Introduction
Glucagonoma is a rare neuroendocrine tumor (NET)
that arises from α-islet cells, representing 1% of all pancreatic
NETs; its annual incidence worldwide is only ~1 per 20 million
(1,2). Overproduction of glucagon, with
necrolytic migratory erythema (NME), is the hallmark of glucagonoma
and typically the first observed symptom. Other clinical findings
include diabetes mellitus, anemia, weight loss, fat leakage and
diarrhea (2,3). The clinical course may also be
complicated by venous thrombosis, pulmonary embolism (30–50%) and
various neuropsychiatric disorders, namely depression, psychosis,
agitation, dementia, paranoid delusions, ataxia, hyperreflexia and
optic atrophy (4,5). Despite a benign nature for some, the
rate of malignant transformation is substantial (50–80%), with
metastases generally present at the time of diagnosis (3,6). The
liver and lymph nodes are the usual sites of spread (6).
Early and accurate diagnosis of glucagonoma may
ensure proper management and improve the prognosis. Currently,
surgical resection is the chief consideration (3), whereas the treatment of metastasis
remains controversial. Thus far, available options [i.e., medical
management, palliative surgery, chemotherapy, somatostatin analog
(SSA) use and others] have yielded poor results in terms of overall
survival and prognosis (7,8). A comprehensive and surgically oriented
approach is perhaps the best means of optimizing long-term
prognosis in instances of metastatic glucagonoma (9).
The present study reports the case of a 32-year-old
female patient with glucagonoma, marked by multiple intrahepatic
metastases and pathognomonic NME. The diagnostic and therapeutic
challenges of managing initially advanced disease and later
postoperative recurrences are also discussed.
Case report
Patient case
A 32-year-old female patient was hospitalized at the
First Hospital of China Medical University (Shenyang, China) in
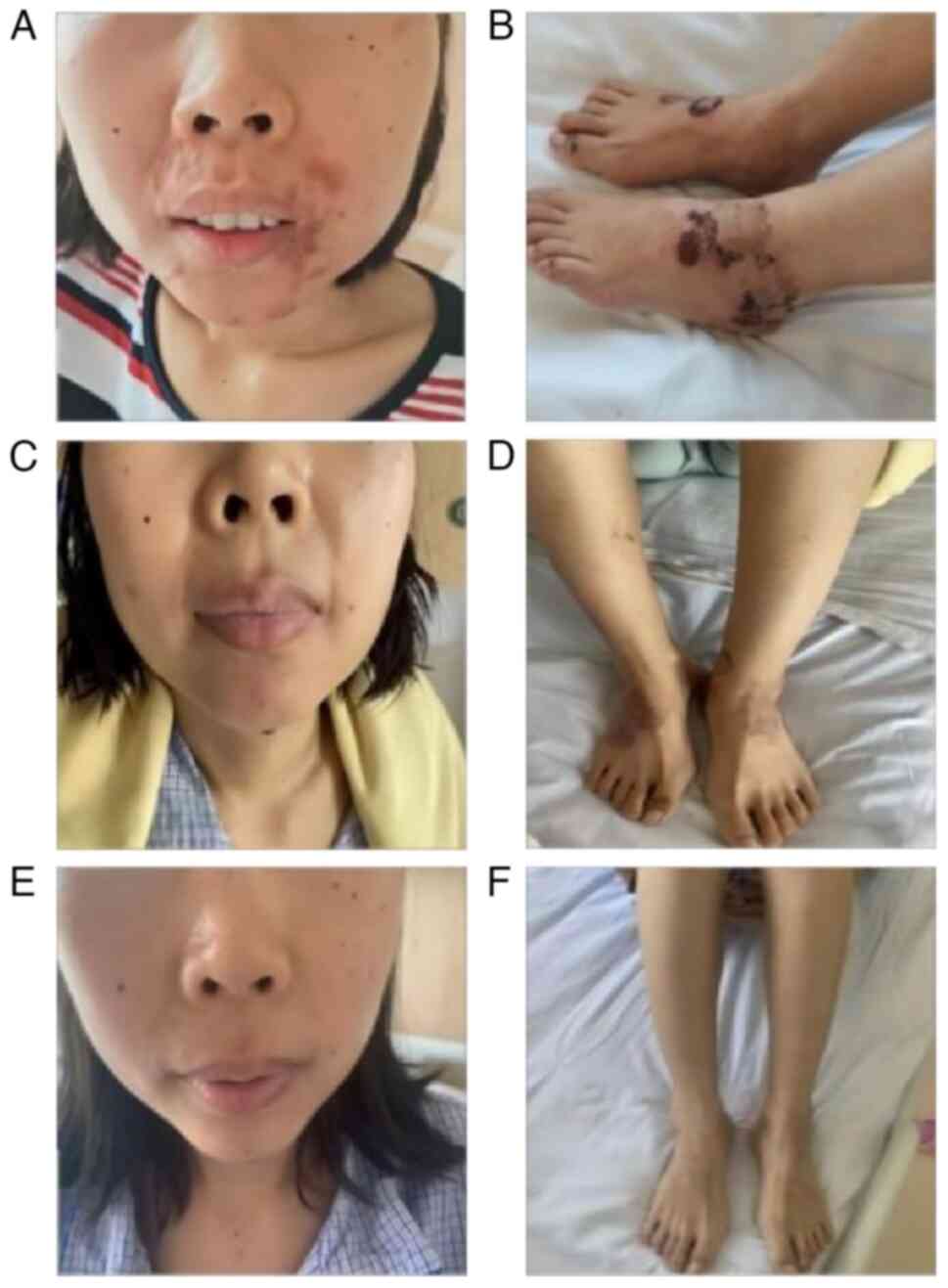
September 2021, primarily for perioral dermatitis (Fig. 1A) and lower-limb NME (Fig. 1B) for a 2-month duration. Glossitis
was also problematic for nearly 6 months. The patient had no family
history of endocrine diseases, particularly diabetes. Laboratory
testing indicated the following: i) mild anemia (Table I); ii) increases in neuron-specific
enolase (NSE) and C-reactive protein (CRP) (Table I); and iii) oral glucose tolerance
test (OGTT) abnormality (Table
II). These findings signaled insulin resistance, despite a
marginally low fasting blood glucose level. Plasma glucagon
analysis indicated >10 times the upper limit of the normal range
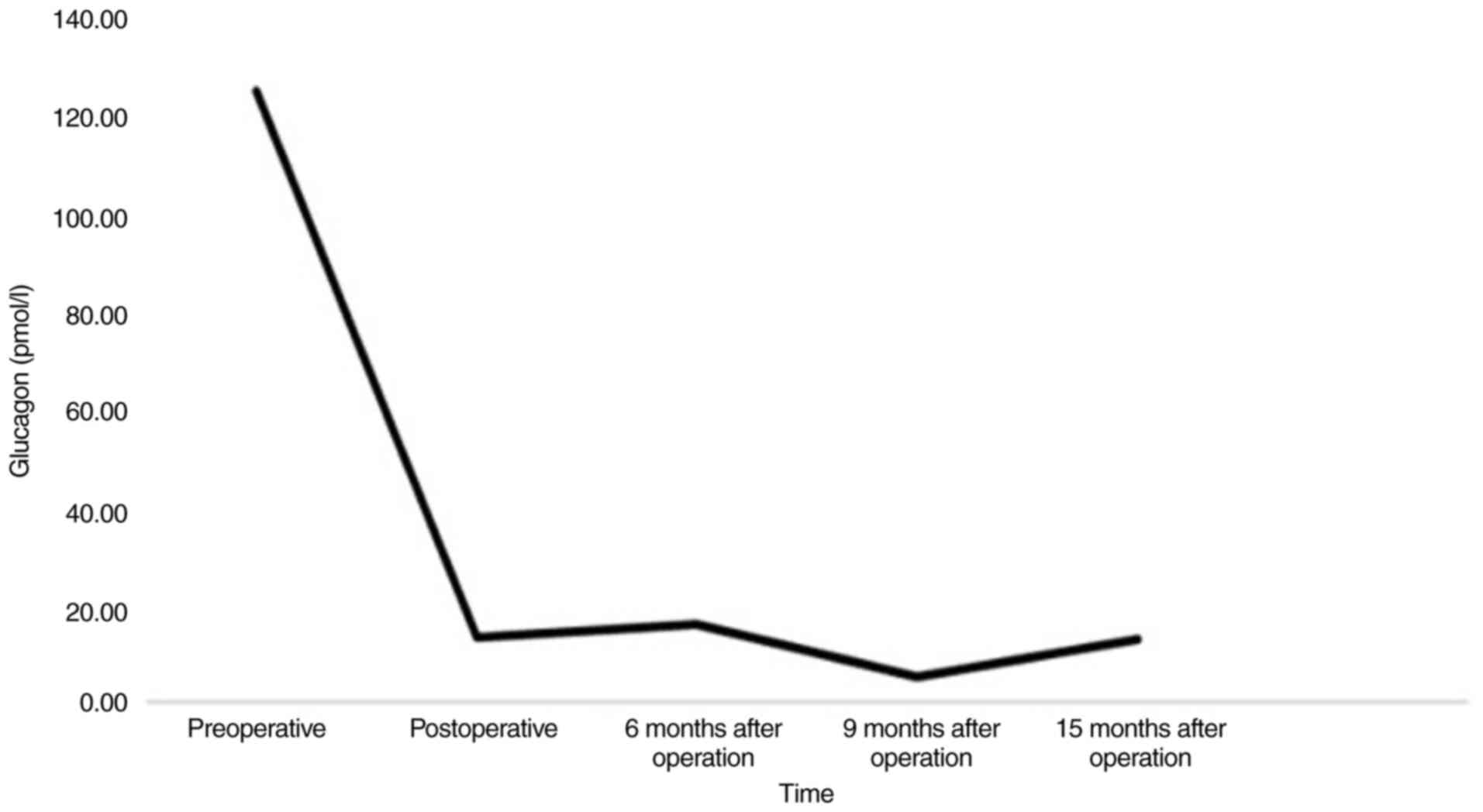
(124.00 pmol/l) (Fig. 2), and serum
prolactin was elevated, but there was no imaging evidence of
adenomas (parathyroid or pituitary) or other related pathology
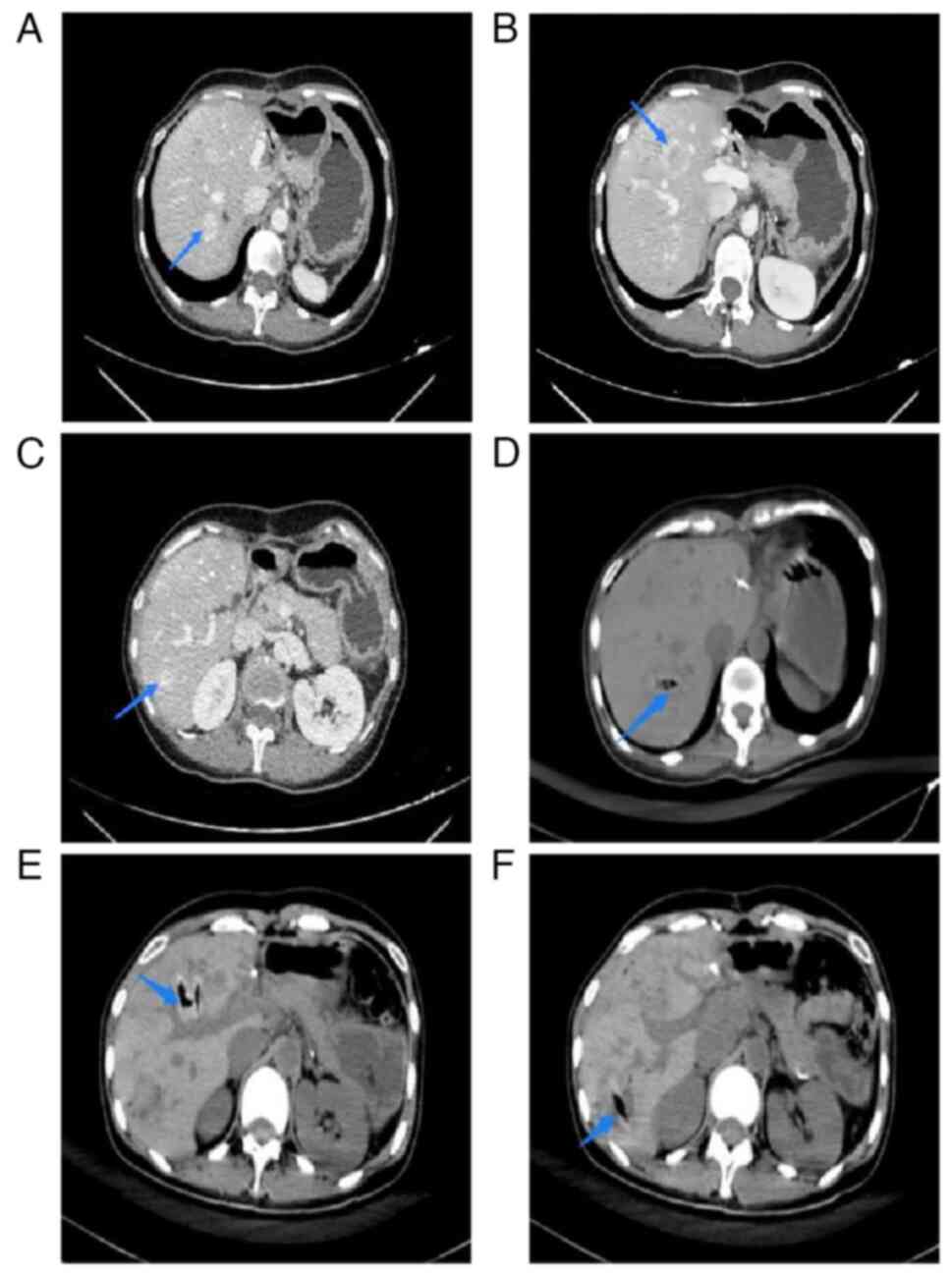
(Table I). On enhanced abdominal
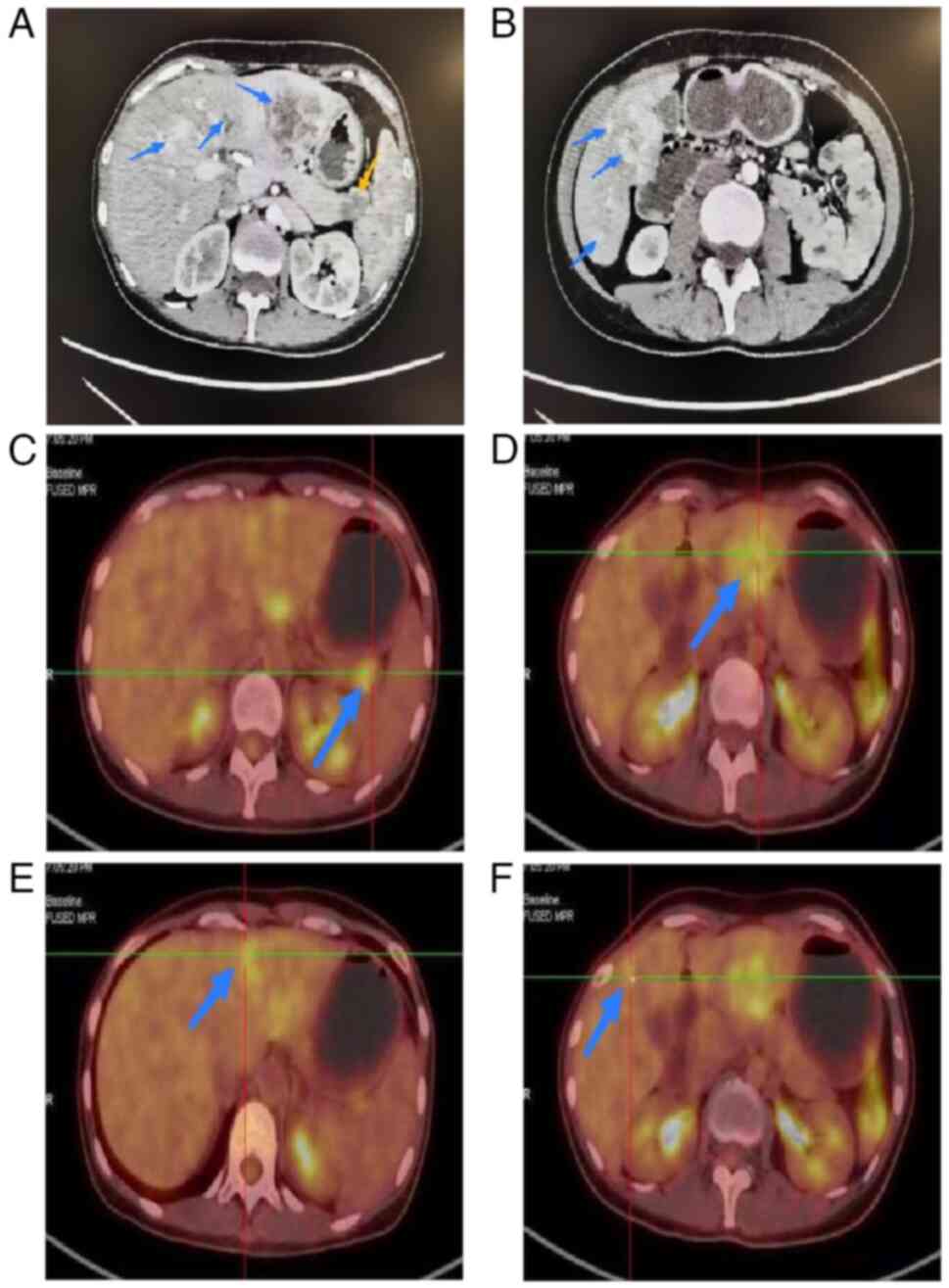
computed tomography (CT), a low-density defect of the pancreatic
tail (~1.9×1.6 cm) and multiple low-density hepatic lesions
(Fig. 3A and B) were visible.
Positron emission tomography-CT confirmed increased metabolic
activity at both pancreatic (Fig.
3C) [maximum standardized uptake value (SUVmax),
3.6] and intrahepatic sites (Fig.
3D-F) SUVmax, 4.5), while excluding involvement
elsewhere. The aforementioned features were interpreted as a
glucagonoma, with multiple intrahepatic metastases.
 | Table I.Clinical data and laboratory
testing. |
Table I.
Clinical data and laboratory
testing.
| Parameter | Value | Reference range |
|---|
| Clinical data |
|
|
| Age,
years | 32 | - |
| Weight,
kg | 45 | - |
|
BMI | 17.58 | - |
| Blood
pressure, mmHg | 125/82 | - |
| Heart
rate | 111 | - |
|
Respiratory rate | 17 | - |
|
Temperature | 36.5 | - |
| Laboratory
data |
|
|
|
K+, mmol/l | 3.50 | 3.50–5.30 |
|
Cl−, mmol/l | 112.0 | 98.0–107.0 |
| RBC,
×1012/l | 3.60 | 3.80–5.10 |
| Hb,
g/l | 99 | 115-150 |
| CRP,
mg/l | 71.00 | 0.00–6.00 |
| GLU,
mmol/l | 3.80 | 3.90–6.10 |
| HbA1C,
% | 5.4 | 3.9–6.1 |
| AST,
U/l | 24 | 13-35 |
| ALT,
U/l | 34 | 7-40 |
| GGT,
U/l | 54 | 7-45 |
| CA19-9,
U/l | 6.29 | 40.00–530.00 |
| CA72-4,
U/l | 2.98 | 0.77–33.03 |
| NSE,
ng/ml | 16.36 | 0.00–16.00 |
| ACTH,
pg/ml | 8.87 | 7.20–63.30 |
| COR,
nmol/l | 471.00 | 171.00–536.00 |
| LH,
mIU/l | 5.51 | 1.10–11.60 |
| FSH,
mIU/l | 10.60 | 2.80–11.30 |
| PRG,
pmol/l | <0.64 | 0.64–3.60 |
| PRL,
mIU/l | 562.00 | 40.00–530.00 |
| E2,
pmol/l | <73.40 | 91.75–275.25 |
 | Table II.Oral glucose tolerance test. |
Table II.
Oral glucose tolerance test.
| Parameter | Normal range | 0 min | 30 min | 60 min | 120 min | 180 min |
|---|
| Glucose,
mmol/l | 3.90–6.10 | 3.97 | 11.70 | 14.42 | 15.00 | 7.27 |
| C-peptide,
pmol/l | 99.90–1242.00 | 836.40 | 2,978.30 | 5,554.80 | 7,652.60 | 6,562.20 |
| Serum insulin,
mIU/l | 4.03–23.46 | 11.65 | 75.26 | 158.60 | 297.30 | 186.70 |
Hepatic spread ordinarily would preclude a complete
resection. However, the patient's liver was functionally intact
(Table I), the patient was young
and in otherwise good health, and no invasion of the main artery
was evident. Consequently, a distal pancreatectomy and splenectomy
(DPS), with palliative resections of the hepatic metastases, was
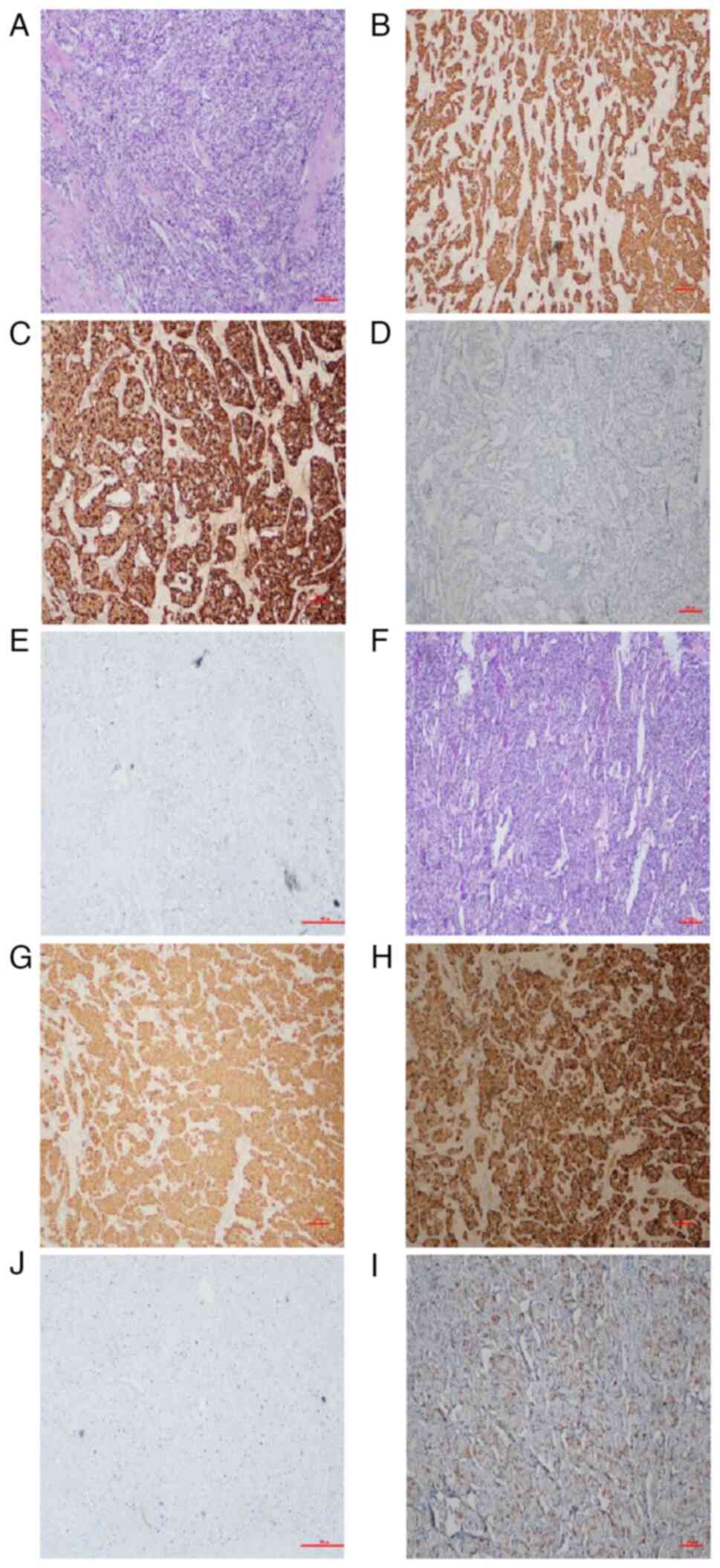
performed. Tissue examination thereafter confirmed a primary
pancreatic neuroendocrine tumor [grade 2 (1)] (Fig.
4A-E), metastatic to the liver (Fig. 4F-J). Both primary and metastatic
lesions proved immunohistochemically positive for glucagon
[pancreas, 40%+ (Fig. 4D); liver,
90%+ (Fig. 4I)], synaptophysin
(Syn) (Fig. 4B and F) and
chromogranin A (CgA) (Fig. 4C and
H). The Ki-67 indices were 15% each (Fig. 4E and J).
After surgery, the patient was administered periodic
intramuscular injections (every 28 days) of a long-acting release
(LAR) octreotide (30 mg) formulation as long-term therapy (a total
of 18 times to date). The erythema of both lower limbs (Fig. 1C and D) resolved by postoperative
day 7, as did the oral manifestations. The serum glucagon level
also normalized (13.34 pmol/l) (Fig.
2), in sharp contrast with the preoperative baseline. However,
multiple low-density hepatic lesions reappeared on the 6-month
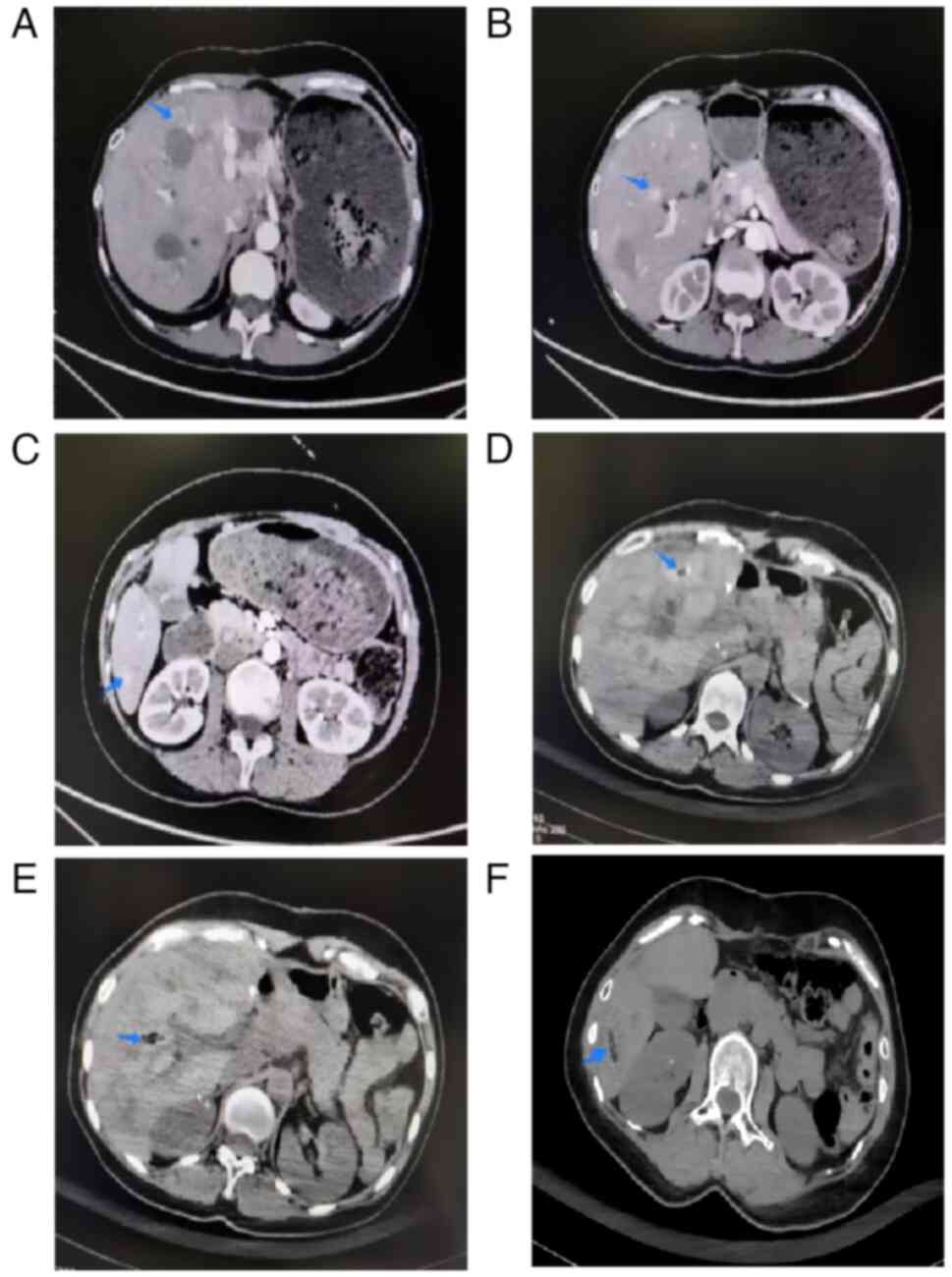
follow-up CT scans. Initially, CT-guided microwave ablation (tumor
in right anterior lobe of liver: 50 W for 8 min; tumor in right
posterior lobe of liver: 60 W for 10 min) was performed, with
post-treatment CT imaging (Before: Fig.
5A-C; after: Fig. 5D-F). CT
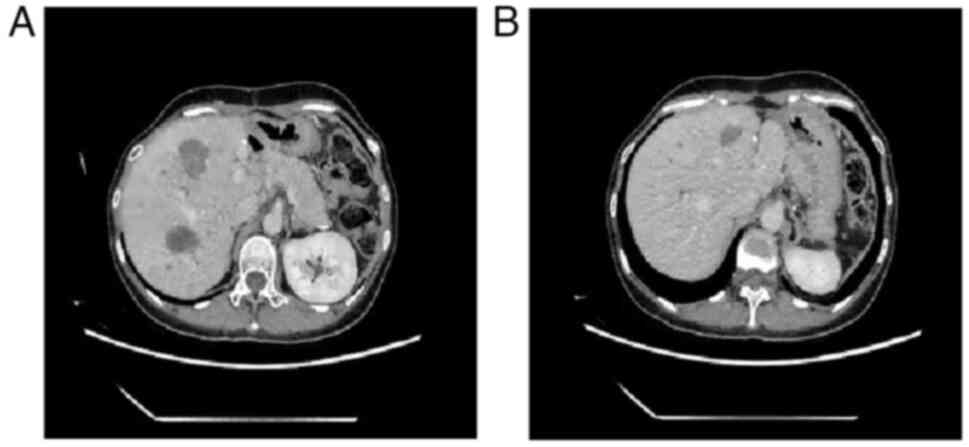
imaging at the patient review performed at 10 months
postoperatively showed that the liver lesions were smaller than
before (Fig. 6A and B). CT-guided
microwave ablation (tumor in right anterior lobe of liver: 70 W for
5 min; tumor in right posterior lobe of liver: 60 W for 8 min;
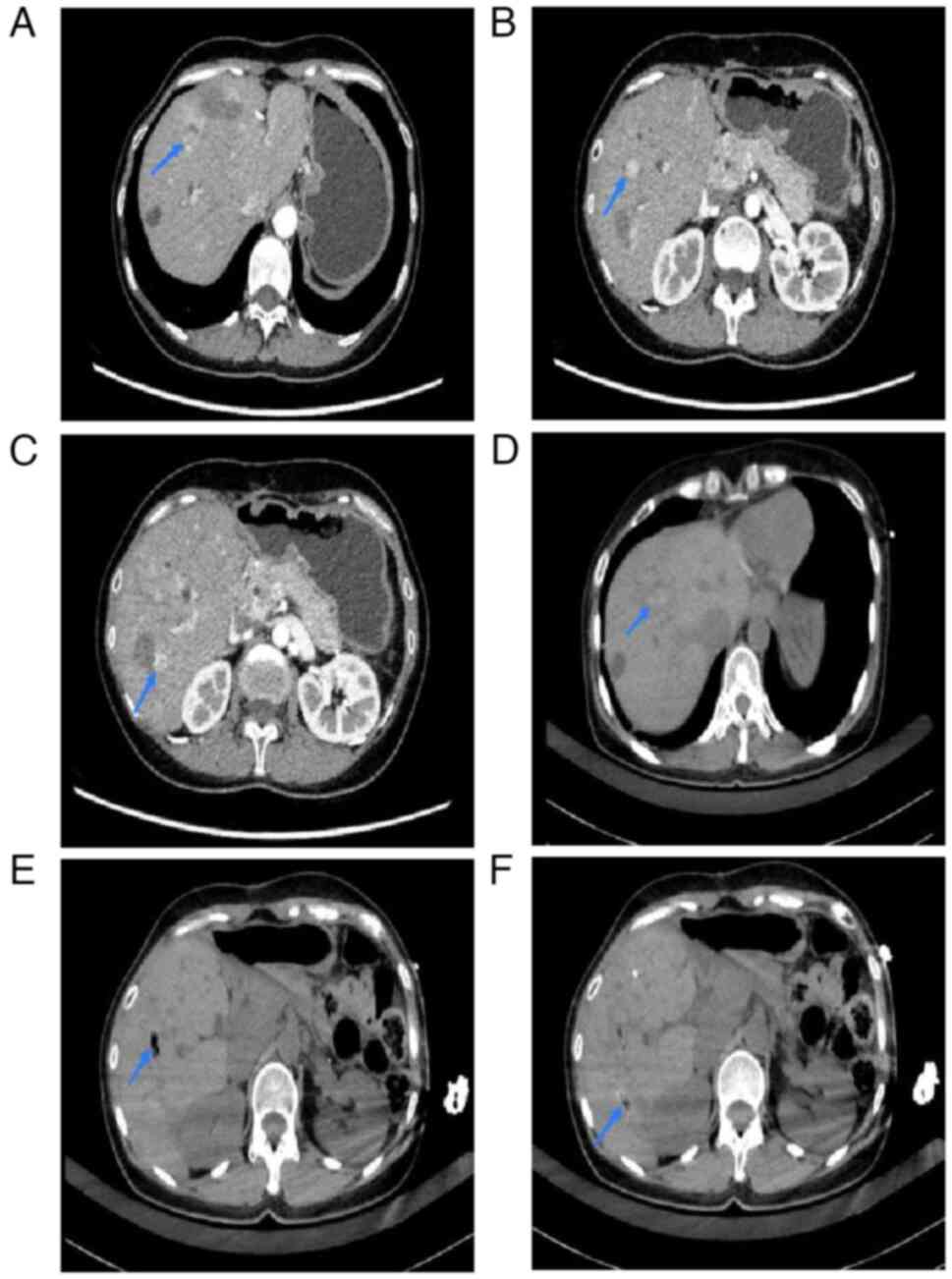
tumor in left medial lobe: 60 W for 5 min) was also undertaken at
18 months postoperatively (before: Fig.
7A-C; after: Fig. 7D-F) and
ablation (lower end of right lobe, top end of diaphragm and right
anterior lobe of liver: 60 W for 8 min each) was performed at 24
months postoperatively (before: Fig.
8A-C; after: Fig. 8D-F),
targeting all tumor recurrences. A percutaneous needle biopsy
obtained prior to ablation disclosed tumor angiogenesis; but the
residual neuroendocrine tumor (grade 2) was no longer positive for
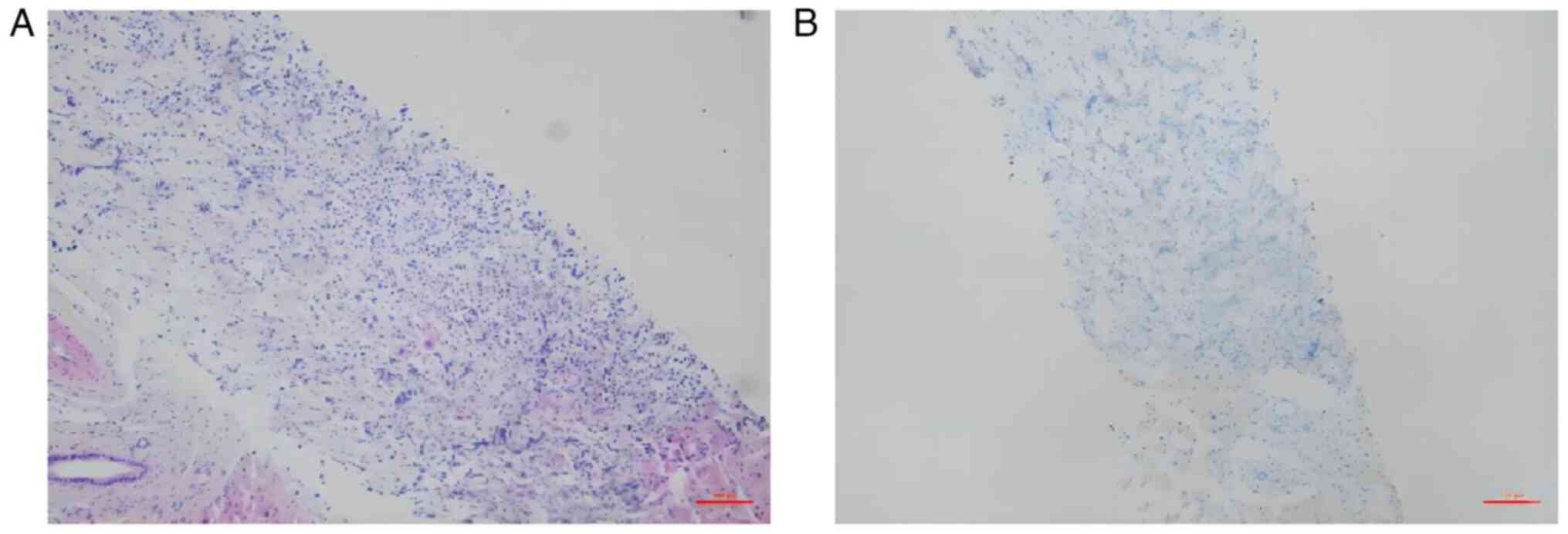
glucagon (Fig. 9), and serum
glucagon levels had stabilized, falling within the normal range
(5.35 pmol/l) (Fig. 2). To date,
intramuscular administration of LAR octreotide has continued every
28 days at the same dose, without complications or adverse
reactions. The patient was treated every 28 days and followed up at
the same time. The last visit was in mid-January 2024. There was no
evidence of local or systemic recurrence.
Pathology
Hematoxylin and eosing staining
Tissues was fixed with 10% neutral formalin at room
temperature for 16–18 h, and then cut into 4-µm thickness. The
sections were dewaxed at 45°C for ~5 min. The sections were stained
with hematoxylin and eosin at room temperature for 3.75 min using
the Roche Ventana HE 600 automatic staining system (Roche
Diagnostics), and then sealed with neutral gum. The staining was
evaluated under a light microscope at ×100 magnification.
Immunohistochemical staining
The sections were dewaxed at 45°C for ~5 min, and
then repaired with immunohistochemical antigen repair solution
(neutral) at 99°C for 20 min. The antibodies (immediate-use Syn
antibody reagent; cat. no. 20180177; immediate-use CgA antibody
reagent; cat. no. 20180186; immediate-use glucagon antibody
reagent; cat. no. 20180317; immediate-use Ki-67 antibody reagent;
cat. no. 20180160) (all Fuzhou Maixin Biotech Co., Ltd.) were
separately added to the BenchMark XT on the Roche Ventana platform
at 32°C for 30 min. After washing with PBS, DAB staining was
performed at 36°C using 25 ml ultraView Universal DAB Inhibitor (3%
H2O2) for 8 min, 25 ml ultraView Universal
HRP Multimer (55 µg/ml) for 8 min, 25 ml ultraView Universal DAB
Chromogen (0.2% w/v DAB) plus 25 ml ultraView Universal DAB
H2O2 (0.04% H2O2) for a total of 8 min, and
25 ml ultraView Universal DAB Copper (5 g/l CuSO4) for 4
min. Washing with PBS was performed between each step. The sections
were then mounted. Evaluation of staining was performed under a
light microscope at ×100 magnification.
Discussion
Glucagonomas are particularly rare neuroendocrine
neoplasms (5,10). The neoplasms manifest clinically as
glucagonoma syndrome, the hallmark of which is NME (10,11).
Most of those previously reported had spread to the liver or lymph
nodes and were overtly malignant, underscoring the importance of
prompt detection (12,13). The patient treated in the present
study was hospitalized primarily for NME, so it is apparent that
clinical factors figure prominently in diagnostic accuracy
(10,14). However, such determinations are not
without difficulties. Most patients are diagnosed with diabetes or
skin disease and the real lesion is missed (15,16).
In our experience, a combination of clinical and laboratory
findings works best, applying present-day diagnostic criteria for
glucagonoma as follows: i) Elevated serum glucagon level by
radioimmunoassay; ii) radiographic or histological evidence of a
neuroendocrine tumor; and iii) characteristic clinical features
(NME) (10,13,17).
The relapse of NME is therefore a pivotal and telling development.
In addition, the patients with pancreatic glucagonomas sometimes
exhibit complications of pituitary and parathyroid tumors (13,15,16).
In the present case, although the enhanced abdominal CT scan of the
patient showed the pancreatic glucagonoma to be hypervascular, the
case was not complicated by pituitary and parathyroid tumors.
Although benign on occasion, glucagonomas are often
malignant and possibly have already disseminated at the time of
discovery. The long-term prognosis is subsequently poor, despite an
array of available therapeutic options (7,8).
Conventional or laparoscopic resection is safe and effective,
associated with low rates of recurrence; however, only 10–20% of
patients are surgically curable, given the propensity for
multicentric tumor dissemination (18). Ultimately, the benefits of surgery
must be weighed against potential complications and mortality risks
(19–21). In instances of liver metastasis, a
surgical solution remains controversial, given the protracted and
unpredictable course of glucagonomas. For the most part, surgical
resection is still the mainstay of treatment for localized disease,
whereas palliative cytoreductive surgery may help relieve symptoms
and effectively confer prognostic improvement. Even with known
metastasis, resecting the primary tumor prolongs patient survival
(21,22).
Hormonal secretion by functional glucagonomas is
most often suppressed through SSA use. These first-line agents for
symptom control also exert certain anti-proliferative tumor
effects, thus prolonging disease-free survival in some patients
(23,24). While undergoing systemic treatment,
patients with liver metastases <5 cm maximally (preferably <3
cm) may qualify for ablative treatments as well (25,26).
Ablative interventions seem to boost symptom relief in this setting
(lasting 14–27 months) and have generated 5-year survival rates of
57–80% (27).
The present patient harbored multiple metastases
upon presentation. However, a younger age and favorable
preoperative status permitted a DPS procedure, with palliative
resections of existing hepatic nodules. Afterwards, the patchy
changes to the facial and lower-leg skin gradually resolved. Serum
glucagon levels were also monitored at intervals and marked
improvement was found postoperatively. After 6 months, several
hepatic lesions were again discovered, and the larger growths were
subjected to percutaneous ablation. For the treatment of
postoperative liver metastases in this patient, ablation therapy
was more desirable than transarterial therapy (25,27).
The patient continued to receive intramuscular injections of LAR
octreotide (30 mg) while undergoing three separate ablative
procedures. All existing hepatic disease was successfully
eradicated as a result. Nonetheless, continued monitoring of serum
glucagon and imaging parameters is obligatory.
To date, the patient's symptoms are gone, and the
postoperative glucagon levels have normalized, aligning with the
results of repeat immunostaining of glucagon expression in a liver
biopsy specimen. This indicates that the patient with multiple
intrahepatic metastases may benefit from palliative surgery,
conducting postoperative ablative treatments as needed during SSA
administration. The prognosis corresponds well with tumor
classification, grading and disease stage. The 5-year overall
survival rate is ~54%, and the 5-year relative survival rates of
localized, locally advanced and metastatic glucagonoma are 93, 77
and 27%, respectively (27,28). Although the expected survival time
in instances of metastatic glucagonoma is ~20 months (28), the clinical course of the present
patient indicates that prolongation is feasible, given a prompt
diagnosis and optimal therapeutic choices.
In the event of multiple liver metastases,
palliative metastasectomies and postoperative ablations may be
beneficial and help prolong survival time, while achieving
hormone-related symptoms control through SSA use (29–32).
In the present study, the LAR formulation of octreotide was found
to be an important and effective long-term therapy, although its
merit may be debated due to the scarcity of data.
In conclusion, in patients with glucagonomas, the
comprehensive treatment of advanced disease is a complex process,
guided by overall patient fitness and tumor characteristics. A
multimodal effort would be ideal, gathering as many patients as
possible for analysis and exploring the full scope of
individualized therapy. Managing these neoplastic oddities may then
become more systematic and uniform to optimize patient
outcomes.
Acknowledgements
The authors would like to thank Professor Chunlin Ge
(Department of Hepatobiliary and Pancreatic Surgery, The First
Hospital of China Medical University, Shenyang, China) for
performing the surgical resections, Professor Yonghui Xia
(Department of Interventional Radiology, The First Hospital of
China Medical University) for performing the percutaneous ablative
procedures, Professor Jin Wang (Department of Medical Oncology, The
First Hospital of China Medical University) for providing the
postoperative therapeutics and Dr Hongjiu Ren (Department of
Clinical Pathology, The First Hospital of China Medical University)
for providing the pathology-related data.
Funding
This study was funded by the Youth Talent Support Program of
China Medical University (grant no. QGZD2018014).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
SY and MG communicated with various departments,
collected patient information, and carried out clinical management
and prognostic follow-up. CZ was responsible for obtaining medical
images and analyzing data related to patient laboratory tests and
imaging findings. LC provided and analyzed disease-related
diagnostic and treatment information. LZ was responsible for the
treatment of the patient, the preoperative clinical management, the
formulation of treatment plans, the completion of the operation
with Professor Chunlin Ge, and the comprehensive treatment in the
perioperative period. SY and LZ confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The patient provided written informed consent to
participate.
Patient consent for publication
The patient provided written informed consent for
publication of this report and the attached images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nagtegaal ID, Odze RD, Klimstra D, Paradis
V, Rugge M, Schirmacher P, Washington KM, Carneiro F and Cree IA;
WHO Classification of Tumours Editorial Board, : The 2019 WHO
classification of tumours of the digestive system. Histopathology.
76:182–188. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schwartz RA: Glucagonoma and
pseudoglucagonoma syndromes. Int J Dermatol. 36:81–89. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stacpoole PW: The glucagonoma syndrome:
Clinical features, diagnosis, and treatment. Endocr Rev. 2:347–361.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yusuf MA, Mehmood S, Iftikhar J, Saqib M,
Siddique MZ and Imtiaz W: Glucagonoma syndrome: A Rare
paraneoplastic disorder due to neuroendocrine tumor of the
pancreas. J Coll Physicians Surg Pak. 32 (Suppl):S147–S149. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eldor R, Glaser B, Fraenkel M, Doviner V,
Salmon A and Gross DJ: Glucagonoma and the glucagonoma
syndrome-cumulative experience with an elusive endocrine tumour.
Clin Endocrinol (Oxf). 74:593–598. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Metz DC and Jensen RT: Gastrointestinal
neuroendocrine tumors: Pancreatic endocrine tumors.
Gastroenterology. 135:1469–1492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toberer F, Hartschuh W and Wiedemeyer K:
Glucagonoma-Associated necrolytic migratory erythema: The broad
spectrum of the clinical and histopathological findings and clues
to the diagnosis. Am J Dermatopathol. 41:e29–e32. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li W, Yang X, Deng Y, Jiang Y, Xu G, Li E,
Wu Y, Ren J, Ma Z, Dong S, et al: Necrolytic migratory erythema is
an important visual cutaneous clue of glucagonoma. Sci Rep.
12:90532022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Doi R: Surgical management of pancreatic
endocrine tumors. Nihon Rinsho. 69 (Suppl 2):S611–S666, (In
Japanese).
|
|
10
|
John AM and Schwartz RA: Glucagonoma
syndrome: A review and update on treatment. J Eur Acad Dermatol
Venereol. 30:2016–2022. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cui M, Wang R and Liao Q: Necrolytic
migratory erythema: An important sign of glucagonoma. Postgrad Med
J. 97:1992021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wermers RA, Fatourechi V, Wynne AG, Kvols
LK and Lloyd RV: The glucagonoma syndrome. Clinical and pathologic
features in 21 patients. Medicine (Baltimore). 75:53–63. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chastain MA: The glucagonoma syndrome: A
review of its features and discussion of new perspectives. Am J Med
Sci. 321:306–320. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tolliver S, Graham J and Kaffenberger BH:
A review of cutaneous manifestations within glucagonoma syndrome:
Necrolytic migratory erythema. Int J Dermatol. 57:642–645. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Song X, Zheng S, Yang G, Xiong G, Cao Z,
Feng M, Zhang T and Zhao Y: Glucagonoma and the glucagonoma
syndrome. Oncol Lett. 15:2749–2755. 2018.PubMed/NCBI
|
|
16
|
He S, Zeng W, Geng S and Jia J:
Glucagonoma syndrome with atypical necrolytic migratory erythema.
Indian J Dermatol Venereol Leprol. 87:49–53. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang ZX, Wang F and Zhao JG: Glucagonoma
syndrome with severe erythematous rash: A rare case report.
Medicine (Baltimore). 98:e171582019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sarmiento JM and Que FG: Hepatic surgery
for metastases from neuroendocrine tumors. Surg Oncol Clin N Am.
12:231–242. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Madoff DC, Gupta S, Ahrar K, Murthy R and
Yao JC: Update on the management of neuroendocrine hepatic
metastases. J Vasc Interv Radiol. 17:1235–1250. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ito T, Igarashi H and Jensen RT: Therapy
of metastatic pancreatic neuroendocrine tumors (pNETs): Recent
insights and advances. J Gastroenterol. 47:941–960. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saeed A, Buell JF and Kandil E: Surgical
treatment of liver metastases in patients with neuroendocrine
tumors. Ann Transl Med. 1:62013.PubMed/NCBI
|
|
22
|
Vaghaiwalla T and Keutgen XM: Surgical
management of pancreatic neuroendocrine tumors. Surg Oncol Clin N
Am. 29:243–252. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kvols LK, Moertel CG, O'Connell MJ, Schutt
AJ, Rubin J and Hahn RG: Treatment of the malignant carcinoid
syndrome. Evaluation of a long-acting somatostatin analogue. N Engl
J Med. 315:663–666. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruszniewski P, Ish-Shalom S, Wymenga M,
O'Toole D, Arnold R, Tomassetti P, Bax N, Caplin M, Eriksson B,
Glaser B, et al: Rapid and sustained relief from the symptoms of
carcinoid syndrome: Results from an open 6-month study of the
28-day prolonged-release formulation of lanreotide.
Neuroendocrinology. 80:244–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Farley HA and Pommier RF: Treatment of
neuroendocrine liver metastases. Surg Oncol Clin N Am. 25:217–225.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kose E, Kahramangil B, Aydin H, Donmez M,
Takahashi H, Aucejo F, Siperstein A and Berber E: Outcomes of
laparoscopic tumor ablation for neuroendocrine liver metastases: A
20-year experience. Surg Endosc. 34:249–256. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mohan H, Nicholson P, Winter DC, O'Shea D,
O'Toole D, Geoghegan J, Maguire D, Hoti E, Traynor O and Cantwell
CP: Radiofrequency ablation for neuroendocrine liver metastases: A
systematic review. J Vasc Interv Radiol. 26:935–942.e1. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dasari A, Shen C, Halperin D, Zhao B, Zhou
S, Xu Y, Shih T and Yao JC: Trends in the incidence, prevalence,
and survival outcomes in patients with neuroendocrine tumors in the
United States. JAMA Oncol. 3:1335–1342. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chan JA and Kulke MH: Medical management
of pancreatic neuroendocrine tumors: Current and future therapy.
Surg Oncol Clin N Am. 25:423–437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Saltz L, Trochanowski B, Buckley M,
Heffernan B, Niedzwiecki D, Tao Y and Kelsen D: Octreotide as an
antineoplastic agent in the treatment of functional and
nonfunctional neuroendocrine tumors. Cancer. 72:244–248. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lo CH, Ho CL and Shih YL: Glucagonoma with
necrolytic migratory erythema exhibiting responsiveness to
subcutaneous octreotide injections. QJM. 107:157–158. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kimbara S, Fujiwara Y, Toyoda M, Chayahara
N, Imamura Y, Kiyota N, Mukohara T, Fukunaga A, Oka M, Nishigori C
and Minami H: Rapid improvement of glucagonoma-related necrolytic
migratory erythema with octreotide. Clin J Gastroenterol.
7:255–259. 2014. View Article : Google Scholar : PubMed/NCBI
|