Introduction
Breast cancer is the most frequently diagnosed
cancer worldwide (1). Although
various systemic therapies have been used in combination with local
treatments in recent years, surgery and postoperative radiation
therapy remain important treatment modalities for localized breast
cancer (2). However, the role of
internal mammary node (IMN) irradiation as a component is a
controversial subject (3).
The IMN is known as an important lymphatic drainage
pathway in breast cancer (4). The
frequency of IMN metastases increases with the number of axillary
lymph node metastases (n=0, 3–6%; n=1-3, 14–26%; n=4-, 20–43%)
(5). However, in clinical practice,
IMN metastatic recurrence is rare (10-year IMN recurrence rate,
1.5%) (6). The National
Comprehensive Cancer Network (NCCN) guidelines recommend IMN
irradiation for patients with breast cancer, whereas the Japanese
Breast Cancer Society Practice Guidelines weakly recommend IMN
irradiation for patients requiring regional lymph node irradiation
(7,8).
Several studies have indicated that in certain
cases, such as those with the presence of IMN metastasis, the
number of axillary node metastases ≥4, or the number of axillary
node metastasis=1-3 with central/medial primary location, may
benefit from IMN irradiation (9–11).
However, it remains uncertain whether mild IMN enlargement
(Mild-IMN), defined as IMN enlargement (<0.5 cm) without
fluorine-18 fluorodeoxyglucose (FDG) uptake and larger than the
contralateral IMN, is a high-risk factor. Although FDG-positron
emission tomography/computed tomography (FDG-PET/CT) has a high
detection power for lymph node metastasis evaluation, some
Mild-IMNs without FDG uptake actually demonstrate IMN metastases
(12–14). Therefore, this study aimed to
investigate the relationship between treatment outcomes and IMN
status in patients with breast cancer treated with postmastectomy
radiation therapy (PMRT).
Materials and methods
Study population
Between January 2011 and December 2018, a total of
296 initial patients with breast cancer (cancer center, 243;
community hospital, 53) were treated with PMRT, which is performed
for the patients with large tumor size (≥T3, Union for
International Cancer Control 8th (15)) and large number of axillary lymph
node metastases (≥4). Patients with the following characteristics
were excluded from the study: (1)
bilateral breast cancer (n=9), (2)
no available follow-up CT images (n=13), and (3) no neoadjuvant chemotherapy (NAC) or
adjuvant chemotherapy (AC) (n=24). Subsequently, we retrospectively
evaluated the remaining 250 breast cancer patients who underwent
PMRT. The present study was approved (approval. no. 2023-526 and
gai 2023-13) by the Ethics Committee of our institutions (National
Hospital Organization Shikoku Cancer Center, Matsuyama, Japan;
Ehime Prefectural Central Hospital, Matsuyama, Japan), and the
opt-out consent was applied because of the retrospective nature of
this study.
Imaging evaluation
Imaging follow-ups with FDG-PET/CT or CT were
performed between 6-month and 1-year after PMRT and subsequently at
approximately 1-year intervals, as determined by the attending
physicians.
Clinically metastatic IMN (cMet-IMN) was defined as
that with a size of ≥0.5 cm or that with FDG uptake (16–18).
Mild-IMN was defined as that with a size of <0.5 cm, lacking FDG
uptake and that larger than the contralateral IMN, a condition not
identified during PMRT planning. Clinically normal IMN (Normal-IMN)
constituted the remaining category. Based on this detailed IMN
status evaluation, patients with breast cancer were divided into
three groups (cMet-IMN, Mild-IMN, and Normal-IMN).
Treatment
All 250 patients underwent mastectomy with axillary
lymph node dissection or sentinel lymph node biopsy. All patients
received neoadjuvant chemotherapy (NAC) or AC before PMRT. A PMRT
dose of 50 Gy in 25 fractions was administered to the chest wall
encompassing the supraclavicular or infraclavicular region and
excluding the axillary region from the treatment region. The IMN
region received additional irradiation in all the 39 patients with
cMet-IMNs. Among these, 25 patients with cMet-IMN received an
additional IMN boost of 10 Gy in 5 fractions, specifically directed
at highly suspected ipsilateral IMN metastasis detected on imaging
examination. The remaining 14 patients with cMet-IMNs did not
receive an additional IMN boost. The cMet-IMN boost was selected
based on the preference of the radiation oncologists (one radiation
oncologist, planning without an IMN boost; the other radiation
oncologists, planning with an IMN boost) and not based on the IMN
size after systemic therapy. Eight patients received an additional
tumor bed boost of 10 Gy in 5 fractions due to a positive surgical
margin. Five patients received a supraclavicular boost of 10 Gy in
five fractions because imaging examinations strongly indicated
ipsilateral supraclavicular node metastasis, and lymph node
resections were not performed.
All the patients were treated with three-dimensional
conformal radiation therapy. Two photon-tangential fields of 4–6 MV
using the field-in-field technique were applied on the chest wall,
with or without the IMN region. Two photon-opposing fields of 4–6
and 10 MV were used in the supraclavicular and infraclavicular
regions, respectively. A single-electron filed of 4–12 MeV was used
for the IMN boost and positive surgical margin boost. Two 10 MV
photon-opposing fields were used in the supraclavicular boost
plan.
NAC or AC was administered to all the patients. The
anthracycline with or without taxane regimen, such as EC
(epirubicin and cyclophosphamide) ± DTX/PTX/nab-PTX (docetaxel,
paclitaxel, or nab-paclitaxel) ± HER (trastuzumab) (n=48), FEC
(5-fluorouracil, epirubicin, and cyclophosphamide) ± DTX/PTX ± HER
(n=55), AC (doxorubicin and cyclophosphamide) ± DTX/PTX ± HER
(n=18), were used in NAC. Similarly, the anthracycline with or
without taxane regimen, such as EC ± DTX/PTX/nab-PTX ± HER (n=47),
FEC ± DTX/PTX ± HER (n=38), AC ± DTX ± HER (n=28), were used in AC.
Taxane-based regimens such as TC (taxane and cyclophosphamide) +
HER (n=1) were used for NAC. Similarly, taxane-based regimens such
as TC ± HER (n=13) were used in AC. Additionally,
tegafur/gimeracil/oteracil (n=2) was used in AC. After PMRT, 36
patients were treated with HER ± hormonal therapy, four patients
were treated with chemotherapy, such as tegafur/gimeracil/oteracil
(n=3) or capecitabine (n=1), and 154 patients were treated with
hormonal therapy. Of these, the most commonly used regimens were EC
(epirubicin 90 mg/m2 i.v./cyclophosphamide 600
mg/m2 i.v. q21 for 4 cycles) or FEC (5-fluorouracil 500
mg/m2 i.v./epirubicin 60–100 mg/m2
i.v./cyclophosphamide 500 mg/m2 i.v. q21 for 4
cycles).
Breast cancer was classified into four groups
according to estrogen receptor (ER), progesterone receptor (PR) and
human epidermal growth factor receptor 2 (HER2) status. Based on
the immunohistochemistry (IHC), ER-positive (≥1%) and PR-positive
(≥1%) were determined. HER2-positive was determined by IHC or
fluorescence in situ hybridization (FISH). In detail, based on IHC,
HER2 protein expression rates were classified into four groups (0
to 3+). The cases of 2+ HER2 protein expression rates were reviewed
by FISH. Finally, the cases that were positive by FISH and 3+ by
IHC were determined to be HER2-positive. Luminal A-like breast
cancer is ER-positive and PR-positive, HER2-negative. Luminal
B-like HER2-positive breast cancer is ER-positive and
HER2-positive. Luminal B-like HER2-negative breast cancer is
ER-positive and HER2-negative. Non-luminal HER2-positive breast
cancer is ER-negative, PR-negative, and HER2-positive.
Triple-negative breast cancer is ER-negative, PR-negative, and
HER2-negative. The number of patients with luminal A-like, luminal
B-like HER2-positive, luminal B-like HER2-negative, non-luminal
HER2-positive, and triple-negative tumors was 98 (39.2%), 27
(10.8%), 40 (16.0%), 34 (13.6%), and 51 (20.4%), respectively. In
this study, treatment outcomes were analyzed by these ER, PR, and
HER2 status. In addition, nuclear grade was evaluated according to
the criteria of the National Surgical Adjuvant Study of Breast
Cancer (NSAS-B) protocol (19).
Statistical analysis
Survival and recurrence-free times were calculated
from the initiation of PMRT for breast cancer. The Kaplan-Meier
method was used to generate curves for overall survival (OS),
disease-free survival (DFS), and IMN recurrence-free survival (IRF)
rates. Univariate and multivariate Cox proportional hazards models
were used to determine hazard ratios (HRs), 95% confidence
intervals (CIs), and p values. Statistical significance was defined
as p ≤0.05. Statistical analyses were performed using the JMP
software (JMP version 14.3.0; SAS Institute, Cary, NC, USA).
Results
Clinical characteristics
A total of 217 (86%) patients had ductal carcinoma,
with 188 classified as scirrhous, 20 as solid tubular, and 9 in
other categories. All 39 patients with cMet-IMN showed FDG uptake,
with a median cMet-IMN size of 1.1 cm (range, 0.6–2.1 cm). These
patients received radiation to the IMN region in addition to
radiation to the chest wall, supraclavicular, or infraclavicular
regions. Among them, 25 (64.1%) patients received boost irradiation
in the IMN region (10 Gy in 5 fractions). Thirty-nine patients with
Mild-IMN did not exhibit FDG uptake, whereas the remaining 172
patients had Normal-IMN. General condition was assessed using the
Eastern Cooperative Oncology Group Performance Status (ECOG-PS),
with PS=0 in 94.4% (n=236) of the patients (20). Further details regarding the patient
characteristics are presented in Table
I.
 | Table I.Characteristics. |
Table I.
Characteristics.
| Characteristics | Value |
|---|
| Age, years |
|
| Median
(range) | 55 (30–86) |
| <55, n
(%) | 129 (48.4) |
| ≥55, n
(%) | 121 (51.6) |
| ECOG-PS, n (%) |
|
| 0 | 236 (94.4) |
| 1 | 14 (5.6) |
| cT stage (UICC 8th),
n (%) |
|
| 1 | 31 (12.4) |
| 2 | 140 (56.0) |
| 3 | 47 (18.8) |
| 4 | 32 (12.8) |
| cN stage (UICC 8th),
n (%) |
|
| 0 | 40 (16.0) |
| 1 | 117 (46.8) |
| 2 | 34 (13.6) |
| 3 | 59 (23.6) |
| cTNM stage (UICC
8th), n (%) |
|
| 1 | 14 (5.6) |
| 2 | 114 (45.6) |
| 3 | 122 (48.8) |
| Histologic type, n
(%) |
|
| Invasive
ductal carcinoma | 217 (86.8) |
| Invasive
lobular carcinoma | 16 (6.4) |
|
Others | 17 (6.8) |
| Nuclear grade, n
(%) |
|
| 1 | 38 (15.2) |
| 2 | 81 (32.4) |
| 3 | 92 (36.8) |
|
Unknown | 39 (15.6) |
| Laterality, n
(%) |
|
| Left | 141 (56.4) |
|
Right | 109 (43.6) |
| Tumor location, n
(%) |
|
|
Medial/central | 119 (47.6) |
|
Lateral | 131 (52.4) |
| ER status, n (%) |
|
|
Positive | 182 (72.8) |
|
Negative | 68 (27.2) |
| PR status, n (%) |
|
|
Positive | 146 (58.4) |
|
Negative | 104 (41.6) |
| HER2, n (%) |
|
|
Positive | 61 (24.4) |
|
Negative | 189 (75.6) |
| IMN status, n
(%) |
|
|
cMet-IMN | 39 (15.6) |
|
Mild-IMN | 39 (15.6) |
|
Normal-IMN | 172 (68.8) |
| RT schedule, n
(%) |
|
| PMRT
alone | 212 (84.8) |
| PMRT +
boost | 38 (15.2) |
|
IMN boost | 25 (10.0) |
|
Positive surgical
margin boost | 8 (3.2) |
|
Supraclavicular
lymph node boost | 5 (2.0) |
| NAC or AC, n
(%) |
|
|
NAC | 122 (48.8) |
|
Anthracycline with
or without taxane regimen | 121 (48.4) |
|
Taxane-based
regimen | 1 (0.4) |
| AC | 128 (51.2) |
|
Anthracycline with or without
taxane regimen | 113 (45.2) |
|
Taxane-based regimen | 13 (5.2) |
|
Tegafur/gimeracil/oteracil | 2 (0.8) |
| Systemic therapy
after PMRT, n (%) |
|
|
Trastuzumab and/or hormonal
therapy | 36 (14.4) |
|
Hormonal therapy | 154 (61.6) |
|
Others | 4 (1.6) |
| No | 56 (22.4) |
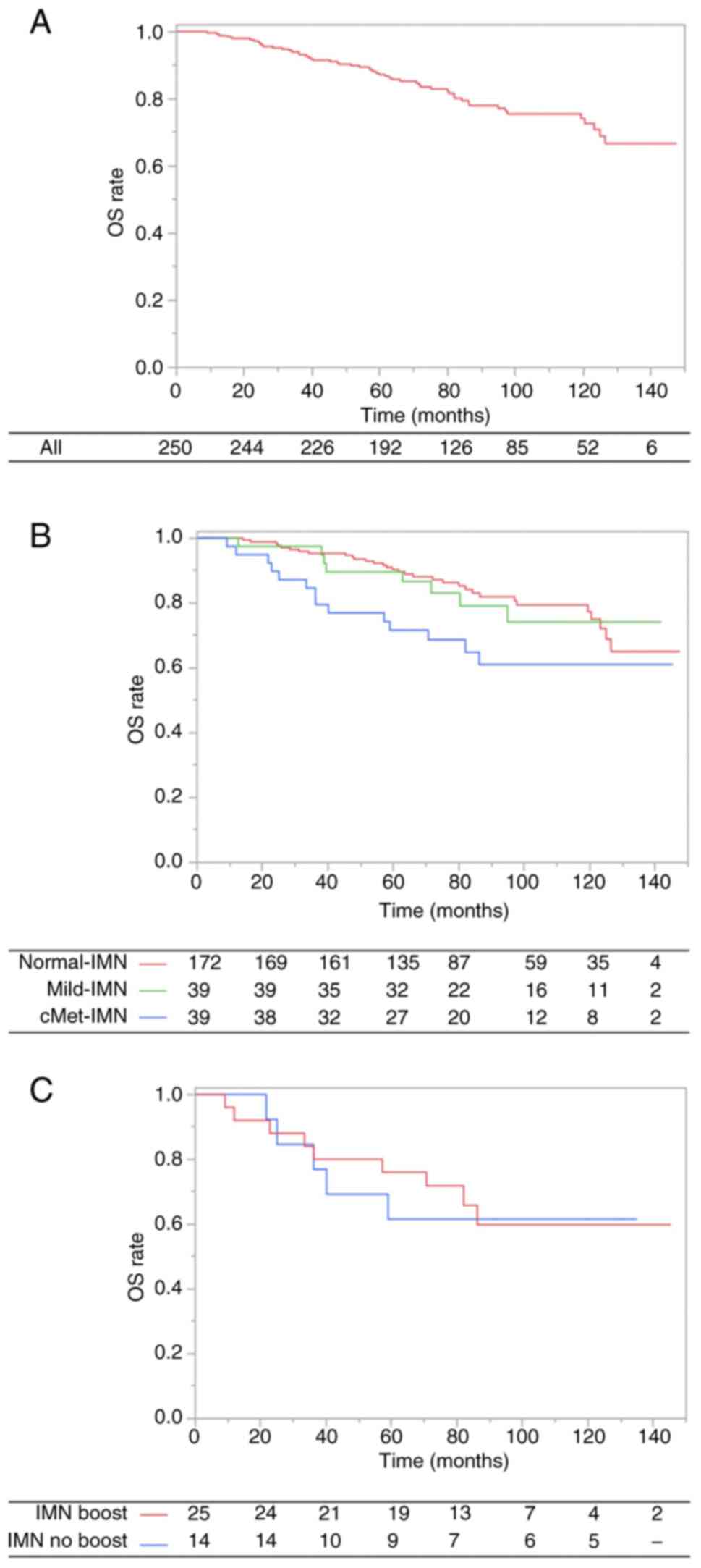
Overall survival
The median follow-up time for OS was 80.0 months
(range, 7.2–147.6 months). At the time of analysis, 54 patients
(Normal-IMN, 32; Mild-IMN, 8; cMet-IMN, 14) had died. Thirty-three
patients experienced cause-specific death was 33 (84.6%) patients
(Table SI). The 7-year OS rate was
80.2% (Normal-IMN, 84.2%; Mild-IMN, 79.1%; cMet-IMN, 64.8%;
Fig. 1A and B).
In univariate analysis, age (<55 years vs. ≥55
years; HR, 1.79; 95% CI, 1.03–3.10; P=0.04), progesterone receptor
(PR) status (positive vs. negative; HR, 1.70; 95% CI, 1.00–2.90;
P=0.05), and IMN status (Normal-IMN vs. cMet-IMN; HR, 2.08; 95% CI,
1.11–3.90; P=0.02) were identified as significant factors for OS.
However, in IMN status, Mild-IMN did not have an impact on OS
(Normal-IMN vs. Mild-IMN; HR, 1.04, 95% CI, 0.48–2.27; P=0.92). In
multivariate analysis, age (HR, 1.90; 1.03–3.50; P=0.04) and IMN
status (Normal-IMN vs. cMet-IMN; HR, 1.93; 95% CI, 1.01–3.68;
P=0.05) remained significant factors for OS. These findings are
presented in Table II.
 | Table II.UVA and MVA for overall survival. |
Table II.
UVA and MVA for overall survival.
|
| UVA | MVA |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<55 years
vs. ≥55 years) | 1.79
(1.03–3.10) | 0.04 | 1.90
(1.03–3.50) | 0.04 |
| cT stage (UICC 8th)
(1–2 vs. 3–4) | 1.29
(0.74–2.26) | 0.37 | - | - |
| cN stage (UICC 8th)
(0–1 vs. 2–3) | 1.67
(0.98–2.84) | 0.06 | - | - |
| Nuclear grade (1–2
vs. 3) | 1.39
(0.80–2.41) | 0.24 | - | - |
| Laterality (left
vs. right) | 1.11
(0.65–1.92) | 0.70 | - | - |
| Tumor location
(medial/central vs. lateral) | 0.91
(0.53–1.55) | 0.72 | - | - |
| ER status (positive
vs. negative) | 1.50
(0.85–2.64) | 0.16 | - | - |
| PR status (positive
vs. negative) | 1.70
(1.00–2.90) | 0.05 | 1.44
(0.79–2.62) | 0.23 |
| HER2 status
(positive vs. negative) | 1.98
(0.96–4.07) | 0.06 | - | - |
| IMN size
(Normal-IMN vs. Mild-IMN) | 1.04
(0.48–2.27) | 0.92 | - | - |
| IMN size
(Normal-IMN vs. cMet-IMN) | 2.08
(1.11–3.90) | 0.02 | 1.93
(1.01–3.68) | 0.05 |
Furthermore, for the patients with cMet-IMN, the use
of IMN boost did not yield a significant impact on OS (IMN boost
vs. IMN no boost; HR, 1.12; 95% CI, 0.37–3.34; P=0.84; Fig. 1C). Similarly, among patients with
large cMet-IMN (size of ≥1.0 cm), the IMN boost did not
significantly impact OS (IMN boost vs. IMN no boost; HR, 2.02; 95%
CI, 0.25–16.50; P=0.51).
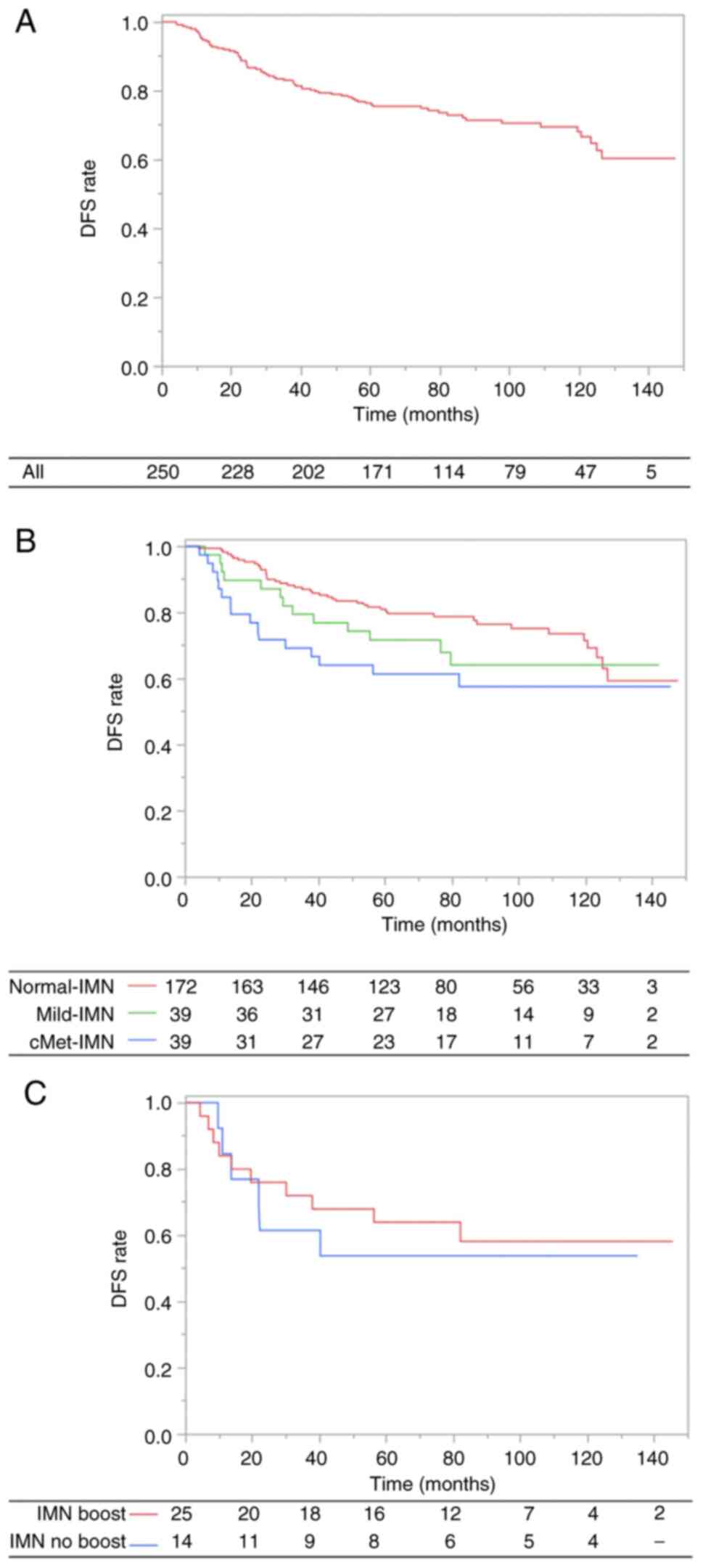
Disease-free survival and IMN
The median follow-up duration for DFS was 74.1
months (range, 4.0–147.6 months). The 7-year DFS rate was 73.0%
(Normal-IMN, 78.8%; Mild-IMN, 64.2%; cMet-IMN, 57.6%; Fig. 2A and B). Sixty-three patients
experienced recurrence and 57 experienced simultaneous recurrence
in multiple locations. The most frequent site of recurrence was
distant metastases (n=54).
In univariate analysis, HER2 status (positive vs.
negative) and IMN status (Normal-IMN vs. cMet-IMN) were identified
as significant factors for DFS. However, in terms of IMN status,
Mild-IMN did not have an influence on the DFS (Normal-IMN vs.
Mild-IMN; HR, 1.34, 95% CI, 0.72–2.49; P=0.36). In multivariate
analysis, IMN status (Normal-IMN vs. cMet-IMN; HR, 1.91; 95% CI,
1.08–3.39; P=0.03) remained a significant factor for DFS. These
findings are presented in Table
III.
 | Table III.UVA and MVA for disease-free
survival. |
Table III.
UVA and MVA for disease-free
survival.
|
| UVA | MVA |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<55 years
vs. ≥55 years) | 1.21
(0.77–1.93) | 0.41 | - | - |
| cT stage (UICC 8th)
(1–2 vs. 3–4) | 1.25
(0.77–2.03) | 0.37 | - | - |
| cN stage (UICC 8th)
(0–1 vs. 2–3) | 1.55
(0.97–2.45) | 0.06 | - | - |
| Nuclear grade (1–2
vs. 3) | 1.35
(0.84–2.15) | 0.21 | - | - |
| Laterality (left
vs. right) | 1.16
(0.73–1.84) | 0.54 | - | - |
| Tumor location
(medial/central vs. lateral) | 0.88
(0.55–1.39) | 0.58 | - | - |
| ER status (positive
vs. negative) | 1.13
(0.67–1.88) | 0.65 | - | - |
| PR status (positive
vs. negative) | 1.29
(0.81–2.05) | 0.28 | - | - |
| HER2 status
(positive vs. negative) | 1.92
(1.03–3.58) | 0.04 | 1.71
(0.87–3.38) | 0.12 |
| IMN size
(Normal-IMN vs. Mild-IMN) | 1.34
(0.72–2.49) | 0.36 | - | - |
| IMN size
(Normal-IMN vs. cMet-IMN) | 1.90
(1.07–3.37) | 0.03 | 1.91
(1.08–3.39) | 0.03 |
In addition, for the patients with cMet-IMN, IMN
boost did not have a significant impact on DFS (IMN boost vs. IMN
no boost; HR, 1.20; 95% CI, 0.44–3.30; P=0.73; Fig. 2C). Similarly, for the patients with
large cMet-IMN (size of ≥1.0 cm), the IMN boost did not have a
significant impact on DFS (IMN boost vs. IMN no boost; HR, 1.13;
95% CI, 0.24–5.31; P=0.88).
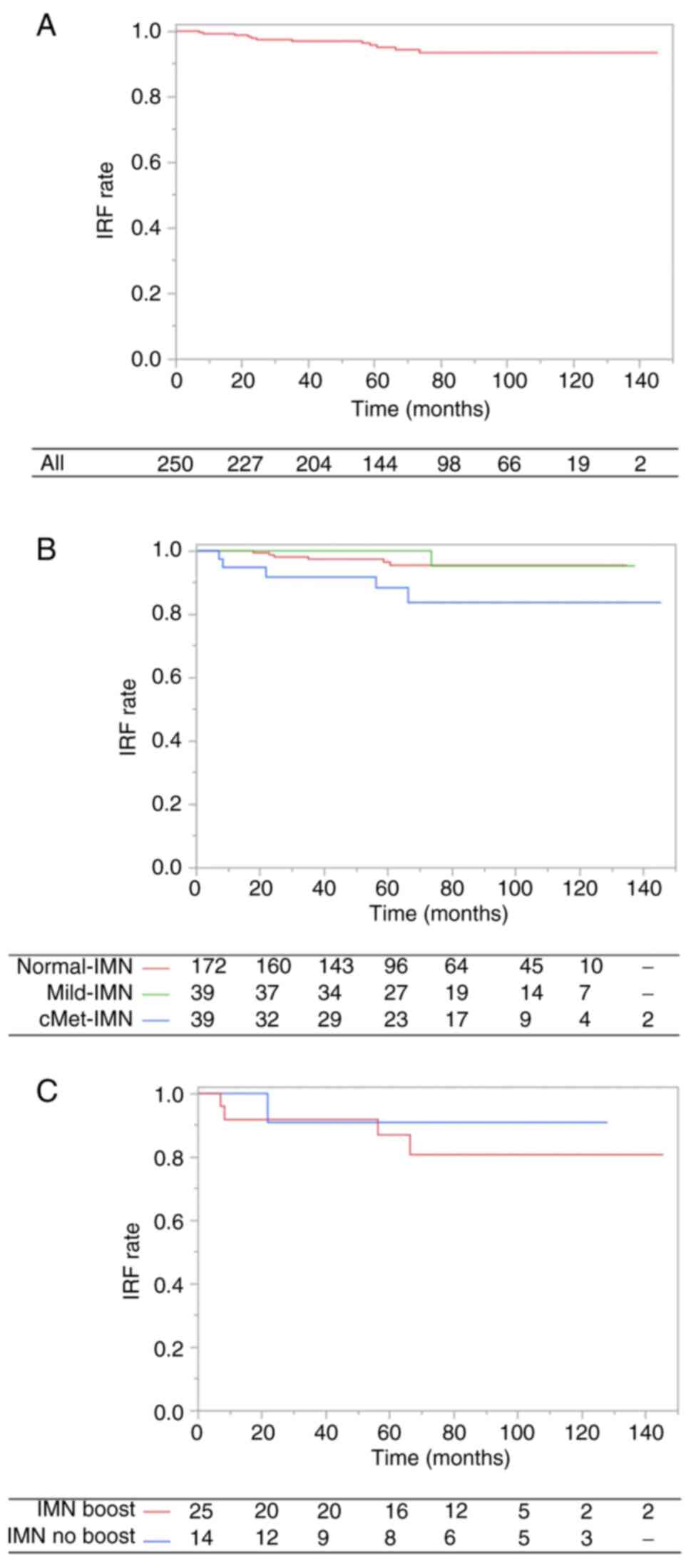
IMN recurrence-free survival
The median follow-up duration for IRF was 65.4
months (range, 1.0–145.4 months). The 7-year IRF rate was 93.4%
(Normal-IMN, 95.5%; Mild-IMN, 95.2%; cMet-IMN, 83.7%; Fig. 3A and B). The number of first
recurrences with IMN was 13.
In univariate analysis, clinical N stage (0–1 vs.
2–3; HR, 5.42; 95% CI, 1.47–20.01; P=0.01), ER status (positive vs.
negative; HR, 4.45; 95% CI, 1.41–14.03; P=0.01), and IMN status
(Normal-IMN vs. cMet-IMN; HR, 3.84; 95% CI, 1.17–12.61; P=0.03)
were significant factors for IRF. However, concerning IMN status,
Mild-IMN did not impact IRF (Normal-IMN vs. Mild-IMN; HR, 1.47; 95%
CI, 0.18–12.24; P=0.72). In the multivariate analysis, ER status
(positive vs. negative; HR, 4.18; 95% CI, 1.20–14.53; P=0.02)
remained a significant factor for IRF. These results are presented
in Table IV.
 | Table IV.UVA and MVA for IMN recurrence-free
survival. |
Table IV.
UVA and MVA for IMN recurrence-free
survival.
|
| UVA | MVA |
|---|
|
|
|
|
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (<55 years
vs. ≥55 years) | 1.01
(0.32–3.12) | 0.99 | - | - |
| cT stage (UICC 8th)
(1–2 vs. 3–4) | 2.00
(0.44–9.14) | 0.37 | - | - |
| cN stage (UICC 8th)
(0–1 vs. 2–3) | 5.42
(1.47–20.01) | 0.01 | 2.85
(0.59–13.80) | 0.19 |
| Nuclear grade (1–2
vs. 3) | 1.33
(0.42–4.20) | 0.63 | - | - |
| Laterality (left
vs. right) | 1.02
(0.32–3.22) | 0.98 | - | - |
| Tumor location
(medial/central vs. lateral) | 0.80
(0.26–2.49) | 0.70 | - | - |
| ER status (positive
vs. negative) | 4.45
(1.41–14.03) | 0.01 | 4.18
(1.20–14.53) | 0.02 |
| PR status (positive
vs. negative) | 3.22
(0.97–10.71) | 0.06 | - | - |
| HER2 status
(positive vs. negative) | 3.97
(0.51–30.80) | 0.19 | - | - |
| IMN status
(Normal-IMN vs. Mild-IMN) | 1.47
(0.18–12.24) | 0.72 | - | - |
| IMN status
(Normal-IMN vs. cMet-IMN) | 3.84
(1.17–12.61) | 0.03 | 1.66
(0.41–6.78) | 0.48 |
Furthermore, among patients with cMet-IMN, the
application of an IMN boost (10 Gy in 5 fractions) did not yield a
significant impact on IRF (IMN boost vs. IMN no boost; HR, 1.94;
95% CI, 0.22–17.47; P=0.55; Fig.
3C). Similarly, for the patients with large cMet-IMN (size of
≥1.0 cm), the application of an IMN boost (10 Gy in 5 fractions)
did not significantly impact IRF (IMN boost vs. IMN no boost; HR,
1.38; 95% CI, 0.14–13.46; P=0.78).
Discussion
In patients with breast cancer treated with systemic
therapy and PMRT, the use of Mild-IMN without FDG uptake was not a
significant adverse factor for OS and DFS. By contrast, cMet-IMN
with FDG uptake emerged as a significant adverse factor for both OS
and DFS. In addition, the application of an IMN boost (10 Gy in 5
fractions) for cMet-IMN did not lead to improvements in the OS,
DFS, and IRF.
Although the diagnostic accuracy of lymph node
metastasis by magnetic resonance (MR) or FDG-PET/CT is very high,
there is not always complete concordance between the clinical N
stage and pathological N stage (21). In some studies, the size of IMN of
≥0.5 cm has been considered indicative of IMN metastasis (16–18).
Therefore, the cut-off size for IMN metastasis was notably small.
Mild-IMN, characterized by IMN enlargement (<0.5 cm) without FDG
uptake and larger size compared to the contralateral IMN, is
occasionally identified in clinical practice. Distinguishing
whether this Mild-IMN represents a microscopic metastatic lymph
node or a reactive enlargement is difficult to diagnose in imaging
studies alone. In our study, patients with Mild-IMN achieved
similar treatment outcomes as patients with Normal-IMN, even
without IMN irradiation. This suggests that Mild-IMN may be a
reactive enlargement or could be effectively managed by systemic
therapy without IMN irradiation even if it harbors
micro-metastasis.
Furthermore, in our study, the application of an IMN
boost (10 Gy in 5 fractions) did not improve the OS, DFS, and IRF
for patients with cMet-IMN. Limited studies have explored the
optimal RT dose for the IMN region (21–23).
Yang et al (24) suggested
that a higher RT dose (biologically equivalent dose in 2 Gy
fractions of >63.5 Gy) might improve the DFS, particularly for
IMN size ≥1.0 cm. In contrast, our study found that the IMN boost
(10 Gy in 5 fractions IMN boost; total biologically equivalent dose
in 2 Gy fractions of 60 Gy) did not improve OS, DFS, and IRF for
patients with IMN size of ≥1.0 cm and ≥0.5 cm. Given that the RT
doses required for cMet-IMN were higher than those used in our
study, it is possible that the IMN boost dose in our study may have
been insufficient to improve treatment outcomes. Considering the
only factor affecting IRF was ER status, it could be an option to
irradiate cMet-IMN boost with higher RT dose may be an option in
ER-negative cases in clinical practice. Further large-scale studies
are needed to assess the impact of the IMN boost dose on enhancing
treatment outcomes.
This study has some limitations due to its
retrospective nature. First, the limited number of patients with
Mild-IMN and cMet-IMN reduced the statistical power of our study.
Second, we only assessed the clinical T and N stages, as obtaining
pathological T and N stages was not possible for patients treated
with NAC. Additionally, we were unable to evaluate the prognostic
impact of the NAC response on OS, DFS, and IRF as many patients in
our study received AC without NAC. Third, differences by surgeon's
surgical skills could not be analyzed. However, at our
institutions, because total mastectomy is generally performed by
breast surgeons, we believe that the quality of surgical procedures
is adequate. Finally, in our study, because of the wide age range,
the median age was used as a cutoff value to examine the impact on
treatment outcome. Because hormone therapy for breast cancer
depends not only on estrogen/progesterone status but also on
menopausal status, this may not be the optimal age cutoff value. In
the future, a prospective study adjusting for age will be
warranted. Despite these limitations, as the first study to examine
the clinical significance of Mild-IMN, these results are meaningful
for optimizing IMN irradiation in routine clinical practice. Future
large-scale studies are needed to determine the appropriate IMN
irradiation and IMN boost dose.
In conclusion, the impact of Mild-IMN on OS, DFS,
and IRF was minor. The presence of Mild-IMN does not significantly
warrant IMN irradiation. Furthermore, while irradiating cMet-IMN is
important, an IMN boost of 10 Gy in 5 fractions may not
significantly improve treatment outcomes, and only ER status
appears to be a factor influencing cMet-IMN control.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KM, YH, HK, KN and KA had full access to the data in
the study, confirm the authenticity of all the raw data, and take
responsibility for the integrity of the data and the accuracy of
the data analysis. KM, YH, HK, KN and KA designed the study. KM,
YH, HK, KN and KA collected patient data, and collaborated on
discussions. KM prepared the manuscript and YH edited the
manuscript. KM, YH, HK, KN and KA drafted the manuscript. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study were
in accordance with the ethical standards of the Institutional
Research Committee and the 1964 Declaration of Helsinki and its
later amendments. The patients treated at our institutions
consented in writing to the use of their anonymous data for
research in general. Opt-out consent was applied due to the
retrospective nature of the present study, and there was no
non-consent for the present study. The present study was approved
by the Ethics Committee of National Hospital Organization Shikoku
Cancer Center (Matsuyama, Japan; approval. no. 2023-526) and the
Ethics Committee of Ehime Prefectural Central Hospital (Matsuyama,
Japan; approval. no. gai 2023-13).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwamoto T, Kumamaru H, Niikura N, Sagara
Y, Miyashita M, Konishi T, Sanuki N, Tanakura K, Nagahashi M,
Hayashi N, et al: Survival trends and patient characteristics
between 2004 and 2016 for breast cancer in Japan based on the
national clinical database-breast cancer registry. Breast Cancer.
31:185–194. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen RC, Lin NU, Golshan M, Harris JR and
Bellon JR: Internal mammary nodes in breast cancer: Diagnosis and
implications for patient management-a systematic review. J Clin
Oncol. 26:4981–4989. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hassiotou F and Geddes D: Anatomy of the
human mammary gland: Current status of knowledge. Clin Anat.
26:29–48. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang O, Wang L, Shen K, Lin H, Hu Z, Liu
G, Wu J, Lu J, Shao Z, Han Q and Shen Z: Breast cancer
subpopulation with high risk of internal mammary lymph nodes
metastasis: Analysis of 2,269 Chinese breast cancer patients
treated with extended radical mastectomy. Breast Cancer Res Treat.
107:379–387. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katz A, Strom EA, Buchholz TA, Thames HD,
Smith CD, Jhingran A, Hortobagyi G, Buzdar AU, Theriault R,
Singletary SE and McNeese MD: Locoregional recurrence patterns
after mastectomy and doxorubicin-based chemotherapy: Implications
for postoperative irradiation. J Clin Oncol. 18:2817–2827. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gradishar WJ, Moran MS, Abraham J, Aft R,
Agnese D, Allison KH, Anderson B, Burstein HJ, Chew H, Dang C, et
al: Breast cancer, version 3.2022, NCCN clinical practice
guidelines in oncology. J Natl Compr Canc Netw. 20:691–722. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Yamauchi C, Yoshimura M, Sekiguchi K,
Hamamoto Y, Nakajima N, Sanuki N, Ogo E, Oguchi M, Saji S and Iwata
H: The Japanese breast cancer society clinical practice guideline
for radiation treatment of breast cancer, 2018 edition. Breast
Cancer. 27:9–16. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aleknavičius E, Atkočius V, Kuzmickienė I
and Steponavičienė R: Postmastectomy internal mammary nodal
irradiation: A long-term outcome. Medicina (Kaunas). 50:230–236.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thorsen LB, Offersen BV, Danø H, Berg M,
Jensen I, Pedersen AN, Zimmermann SJ, Brodersen HJ, Overgaard M and
Overgaard J: DBCG-IMN: A population-based cohort study on the
effect of internal mammary node irradiation in early node-positive
breast cancer. J Clin Oncol. 34:314–320. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim YB, Byun HK, Kim DY, Ahn SJ, Lee HS,
Park W, Kim SS, Kim JH, Lee KC, Lee IJ, et al: Effect of elective
internal mammary node irradiation on disease-free survival in women
with node-positive breast cancer: A randomized phase 3 clinical
trial. JAMA Oncol. 8:96–105. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang X, Liu Y, Luo H and Zhang J: PET/CT
and MRI for identifying axillary lymph node metastases in breast
cancer patients: Systematic review and meta-analysis. J Magn Reson
Imaging. 52:1840–1851. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Riegger C, Herrmann J, Nagarajah J,
Hecktor J, Kuemmel S, Otterbach F, Hahn S, Bockisch A, Lauenstein
T, Antoch G and Heusner TA: Whole-body FDG PET/CT is more accurate
than conventional imaging for staging primary breast cancer
patients. Eur J Nucl Med Mol Imaging. 39:852–863. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jochelson MS, Lebron L, Jacobs SS, Zheng
J, Moskowitz CS, Powell SN, Sacchini V, Ulaner GA, Morris EA and
Dershaw DD: Detection of internal mammary adenopathy in patients
with breast cancer by PET/CT and MRI. AJR Am J Roentgenol.
205:899–904. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalli S, Semine A, Cohen S, Naber SP,
Makim SS and Bahl M: American joint committee on cancer's staging
system for breast cancer, eighth edition: What the radiologist
needs to know. Radiographics. 38:1921–1933. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang YJ, Oh JL, Whitman GJ, Iyengar P, Yu
TK, Tereffe W, Woodward WA, Perkins G, Buchholz TA and Strom EA:
Clinically apparent internal mammary nodal metastasis in patients
with advanced breast cancer: Incidence and local control. Int J
Radiat Oncol Biol Phys. 77:1113–1119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee HW and Kim SH: Breast magnetic
resonance imaging for assessment of internal mammary lymph node
status in breast cancer. J Breast Cancer. 19:191–198. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kinoshita T, Odagiri K, Andoh K, Doiuchi
T, Sugimura K, Shiotani S and Asaga T: Evaluation of small internal
mammary lymph node metastases in breast cancer by MRI. Radiat Med.
17:189–193. 1999.PubMed/NCBI
|
|
19
|
Tsuda H, Akiyama F, Kurosumi M, Sakamoto G
and Watanabe T: Establishment of histological criteria for
high-risk node-negative breast carcinoma for a multi-institutional
randomized clinical trial of adjuvant therapy. Japan national
surgical adjuvant study of breast cancer (NSAS-BC) pathology
section. Jpn J Clin Oncol. 28:486–491. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the eastern cooperative oncology group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liang X, Yu J, Wen B, Xie J, Cai Q and
Yang Q: MRI and FDG-PET/CT based assessment of axillary lymph node
metastasis in early breast cancer: A meta-analysis. Clin Radiol.
72:295–301. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sachdev S, Goodman CR, Neuschler E,
Kalakota K, Cutright D, Donnelly ED, Hayes JP, Prescott AE,
Mirabelli G and Strauss JB: Radiotherapy of MRI-detected involved
internal mammary lymph nodes in breast cancer. Radiat Oncol.
12:1992017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim J, Chang JS, Choi SH, Kim YB, Keum KC,
Suh CO, Yang G, Cho Y, Kim JW and Lee IJ: Radiotherapy for initial
clinically positive internal mammary nodes in breast cancer. Radiat
Oncol. 37:91–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang K, Kim H, Choi DH, Park W, Noh JM and
Cho WK: Optimal radiotherapy for patients with internal mammary
lymph node metastasis from breast cancer. Radiat Oncol. 15:162020.
View Article : Google Scholar : PubMed/NCBI
|