Introduction
Acute myeloid leukaemia (AML) is a malignant clonal
disease originating from haematopoietic stem cells and primarily
affects older patients, with a median age >68 years at diagnosis
(1). In 2018, the U.S. Food and
Drug Administration approved venetoclax for treating patients with
AML who were unfit or >65 years old. Combination of azacitidine
and standard-dose venetoclax has been confirmed to achieve a higher
remission rate and longer overall survival (OS) time in older
patients who cannot tolerate conventional chemotherapy, and the
incidence range of grade 3/4 adverse reactions is 42–73% (2–8).
According to a real-world report (9), patients with newly diagnosed AML were
treated with azacitidine combined with venetoclax. However, owing
to chemotherapy toxicity, 59.8% of patients needed to adjust the
chemotherapy regimen, and 34.9% needed to adjust the chemotherapy
dose when a standard 400 mg venetoclax dose is used. In China, only
a few studies (7,8) have focused on azacitidine combined
with low-dose venetoclax for treating older patients with newly
diagnosed AML at the time of diagnosis. Moreover, considering that
the standard dose is poorly tolerated in the Chinese population,
the incidence of serious adverse reactions reported upon using the
existing standard-dose regimen is high (2–8).
Therefore, in the present study, the aim was to investigate the
short-term clinical efficacy and safety of azacitidine combined
with a low-dose venetoclax regimen for patients with AML.
Materials and methods
Clinical data
The clinical data of 26 older patients with AML who
received the azacitidine and venetoclax regimen at Yuyao People's
Hospital (Yuyao, China) between January 2021 and May 2023 were
retrospectively analysed. All patients were diagnosed based on bone
marrow cell morphology, flow cytometry typing, cytogenetics and
molecular biology typing criteria. Prognostic risk stratification
was performed according to the National Comprehensive Cancer
Network 2021 3rd Edition criteria (10). Relevant patient data were collected
and followed up. Owing to various reasons such as age, financial
situation and physical condition, none of the enrolled patients
were eligible for conventional chemotherapy. The inclusion criteria
were as follows: i) ≥75 years old or 65–75 years old with an
Eastern Cooperative Oncology Group physical fitness score of 2–4
(11); ii) presence of severe
heart, lung, liver and kidney diseases; and iii) presence of any
comorbidities deemed unsuitable for intensive chemotherapy by the
treating physician (4). The
exclusion criteria were as follows: i) Previous treatment with
methylated drug decitabine or azacitidine and chemotherapy (except
hydroxyurea); and ii) presence of other malignant tumours. The
present study was approved by the Ethics Committee of Yuyao
People's Hospital (Yuyao, China), and all patients or their legal
guardians provided written informed consent.
Therapeutic regimen
According to the patient's wishes, the treatment was
started after excluding chemotherapy connexion. The specific dosage
for each regimen was as follows: i) Regimen 1, subcutaneous
injection of 100 mg azacitidine on days 1–5 and 100 mg oral
venetoclax on days 3–16; and ii) regimen 2, subcutaneous injection
of 100 mg azacitidine on days 1–5 and 100 mg oral venetoclax on day
3 plus 200 mg oral venetoclax on days 4–30. The administration was
scheduled to be repeated once every 28 days, with an appropriate
extension of time if the patient did not recover haematopoietic
function [recovery considered as a platelet (PLT) count of
>1×1011/l and an absolute neutrophil count (ANC) of
>1×109/l].
Therapeutic evaluation
Bone marrow examination was performed at the end of
every course. According to haematologic diagnosis and therapeutic
efficacy criteria (10), complete
response (CR) was defined as the disappearance of AML symptoms and
signs, ANC value in peripheral blood ≥1.5×109 cells/l,
PLT count ≥100×109 cells/l, leukocyte classification
without leukaemia cells, bone marrow image showing granulocyte type
I + II ≤5% and no extramedullary leukaemia invasion. CR with
incomplete haematological recovery (CRi) occurred when all the
criteria for CR were met except for neutropenia
(<1.0×109 cells/l) or thrombocytopenia
(<100×109 cells/l). Partial response (PR) was
characterized by bone marrow granulocyte type I + II >5% but
≤20%, with one clinical and haematologic response not meeting the
CR standard. No response (NR) was defined as bone marrow and blood
images not meeting the aforementioned criteria. Finally, overall
response (OR) was calculated as follows: OR=CR + PR.
Adverse reactions and treatment
principles
Adverse event severity was graded following the
National Cancer Institute Common Adverse Event Evaluation Criteria
version 5.0 (12). The patients'
blood, liver and kidney functions were regularly monitored during
treatment. When the patient's ANC was <1.0×109
cells/l, patients were administered subcutaneous injections of
granulocyte colony-stimulating factor (range, 200–400) µg/day. When
the patient's haemoglobin level was <60 g/l, a red blood cell
suspension was transfused. PLTs were injected when the patient's
PLT was <20×109 cells/l; coinfected patients were
treated with active anti-infection treatment (antibiotics). If the
patient was co-infected with a fungal infection, CYP3A inhibitors
were not used to avoid increasing the venetoclax concentration.
Nausea, vomiting, diarrhoea and other gastrointestinal reactions
were actively managed with symptomatic treatments.
Follow-up visit
Patients were followed up until December 2023,
mainly through in-patient and out-patient assessments, and
telephone follow-ups. The follow-up is part of the standard
procedure after treatment.
Statistical analysis
The results were analysed using SPSS (version 26;
IBM Corp.). Categorical variables are presented as proportions,
whereas continuous variables are presented as medians (P25, P75).
The Kaplan-Meier method was used to analyze OS and progression-free
survival (PFS), with the log-rank test used to assess statistical
associations, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical features
Overall, 26 patients diagnosed with AML were
included in the present study. In the all-comers cohort, the median
age at diagnosis was 73 years, including 11 patients aged ≥75
years. The median ratio of original cells was 60% (range, 21–97%),
and the median treatment cycle was seven (range, 1–18 cycles). A
total of 13 patients underwent genetic testing of bone marrow
samples, using next-generation sequencing for 278 genes associated
with leukemia, as performed by ADICON Medical Laboratory. In
European Leukemia Network (ELN) risk stratification, five cases had
a good prognosis, 13 cases had a moderate prognosis and eight cases
had a poor prognosis. The clinical characteristics of both patient
groups are presented in Table
I.
 | Table I.Basic characteristics of the 26
patients with newly diagnosed AML. |
Table I.
Basic characteristics of the 26
patients with newly diagnosed AML.
| Basic
characteristics | 100 mg Venetoclax on
days 3–16 (n=15) | 100 mg Venetoclax on
day 3 + 200 mg on days 4–30 (n=11) |
|---|
| Age, years | 69.0 (65.0–82.0) | 74.0(65.0–82.0) |
| Sex, n |
|
|
| Male | 9 | 5 |
|
Female | 6 | 6 |
| WHO classification,
n |
|
|
|
AML-M0 | 2 | 0 |
|
AML-M2 | 7 | 3 |
|
AML-M4 | 5 | 4 |
|
AML-M5 | 1 | 4 |
| Proportion of bone
marrow original cells, % | 36.0 (21.5–97.0) | 67.3 (21.0–94.5) |
| WBCs, ×109
cells/l | 7.6 (1.3–63.6) | 2.4 (0.8–54.5) |
| HB, g/l | 71.0
(68.0–110.0) | 86.0
(58.0–102.0) |
| PLTs, ×109
cells/l | 66.0
(20.0–298.0) | 71.5
(28.0–214.0) |
| ECOG
scorea |
|
|
| 2 | 5 | 1 |
| 3 | 7 | 7 |
| 4 | 3 | 3 |
| ELN risk
stratificationb,
n |
|
|
|
Favourable | 2 | 3 |
|
Intermediate | 8 | 5 |
|
Adverse | 5 | 3 |
| Other systemic
diseasesc, n | 15 | 11 |
Clinical efficacy
At the end of the first treatment course (29 days
after the beginning of chemotherapy), 17 (65.4%), five (19.2%) and
three (11.5%) cases achieved CR, CRi and PR, respectively. The CR +
CRi rate was 84.6%, and the objective response rate (ORR) was 96.2%
(Table II).
 | Table II.Clinical efficacy and survival of
patients with newly diagnosed AML. |
Table II.
Clinical efficacy and survival of
patients with newly diagnosed AML.
| Clinical
efficacy | 100 mg Venetoclax
on days 3–16 (n=15) | 100 mg Venetoclax
on day 3 + 200 mg on days 4–30 (n=11) |
|---|
| OR, n (%) | 15 (100.0) | 10 (90.9) |
| CR + CRi, n
(%) | 13 (86.7) | 9 (81.8) |
| PR, n (%) | 2 (13.3) | 1 (9.09) |
| NR, n (%) | 0 (0.0) | 1 (9.09) |
The median number of sessions for all patients was
seven (range, 1–18 sessions). Of the 26 patients, nine (34.6%)
patients relapsed within a mean of 6.7 months (range, 4.7–14.1
months). Two patients did not undergo the second phase of
chemotherapy owing to their critical condition, and one patient
showed no response.
Survival analysis
By December 2023, the median follow-up time was 10.5
months (range, 1.9–26.0 months), and no patient died during the
first course of treatment, with 12 (46.2%) patients surviving, five
(9.2%) patients having minimal residual disease negative and 14
(53.8%) patients dying. Three, five and six cases of multiple organ
failures, primary disease progression and severe infection,
respectively were observed. Among them, a patient with multiple
organ failure was admitted to the Intensive Care Unit and strongly
requested chemotherapy. After full communication and understanding
from the family members, chemotherapy was administered according to
protocol 1, and bone marrow re-examination indicated partial
remission. Subsequently, the patient became unconscious, and
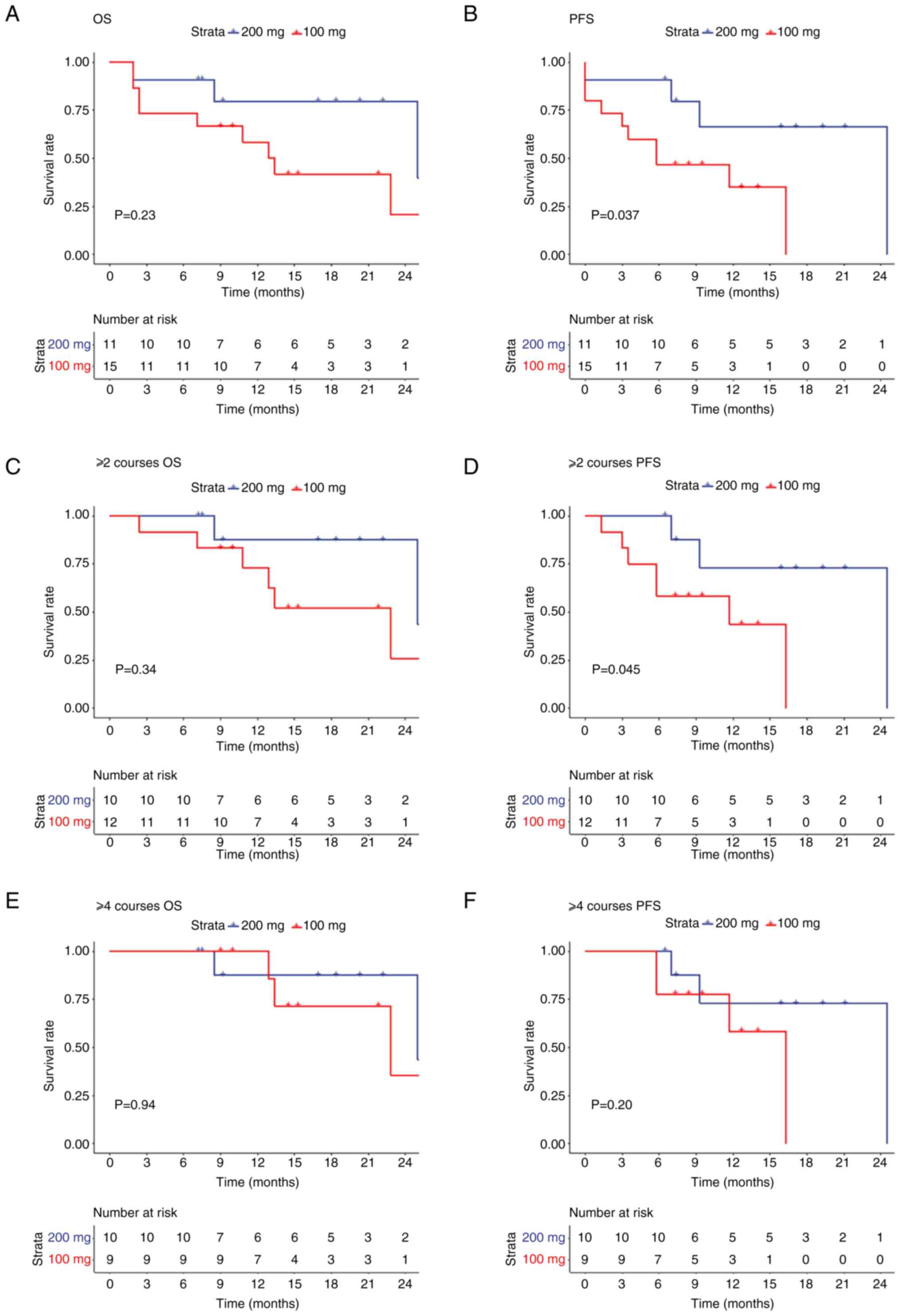
treatment was discontinued. The OS and PFS of the patients in two
groups were preliminarily analyzed (Fig. 1). The survival analysis of the two
groups revealed no significant differences in OS between the two
groups (P=0.23; Fig. 1A); however,
the PFS rate in the 200 mg group was significantly improved
compared with that in the 100 mg group (P=0.037; Fig. 1B). Furthermore, the rate of PFS in
the 200 mg group was significantly higher than that in the 100 mg
group treated with ≥2 treatment courses (P=0.045), while no
significant difference was observed in OS (P=0.34) between the two
groups (Fig. 1C and D). By
contrast, no significant difference in PFS (P=0.20) and OS (P=0.94)
was observed between the two groups after ≥4 treatment courses
(Fig. 1E and F).
Adverse reactions
The most common adverse event during treatment was
haematological. All patients had varying degrees of decreased white
blood cell counts and neutropenia. In the first course of
treatment, the median leukocyte hypoplasia was 1.0×109
cells/l (range, 0.3–17.7×109 cells/l), and the median
neutrophil hypoplasia was 0.3×109 cells/l (range,
0.0–13.1×109 cells/l). A total of 15 (57.7%) patients
had grade 3/4 febrile agranulocytosis.
All patients had thrombocytopenia, with low PLT
values of 20.0×109 cells/l (range,
2.0–231.0×109 cells/l) the first course of treatment; 10
patients had grade 3/4 thrombocytopenia, 13 (>50%) patients did
not need PLT infusion during chemotherapy and 11 patients had PLT
counts >40×109 cells/l before chemotherapy. Only two
patients had PLT counts <40×109 cells/l.
All 26 patients had anaemia, with haemoglobin levels
of 55.0 g/l (range, 32.0–99.0 g/l) in the first course of
treatment, and 11 (42.3%) patients had grade 3/4 anaemia.
The most common non-haematological adverse reactions
were infection and grade 3/4 febrile neutropenia, occurring in
eight (30.8%) cases. Pulmonary infection occurred in 22 patients,
with 9 (34.6%) patients experiencing grade 3/4 severity. All
patients clinically diagnosed with pulmonary fungal infections were
treated with drugs such as caspofungin (70 mg first dose, 50 mg
subsequent daily dose, for 14 days) and not with strong CYP3A
inhibitors such as posaconazole and voriconazole or moderate CYP3A
inhibitors such as esaconazole and fluconazole. Gastrointestinal
reactions, followed with nausea and vomiting, were observed in
three patients (Tables III and
IV).
 | Table III.Number of grade 3/4 adverse events in
26 patients during the first treatment course. |
Table III.
Number of grade 3/4 adverse events in
26 patients during the first treatment course.
| Grade 3/4 adverse
events | 100 mg Venetoclax
on days 1–14 (n=15) | 100 mg Venetoclax
on day 3 + 200 mg on days 4–30 (n=11) |
|---|
| Leukocytopenia,
n | 5 | 8 |
| Agranulocytosis,
n | 7 | 8 |
| Pulmonary
infection, n | 3 | 6 |
| Febrile
neutropenia, n | 3 | 5 |
| Thrombocytopenia,
n | 4 | 7 |
| Anaemia, n | 5 | 6 |
| Nausea, n | 1 | 2 |
| Emesis, n | 0 | 0 |
| Diarrhoea, n | 0 | 0 |
| Lacking in
strength, n | 1 | 1 |
| Tumour cytolysis
syndrome, n | 0 | 1 |
 | Table IV.Number of low blood cell counts in 26
patients during the first treatment course. |
Table IV.
Number of low blood cell counts in 26
patients during the first treatment course.
| Low blood cell
values | 100 mg Venetoclax
on days 3–16 (n=15) | 100 mg Venetoclax
on day 3 + 200 mg on days 4–30 (n=11) |
|---|
| Minimum leukocyte
value, ×109 cells/l | 1.1 (0.3–2.9) | 0.9 (0.3–17.7) |
| Minimum neutrophil
value, ×109 cells/l | 0.3
(0.00–2.57) | 0.2 (0.1–13.1) |
| Minimum Hb value,
g/l | 57.5
(32.0–75.0) | 57.0
(47.0–99.0) |
| Minimum PLT value,
×109 cells/l | 27.0
(2.0–72.0) | 23.0
(7.0–204.0) |
Only one patient (regimen 2) developed tumour lysis
syndrome, and none was observed in the 100-mg group.
Discussion
AML is the most common haematological malignancy in
older patients, with a median age of 67 years (1). Recently, its incidence has been
increasing annually, and patients often face rapid mortality owing
to severe complications such as anaemia, bleeding and infection
(13). The median survival of older
patients who forego conventional chemotherapy is only 2 months
(14). Older patients with AML
cannot tolerate strong chemotherapy or low-dose chemotherapy
(low-dose cytarabine), with a CR rate of 13.3% and a median
survival time of 5.2 months (15).
The response rate and duration of remission of monotherapy with
demethylated drug treatment were short; the remission rate of
hypomethylating agent (HMA) monotherapy was <30.0%, and the
median survival time was <12 months (16–18).
Demethylated drugs combined with different pre-activation regimens
such as aclacinomycin + cytarabine + recombinant human granulocyte
colony-stimulating/homoharringtonine + cytarabine + recombinant
human granulocyte colony-stimulating factor are safe and feasible
for the treatment of older patients with AML, with a CR rate of
40.0–70.0% and a median survival time of ~10 months (19–22).
With the development of molecular biology, increasing attention has
shifted towards molecular-targeted drugs and combinations of
targeted drugs.
The combination of azacitidine and venetoclax can
achieve a higher remission rate and prolong OS in older patients
with AML who cannot tolerate conventional chemotherapy. Domestic
and foreign studies have confirmed that the CR + CRi rate of older
patients with AML treated with azacitidine combined with a
standard-dose venetoclax regimen can reach 60–88% (2–8). The
median survival time range is 5.0–28.9 months, which greatly
improved the prognosis of older patients with AML.
In 2014, DiNardo et al (2) conducted a multicentre phase I clinical
trial for treating older patients with AML using venetoclax
combined with demethylated drugs. In this trial, the maximum dose
of venetoclax used once a day was 1,200 mg, and the study
determined that 400 mg/day was the best dose for sensitivity to
venetoclax combined with demethylated drugs. DiNardo et al
(3) conducted a large, multicentre,
phase 1b dose-escalation and scale-up study to further evaluate 400
mg venetoclax plus HMA as the optimal therapeutic dose in a larger
population. However, in clinical practice, the standard dose (400
mg) of venetoclax combined with azacitidine leads to greater side
effects, with an incidence range of serious adverse reactions of
42–73% (2–8) (Table
V). According to the Real World Report (9), among patients treated with
standard-dose venetoclax combined with azacitidine, 59.8% needed to
adjust the chemotherapy regimen, and 34.9% required modifications
in the chemotherapy dose.
 | Table V.Summary of studies on azacitidine
combined with venetoclax in the treatment of elderly patients with
newly diagnosed AML. |
Table V.
Summary of studies on azacitidine
combined with venetoclax in the treatment of elderly patients with
newly diagnosed AML.
| First author(s),
year | Cases, n | Age, years | Treatment plan | Rate of CR + Cri,
% | Median survival
time, months | Incidence of
thrombo-cytopenia, % | Incidence of fever
with neutro-penia, % | Incidence of
neutro-philia, % | Incidence of
anaemia, % | (Refs.) |
|---|
| DiNardo et
al, 2018 | 57 | ≥65 | 75 mg/m2
azacitidine on days 1–7 + 400 mg venetoclax | 61.4 | 12.4 | 47.4 | 42.1 | 40.3 | N/A | (2) |
| DiNardo et
al, 2019 | 145 | 74 | 75 mg/m2
azacitidine on days 1–7 or 20 mg/m2 decitabine on days
1–5 + 400 mg venetoclax | 73.0 | 12.5 | 21.4 | 42.7 | 17.2 | 24.8 | (3) |
| DiNardo et
al, 2020 | 431 | ≥75 | 75 mg/m2
azacitidine on days 1–7 + 400 mg venetoclax on days 1–28 | 66.4 | 20.5 | 45.1 | 41.9 | 41.9 | N/A | (4) |
| Winter et
al, 2019 | 33 | 72 | 75 mg/m2
azacitidine on days 1–7 + 400 mg venetoclax on days 1–28 | 84.9 | 28.9 | 81.8 | 42.1 | 84.8 | 81.8 | (5) |
| Morsia et
al, 2020 | 44 | 73.5 | 75 mg/m2
azacitidine on days 1–7 or 20 mg/m2 decitabine on days
1–5 + 400 mg venetoclax (adjusted based on the patient's
condition) | 50.0 | 7.0 | N/A | N/A | N/A | 9.1 | (6) |
| Lou et al,
2022 | 27 | 70 | 75 mg/m2
azacitidine on days 1–7 + 100 mg venetoclax on day 1, 200 mg on day
2 and 400 mg on days 3–28 | 51.9 | 10.8 | 81.5 | 26 | N/A | 74.1 | (7) |
| Zhang et al,
2022 | 11 | 68 | 75 mg/m2
azacitidine on days 1–7 + 100 mg venetoclax on day 1, 200 mg on day
2 and 400 mg on days 3–28 | 45.5 | 5.0 | 100.0 | 18.2 | 100.0 | N/A | (8) |
| Present study | 11 | 74 | 100 mg azacitidine
on |
|
|
|
|
|
|
|
| (200 mg group) |
|
| days 1–5 + 100 mg
venetoclax on day 3 + 200 mg on days 4–30 | 81.8 | 10.8 | 63.6 | 45.5 | 72.7 | 54.5 | N/A |
| Present study (100
mg group) | 15 | 72 | 100 mg azacitidine
on days 1–5 + 100 mg venetoclax on days 3–16 | 86.7 | 8.5 | 26.7 | 20.0 | 46.7 | 33.3 | N/A |
| Present study
(total regimens) | 26 | 73 | 100 mg azacitidine
on days 1–5 + 100 mg venetoclax on days 3–16/100 mg on day 3 + 200
mg on days 4–30 | 84.6 | 9.5 | 42.3 | 30.8 | 57.7 | 42.3 | N/A |
In the present study, a low-dose regimen (100 mg
azacitidine on days 1–5 and 100 mg venetoclax on days 3–16 or 200
mg venetoclax on days 3–30) was administered to 26 patients with a
median age of 73 years. At the end of the first course of
treatment, the CR + Cri, and ORR rates were 84.6 and 96.2%,
respectively. The CR + CRi rate in the 100 and 200 mg group was
86.7 and 81.8%, respectively. Compared with the previous standard
protocol, the remission rate was similar to that reported in
studies in other countries (2–8). From
the survival analysis of the two groups, the PFS rate in the 200 mg
group was higher than that in the 100 mg group. Still, the two
groups had no statistically significant difference in OS.
The incidence of grade 3/4 serious adverse reactions
was 31.7 and 59.1% in the 100 and 200 mg group, respectively. The
overall incidence of grade 3/4 haematological adverse reactions was
43.3% in both groups, which was lower than the reported incidence
of adverse reactions in China (7,8). The
results showed that adverse reactions were lower in the 100 mg
group than those in the 200 mg group, and the overall efficacy of
the two groups was comparable. No deaths occurred during the first
course of treatment, which was relatively rare in previous reports
(2–8). Half of the patients could not receive
PLT transfusion during the first course of chemotherapy, including
11 patients with PLT counts >40×109 cells/l before
chemotherapy, and only one patient needed a PLT transfusion.
Clinically, patients often experience severe myelosuppression
during treatment, and bone marrow aspiration can be performed
within ~14 days. Tumour load can be assessed based on cell
morphology and immune typing. If no evident original cells are
observed in bone marrow cell morphology or immunotyping, the
treatment course can be appropriately shortened based on the
patient's preferences.
Specific genetic variants may be closely associated
with the prognosis of senile acute leukaemia. Some genetic
abnormalities may cause the disease to become more aggressive,
whereas others may be associated with better treatment response and
survival. AML with gene mutations associated with myeloid
dysplasia, including ASXL1, BCOR, EZH2, RUNX1, SF3B1, SRSF2, STAG2,
U2AF1 and ZRSR2, was classified as having a poor prognosis. All
patients with CEBPA bZIP mutations, whether biallelic or
monoallelic, were associated with a good prognosis (23). Therefore, understanding a patient's
genetic variations can help predict disease progression and
prognosis. The effects of genetic changes on treatments in a larger
patient cohort will be further analysed in future studies. In the
present study, genetic testing was conducted only on 13 patients,
as some patients refused genetic testing. Increasing the number of
patients that undergo genetic testing would provide a more
comprehensive stratification of risk. In ELN risk stratification,
five cases had a good prognosis, 13 cases had a moderate prognosis
and eight cases had a poor prognosis.
The current study had some limitations. A small
sample size was included and a short follow-up period followed.
Thus, for older patients with newly diagnosed AML who cannot
tolerate conventional chemotherapy, it is necessary to expand the
sample size and extend the follow-up period. Additionally, patients
may not achieve deep remission after reduction due to factors such
as comorbidities, poor physical condition and patient compliance.
Therefore, after disease remission, switching regimens or other
low-dose chemotherapy regimens can further improve the survival
rate of patients.
In conclusion, the preliminary results of the
current study indicated that low-dose venetoclax combined with
azacitidine is an effective and safe new treatment option for older
and frail patients with newly diagnosed AML.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural Science
Foundation Project of Ningbo Science and Technology Bureau,
Zhejiang, China (grant no. 2022J040).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YX conceived and designed the study. CR drafted the
manuscript. CA, MG, YL and YW collected the data. WH, MW, YC and PG
analysed the data. CA and FY discussed the results. CR, FY, YC, MW,
CA, YL, PG, YW, XH, MG, WH and YX helped design the study and
confirm the authenticity of all the raw data. All authors have
reviewed and edited the manuscript. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the local Ethics
Committee of Yuyao People's Hospital (Yuyao, China), to ensure
compliance with ethical standards (approval no. 2023-07-009).
Patient consent for publication
Written informed consent for publication of the
article was obtained from the patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AML
|
acute myeloid leukemia
|
|
ANC
|
absolute neutrophil count
|
|
CR
|
complete remission
|
|
CRi
|
CR with incomplete hematological
recovery
|
|
OS
|
overall survival
|
|
PR
|
partial response
|
|
PLT
|
platelet
|
|
NR
|
no response
|
|
OR
|
overall response
|
References
|
1
|
Mangaonkar AA and Patnaik MM: Patterns of
care and survival for elderly acute myeloid leukemia-challenges and
opportunities. Curr Hematol Malig Rep. 12:290–299. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
DiNardo CD, Pratz KW, Letai A, Jonas BA,
Wei AH, Thirman M, Arellano M, Frattini MG, Kantarjian H, Popovic
R, et al: Safety and preliminary efficacy of venetoclax with
decitabine or azacitidine in elderly patients with previously
untreated acute myeloid leukaemia: A non-randomised, open-label,
phase 1b study. Lancet Oncol. 19:216–228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
DiNardo CD, Pratz K, Pullarkat V, Jonas
BA, Arellano M, Becker PS, Frankfurt O, Konopleva M, Wei AH,
Kantarjian HM, et al: Venetoclax combined with decitabine or
azacitidine in treatment-naive, elderly patients with acute myeloid
leukemia. Blood. 133:7–17. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DiNardo CD, Jonas BA, Pullarkat V, Thirman
MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, et
al: Azacitidine and venetoclax in previously untreated acute
myeloid leukemia. N Engl J Med. 383:617–629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winters AC, Gutman JA, Purev E, Nakic M,
Tobin J, Chase S, Kaiser J, Lyle L, Boggs C, Halsema K, et al:
Real-world experience of venetoclax with azacitidine for untreated
patients with acute myeloid leukemia. Blood Adv. 3:2911–2919. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Morsia E, McCullough K, Joshi M, Cook J,
Alkhateeb HB, Al-Kali A, Begna K, Elliott M, Hogan W, Litzow M, et
al: Venetoclax and hypomethylating agents in acute myeloid
leukemia: Mayo clinic series on 86 patients. Am J Hematol.
95:1511–1521. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lou D, Liu L and Qin WW: Clinical analysis
of venetoclax combined with azacitidine in elderly patients with
newly diagnosed acute myeloid leukemia. Chin J Clin Oncol.
49:775–780. 2022.(In Chinese).
|
|
8
|
Zhang B, Ji JM, Wu Y, Ji O, Lin L and Zhu
G: Clinical efficacy and safety analysis of azacytidine combined
with venetoclax in patients with newly diagnosed acute myeloid
leukemia who cannot tolerate conventional chemotherapy. J Clin
Intern Med. 39:632–634. 2022.(In Chinese).
|
|
9
|
Vachhani P, Flahavan EM, Xu T, Ma E,
Montez M, Gershon A, Onishi M, Jin H, Ku G, Flores B, et al:
Venetoclax and hypomethylating agents as first-line treatment in
newly diagnosed patients with AML in a predominately community
setting in the US. Oncologist. 27:907–918. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pollyea DA, Bixby D, Perl A, Bhatt VR,
Altman JK, Appelbaum FR, de Lima M, Fathi AT, Foran JM, Gojo I, et
al: NCCN guidelines insights: Acute myeloid leukemia, version
2.2021. J Natl Compr Canc Netw. 19:16–27. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Young J, Badgery-Parker T, Dobbins T,
Jorgensen M, Gibbs P, Faragher I, Jones I and Currow D: Comparison
of ECOG/WHO performance status and ASA score as a measure of
functional status. J Pain Symptom Manage. 49:258–264. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Freites-Martinez A, Santana N,
Arias-Santiago S and Viera A: Using the common terminology criteria
for adverse events (CTCAE-version 5.0) to evaluate the severity of
adverse events of anticancer therapies. Actas Dermosifiliogr (Engl
Ed). 112:90–92. 2021.(In English, Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dhopeshwarkar N, Iqbal S, Wang X and Salas
M: A retrospective study of comorbidities and complications in
elderly acute myeloid leukemia patients in the United States. Clin
Lymphoma Myeloma Leuk. 19:e436–e456. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oran B and Weisdorf DJ: Survival for older
patients with acute myeloid leukemia: A population-based study.
Haematologica. 97:1916–1924. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Döhner H, Lübbert M, Fiedler W, Fouillard
L, Haaland A, Brandwein JM, Lepretre S, Reman O, Turlure P, Ottmann
OG, et al: Randomized, phase 2 trial of low-dose cytarabine with or
without volasertib in AML patients not suitable for induction
therapy. Blood. 124:1426–1433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dombret H, Seymour JF, Butrym A,
Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC,
Candoni A, et al: International phase 3 study of azacitidine vs
conventional care regimens in older patients with newly diagnosed
AML with >30% blasts. Blood. 126:291–299. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Al-Ali HK, Jaekel N, Junghanss C,
Maschmeyer G, Krahl R, Cross M, Hoppe G and Niederwieser D:
Azacitidine in patients with acute myeloid leukemia medically unfit
for or resistant to chemotherapy: A multicenter phase I/II study.
Leuk Lymphoma. 53:110–117. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He PF, Zhou JD, Yao DM, Ma JC, Wen XM,
Zhang ZH, Lian XY, Xu ZJ, Qian J and Lin J: Efficacy and safety of
decitabine in treatment of elderly patients with acute myeloid
leukemia: A systematic review and meta-analysis. Oncotarget.
8:41498–41507. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hong M, Zhu H, Sun Q, Zhu Y, Miao Y, Yang
H, Qiu HR, Li JY and Qian SX: Decitabine in combination with
low-dose cytarabine, aclarubicin and G-CSF tends to improve
prognosis in elderly patients with high-risk AML. Aging (Albany
NY). 12:5792–5811. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang J, Hong M, Zhu Y, Zhao H, Zhang X,
Wu Y, Lian Y, Zhao X, Li J and Qian S: Decitabine in combination
with G-CSF, low-dose cytarabine and aclarubicin is as effective as
standard dose chemotherapy in the induction treatment for patients
aged from 55 to 69 years old with newly diagnosed acute myeloid
leukemia. Leuk Lymphoma. 59:2570–2579. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie M, Jiang Q, Li L, Zhu J, Zhu L, Zhou
D, Zheng Y, Yang X, Zhu M, Sun J, et al: HAG (homoharringtonine,
cytarabine, G-CSF) regimen for the treatment of acute myeloid
leukemia and myelodysplastic syndrome: A meta-analysis with 2,314
participants. PLoS One. 11:e01642382016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Suzushima H, Wada N, Yamasaki H, Eto K,
Shimomura T, Kugimiya MH, Horikawa K, Nishimura S, Tsuda H, Mitsuya
H and Asou N: Low-dose cytarabine and aclarubicin in combination
with granulocyte colony-stimulating factor for elderly patients
with previously untreated acute myeloid leukemia. Leuk Res.
34:610–614. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hackl H, Astanina K and Wieser R:
Molecular and genetic alterations associated with therapy
resistance and relapse of acute myeloid leukemia. J Hematol Oncol.
10:512017. View Article : Google Scholar : PubMed/NCBI
|