Introduction
According to the World Health Organization, renal
cell carcinoma (RCC) is considered to be the 16th most commonly
diagnosed cancer in the world, with RCC-related deaths surpassing
170,000 annually (1). The presence
of a pseudo-capsule (PC) in RCC is widely known, and
radiographically detectable PC is a distinctive feature in the
imaging diagnosis of RCC (2). A PC
is located at the border between cancer tissue and normal kidney
tissue; therefore, it can be a useful indicator during
nephron-sparing surgery (NSS) (3).
However, little is currently known about the clinical and
biological role of PCs. Pickhardt et al (2) suggested that the formation of a PC is
derived from tumor growth in an organ, which causes compression and
necrosis of the adjacent normal parenchyma resulting in the
deposition of fibrous tissue. Wang et al (4) reported that the constituent components
of a PC in clear cell RCC (ccRCC) include collagen fibers, smooth
muscle bundles and some fibroblasts.
PCs have been detected in >90% of RCC cases
worldwide, regardless of histopathological subtype, such as clear
cell, papillary or chromophobe RCC (5–7). Among
these subtypes, ccRCC is most likely to form a thick PC (6,8). By
contrast, benign renal neoplasms, such as papillary adenoma and
oncocytoma, usually do not have a PC or, if one is present, it
tends to be thin (9,10). RCC has the potential of invasion to
the PC and, subsequently, to the normal tissues (NTs) beyond the
PC. Although RCC invasion to PCs is considered a poor prognostic
factor (11,12), a detailed molecular mechanism
underlying the formation and destruction of PCs in RCC has not yet
been provided, to the best of our knowledge. The present study
investigated the potential mechanisms underlying the formation and
destruction of a PC in localized ccRCC using clinical human tissues
and a rat model of carcinogenesis.
Patients and methods
Inclusion criteria, patient cohort and
evaluation of PCs in ccRCC
The present study was approved by the Institutional
Review Board of Nara Medical University (Kashihara, Japan; approval
no. NMU-1256) and complied with the 1964 Declaration of Helsinki
and its later amendments. All participants provided written
informed consent for the present study. Surgical specimens from 169
consecutive patients with localized ccRCC who underwent radical
nephrectomy (RN) or NSS with an adequate (≥5 mm) resection margin
at the Department of Urology, Nara Medical University Hospital
between January 2007 and December 2014 were included in the
analysis. The patients did not have any intraoperative capsular
damage and did not undergo enucleation. The clinicopathological and
follow-up data of the patients were obtained through a
retrospective chart review. The extent of the formation and
destruction of the PC was evaluated based on the invasion of PC
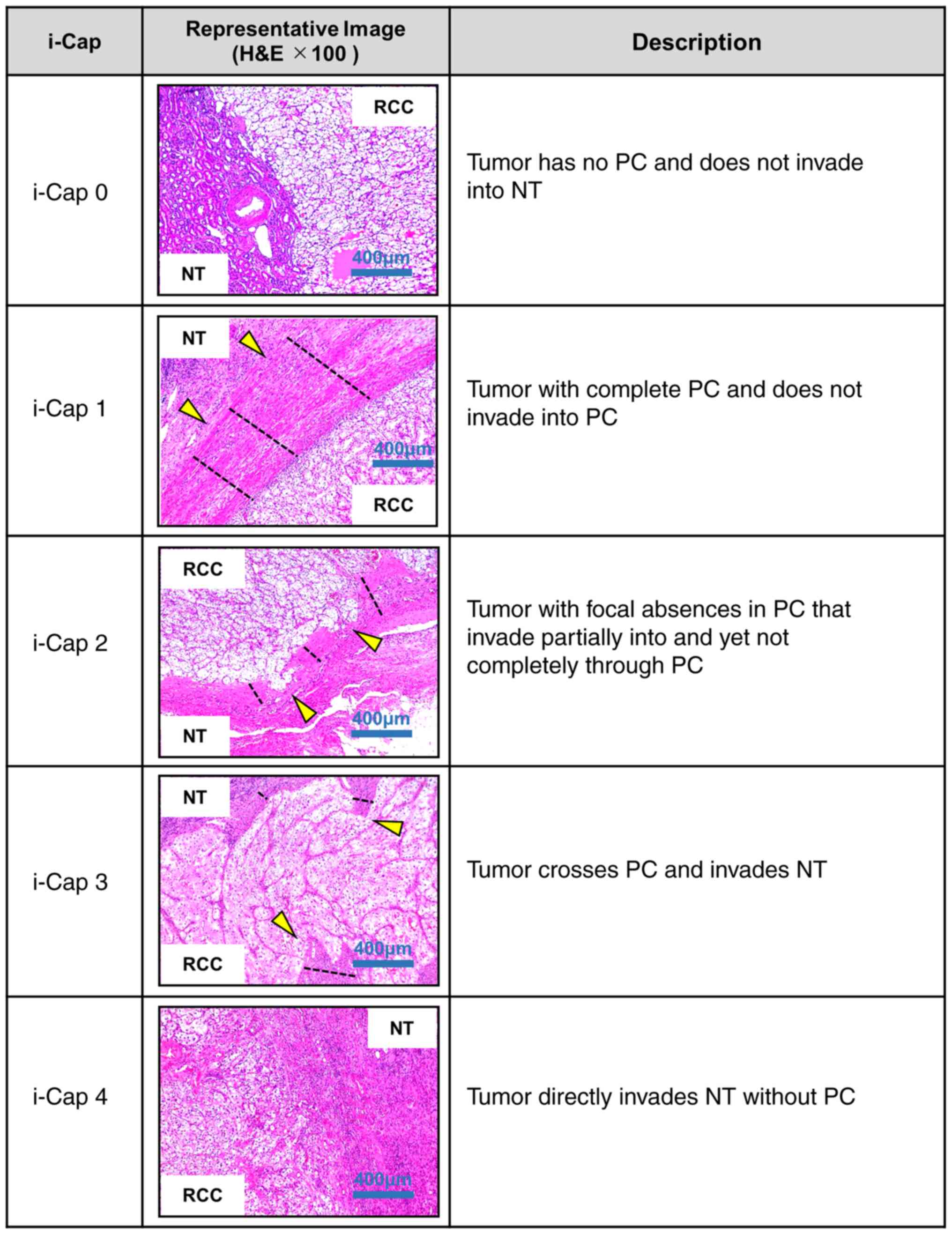
(i-Cap) scoring system (three categories), previously reported by
Snarskis et al (12). In the
present study, a modified i-Cap scoring system was used as follows:
i-Cap 0, tumor has no PC and does not invade NT; i-Cap 1, tumor has
a complete PC and does not invade into the PC; i-Cap 2, tumor with
focal absences in the PC, which partially invades the PC but not
completely through the PC; i-Cap 3, tumor crosses the PC and
invades the NT; i-Cap 4, tumor directly invades the NT without a PC
(Fig. 1). A uropathologist with
expertise in RCC pathological diagnosis (FT), blinded to the
clinical outcome of the patients, reviewed each hematoxylin &
eosin (H&E)-stained specimen. For H&E staining, the
specimens were stained with 1.5 g/l hematoxylin for 10 min, washed
and then stained with eosin for 2 min at room temperature. Results
were observed using a light microscope. Tumors were staged
according to the pathological tumor-node-metastasis (TNM)
guidelines in the Union for International Cancer Control Staging
Manual, 8th edition (13), and were
graded according to the criteria set out by the Fuhrman grading
system (14). All of the tumors
were scored by the i-Cap scoring system, ranging from 0 to 4. The
i-Cap classification was heterogeneous within tumors, with the
highest values assigned to areas in contact with normal renal
tissue (i.e. not areas of the fibrous septum between tumor
nodules). Regarding the thickness of the PC, it was also measured
at the region with the highest i-Cap score. The thickness of the PC
was measured and the mean of two observer results was taken using
scan images from a fluorescence microscope (EVOS FL Auto,
AMAFD1000; Thermo Fisher Scientific, Inc.) (Fig. 1).
Evaluation of PC formation of
metastatic lesions
Out of the 169 patients, a total of 15 specimens of
metastatic lesions from 14 patients who underwent metastasectomy
for metastatic ccRCC were evaluated for i-Cap and PC formation.
Surgical resection of metastases aimed to reduce the cancer burden,
control pain or prevent paralysis.
Identification of genes involved in PC
destruction with ccRCC rat models
N-diethylnitrosamine (DEN)-initiated and ferric
nitrilotriacetate (FeNTA)-promoted rat models of ccRCC
An in vivo rat carcinogenic model of ccRCC
was created via intraperitoneal administration of DEN and FeNTA
(both Tokyo Chemical Industry Co., Ltd.) according to reports by
Toyokuni et al (15) and
Vargas et al (16). A total
of 32 female Wistar rats (age, 2 weeks) were purchased from
Oriental Bio Service Ltd. The experiment started from 4 weeks after
birth, and the mean weight at the beginning of the experiment was
110 g (range, 98–130 g). All animal studies were approved by the
institutional animal care and use committee of Nara Medical
University and were conducted in accordance with local humane
animal care standards (approval no. 12211). This animal study was
conducted at Nara Medical University between February and September
2019. Animal care was conducted in compliance with the
recommendations of The Guide for Care and Use of Laboratory Animals
(National Research Council) (17).
All rats were maintained under pathogen-free conditions, were
provided with free access to sterile food and water, and were kept
under controlled, stable ambient conditions (23±3°C; 12-h
light/dark cycle; 50±20% humidity). The dietary intake and body
weight of rats were monitored every week, and termination of the
experiment was considered if the rats refused food and significant
weight loss was observed. Rats were also visually inspected daily
to check whether the tumor was large enough to be visible on the
body surface or whether the rats were exhibiting significant
ascites. If these conditions were suspected, euthanasia was
considered. During the experiment, if the orthotopic tumor grew to
a size where it could be seen from the body surface, or weight loss
of ≥20% occurred within 2 to 3 days or weight loss of ≥25% occurred
within 7 days, euthanasia was performed. The greatest weight loss
observed was 16 g (from 498 to 482 g) in 1 week.
The control group and ccRCC model group of 12 and 20
rats were prepared, respectively. In the ccRCC model group, DEN was
administered intraperitoneally at a dose of 200 mg/kg, followed by
intraperitoneal administration of FeNTA at a dose of 9 mg/kg twice
a week for 12, 16, 20 and 24 weeks. All rats were euthanized by
cervical dislocation under anesthesia with isoflurane (induction
4%, maintenance 2–3%) 8 weeks after the complete administration of
carcinogens. The control group also underwent euthanasia at the
same time and in the same manner as the test group. Subsequently,
the kidneys were removed, placed on filter paper and fixed in 10%
neutral buffered formalin for 18 h at room temperature. The
paraffin-embedded tissues were cut into 5-µm pieces and subjected
to H&E staining on glass slides. For H&E staining, the
specimens were stained with 1.5 g/l hematoxylin for 10 min, washed
and then stained with eosin for 2 min at room temperature. The
results were then observed using a light microscope. The
step-sections of the kidneys were observed under a light
microscope, and the relationship between the PC and the tumor was
investigated. The kidneys were fixed in formalin immediately after
removal so that the gap between the tumor and normal kidney tissue
could be observed; therefore, only the tumor was removed and the
tumor size and weight were not measured. Evaluation of i-Cap in rat
models was also performed by the same pathologist (FT) that
evaluated the human specimens. The lungs and livers were also
removed and treated in the same way as the kidneys to assess
whether tumors were present outside of the kidneys, but no
metastatic tumors were identified.
Identification of genes involved in PC
destruction
Reverse transcription-quantitative PCR (RT-qPCR) was
performed to measure the expression levels of mRNA. Total RNA was
extracted using a miRNeasy FFPE kit (Qiagen GmbH), according to the
manufacturer's instructions. Conversion to cDNA was performed using
an RT2 First Standard kit (Qiagen GmbH), according to the
manufacturer's instructions. cDNA was added to RT2 SYBR
Green qPCR Mastermix (Qiagen GmbH) and the mRNA expression of ~400
genes was measured using three RT2 Profiler PCR Array
panels as follows: Rat Extracellular Matrix & Adhesion
Molecules (cat. no. PARN-013ZD), Rat Tumor Metastasis (cat. no.
PARN-028ZD) and Rat Fibrosis (cat. no. PARN-120ZD) (all from Qiagen
GmbH) in NT around the PC and tumor tissue around the PC to
identify genes that were upregulated or downregulated in the
formation and destruction of the PC. RT-qPCR and Heat map analysis
were performed using the CFX96 Touch Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.), in a manner similar to that reported
in our previous study (18).
RT-qPCR was performed under the following conditions: Denaturation
at 95°C for 10 min; 40 cycles of denaturation at 95°C for 15 sec;
and annealing and extension at 60°C for 1 min. Primer sequences
were not available due to trade secrets. mRNA expression was
compared between the i-Cap 1 group and the i-Cap 2–3 group.
Relative expression was normalized to Actb, B2m, Hprt1, Ldha and
Rplp1 expression, as the use of multiple housekeeping genes is
known to increase reliability (19), and estimated using the
2−ΔΔCq method (20).
Results were presented as the fold-change relative to the
control.
Confirmation of the relevant gene
groups in human ccRCC specimens via immunohistochemistry (IHC)
IHC was used to assess whether the genes identified
in the rat model were involved in PC formation and destruction in
human ccRCC specimens. Resected tissue specimens were fixed in 10%
formalin, incubated overnight at room temperature and embedded in
paraffin. Paraffin-embedded blocks were then cut into 3-µm sections
and placed on Superfrost Plus microslides (Thermo Fisher
Scientific, Inc.). Sections were deparaffinized in xylene and
hydrated in decreasing concentrations of ethyl alcohol, and antigen
retrieval was carried out via autoclaving with citric acid buffer
(pH 6.0) for 20 min at 120°C. Next, the sections were incubated
with 3% hydrogen peroxide for 15 min at room temperature to block
endogenous peroxidase activity. IHC staining was performed using
the Histofine SAB-PO (Multi) kit (cat. no. 424043; Nichirei
Biosciences, Inc.) according to the manufacturer's instructions.
Non-specific binding was blocked by incubating the sections with
10% normal goat serum for 10 min. The sections were incubated with
monoclonal antibodies against collagen type 4A2 (COL4A2; cat. no.
ab125208; 1:500 dilution; Abcam), matrix metalloproteinase-7
(MMP-7; cat. no. MAB9071; 1:200 dilution; R&D Systems, Inc.),
endoglin (ENG; cat. no. AF1097; 1:100 dilution; R&D Systems,
Inc.) and l-selectin (SELL; cat. no. sc-390756; 1:50 dilution;
Santa Cruz Biotechnology, Inc.) overnight at 4°C. The secondary
antibody reaction was performed using Histofine SAB-PO (Multi) kit
(cat. no. 424043, Nichirei Biosciences, Inc.) according to the
manufacturer's instructions. The slides were developed with DAB
(Histofine, cat. no. 415172, Nichirei Biosciences, Inc.) until the
signal clearly appeared, and the nuclei were stained with Mayer's
hematoxylin for 1 min at room temperature, dehydrated and sealed
with a cover slip. Images were obtained using a fluorescence
microscope (EVOS FL Auto, AMAFD1000; Thermo Fisher Scientific,
Inc.). All stained tissue samples were evaluated by two
investigators (YI and TM) without knowledge of the patient data.
The tumor tissues and NTs around the PC from the region in which
the i-Cap score was assigned were evaluated by immunostaining.
The sections were analyzed and staining was assessed
using a semiquantitative grading system based on a previous report
by Allred et al (21).
Briefly, the expression level of each marker was scored by
assigning a proportion score and an intensity score. The proportion
score represents the estimated proportion of immunoreactive cells
or stroma: 0, 0% of cells; 1, 0–1%; 2, 1–10%; 3, 10–33%; 4, 33–67%;
5, 67–100%. The intensity score represents the average intensity of
positive cells or stroma: 0, none; 1, weak; 2, intermediate; 3,
strong. The proportion and intensity scores were added to obtain a
combined immunostaining score for the expression of each marker,
which ranged from 0 to 8: 0, none; 1–2, low; 3–4, moderate; 5–6,
high.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism 5.0 (Dotmatics). The associations between i-Cap and tumor
clinicopathological variables or IHC results were evaluated by
Kruskal-Wallis test and the Dunn's multiple comparison test or
Fisher's exact test. Cancer-specific survival (CSS) or disease-free
survival (DFS) were estimated using the Kaplan-Meier method. CSS
endpoints were defined as death due to RCC after surgery. DFS
endpoints were defined as distant metastasis, local recurrence or
death from any cause after surgery. CSS or DFS were calculated from
the day when nephrectomy or NSS was performed until the last
follow-up or death by RCC, or when RCC recurrence or metastasis
were diagnosed. The differences between each group were compared
using the log-rank test. Multivariate logistic and Cox regression
analyses were performed using SPSS software version 21 (IBM Corp.)
to identify factors that predict postoperative DFS and CSS. All
tests were two-sided and P<0.05 was considered to indicate a
statistically significant difference.
Results
Relationship between PCs and
clinicopathological characteristics in ccRCC
Table I shows the
clinicopathological information of 169 patients who underwent
surgery at Nara Medical University between 2007 and 2014. The
median follow-up period was 91 months (interquartile range, 60–114
months). During the follow-up period, 39 patients (23.1%)
experienced metastasis and 4 patients (2.4%) had local recurrence.
Among them, 1 patient showed both local recurrence and metastasis.
A total of 33 patients (19.5%) died; of these, 16 (9.5%) died due
to ccRCC. Table I also summarizes
the relationship between i-Cap and PC thickness, tumor size and
Fuhrman grade. Patients with i-Cap 3 had a significantly thinner PC
than those with i-Cap 1. Notably, there was no significant
difference between i-Cap 2 and i-Cap 1. In addition, patients with
i-Cap 2, 3 and 4 had significantly larger tumor diameters than
those with i-Cap 1. In addition, patients with i-Cap 3 and 4 had a
higher proportion of high Fuhrman grades than those with i-Cap
1.
 | Table I.Clinicopathological information of
patients in each i-Cap score group. |
Table I.
Clinicopathological information of
patients in each i-Cap score group.
| Variable | Total | i-Cap 0 | i-Cap 1 | i-Cap 2 | i-Cap 3 | i-Cap 4 |
|---|
| Cases, n | 169 | 5 | 89 | 41 | 25 | 9 |
| Age, years |
|
|
|
|
|
|
| Median
(IQR) | 64 (56–74) | 73 (71–75) | 65 (53–73) | 67 (56–74) | 67 (62–74) | 63 (62–64) |
| Sex, n |
|
|
|
|
|
|
|
Male | 126 | 3 | 67 | 39 | 19 | 8 |
|
Female | 43 | 2 | 22 | 2 | 6 | 1 |
| Sugery, n |
|
|
|
|
|
|
| RN | 134 | 2 | 60 | 41 | 22 | 9 |
|
NSS | 35 | 3 | 29 | 0 | 3 | 0 |
| Tumor size, mm |
|
|
|
|
|
|
| Median
(IQR) | 45.0 | 23.5 | 34.0 | 48.0 | 64.0 | 60 |
|
| (26.0–65.0) | (16.5–32.8) | (20.0–50.8) | (36.5–60.0) | (45.8–100.5) | (51.5–120) |
|
P-value |
| ns | Refa |
<0.05a |
<0.001a |
<0.01a |
| Serum CRP,
mg/l |
|
|
|
|
|
|
| Median
(IQR) | 0.1 (0.1–0.3) | 0.1 (0.1–0.4) | 0.1 (0.1–0.3) | 0.1 (0.0–0.2) | 0.1 (0.1–1.1) | 1.3 (0.1–4.6) |
| Serum Alb,
g/dl |
|
|
|
|
|
|
| Median
(IQR) | 4.3 (4.0–4.6) | 4.6 (4.5–4.6) | 4.3 (4.0–4.5) | 4.3 (4.1–4.7) | 4.2 (4.0–4.5) | 4.0 (4.0–4.4) |
| Pathological T
stage, n |
|
|
|
|
|
|
| 1 | 99 | 2 | 65 | 25 | 6 | 1 |
| 2 | 6 | 0 | 4 | 1 | 1 | 0 |
| 3 | 61 | 3 | 18 | 15 | 18 | 7 |
| 4 | 3 | 0 | 2 | 0 | 0 | 1 |
| INF, n |
|
|
|
|
|
|
| a | 77 | 4 | 59 | 11 | 1 | 2 |
| b and
c | 92 | 1 | 30 | 30 | 24 | 7 |
| PC thickness,
mm |
|
|
|
|
|
|
| Median
(IQR) | 0.62 |
| 0.72 | 0.57 | 0.37 |
|
|
| (0.36–1.02) |
| (0.39–1.26) | (0.42–0.71) | (0.25–0.70) |
|
| Mean ±
SD | 0.74±0.49 |
| 0.85±0.55 | 0.69±0.39 | 0.49±0.31 |
|
|
P-value |
| NA | Refa | nsa |
<0.05a | NA |
| Fuhrman grade
maximum, n |
|
|
|
|
|
|
| 1 and
2 | 122 | 5 | 72 | 31 | 12 | 2 |
| 3 and
4 | 47 | 0 | 17 | 10 | 13 | 7 |
| P-value |
| 0.58b | Refb | 0.49b |
<0.01b |
<0.01b |
| Disease recurrence
after surgery, n |
|
|
|
|
|
|
| Distant
metastasis | 39 | 0 | 10 | 12 | 12 | 5 |
| Local
recurrence | 4 | 0 | 1 | 0 | 1 | 2 |
| Follow-up,
months |
|
|
|
|
|
|
| Median
(IQR) | 91 (60–114) | 105 (68–107) | 93 (63–114) | 85 (68–127) | 95 (62–117) | 49 (15–84) |
Prognostic factors after surgery
The Kaplan-Meier curves for DFS and CSS based on
i-Cap score are displayed in Fig.
2. CSS and DFS were lower as the i-Cap score increased
(Fig. 2A and B). Patients with PC
invasion (i-Cap 2–4) had significantly worse DFS and CSS compared
with those without PC invasion (i-Cap 0 and 1) [hazard ratio (HR)
4.13, 95% confidence interval (CI) 2.27–7.67, P<0.001; HR 6.16,
95% CI 2.29–16.6, P<0.001] (Fig. 2C
and D). Multivariate analysis revealed that i-Cap, Fuhrman
grade and tumor size were negative prognostic factors for DFS, and
i-Cap and tumor size were negative prognostic factors for CSS
(Table II).
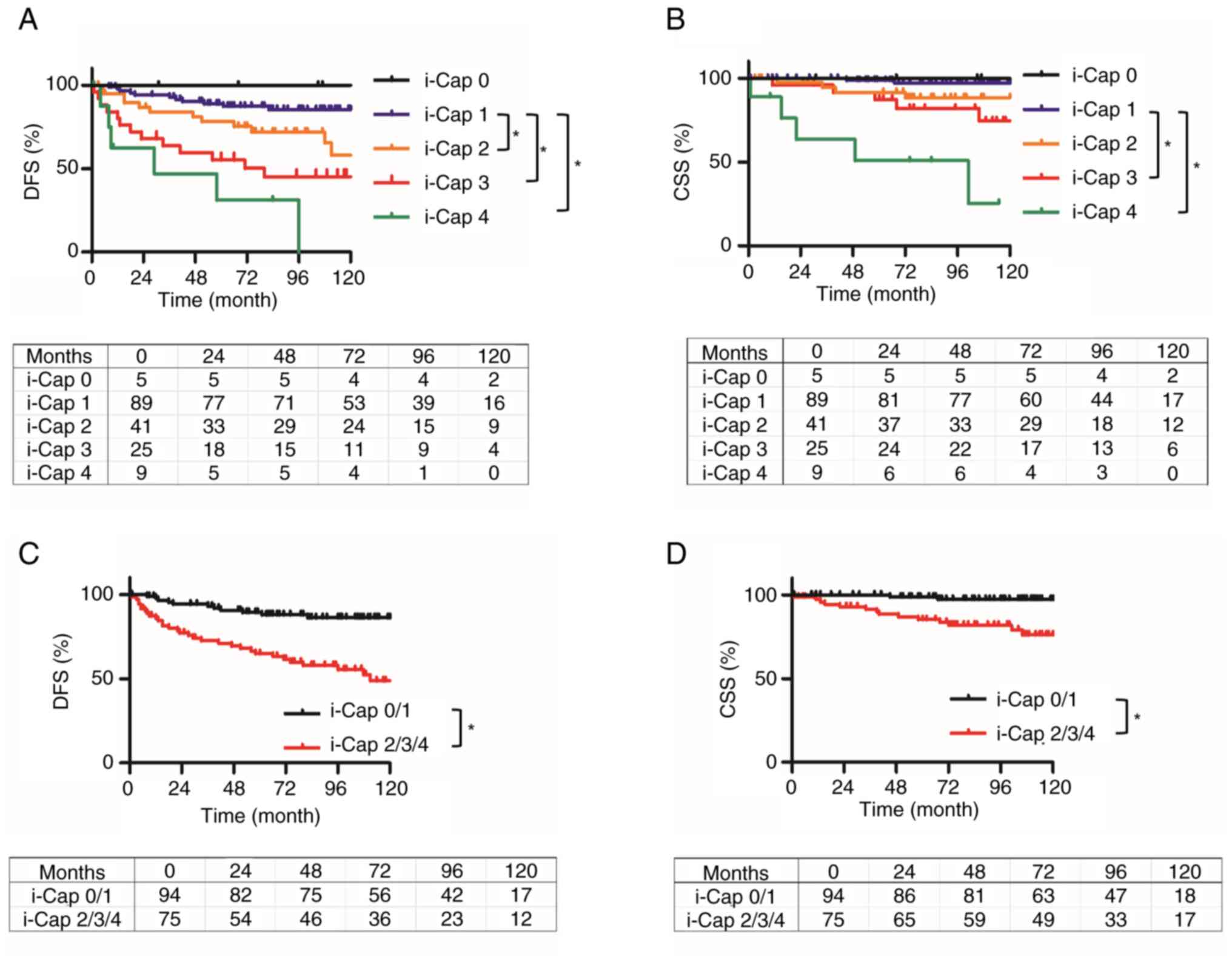 | Figure 2.Kaplan-Meier curves of DFS and CSS
for each i-Cap score, and for patients with or without invasion of
the PC. DFS and CSS were estimated using the Kaplan-Meier method.
(A) DFS of patients in each i-Cap group. Compared with in patients
with i-Cap 1, those with i-Cap 2, 3 and 4 had a significantly worse
DFS. (B) CSS of patients in each i-Cap group. Compared with in
patients with i-Cap 1, those with i-Cap 3 and 4 had a significantly
worse CSS. (C) DFS was compared between two groups, those which
exhibited invasion into the PC or normal renal tissue (i-Cap 2/3/4)
and those that did not (i-Cap 0/1). Compared with in patients with
i-Cap 0/1, those with i-Cap 2/3/4 had a significantly worse DFS.
(D) CSS compared between two groups, those which exhibited invasion
into the PC or normal renal tissue (i-Cap 2/3/4) and those that did
not (i-Cap 0/1). Compared with in patients with i-Cap 0/1, those
with i-Cap 2/3/4 had a significantly worse CSS. *P<0.05. DFS,
disease-free survival; CSS, cancer-specific survival; PC,
pseudo-capsule; i-Cap, invasion of PC. |
 | Table II.Multivariate analysis for DFS and
CSS. |
Table II.
Multivariate analysis for DFS and
CSS.
|
| DFS | CSS |
|---|
|
|
|
|
|---|
|
| Multivariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| UICC 8th pT stage
(pT1/2/3/4)a | 1.05 | 0.69–1.59 | 0.82 | 1.09 | 0.49–2.41 | 0.84 |
| Fuhrman grade
(G1-4)a | 1.95 | 1.17–3.25 | 0.010 | 1.44 | 0.65–3.23 | 0.37 |
| Size
(mm)b | 1.02 | 1.00–1.27 | 0.010 | 1.02 | 1.00–1.04 | 0.02 |
| INF
(a/b/c)a | 1.34 | 0.64–2.78 | 0.44 | 1.19 | 0.29–4.87 | 0.81 |
| Serum CRP
(mg/l)b | 0.95 | 0.86–1.06 | 0.39 | 1.00 | 0.85–1.17 | 0.39 |
| Serum Alb
(g/dl)b | 0.80 | 0.39–1.66 | 0.55 | 0.60 | 0.85–1.17 | 0.55 |
| i-Cap
(0–4)a | 1.60 | 1.13–2.25 | <0.01 | 2.20 | 1.20–4.01 | 0.01 |
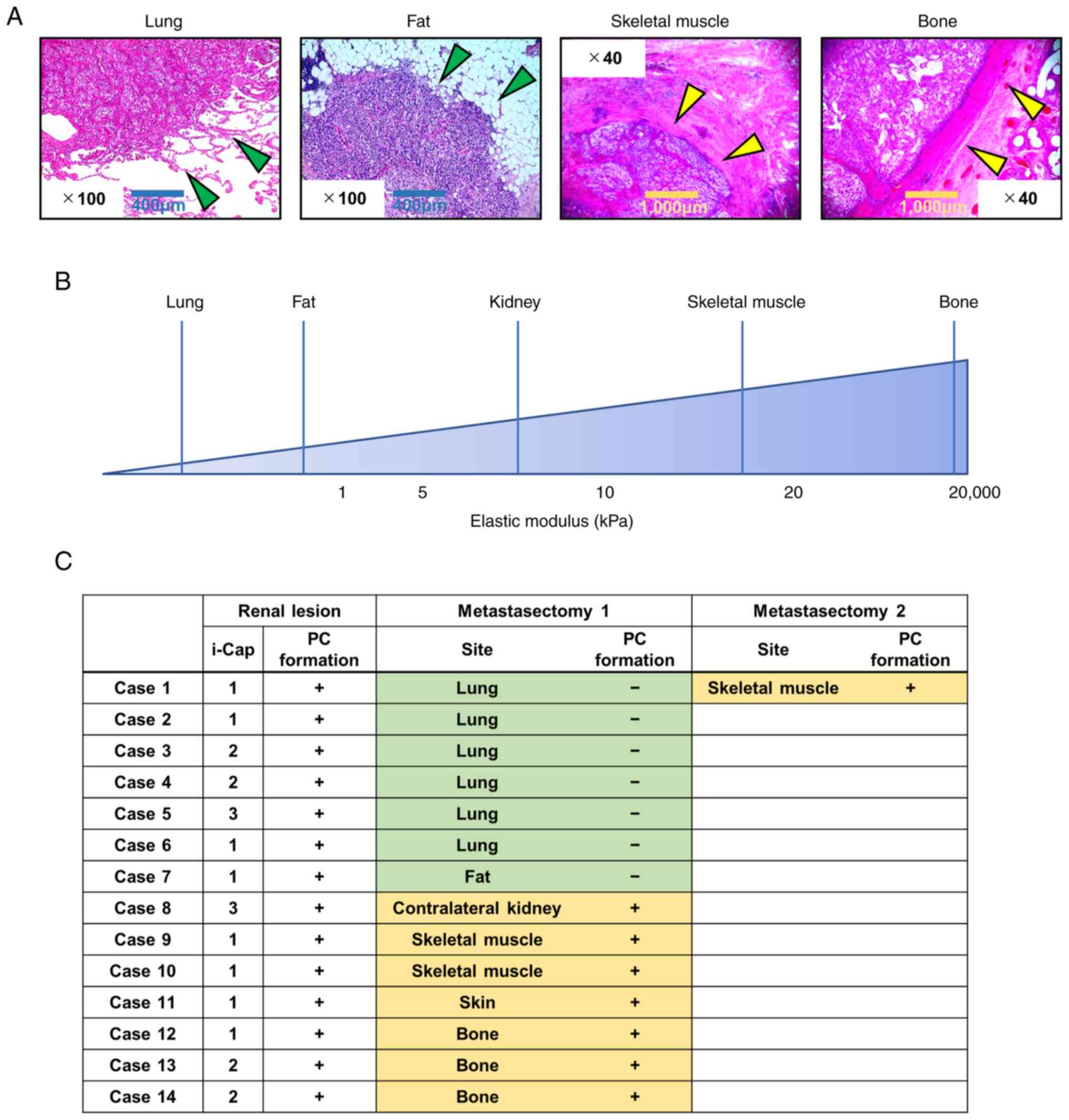
Evaluation of PC formation in each
metastatic lesion
The present study evaluated 15 specimens from 14
patients who underwent resection of metastatic ccRCC at Nara
Medical University. The 15 specimens consisted of 6 from the lung,
3 from skeletal muscle and bone, and 1 from the skin, contralateral
kidney and fat (adipose tissue in the abdomen). As shown in
Fig. 3, in all cases, PC formation
was observed in the primary kidney tumor, although there was a
difference in the i-Cap score. In addition, there was a difference
in PC formation depending on the metastatic site. Specifically, PC
formation was not observed in organs that are considered to lack an
epithelial component and have a lower elastic modulus than that of
the kidney (22,23).
Identification of genes involved in PC
formation and destruction in ccRCC rat models
A total of 3 rats from the control group and 5 rats
from the ccRCC rat model group were sacrificed at each time point 8
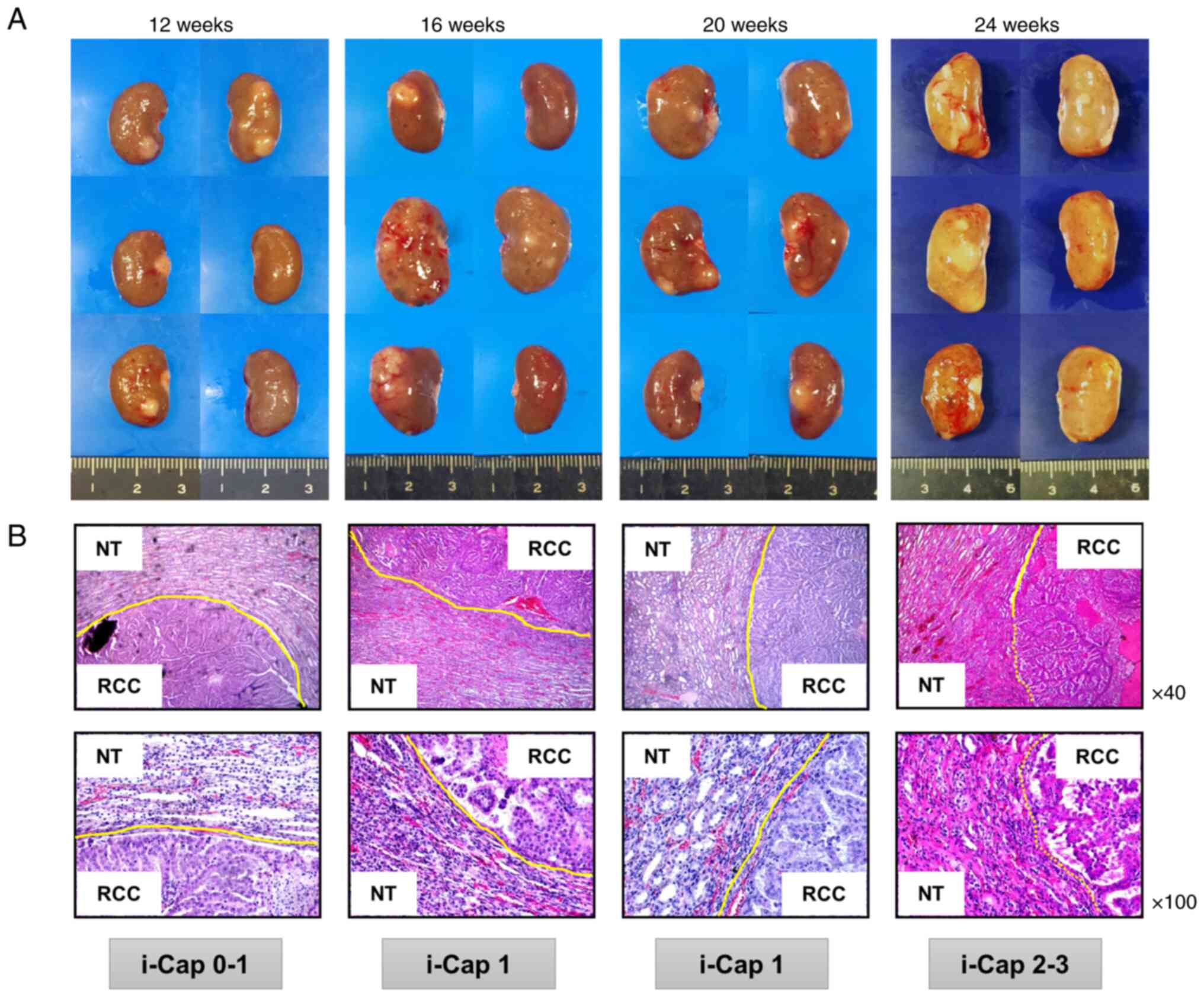
weeks after the end of FeNTA administration. Macroscopic images of
kidneys from rats with ccRCC at each time point of FeNTA
administration (12, 16, 20 and 24 weeks) showed a tendency for
renal tumors to grow with multiple occurrences (Fig. 4A). Microscopic images of renal
tumors at each time point of FeNTA administration exhibited a trend
towards an increase in i-Cap score as the administration period
increased (Fig. 4B).
Heat map analysis compared mRNA expression levels
between rats with ccRCC in the i-Cap 1 and i-Cap 2–3 groups
(Fig. 5A). The areas shown in red
are upregulated, and the areas shown in green are downregulated.
Also, the areas displayed in black are not regulated. Black text
with a white cross indicates that there is no calculated value. The
present study paid attention to the extracellular matrix,
angiogenesis and immune-related markers among the genes that had a
difference of >2-fold in RT-qPCR results. The expression levels
of COL4A2, ENG, MMP-7 and SELL were enhanced in the
i-Cap 2–3 group compared with those in the i-Cap 1 group, with a
>2-fold difference.
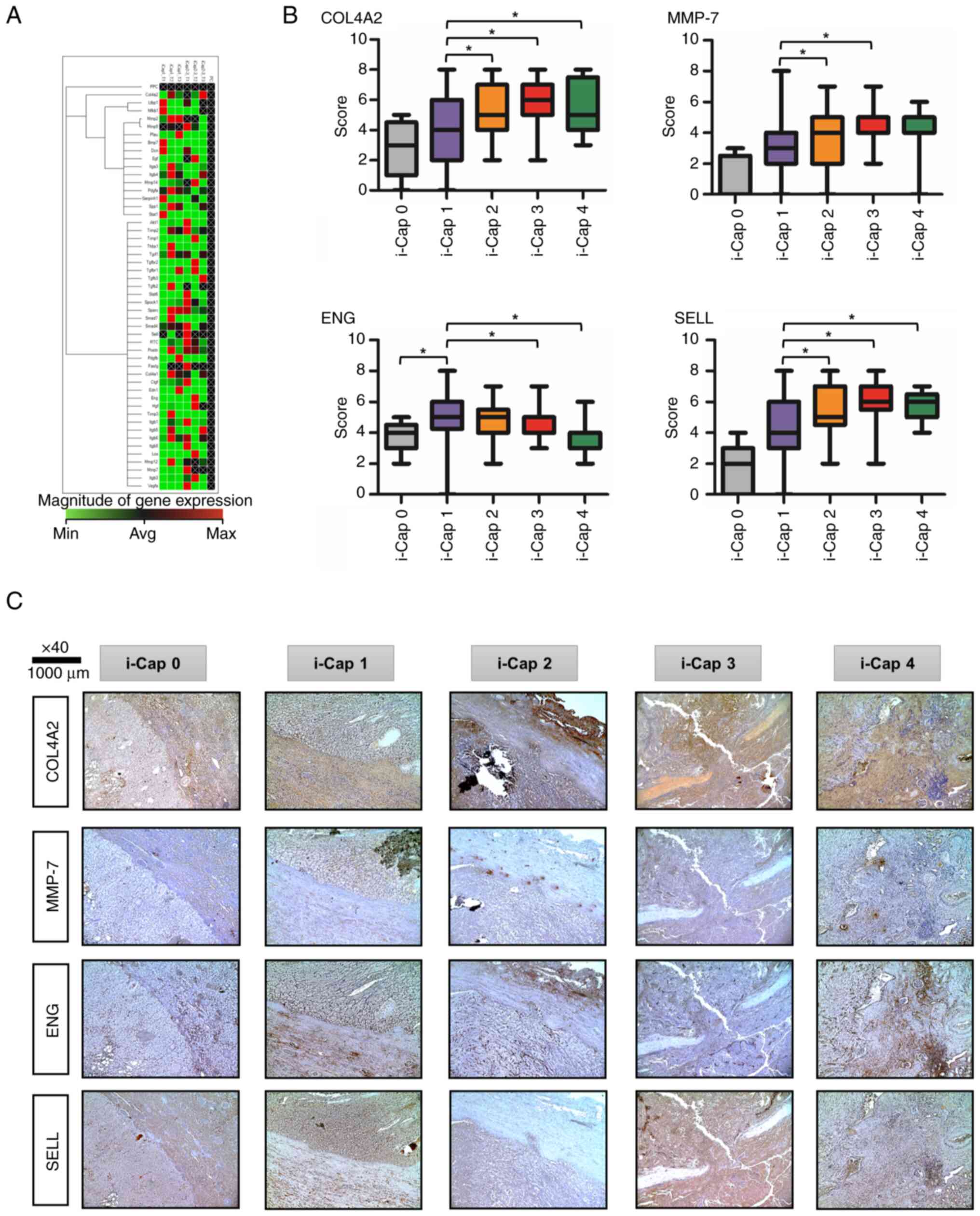 | Figure 5.Factors associated with i-Cap score,
and their immunohistochemical staining and score comparison. (A)
Heat map demonstrating the differences in mRNA expression levels
between the i-Cap 1 and i-Cap 2/3 groups in a rat model of ccRCC,
as revealed through PCR panel analysis. (B) Immunostaining scores
of each protein in each i-Cap score group. *P<0.05
(Kruskal-Wallis and Dunn's post hoc test). (C) Representative
images of immunohistochemical staining of human specimens for four
proteins in each i-Cap score group (×40 magnification). COL4A2,
collagen type 4A2; MMP-7, matrix metalloproteinase-7; ENG,
endoglin; SELL, l-selectin; i-Cap, invasion of psuedo-capsule; min,
minimum; avg, average; max, maximum. |
Evaluation of four genes identified in
a rat model of ccRCC in human specimens
The semi-quantified scores are shown in Fig. 5B. Representative IHC images of
COL4A2, MMP-7, ENG and SELL immunostaining for each i-Cap score are
shown (Fig. 5C). For COL4A2, MMP-7
and SELL, it was indicated that the expression levels of these
proteins increased as i-Cap progressed, that is, as PC destruction
progressed. By contrast, the opposite was true for ENG, indicating
that protein expression decreased as i-Cap progressed.
Discussion
The present study investigated the processes
involved in the formation and destruction of a PC in ccRCC. To the
best of our knowledge, no similar study has yet been published.
Firstly, the present study confirmed the presence of tumors in
which a PC was not formed in local ccRCC, and these tumors were
classified as i-Cap 0. Only 5 out of 169 cases (3%) were classified
as i-Cap 0, with smaller tumor size and lower Fuhrman grade
compared with the others. Additionally, in the evaluation of
metastatic lesions, PC formation was observed in the primary tumor
site (i.e. the kidney) in all cases; however, although this
information was only available from a sample size of 14 cases, no
evidence of PC formation in soft tissues, such as fat and lungs,
which are known to have low elastic moduli, was identified.
Evaluation of the elastic modulus of each tissue by Butcher et
al (22) and Handorf et
al (23) reported that fat and
lung tissues are less stiff than the kidney, whereas muscle and
bone are stiffer than the kidney. A plausible hypothesis derived
from the present metastasectomy findings is that a PC does not form
in ccRCC when normal tissue stiffness is lower than that of tumors,
especially when the epithelial component is absent. In addition,
i-Cap 0 tumors were characterized by very small diameters and
low-grade tumors. Previous research has indicated that low-grade
ccRCC tumors exhibit a significantly slower growth rate compared
with high-grade ccRCC tumors (24,25).
This suggests that i-Cap 0 tumors may also possess a very slow
growth rate. Given their small size and slow proliferation, it is
possible that the normal renal parenchyma is not compressed,
leading to the absence of PC formation. Evaluation of PC formation
in these metastases and the pathological features of i-Cap 0 tumors
indicated that a PC is caused by the physical exclusion of normal
parenchymal components, as reported by Pickhardt et al
(2).
The present study also focused on the destruction of
PCs and used the i-Cap classification reported by Snarskis et
al (12) as a reference. The
difference between this previous study and the present study is
that the current study used the classification i-Cap 0 when there
was no PC formation, whereas the i-Cap classification was the same
as Snarskis et al when a PC was present. The present study
examined the relationship between PC thickness, tumor size and
Fuhrman grade for each i-Cap group. As the i-Cap score increased,
the PC became thinner, the tumor diameter became larger and the
degree of malignancy also increased. In addition, the i-Cap score
also increased as tumors became more aggressive. Notably, i-Cap was
associated with oncological prognosis according to the results of a
univariate analysis, and i-Cap and tumor size were identified as
prognostic factors for both DFS and CSS in multivariate analyses in
the present study. The present finding that PC invasion is a factor
of poor oncological prognosis is consistent with the findings of
Cho et al (11). In the
present study, only 4 of 169 patients had local recurrence. Of
these, only 1 patient was treated with NSS; this patient was 1 of
29 classified as i-Cap 1 and 1 out of 35 who underwent NSS. None of
the 3 patients who underwent NSS and were classified as i-Cap 3
experienced local recurrence. Therefore, it was difficult to assess
the association between NSS, i-Cap and local recurrence in the
present study.
In the FeNTA-administered ccRCC rat model, it was
confirmed that the tumors occurred more frequently and growth
increased as the administration period progressed. In addition, the
tendency of a PC to collapse with the extension of the
administration period was confirmed. This suggests that the
destruction of a PC is caused by tumor growth and exacerbation. To
identify the molecules involved in PC destruction, comprehensive
RNA analysis was performed using tumors obtained from rats with
ccRCC from the i-Cap 2–3 and i-Cap 1 groups. As a result, the
present study paid attention to the extracellular matrix,
angiogenesis and immune-related markers, which had a fold
difference of >2. Subsequently, immunohistochemical staining was
performed for the PC destruction-associated molecules in the tumor
margin of human localized RCC specimens and their expression was
evaluated in each i-Cap group.
COL4 is a major component of the basement membrane
(BM) in the extracellular matrix. In renal tumors, COL4A1 and
COL4A2 chains have been detected in the BM (26). Disturbance of BM structure and an
increase in density are seen with increasing malignancy in RCC
(27). Furthermore, Provenzano
et al (28) showed that
collagen rearrangement and densification in breast cancer can
promote tumorigenesis and invasion into surrounding tissues. These
findings support the present finding that the expression of COL4 is
enhanced with exacerbation of i-Cap.
MMP-7 is a member of the MMP family of extracellular
matrix-degrading enzymes. It is well known that MMPs are
upregulated in various types of cancer, and play important roles in
cancer invasion and metastasis (29–31).
Among them, MMP-7 is considered to be produced primarily by
fibroblasts, inflammatory cells and cancer cells, and to degrade
proteoglycans, elastin, COL4 and fibronectin (32). MMP-7 has been shown to be enhanced
at the invasion front of malignant tumors of esophageal squamous
cell carcinoma (29) and colorectal
carcinoma (31), indicating a
direct role in cancer cell invasion. In addition, in RCC, Miyata
et al (30) reported
enhanced expression at the invasion tip. In the present study of PC
rupture, MMP-7 expression increased in response to PC destruction,
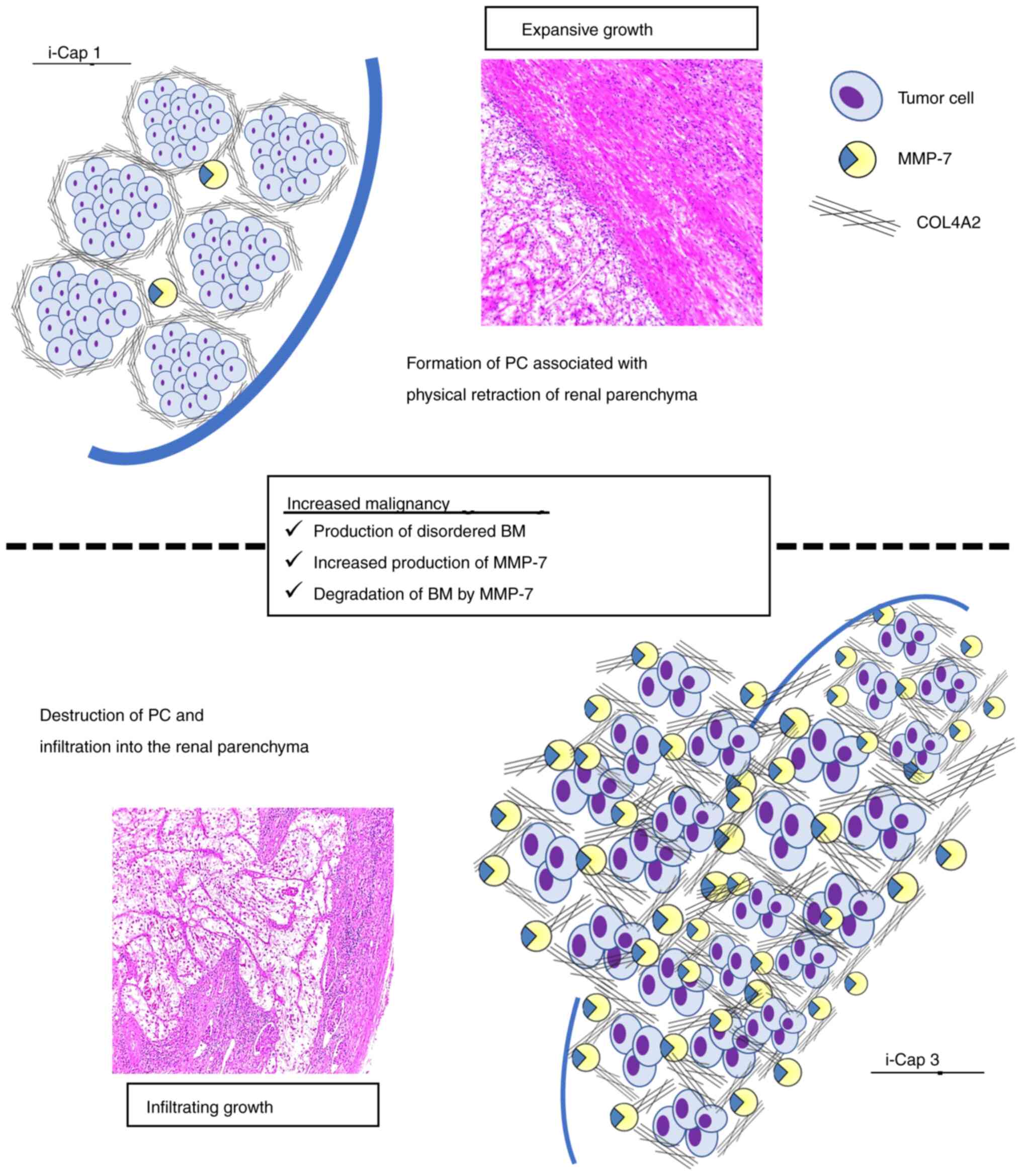
suggesting that MMP-7 serves a role in RCC peri-invasion. Fig. 6 schematically shows the differences
in extracellular matrix reconstruction by COL4 and MMP-7 between
i-Cap 1 and 3. MMP-7 cleaves the cancer cell membrane protein
hepatocyte growth factor activator inhibitor type 1 (HAI-1) to
produce a soluble HAI-1 (sHAI-1) fragment. It has been shown that
sHAI-1 and MMP-7 cooperate to induce cancer cell aggregation and
metastasis, and therapeutics targeting sHAI-1 have attracted
attention (33). Although the
current study did not detect sHAI-1 expression, it was confirmed
that expression of MMP-7 at the site of invasion was high, which
may benefit from sHAI-1-targeted therapy.
RCC is known to be hypervascular and rich in
neovascularization, but is also a highly heterogeneous tumor.
Unsupervised transcriptome analysis of 823 tumors from patients
with advanced RCC by Motzer et al (34) revealed that the combination of
angiogenesis, immunity, cell cycle, metabolism and stromal programs
are classified into seven distinct molecular subsets. Tyrosine
kinase inhibitors are effective in subsets with high angiogenesis,
and immune checkpoint inhibitors improve clinical benefit in tumors
with high T effector and/or cell cycle transcription. These subset
classifications were performed for each international metastatic
RCC database consortium risk classification used to classify the
prognosis of metastatic renal cancer, and it was shown that the
classification of the immune system subset gradually increases and
that of the angiogenic system subset gradually decreases while
exacerbating from favorable risk to intermediate and poor risk. Ohe
et al (35) and Cioca et
al (36) also showed that
decreased blood vessel density in RCC is associated with
exacerbation of cancer malignancy. The present finding that higher
i-Cap was associated with a poorer prognosis and decreased ENG
expression is consistent with these findings.
SELL is a cell adhesion molecule involved in
lymphocyte migration, which is expressed on most circulating
leukocytes. Notably, loss of SELL is indicative of T-cell
activation as it occurs upon cell activation. ccRCC has been
characterized as having one of the highest immune infiltration
scores in pan-cancer analyses (37,38).
In recent years, the immunoscore has attracted attention in the
field of colon cancer, as it reflects the oncological prognosis.
The immunoscore ranges from I0, the so-called ‘cold’ tumor (no or
low density of immune cells both at the periphery and center of the
tumor), to I4, the so-called ‘hot’ tumor (high immune cell density
at both the periphery and center of the tumor), and is used to
classify cancer according to immune infiltration (39). Page et al (40) also reported that the infiltration of
immune cells at the infiltration site of the tumor margin is
related to prognosis. Notably, ccRCC is considered to have a poor
prognosis as immune cell infiltration increases (41). In the present study, the expression
of SELL was detected, focusing on the infiltration of PC. As a
result, it was confirmed that the expression of SELL was enhanced
as the i-Cap score increased. This suggests the possibility that
immune cell infiltration occurs along with PC destruction.
As aforementioned, it has been confirmed that tumor
infiltration into the PC, which is associated with exacerbation of
tumor malignancy, is accompanied by decreased angiogenesis,
destruction of the extracellular matrix by MMP-7 and reconstruction
by COL4, and infiltration of immune cells. This finding may be the
key to identifying the PC features that accompany most cases of
ccRCC.
The present study has various limitations. First,
prognostic factors were retrospectively examined and patients who
received adjuvant treatment were not included. Pathological scoring
was also performed retrospectively by a single urological
pathologist. However, this issue is minimized as the pathologist
that performed the scoring did not know the clinical information of
the patients. Moreover, some i-Cap scores may have been upgraded
secondary to surgical removal and/or iatrogenic disruption of the
PC during specimen processing. There may also have been
inter-observer variability and institutional bias among the
investigators who graded the immunostaining score. Furthermore,
there were only five cases of i-Cap 0 in the present study. In our
other study (unpublished data) of only NSS, it was revealed that
ccRCC did not form a PC in some cases (3 out of 11 cases) when the
tumor size was <2 cm. The majority of the cases in the present
study were nephrectomies, and there were few cases <4 cm that
were eligible for NSS, which may be one of the reasons why only
five cases of i-Cap 0 were identified. Of the 39 patients in which
postoperative metastasis was observed, 14 patients underwent
resection of the metastasis. Although it would have been best to
evaluate the PCs in the metastatic lesions of all patients, there
were cases in which drug therapy was preferred. A feature of the
present study is that by using a rat model, the genetic background
and tumor background are uniform; therefore, it is possible to
identify a group of genes that are likely to have some significance
with a limited number of samples. Subsequently, the genes
identified using the rat model were assessed in human samples.
However, in the rat model of ccRCC, the expression levels of
ENG increased as i-Cap score increased, but the opposite
result was obtained in human specimens. It was hypothesized that
this may be due to species differences or simply due to the smaller
numbers assessed in the rat model. Additionally, in the animal
model, the kidneys were fixed in formalin immediately after removal
so that the gap between the tumor and normal renal tissue could be
observed; therefore, another limitation is that it was not possible
to remove only the tumor or measure the tumor weight.
In conclusion, the present study investigated the
formation and destruction of PCs in ccRCC. PCs were formed by
physical compression and tended to collapse as the tumor became
malignant. It was revealed that tumor invasion into the PC, that
is, disruption of the PC, can be a prognostic factor in ccRCC.
Furthermore, PC breakdown was accompanied by degradation of the
extracellular matrix by MMP-7, reconstitution by COL4, decreased
angiogenesis and infiltration of immune cells.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The PCR array data generated in the present study
may be found in the NCBI Gene Expression Omnibus (42) under accession numbers GSE255816,
GSE255820 and GSE255822 or at the following URLs: https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE255816,
https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE255820
and https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE255822.
The other data generated in the present study may be requested from
the corresponding author.
Authors' contributions
TS, MM and KF contributed to the design of the study
and writing of the manuscript. KoI, SO, TF, YI, KaI, CO and TM
conducted the molecular biology studies. TS and NT performed the
statistical tests. FM and MT contributed to the acquisition of data
and confirm the authenticity of all the raw data. MM, NT and KF
assisted with the writing of the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of Nara Medical University
approved this protocol (approval no. NMU-1256). All subjects gave
their written informed consent for inclusion before they
participated in the study. The institutional animal care and use
committee of Nara Medical University approved the animal study
protocol (project identification code: 12211).
Patient consent for publication
Not applicable.
Competing interests
All authors confirm that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PC
|
pseudo-capsule
|
|
RCC
|
renal cell carcinoma
|
|
ccRCC
|
clear cell RCC
|
|
NSS
|
nephron-sparing surgery
|
|
RN
|
radical nephrectomy
|
|
i-Cap
|
invasion of pseudo-capsule
|
|
NT
|
normal tissue
|
|
TNM
|
Tumor-Node-Metastasis
|
|
UICC
|
Union for International Cancer
Control
|
|
DEN
|
N-diethylnitrosamine
|
|
FeNTA
|
ferric nitrilotriacetic acid
|
|
H&E
|
hematoxylin and eosin
|
|
IHC
|
immunohistochemistry
|
|
COL4A2
|
collagen type 4A2
|
|
ENG
|
endoglin
|
|
MMP
|
matrix metalloproteinase
|
|
SELL
|
l-selectin
|
|
CSS
|
cancer-specific survival
|
|
DFS
|
disease-free survival
|
|
IQR
|
interquartile range
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
|
HAI-1
|
hepatocyte growth factor activator
inhibitor type 1
|
|
sHAI-1
|
soluble HAI-1
|
References
|
1
|
World Health Organization (WHO), .
International Agency for Research on Cancer. WHO; Geneva: 2020
|
|
2
|
Pickhardt PJ, Lonergan GJ, Davis CJ Jr,
Kashitani N and Wagner BJ: From the archives of the AFIP.
Infiltrative renal lesions: Radiologic-pathologic correlation.
Armed forces institute of pathology. Radiographics. 20:215–243.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Minervini A, Carini M, Uzzo RG, Campi R,
Smaldone MC and Kutikov A: Standardized reporting of resection
technique during nephron-sparing surgery: The
surface-intermediate-base margin score. Eur Urol. 66:803–805. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang L, Feng J, Alvarez H, Snarskis C,
Gupta G and Picken MM: Critical histologic appraisal of the
pseudocapsule of small renal tumors. Virchows Arch. 467:311–317.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Minervini A, di Cristofano C, Lapini A,
Marchi M, Lanzi F, Giubilei G, Tosi N, Tuccio A, Mancini M, della
Rocca C, et al: Histopathologic analysis of peritumoral
pseudocapsule and surgical margin status after tumor enucleation
for renal cell carcinoma. Eur Urol. 55:1410–1418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Azhar RA, de Castro Abreu AL, Broxham E,
Sherrod A, Ma Y, Cai J, Gill TS, Desai M and Gill IS: Histological
analysis of the kidney tumor-parenchyma interface. J Urol.
193:415–422. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho S, Lee JH, Jeon SH, Park J, Lee SH,
Kim CH, Sung JY, Kim JH, Pyun JH, Lee JG, et al: A prospective,
multicenter analysis of pseudocapsule characteristics: Do all
stages of renal cell carcinoma have complete pseudocapsules? Urol
Oncol. 35:370–378. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kryvenko ON: Characteristics of the
peritumoral pseudocapsule vary predictably with histologic subtype
of T1 renal neoplasms. Jacob JM, Williamson SR, Gondim DD, Leese
JA, Terry C, Grignon DJ, Boris RS.Urology. November
2015;86(5):956-961. Urol Oncol. 35:453–454. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mantoan Padilha M, Billis A, Allende D,
Zhou M and Magi-Galluzzi C: Metanephric adenoma and solid variant
of papillary renal cell carcinoma: Common and distinctive features.
Histopathology. 62:941–953. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kryvenko ON, Haley SL, Smith SC, Shen SS,
Paluru S, Gupta NS, Jorda M, Epstein JI, Amin MB and Truong LD:
Haemangiomas in kidneys with end-stage renal disease: A novel
clinicopathological association. Histopathology. 65:309–318. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cho HJ, Kim SJ, Ha US, Hong SH, Kim JC,
Choi YJ and Hwang TK: Prognostic value of capsular invasion for
localized clear-cell renal cell carcinoma. Eur Urol. 56:1006–1012.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Snarskis C, Calaway AC, Wang L, Gondim D,
Hughes I, Idrees MT, Kliethermes S, Maniar V, Picken MM, Boris RS
and Gupta GN: Standardized reporting of microscopic renal tumor
margins: introduction of the renal tumor capsule invasion scoring
system. J Urol. 197:23–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brierley J, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumours. Eigth edition. Wiley
Blackwell/John Wiley & Sons, Inc; Chichester, UK: pp. 199–201.
2017
|
|
14
|
Fuhrman SA, Lasky LC and Limas C:
Prognostic significance of morphologic parameters in renal cell
carcinoma. Am J Surg Pathol. 6:655–663. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Toyokuni S, Uchida K, Okamoto K,
Hattori-Nakakuki Y, Hiai H and Stadtman ER: Formation of
4-hydroxy-2-nonenal-modified proteins in the renal proximal tubules
of rats treated with a renal carcinogen, ferric nitrilotriacetate.
Proc Natl Acad Sci USA. 91:2616–2620. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vargas-Olvera CY, Sánchez-González DJ,
Solano JD, Aguilar-Alonso FA, Montalvo-Muñoz F, Martínez-Martínez
CM, Medina-Campos ON and Ibarra-Rubio ME: Characterization of
N-diethylnitrosamine-initiated and ferric
nitrilotriacetate-promoted renal cell carcinoma experimental model
and effect of a tamarind seed extract against acute nephrotoxicity
and carcinogenesis. Mol Cell Biochem. 369:105–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council, . Guide for the
care and use of laboratory animals: Eighth edition. The National
Academies Press; Washington, DC, USA: pp. 11–18. 2011
|
|
18
|
Miyake M, Tanaka N, Hori S, Ohnishi S,
Takahashi H, Fujii T, Owari T, Ohnishi K, Iida K, Morizawa Y, et
al: Dual benefit of supplementary oral 5-aminolevulinic acid to
pelvic radiotherapy in a syngenic prostate cancer model. Prostate.
79:340–351. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vandesompele J, De Preter K, Pattyn F,
Poppe B, Van Roy N, De Paepe A and Speleman F: Accurate
normalization of real-time quantitative RT-PCR data by geometric
averaging of multiple internal control genes. Genome Biol.
3:research0034.0031. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Allred DC, Harvey JM, Berardo M and Clark
GM: Prognostic and predictive factors in breast cancer by
immunohistochemical analysis. Mod Pathol. 11:155–168.
1998.PubMed/NCBI
|
|
22
|
Butcher DT, Alliston T and Weaver VM: A
tense situation: Forcing tumour progression. Nat Rev Cancer.
9:108–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Handorf AM, Zhou Y, Halanski MA and Li WJ:
Tissue stiffness dictates development, homeostasis, and disease
progression. Organogenesis. 11:1–15. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fujimoto N, Sugita A, Terasawa Y and Kato
M: Observations on the growth rate of renal cell carcinoma. Int J
Urol. 2:71–76. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Oda T, Miyao N, Takahashi A, Yanase M,
Masumori N, Itoh N, Tamakawa M and Tsukamoto T: Growth rates of
primary and metastatic lesions of renal cell carcinoma. Int J Urol.
8:473–477. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lohi J, Korhonen M, Leivo I, Kangas L,
Tani T, Kalluri R, Miner JH, Lehto VP and Virtanen I: Expression of
type IV collagen alpha1(IV)-alpha6(IV) polypeptides in normal and
developing human kidney and in renal cell carcinomas and
oncocytomas. Int J Cancer. 72:43–49. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Best SL, Liu Y, Keikhosravi A, Drifka CR,
Woo KM, Mehta GS, Altwegg M, Thimm TN, Houlihan M, Bredfeldt JS, et
al: Collagen organization of renal cell carcinoma differs between
low and high grade tumors. BMC Cancer. 19:4902019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Provenzano PP, Eliceiri KW, Campbell JM,
Inman DR, White JG and Keely PJ: Collagen reorganization at the
tumor-stromal interface facilitates local invasion. BMC Med.
4:382006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gu ZD, Li JY, Li M, Gu J, Shi XT, Ke Y and
Chen KN: Matrix metalloproteinases expression correlates with
survival in patients with esophageal squamous cell carcinoma. Am J
Gastroenterol. 100:1835–1843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Miyata Y, Iwata T, Ohba K, Kanda S,
Nishikido M and Kanetake H: Expression of matrix
metalloproteinase-7 on cancer cells and tissue endothelial cells in
renal cell carcinoma: Prognostic implications and clinical
significance for invasion and metastasis. Clin Cancer Res.
12:6998–7003. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ogawa M, Ikeuchi K, Watanabe M, Etoh K,
Kobayashi T, Takao Y, Anazawa S and Yamazaki Y: Expression of
matrix metalloproteinase 7, laminin and type IV collagen-associated
liver metastasis in human colorectal cancer: Immunohistochemical
approach. Hepatogastroenterology. 52:875–880. 2005.PubMed/NCBI
|
|
32
|
Liao HY, Da CM, Liao B and Zhang HH: Roles
of matrix metalloproteinase-7 (MMP-7) in cancer. Clin Biochem.
92:9–18. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ishikawa T, Kimura Y, Hirano H and Higashi
S: Matrix metalloproteinase-7 induces homotypic tumor cell
aggregation via proteolytic cleavage of the membrane-bound
Kunitz-type inhibitor HAI-1. J Biol Chem. 292:20769–20784. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Motzer RJ, Banchereau R, Hamidi H, Powles
T, McDermott D, Atkins MB, Escudier B, Liu LF, Leng N, Abbas AR, et
al: Molecular subsets in renal cancer determine outcome to
checkpoint and angiogenesis blockade. Cancer Cell. 38:803–817.e4.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ohe C, Yoshida T, Amin MB, Atsumi N, Ikeda
J, Saiga K, Noda Y, Yasukochi Y, Ohashi R, Ohsugi H, et al:
Development and validation of a vascularity-based architectural
classification for clear cell renal cell carcinoma: correlation
with conventional pathological prognostic factors, gene expression
patterns, and clinical outcomes. Mod Pathol. 35:816–824. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cioca A, Muntean D and Bungardean C: CD105
as a tool for assessing microvessel density in renal cell
carcinoma. Indian J Pathol Microbiol. 62:239–243. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rooney MS, Shukla SA, Wu CJ, Getz G and
Hacohen N: Molecular and genetic properties of tumors associated
with local immune cytolytic activity. Cell. 160:48–61. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Şenbabaoğlu Y, Gejman RS, Winer AG, Liu M,
Van Allen EM, de Velasco G, Miao D, Ostrovnaya I, Drill E, Luna A,
et al: Tumor immune microenvironment characterization in clear cell
renal cell carcinoma identifies prognostic and
immunotherapeutically relevant messenger RNA signatures. Genome
Biol. 17:2312016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Galon J, Pagès F, Marincola FM, Angell HK,
Thurin M, Lugli A, Zlobec I, Berger A, Bifulco C, Botti G, et al:
Cancer classification using the immunoscore: A worldwide task
force. J Transl Med. 10:2052012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Pagès F, Mlecnik B, Marliot F, Bindea G,
Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, et al:
International validation of the consensus immunoscore for the
classification of colon cancer: A prognostic and accuracy study.
Lancet. 391:2128–2139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fridman WH, Zitvogel L, Sautès-Fridman C
and Kroemer G: The immune contexture in cancer prognosis and
treatment. Nat Rev Clin Oncol. 14:717–734. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Edgar R, Domrachev M and Lash AE: Gene
expression omnibus: NCBI gene expression and hybridization array
data repository. Nucleic Acids Res. 30:207–210. 2002. View Article : Google Scholar : PubMed/NCBI
|