HR-HPV testing for cervical cancer and
precancerous lesions. |
Table III.
Differential value analysis of
colposcopy combined with HR-HPV testing for cervical cancer and
precancerous lesions.
| Examination
method | Area under the
curve | SEM | 95% CI | Sensitivity, % | Specificity, % |
|---|
| HR-HPV | 0.681a | 0.031 | 0.620–0.742 | 98.25 | 37.94 |
| Colposcopy | 0.820a | 0.037 | 0.747–0.893 | 93.82 | 70.18 |
| Combination | 0.868 | 0.027 | 0.816–0.920 | 70.18 | 93.82 |
Comparison of the detection rate of
different types of precancerous cervical lesions by colposcopy
The detection rate of cervical cancer by colposcopy
was lower than the detection rates for CIN I, CIN II and CIN III
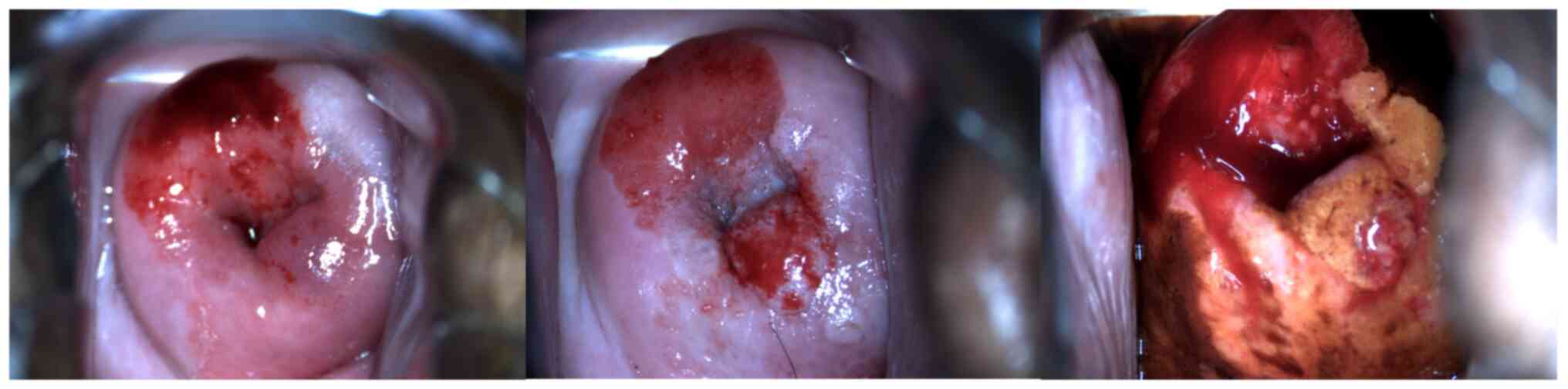
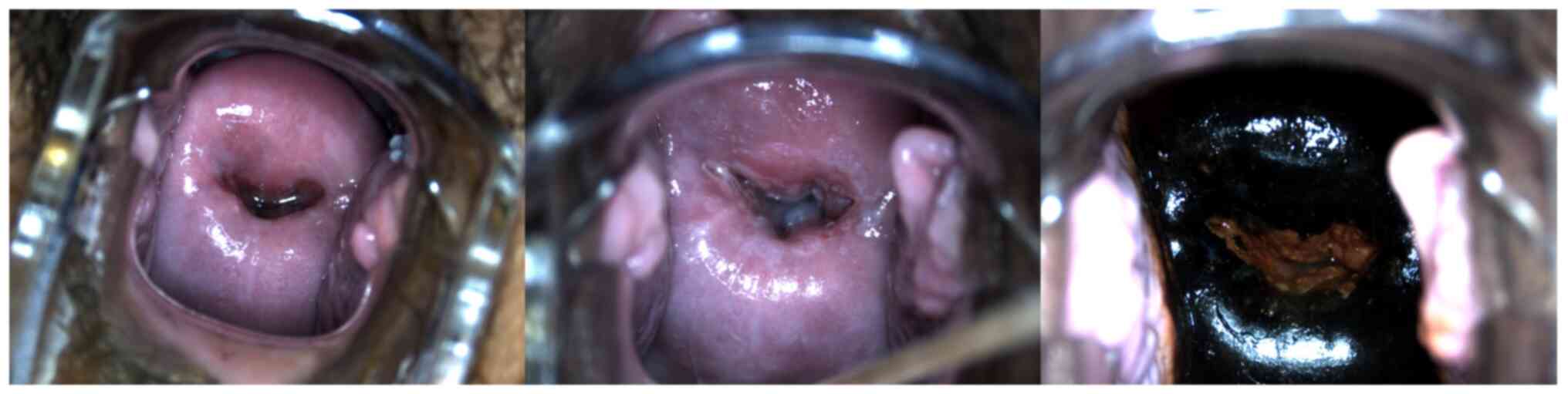
(P<0.05; Table IV; Fig. 2, Fig.
3, Fig. 4, Fig. 5, Fig.
6). This result indicated that the rate of precancerous lesions
detected by colposcopy was higher than that of cervical cancer.
 | Table IV.Comparison of detection rates of
different types of cervical precancerous lesions by colposcopy. |
Table IV.
Comparison of detection rates of
different types of cervical precancerous lesions by colposcopy.
| Group | Patients, n | Detection rate, %
(n) |
|---|
| CIN I | 153 | 92.16
(141)a |
| CIN II | 101 | 95.05
(96)a |
| CIN III | 86 | 95.35
(82)a |
| Cervical
cancer | 57 | 70.18 (40) |
Discussion
Previous studies have reported that HR-HPV infection
was closely associated with cervical cancer and precancerous
lesions, and that HR-HPV could be found in almost all samples from
patients with cervical cancer (27,30).
Therefore, the HR-HPV-positive rates in patients with different
cervical lesions were analyzed in the present study. The results
demonstrated that the HR-HPV-positive rate in patients with
cervical cancer was significantly higher than that in patients with
precancerous lesions, and HR-HPV-positive rate in patients with CIN
I was significantly lower than that in patients with CIN II and CIN
III, which suggested that the HR-HPV-positive rate of patients may
be related to the degree of the cervical lesion.
Further analysis in the present study demonstrated
that the HR-HPV-positive rate was associated with the severity of
cervical precancerous lesions, which indicated that the more severe
the cervical lesions were, the higher the HR-HPV-positive rate
might be, which may be related to the fact that persistent HR-HPV
infection is a major risk factor for cervical cancer. However,
previous studies have reported that HR-HPV quantification is not
related to the severity of cervical lesions, and HR-HPV infection
was not exactly related to the degree of cervical lesions (31,32).
The degree of cervical lesions increased, the differentiation and
maturity of abnormal squamous cells decreased, and the tumor cells
appeared apoptotic and necrotic, followed by HPV loss. However, the
host DNA-integrated virus in cervical cancer cells increased, and
the detection value decreased (33,34).
The results of the present study demonstrated that there was no
significant difference in the HR-HPV-positive rates of patients
with CIN II and CIN III, which indicated that CIN II and CIN III
could not be differentiated by detection of the HR-HPV-positive
rate. This may be because there are other factors, in addition to
HPV infection, that affect the progression of cervical lesions,
such as age at first sexual intercourse, number of sexual partners,
multiparity, oral contraceptives, smoking, obesity, nutrition and
exercise (35).
Patients with cervical precancerous lesions often
have no visible symptoms and lack of characteristic cervical
morphological changes, which make it difficult to diagnose early.
Colposcopy is a non-invasive examination instrument that can
replace biopsy examination, improve the accuracy of biopsy and
reduce the misdiagnosis rate (36).
Colposcopy technology uses strong light to penetrate several layers
of epithelial cells into the stroma, which is then reflected to
form an image. By observing the color, configuration, blood vessels
and iodine staining of the image, the location and severity of
cervical lesions can be determined. For smooth uterus or mild
erosion of the cervix, colposcopy can also be used to find early
underlying issues in a timely manner, and localization and biopsy
can be performed under a microscope (37,38).
Previous study (39) have pointed
out that colposcopy also leads to missed diagnosis of cervical
lesions. Under colposcopy, the mucosa of the cervix and vagina is
magnified 10–40 times, allowing physicians to directly observe the
morphology and structure of cervical blood vessels and surface
epithelium, and to identify suspicious lesion areas that are
difficult to be confirmed with the naked eye. However, colposcopy
has certain limitations. For example, physicians observe the
changes of the cervical epithelium under the action of acetic acid
in just a few minutes during a patient's examination, which is
subjective, and changes in the cervical epithelium may also be
affected by factors such as solution application method,
volatilization degree and action time, which may lead to
misdiagnosis and missed diagnosis (40,41).
The present study demonstrated that the accuracy and positive
predictive value of colposcopy in the diagnosis of cervical cancer
and precancerous lesions were both high, but the negative
predictive value of colposcopy was low, which suggested that false
negative phenomenon could be expected to occur in the diagnosis of
cervical cancer. This is possibly related to its dependence on the
subjectivity of the physician at the time of diagnosis, therefore,
patients need colposcopy and HPV joint diagnosis. Furthermore, the
present study demonstrated that the detection rate of cervical
cancer using colposcopy was lower than that for CIN I, CIN II and
CIN III, which indicated that the diagnostic accuracy of cervical
cancer by colposcopy was lower than that of precancerous
lesions.
Colposcopy provides a basis for the final diagnosis
of cervical lesions by locating suspicious lesions and obtaining
biopsy tissues (42). Previous
reports indicate that colposcopy may lead to misdiagnosis of
cervical precancerous lesions (43,44).
Colposcopy magnifies the cervical lesion site aiding the evaluation
of the surface blood vessels and epithelial morphology, which
enables preliminary judgment on the lesion nature and supports
diagnosis through biopsy sampling. However, the accuracy of the
examination results of this method is affected by the subjective
experience of physicians, which may lead to misdiagnosis (45–47).
HR-HPV testing has become an important screening method for
cervical cancer; it not only improves the sensitivity of
cytological screening, but also predicts the development of disease
in patients with normal cytology or atypical squamous cell lesions,
reduces the number of tests in HPV-negative women with abnormal
cytology and reduces medical waste (48–50).
In the present study, the two examination methods were applied in
the clinical diagnosis of cervical cancer and precancerous lesions.
AUC of combined examination in the identification of cervical
cancer and precancerous lesions was significantly greater than that
of colposcopy or HR-HPV testing alone. The AUC value of the
combined examinations was ≥0.9. which suggested that the combined
examination had a high diagnostic value for cervical cancer and
precancerous lesions and may be used in the differential diagnosis
of cervical cancer and precancerous lesions.
In conclusion, HR-HPV testing combined with
colposcopy has diagnostic value for cervical cancer and
precancerous lesions, and the HR-HPV-positive rate was associated
with the severity of cervical lesions. The limitations of the
present study include: i) A total of 13 types of HR-HPV were
detected in the present study, and it is suggested that future
prospective studies should detect more types of HR-HPV, which will
provide reference for clinical diagnosis of cervical cancer and
precancerous lesions caused by HR-HPV; and ii) colposcopy is
affected by the subjective experience of physicians, which is prone
to false negative results. It is suggested that the final results
should be decided after discussions with the physicians, rather
than drawing arbitrary conclusions.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
PW performed the investigation and data curation and
drafted the original manuscript. DG developed the methodology used
and drafted the original manuscript. XY designed the research,
wrote, reviewed and edited the final manuscript. GΖ conceived the
study, and reviewed and edited the original manuscript. PW and GΖ
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was reviewed and approved by the
hospital ethics committee of Jinan Licheng District Maternal and
Child Health Care Family Planning Service Center, Jinan, Shandong,
and was conducted in accordance to the tenets of the Declaration of
Helsinki. Written informed consent was obtained from all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh D, Vignat J, Lorenzoni V, Eslahi M,
Ginsburg O, Lauby-Secretan B, Arbyn M, Basu P, Bray F and
Vaccarella S: Global estimates of incidence and mortality of
cervical cancer in 2020: A baseline analysis of the WHO global
cervical cancer elimination initiative. Lancet Glob Health.
11:e197–e206. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li M, Du X, Lu M, Zhang W, Sun Z, Li L, Ye
M, Fan W, Jiang S, Liu A, et al: Prevalence characteristics of
single and multiple HPV infections in women with cervical cancer
and precancerous lesions in Beijing, China. J Med Virol.
91:473–481. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lin S, Gao K, Gu S, You L, Qian S, Tang M,
Wang J, Chen K and Jin M: Worldwide trends in cervical cancer
incidence and mortality, with predictions for the next 15 years.
Cancer. 127:4030–4039. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shami S and Coombs J: Cervical cancer
screening guidelines: An update. JAAPA. 34:21–24. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Awolude OA, Oyerinde SO, Ayeni AO and
Adewole IF: Human papillomavirus-based cervical precancer screening
with visual inspection with acetic acid triage to achieve same-day
treatments among women living with human immunodeficiency virus
infection: test-of-concept study in Ibadan, Nigeria. Pan Afr Med J.
40:482021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhao H, He Y, Fan B, Wang Y and Wu YM:
Human papillomavirus E6E7 mRNA and TERC lncRNA in situ detection in
cervical scraped cells and cervical disease progression assessment.
Virol J. 19:182022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dong A, Xu B, Wang Z and Miao X:
Survival-related DLEU1 is associated with HPV infection status and
serves as a biomarker in HPV-infected cervical cancer. Mol Med Rep.
25:772022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lyu Y, Ding L, Gao T, Li Y, Li L, Wang M,
Han Y and Wang J: Influencing factors of high-risk human
papillomavirus infection and DNA load according to the severity of
cervical lesions in female coal mine workers of China. J Cancer.
10:5764–5769. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Horn J, Denecke A, Luyten A, Rothe B,
Reinecke-Lüthge A, Mikolajczyk R and Petry KU: Reduction of
cervical cancer incidence within a primary HPV screening pilot
project (WOLPHSCREEN) in Wolfsburg, Germany. Br J Cancer.
120:1015–1022. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jary A, Teguete I, Sidibé Y, Kodio A, Dolo
O, Burrel S, Boutolleau D, Beauvais-Remigereau L, Sayon S, Kampo M,
et al: Prevalence of cervical HPV infection, sexually transmitted
infections and associated antimicrobial resistance in women
attending cervical cancer screening in Mali. Int J Infect Dis.
108:610–616. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu H, Zhao J, Yu W, Zhao J, Wang Z, Jin L,
Yu Y, Han L, Wang L, Zhu H and Li F: Human papillomavirus DNA, HPV
L1 capsid protein and p16(INK4a) protein as markers to predict
cervical lesion progression. Arch Gynecol Obstet. 299:141–149.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bedell SL, Goldstein LS, Goldstein AR and
Goldstein AT: Cervical cancer screening: past, present, and future.
Sex Med Rev. 8:28–37. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen L, Dong B, Zhang Q, Mao X, Lin W,
Ruan G, Kang Y and Sun P: HR-HPV viral load quality detection
provide more accurate prediction for residual lesions after
treatment: A prospective cohort study in patients with high-grade
squamous lesions or worse. Med Oncol. 37:372020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sargent A, Fletcher S, Bray K, Kitchener
HC and Crosbie EJ: Cross-sectional study of HPV testing in
self-sampled urine and comparison with matched vaginal and cervical
samples in women attending colposcopy for the management of
abnormal cervical screening. BMJ Open. 9:e0253882019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Okunade KS: Human papillomavirus and
cervical cancer. J Obstet Gynaecol. 40:602–608. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hernández-López R, Lorincz AT,
Torres-Ibarra L, Reuter C, Scibior-Bentkowska D, Warman R, Nedjai
B, Mendiola-Pastrana I, León-Maldonado L, Rivera-Paredez B, et al:
Methylation estimates the risk of precancer in HPV-infected women
with discrepant results between cytology and HPV16/18 genotyping.
Clin Epigenetics. 11:1402019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Santesso N, Mustafa RA, Schünemann HJ,
Arbyn M, Blumenthal PD, Cain J, Chirenje M, Denny L, De Vuyst H,
Eckert LO, et al: World health organization guidelines for
treatment of cervical intraepithelial neoplasia 2–3 and
screen-and-treat strategies to prevent cervical cancer. Int J
Gynaecol Obstet. 132:252–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zehua W: Obstetrics and gynecology (5th
edition). Obstetrics Gynecol. (5th Edition). 2004.
|
|
19
|
Perkins RB, Guido RS, Castle PE, Chelmow
D, Einstein MH, Garcia F, Huh WK, Kim JJ, Moscicki AB, Nayar R, et
al: 2019 ASCCP risk-based management consensus guidelines for
abnormal cervical cancer screening tests and cancer precursors. J
Low Genit Tract Dis. 24:102–131. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou Y: Investigation of the clinical
application value of HR-HPV DNA combined with liquid based cytology
in colposcopy of cervical cancer. Contrast Media Mol Imaging.
2022:50545072022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Abdel Aziz MT, Abdel Aziz MZ, Atta HM,
Shaker OG, Abdel Fattah MM, Mohsen GA, Ahmed HH and El Derwi DA:
Screening for human papillomavirus (HPV) in Egyptian women by the
second-generation hybrid capture (HC II) test. Med Sci Monit.
12:MT43–MT49. 2006.PubMed/NCBI
|
|
22
|
Poljak M, Marin IJ, Seme K, Brinovec V,
Maticic M, Meglic-Volkar J, Lesnicar G and Vince A:
Second-generation Hybrid capture test and Amplicor monitor test
generate highly correlated hepatitis B virus DNA levels. J Virol
Methods. 97:165–169. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Knoepp SM, Kuebler DL and Wilbur DC:
Resolution of equivocal results with the Hybrid Capture II
high-risk HPV DNA test: A cytologic/histologic review of 191 cases.
Diagn Mol Pathol. 16:125–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Burness JV, Schroeder JM and Warren JB:
Cervical colposcopy: Indications and risk assessment. Am Fam
Physician. 102:39–48. 2020.PubMed/NCBI
|
|
25
|
Zhou Q, Zhang F, Sui L, Zhang H, Lin L and
Li Y: Application of 2011 international federation for cervical
pathology and colposcopy terminology on the detection of vaginal
intraepithelial neoplasia. Cancer Manag Res. 12:5987–5995. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kudela E, Laucekova Z, Nachajova M,
Visnovsky J, Bielik T, Krivus S, Biringer K, Balharek T and Zubor
P: Colposcopic scoring indexes in the evaluation of cervical
lesions with the cytological result of atypical squamous cells,
cannot exclude high-grade lesion. J Obstet Gynaecol Res.
46:314–319. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
McCluggage WG, Judge MJ, Alvarado-Cabrero
I, Duggan MA, Horn LC, Hui P, Ordi J, Otis CN, Park KJ, Plante M,
et al: Data set for the reporting of carcinomas of the cervix:
Recommendations from the international collaboration on cancer
reporting (ICCR). Int J Gynecol Pathol. 37:205–228. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao X, Song S, Wang Y, Mu X and Zhang L:
Effects of photodynamic therapy in the treatment of high-grade
vaginal intraepithelial lesions following hysterectomy and HPV
infection. Photodiagnosis Photodyn Ther. 42:1033362023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Lu F and Yin Y: Applying logistic
LASSO regression for the diagnosis of atypical Crohn's disease. Sci
Rep. 12:113402022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rajaram S and Gupta B: Screening for
cervical cancer: Choices & dilemmas. Indian J Med Res.
154:210–220. 2021.PubMed/NCBI
|
|
31
|
Ping W: Application value of HPV test in
cervical cancer screening of rural women. J Community Med.
14:22016.
|
|
32
|
Rohner E, Edelman C, Sanusi B, Schmitt JW,
Baker A, Chesko K, Faherty B, Gregory SM, Romocki LS, Sivaraman V,
et al: Extended HPV genotyping to compare HPV type distribution in
self- and provider-collected samples for cervical cancer screening.
Cancer Epidemiol Biomarkers Prev. 29:2651–2661. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jespersen MM, Booth BB and Petersen LK:
Can biopsies be omitted after normal colposcopy in women referred
with low-grade cervical cytology? A prospective cohort study. BMC
Womens Health. 21:3942021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang J, Du Y, Dong J, Zhou Y, Wang P,
Zhang X, Chen Y and He P: Clinical significance of genotyping for
human papillomavirus (HPV) 16 18/45 combined with cytology in
cervical exfoliated cells in HPV oncogenic mRNA-positive women.
Gynecol Oncol. 153:34–40. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhu XH, Li XM, Zhang WL, Liao MM, Li Y,
Wang FF, Shang B, Peng LG, Su YJ, You ZJ, et al: Application of
artificial intelligence-assisted diagnosis for cervical
liquid-based thin-layer cytology. Zhonghua Bing Li Xue Za Zhi.
50:333–338. 2021.(In Chinese). PubMed/NCBI
|
|
36
|
Wittenborn J, Weikert L, Hangarter B,
Stickeler E and Maurer J: The use of micro RNA in the early
detection of cervical intraepithelial neoplasia. Carcinogenesis.
41:1781–1789. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang SK, Luo XP, Li ZF, Su Z, Xia JC, Hu
GY, Zhu YJ, Xie LX, Feng XX, Sun XB, et al: Performance of human
papillomavirus typing test in cervical precancer lesions and
cervical cancer screening. Zhonghua Zhong Liu Za Zhi. 42:252–256.
2020.(In Chinese). PubMed/NCBI
|
|
38
|
Newman H, Hu J, Li X, He J, Bradford L,
Shan S, Wu X, Zhu B, Yang W, Fu B, et al: Evaluation of portable
colposcopy and human papillomavirus testing for screening of
cervical cancer in rural China. Int J Gynecol Cancer. 29:23–27.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cong Q, Song Y, Wang Q, Zhang H, Gao S and
Sui L: A retrospective study of cytology, high-risk HPV, and
colposcopy results of vaginal intraepithelial neoplasia patients.
Biomed Res Int. 2018:58948012018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Luo GP, Zeng X, Cao HY, Tang D and Xi MR:
The clinical significance of HPV L1/PD-L1 tests combined with
colposcopy for cervical precancerous lesions and cervical cancer.
Sichuan Da Xue Xue Bao Yi Xue Ban. 52:516–522. 2021.(In Chinese).
PubMed/NCBI
|
|
41
|
Liu Y, Liao J, Yi X, Pan Z, Pan J, Sun C,
Zhou H and Meng Y: Diagnostic value of colposcopy in patients with
cytology-negative and HR-HPV-positive cervical lesions. Arch
Gynecol Obstet. 306:1161–1169. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luo Q, Lang L, Han N, Liang L, Shen L and
Zhang H: Prevalence and genotype distribution of high-risk human
papillomavirus infection among women with cervical cytological
abnormalities in Chongqing, China, 2014–2020. Diagn Cytopathol.
49:1237–1243. 2021. View
Article : Google Scholar : PubMed/NCBI
|
|
43
|
Painter H, Erlinger A, Simon B, Morroni C,
Ramogola-Masire D and Luckett R: Impact of cervicitis on
performance of cervical cancer screening using HRHPV testing and
visual evaluation in women living with HIV in Botswana. Int J
Gynaecol Obstet. 151:144–146. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu G, Sharma M, Tan N and Barnabas RV:
HIV-positive women have higher risk of human papilloma virus
infection, precancerous lesions, and cervical cancer. Aids.
32:795–808. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vodicka EL, Chung MH, Zimmermann MR,
Kosgei RJ, Lee F, Mugo NR, Okech TC, Sakr SR, Stergachis A,
Garrison LP Jr and Babigumira JB: Estimating the costs of HIV
clinic integrated versus non-integrated treatment of pre-cancerous
cervical lesions and costs of cervical cancer treatment in Kenya.
PLoS One. 14:e02173312019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Husaiyin S, Han L, Wang L, Ma C, Ainiwaer
Z, Rouzi N, Akemujiang M, Simayil H, Aniwa Z, Nurimanguli R and
Niyazi M: Factors associated with high-risk HPV infection and
cervical cancer screening methods among rural Uyghur women aged
>30 years in Xinjiang. BMC Cancer. 18:11622018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Garza-Rodríguez ML, Oyervides-Muñoz MA,
Pérez-Maya AA, Sánchez-Domínguez CN, Berlanga-Garza A,
Antonio-Macedo M, Valdés-Chapa LD, Vidal-Torres D, Vidal-Gutiérrez
O, Pérez-Ibave DC and Treviño V: Analysis of HPV integrations in
mexican pre-tumoral cervical lesions reveal centromere-enriched
breakpoints and abundant unspecific HPV regions. Int J Mol Sci.
22:32422021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Dong B, Chen L, Lin W, Su Y, Mao X, Pan D,
Ruan G, Xue H, Kang Y and Sun P: Cost-effectiveness and accuracy of
cervical cancer screening with a high-risk HPV genotyping assay vs.
a nongenotyping assay in China: An observational cohort study.
Cancer Cell Int. 20:4212020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ferrera A, Valladares W, Cabrera Y, de la
Luz Hernandez M, Darragh T, Baena A, Almonte M and Herrero R:
Performance of an HPV 16/18 E6 oncoprotein test for detection of
cervical precancer and cancer. Int J Cancer. 145:2042–2050. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Suwanthananon C and Inthasorn P: A
comparison of the associations of reid colposcopic index and swede
score with cervical histology. J Obstet Gynaecol Res. 46:618–624.
2020. View Article : Google Scholar : PubMed/NCBI
|