Introduction
Ovarian cancer is the fifth most common cause of
cancer-related mortality worldwide (1). In 2017, the incidence of epithelial
ovarian cancer (EOC) in the USA was 9.4 per 100,000 (2) and in 2020, it was 9.19 per 100,000 in
Taiwan (3). The primary treatment
for advanced EOC involves optimal debulking surgery with the aim of
no residual disease (R0), followed by platinum-paclitaxel
combination chemotherapy (4).
Maintenance therapy with bevacizumab or a poly(ADP-ribose)
polymerase inhibitor has been reported to extend progression-free
survival (PFS) following first-line chemotherapy (5,6).
However, despite advancements in surgery and systemic chemotherapy,
the majority (~80% according to stages) of patients experience
recurrent disease, leading to a 5-year overall survival (OS) rate
of <50% across all stages of EOC (7–9). Early
detection through modern liquid biopsies for new or recurrent
cancer remains one of the primary challenges in managing ovarian
cancers.
The use of blood biomarkers for monitoring cancer
status or recurrence, carcinoembryonic antigen (CEA) (10), carbohydrate antigen 19-9 (11), human epididymis secretory protein 4
(11), apolipoprotein A1 (12), transthyretin (13), transferrin (14) and β2-macroglobulin (15), is well documented. Although these
markers could facilitate earlier detection of recurrence, their
utility is limited by inadequate sensitivity or specificity
(16,17). Considering the high recurrence rate
and poor prognosis following EOC recurrence, identifying effective
methods to stratify patients at elevated risk of recurrence for
further therapy following first line treatment and to enable
earlier detection of recurrence is of importance (18).
Ashworth (19) first
reported a biomarker, the circulating tumor cell (CTC), in the
peripheral blood of a patient with metastatic disease. Studies have
demonstrated that CTCs, shed by ovarian cancer, disseminate to
distant organs through the bloodstream, notably contributing to
ovarian cancer metastasis (20–22).
Although CTCs in EOC have been assessed for their prognostic value,
the results have been inconclusive (23), primarily due to technological
limitations. Consequently, CTC enumeration remains a challenge
because of the scarcity of CTCs in peripheral blood samples
(24). The US Food and Drug
Administration (FDA) has approved only the CellSearch system, which
uses EpCAM antibodies to measure CTCs. However, its establishment
in the clinical treatment of EOC has not occurred (25). The use of CellSearch is limited by
the low availability of devices and a low positive detection rate
(26). We have previously reported
a protocol employing a negative selection strategy followed by flow
cytometry to precisely identify CTCs in blood (27). This method has been effective for
cancers of the head and neck, colon, lung and breast, and for
neuroendocrine tumors. The benefits of negative selection-based CTC
enumeration platforms include: i) Label-free characteristics,
allowing for further molecular analysis; ii) preservation of the
heterogeneity of CTCs that express atypical epithelial markers; and
iii) improved recovery and positive detection rates (28–31).
However, this CTC enumeration platform has not previously been
evaluated in patients with EOC.
The present study employed a novel technique for CTC
enumeration and analysis, and a novel platform for CTC testing in
patients with benign ovarian tumors and those with EOC. The
objectives were to evaluate: i) The accuracy of the technique in
distinguishing malignancy from benign ovarian masses and ii) the
feasibility of using baseline CTC counts and decreased CTC levels
post-anticancer therapy as prognostic factors for oncologic
outcomes, such as survival.
Materials and methods
Patient enrollment
A prospective study was performed at Chang Gung
Memorial Hospital (Linkou, Taiwan), enrolling patients with ovarian
cancer at various stages, including new diagnosis, surveillance,
and recurrent/unresectable or metastatic disease. Additionally,
healthy female subjects without ovarian lesions were enrolled as
controls. The Institutional Review Board of Chang Gung Memorial
Hospital approved the study protocols (approval nos.
201802203B0C502 and 201601461B0). All participants provided written
informed consent. Inclusion criteria for eligible patients were as
follows: i) Age, ≥20 years; ii) understood and consented to the
study protocol voluntarily; iii) had suspected new ovarian cancer
or histologically confirmed EOC; and iv) had adequate (within
normal range) liver and renal function and white blood cell counts
before undergoing surgery or anticancer therapies. Exclusion
criteria included: i) Refusal of anticancer therapy; ii)
non-consent to the blood drawing schedule; or iii) the presence of
metachronous or synchronous double cancers. Physicians staged and
managed the disease according to institutional and National
Comprehensive Cancer Network guidelines (4). Results were reported following the
Reporting Recommendations for Tumor Marker Prognostic Studies
(32). Treatment responses were
evaluated using CA125 measurement and imaging studies, including
computed tomography, magnetic resonance imaging and positron
emission tomography scans, according to version 1.1 of the Response
Evaluation Criteria in Solid Tumors. Responses were categorized as
complete remission, partial response, stable disease or progressive
disease (PD). Diagnoses and treatment plans were reviewed at a
weekly multidisciplinary gynecologic cancer tumor board meeting at
Chang Gung Memorial Hospital, with gynecologic oncologists,
diagnostic radiologists, pathologists, nuclear medicine physicians
and radiation oncologists in attendance.
Sample preparations for circulating
tumor cell testing
Blood samples from patients with EOC (4 ml each for
microscopy and flow cytometry) were collected at enrollment (before
anticancer therapy) and at months 3, 6, 9 and 12 post-treatment,
between August 2019 and May 2021. For patients with suspected
ovarian malignancy (subsequently confirmed as benign by pathology),
blood samples were collected only once before surgery. CTC
enrichment was achieved using red blood cell (RBC) lysis (by mixing
155 mM NH4Cl, 14 mM NaHCO3 and 0.1 mM EDTA at
a 10:1 ratio with whole blood samples) and CD45-positive leukocyte
depletion using EasySep Human CD45 Depletion Kits (cat. no. 18259;
Stemcell Technologies Inc.) according to the manufacturer's
instructions. The methods used for CTC enrichment and counting have
been previously described (27,33,34).
CTCs were not collected from patients experiencing disease
progression or death from cancer, as these were the predefined
endpoints of the study for predicting survival events.
Identification of CTCs by
microscopy
CTCs isolated from 4 ml of whole blood samples were
fixed using 4% paraformaldehyde for 10 min at 25°C. Cells were
permeabilized with 0.1% Triton X-100 in PBS for 10 min at 25°C.
Following a PBS wash, cells were blocked with 2% bovine serum
albumin and a HuFcR binding inhibitor (cat. no. 14-9161-73;
eBioscience; Thermo Fisher Scientific, Inc.) for 30 min at room
temperature. To reduce autofluorescence, 0.0025% Trypan Blue (cat.
no. 15250061; Thermo Fisher Scientific, Inc.) was added before the
antibody reaction. Cells were then incubated with anti-EpCAM
antibody conjugated to Alexa Fluor 488 (1:400 dilution; cat. no.
5198S; Cell Signaling Technology, Inc.) for 1 h at 25°C and
anti-p16 antibody conjugated to Alexa Fluor 647 (1:200 dilution;
cat. no. ab199819; Abcam) overnight at 25°C. Nuclei were stained
with Hoechst (10 µg/ml; cat. no. 62249; Thermo Fisher Scientific,
Inc.) for 10 min at 25°C. Fluorescence images were captured using a
Zeiss Axioskop 2 Plus Fluorescence Microscope (Carl Zeiss AG) and a
Leica TCS SP2 Confocal Laser Scanning Microscope (Leica
Microsystems GmbH). CTCs were defined as cells that: i) Exhibited
definite evidence of epithelial cell differentiation
(EpCAM-positive); ii) lacked characteristics of normal white blood
cells (CD45-negative); and iii) possessed a nucleus
(Hoechst-positive, to exclude non-nucleated blood impurities such
as red blood cells). Throughout the experiment, the HeLa cell line
(purchased from the Bioresource Collection and Research Center
Taiwan; human cervical cancer cell line expected to stain as
Hoechst+CD45-EpCAM-) and the H1975 cell line (purchased from the
Bioresource Collection and Research Center Taiwan; human colon
cancer cell line expected to stain as Hoechst+CD45-EpCAM+),
alongside white blood cells from healthy subjects (Chang Gung
Memorial Hospital IRB approval nos. 201802203B0C502 and
201601461B0; control healthy cells expected to stain as
Hoechst+CD45 +EpCAM-) as an internal control were utilized for
microscopic observation of patient specimens.
Analysis and enumeration of CTCs using
flow cytometry
Cells enriched through RBC lysis and CD45 depletion
were fixed with Fix & Perm Cell Permeabilization Reagents (100
µl both for Fix and Permeabilization reagents; cat. no. GAS003;
Thermo Fisher Scientific, Inc.) for 20 min at 25°C. Subsequently,
cells were incubated with an anti-EpCAM antibody conjugated to
phycoerythrin (1:400 dilution; cat. no. FAB960P-100; R&D
Systems, Inc.) for 1 h at 25°C. To further exclude residual
CD45-positive leukocytes, a goat anti-mouse IgG H&L secondary
antibody conjugated to Alexa Fluor 488 (1:2,000 dilution; cat. no.
ab150113; Abcam) was applied for 30 min at 4°C to label CD45
antibodies from the aforementioned CD45 depletion kit.
Isotype-control antibodies (1:400 dilution; cat. no. IC108P;
R&D Systems, Inc.) applied for 1 h at 25°C served as the
negative control. Following staining, the cell samples were
assessed using a CytoFLEX Flow Cytometer (Beckman Coulter, Inc.).
To conduct CTC counting using the flow cytometer, two-dimensional
displays (dot plots) were used to quantify cells that met
predefined criteria. Briefly, the gating strategy contained six
steps. First, the Hoechst+ cells were gated in 2 ml samples from
all events to avoid cell debris and fragmentations after the
negative selection process (Fig.
S1A). Then, singlet cells were gated to avoid false positive
results due to cell aggregation (Fig.
S1B). CD45+ cells were then excluded to avoid residual white
blood cell contamination (Fig.
S1C). Before CTC enumeration, EpCAM+ (and its isotype+) cells
were independently gated (Fig. S1D and
H). Finally, the CTC count was defined as the number of EpCAM+
cells minus the number of cells gated using its isotype.
Statistical analysis
Descriptive statistics were used to present the
basic characteristics of the enrolled patients. One-way ANOVA with
Bonferroni's correction was used to assess CTC count differences
among groups (malignancy, benign lesion and healthy donors). The
staging criteria utilized in this study adhere to the American
Joint Committee on Cancer 8th edition, incorporating pathologic
staging of tumor (pT), lymph node (pN) and distant metastasis (pM)
(35). PFS was calculated as the
time from the CTC sampling date to cancer-specific progression,
recurrence or death from any cause. To demonstrate the importance
of longitudinal follow-up for CTC counts, patients with
post-treatment CTC counts lower than their baseline at their first
(month 3) sampling were categorized as the ‘CTC decline group’; all
others were placed in the ‘no CTC decline group’. OS was defined as
the time from CTC sampling to death from any cause. Receiver
operating characteristic (ROC) curves and the Youden index were
used to evaluate the differentiating accuracy and cut-off values of
CTC counts. Kaplan-Meier survival plots and the log-rank test were
used to assess factors affecting survival. Patients without disease
progression or death (no event for PFS or overall survival) were
censored but still contributed to the final statistical analysis.
After confirming assumed clinicopathological factors, univariate
and multivariate Cox proportional hazard regression models
identified independent prognostic factors for PFS and OS. The
multivariate analysis included all factors from the univariate
analysis. Statistical analysis was conducted using SPSS (version
18; SPSS Inc.). P<0.05 or 95% CI of hazard ratio (HR)>1 was
considered to indicate a statistically significant difference.
Results
Patient enrollment
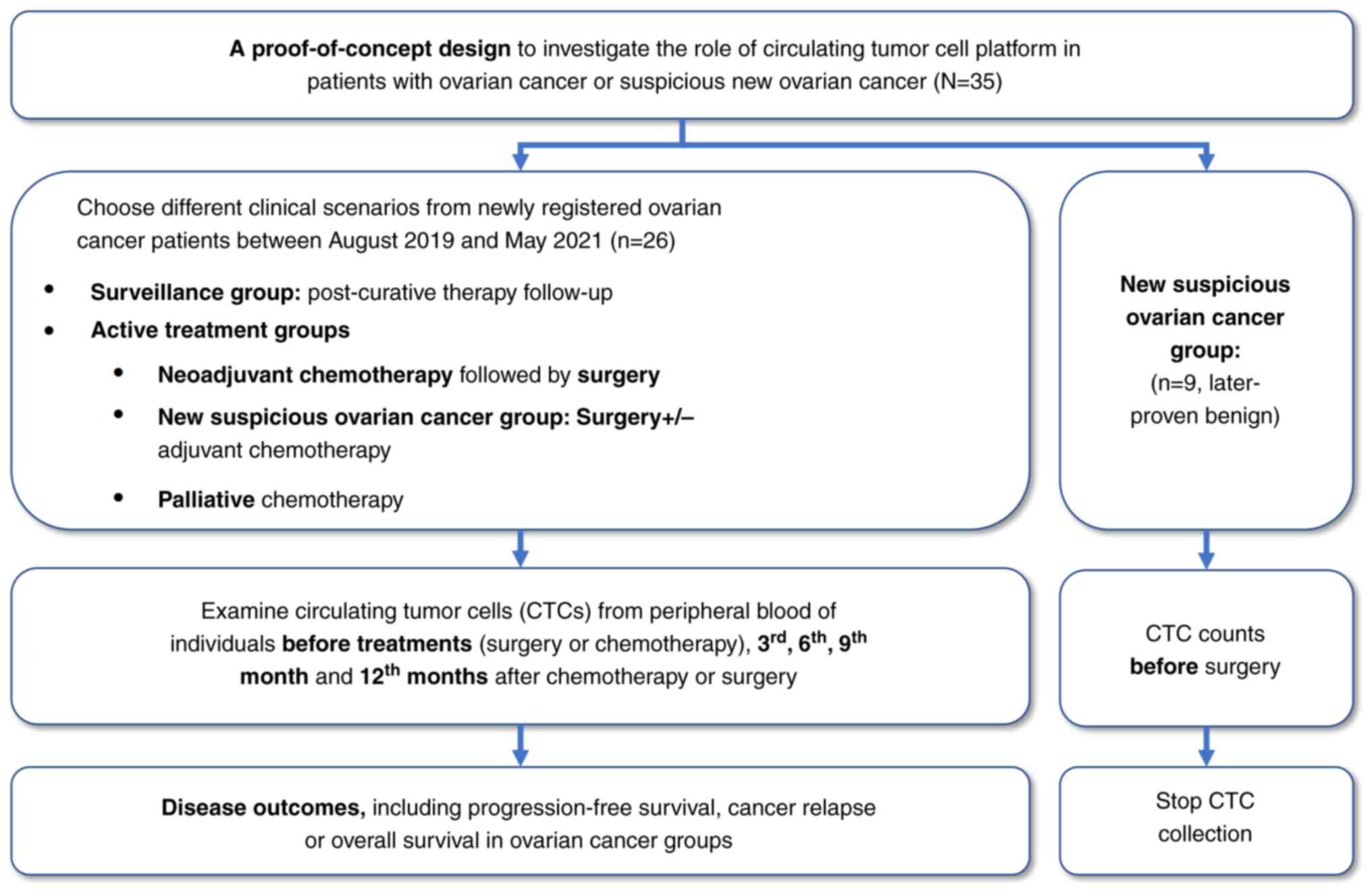
Patient enrollment, according to the prospective
design, is illustrated in Fig. 1.
The characteristics of 26 patients with EOC are presented in
Table I, and nine patients with
benign ovarian lesions are not listed because no cancer staging
information was available. Information of the 29 healthy controls
is not listed because they did not receive any surgery for cancer
or suspicious lesion. Difference in age among the three groups were
evaluated using ANOVA, resulting in a P-value of 0.110 (Table II). Notably, post-hoc comparisons
revealed a difference between cancer [median: 52 (range: 39–76)
years] and healthy donors [median: 45 (range: 27–53) years] with a
P-value of 0.013. However, there was no significant difference
between patients with cancer and benign lesions [median: 46 (range:
23–75) years], as well as between benign lesions and healthy donors
(with P-values of 0.107 and 1.000, respectively), after applying
Bonferroni correction for multiple tests.
 | Table I.Basic characteristics of enrolled
patients with epithelial ovarian cancer (n=26). |
Table I.
Basic characteristics of enrolled
patients with epithelial ovarian cancer (n=26).
| Variable | Value |
|---|
| Age, years | 52 (39–76) |
| Initial symptoms at
diagnosis |
|
|
Yes | 18 (69.2) |
| No | 8 (30.8) |
| CA-125 at baseline,
U/ml |
|
|
≥35 | 10 (38.5) |
|
<35 | 16 (61.5) |
| Stage (FIGO) |
|
|
I–II | 11 (42.3) |
|
III–IV | 15 (57.7) |
| Grade |
|
| 1 | 0 (0.0) |
| 2 | 0 (0.0) |
| 3 | 25 (96.2) |
| Not
available | 1 (3.8) |
| Histology |
|
| Serous
carcinoma | 16 (61.5) |
| Clear
cell carcinoma | 5 (19.2) |
|
Endometrioid carcinoma | 1 (3.9) |
|
Carcinosarcoma | 2 (7.7) |
|
Others | 2 (7.7) |
| Lymph node
status |
|
| N1 | 8 (30.8) |
| N0 | 18 (69.2) |
| Surgery before CTC
testing |
|
|
Yes | 9 (34.6) |
| No | 17 (65.4) |
| Chemotherapy before
CTC testing |
|
|
Yes | 11 (42.3) |
| No | 15 (57.7) |
| Radiotherapy before
CTC testing |
|
|
Yes | 3 (11.5) |
| No | 23 (88.5) |
 | Table II.CTC counts among different
groups. |
Table II.
CTC counts among different
groups.
| Variable | Ovarian cancer
(n=26) | Benign ovarian
lesions (n=9) | Healthy donors
(n=29) |
|---|
| Age median, years
(range) | 52 (39–76) | 46 (23–75) | 45 (27–53) |
| CTC counts,
cells/ml |
|
|
|
|
Mean | 6.8 | 1.1 | 2.4 |
|
Median | 6.3 | 0.5 | 2.0 |
|
Standard deviation | 3.9 | 1.5 | 1.5 |
| Range
(min-max) | (0.0–18.0) | (0.0–4.5) | (0.0–6.0) |
| 95%
CI | (4.9–8.6) | (0.0–2.3) | (1.8–3.0) |
Among 26 patients with cancer, 18 (69.2%) presented
with initial symptoms at diagnosis, which included abdominal
bloating, abdominal pain, constipation, urinary problems and loss
of appetite. A baseline CA125 level ≥35 U/ml was observed in 10
(38.5%) patients. Advanced-stage disease [International Federation
of Gynecology and Obstetrics (FIGO) stages III and IV] (36) was diagnosed in 15 patients (57.7%),
and the majority (96.2%) exhibited grade 3 differentiation. Serous
carcinoma was the most prevalent histology type (61.5%), followed
by clear cell carcinoma (19.2%), carcinosarcoma (7.7%), other types
(7.7%) and endometrioid carcinoma (3.9%). Lymph node involvement
was noted in 8 (30.8%) patients. At the time of diagnosis and
enrollment, a subset of patients had undergone operations (34.6%),
radiotherapy (11.5%) and chemotherapy (42.3%).
Exploratory endpoint-CTC enumeration
and identification
CTCs were captured and quantitatively measured using
flow cytometry, with verification using fluorescence microscopy.
Fig. S1A-D illustrates the gating
processes for counting CTC numbers from a real patient (study
subject #006 with ovarian benign lesion). Fig. S1E-H demonstrates the processes of
gating isotype control from the sample from the same patient (study
subject #006). Fig. S2
demonstrates the images for confirmation of CTC identified. A few
samples were excluded or not collected due to the following
reasons: i) One patient withdrew from the trial, affecting three
samples; ii) disease progression occurred in nine patients at
various points during the trial, resulting in the death of five
patients and the loss of 13 samples; and iii) eight samples were
not collected due to patient-related issues, such as changes in the
outpatient clinic schedule. Consequently, of the 89 samples
expected, which included those from nine individuals with benign
lesions, a total of 56 samples were analyzed. The analysis focused
on the serial measurement of CTCs and the impact of CTC reduction
in the first three months post-treatment, on survival.
CTC testing accurately differentiates
between malignant and benign lesions
Table II
demonstrates that CTC counts were significantly different among
patients with ovarian cancer, those with benign ovarian lesions and
healthy donors (P<0.0001, malignant vs. benign groups;
P<0.0001, malignant vs. healthy group). No significant
difference was demonstrated between patients with benign ovarian
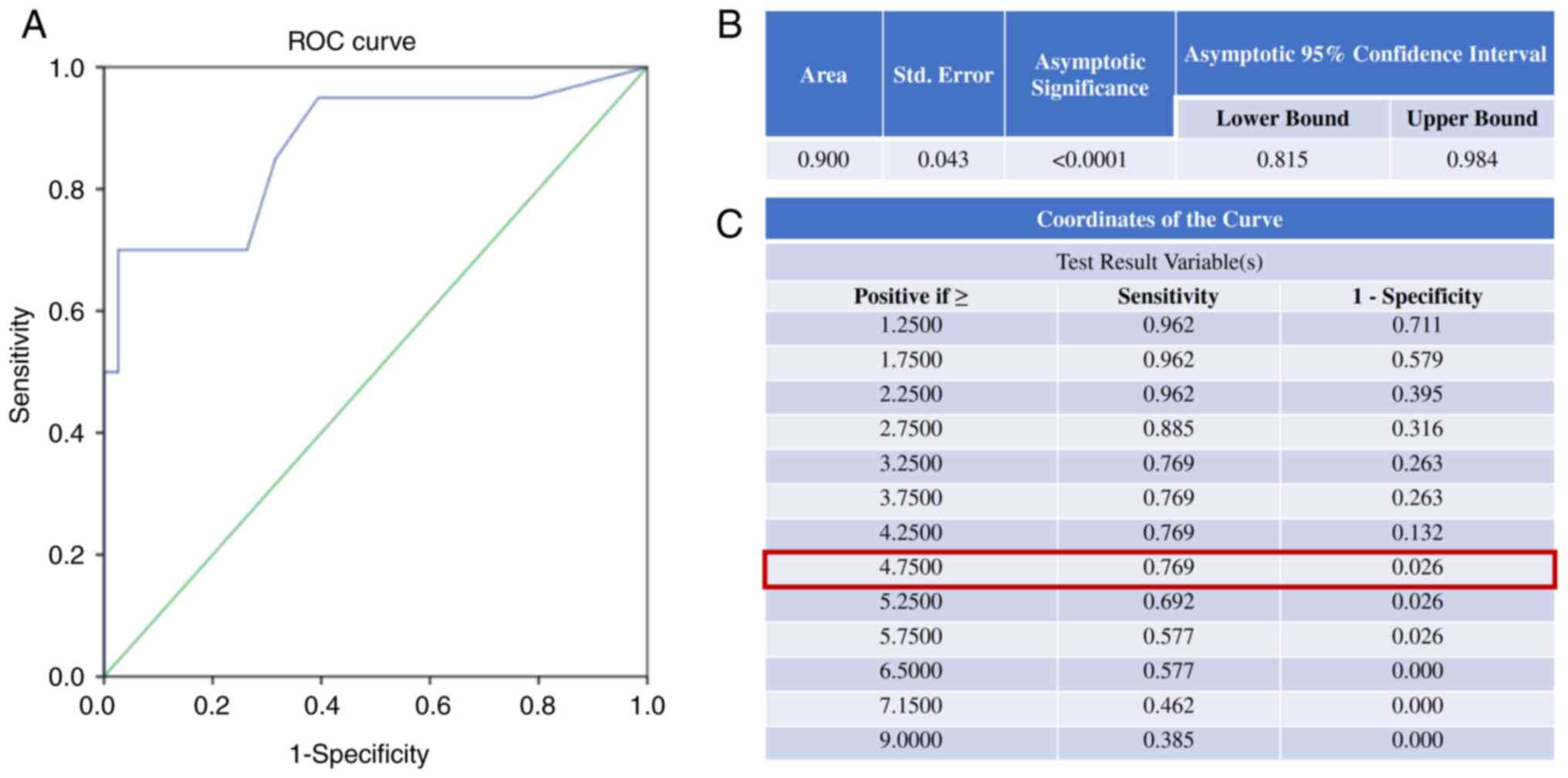
lesions and healthy donors (P=0.283). The area under the curve
(AUC) for the ROC curve for distinguishing patients with cancer
(n=26) from non-cancer individuals (benign ovarian lesions and
healthy donors, n=38) based on CTC number was 0.900, with
P<0.001 (Fig. 2A and B). The
optimal cut-off for CTC number in this cohort, determined using the
Youden index, was 4.75 cells/ml, yielding a sensitivity of 76.9%
and a specificity of 97.4% (Fig.
2C). Using 29 healthy donors as controls, the accuracy,
positive predictive value and negative predictive value were 0.879,
0.933 and 0.860, respectively.
Baseline CTCs and serial CTC testing
predict survival
During the study follow-up period, nine patients
experienced PD, and five died from the disease after a median
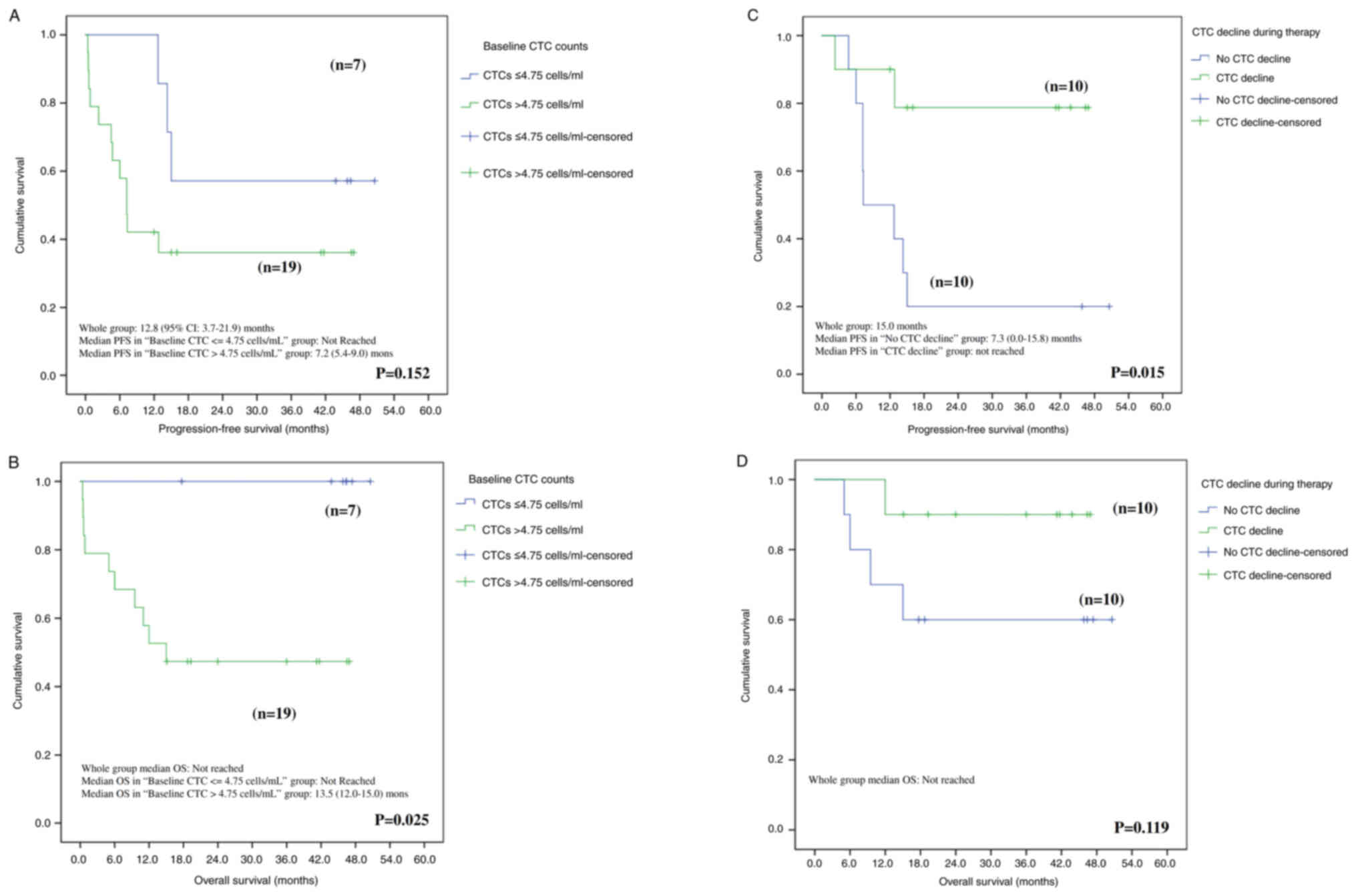
follow-up of 10.6 months (range, 0.4–19.0 months). The median PFS
for the CTCs ≤4.75 cells/ml was not reached, and it was 7.2 months
(95% CI: 5.4–9.0) for patients with baseline CTC counts >4.75
cells/ml. The median OS for the entire population was not reached.
Baseline CTC counts (cut-off value at 4.75 cells/ml) may have a
significant effect on OS rather than PFS with P=0.152 and P=0.025
for PFS and OS, respectively (Fig. 3A
and B). Conversely, a decline in CTC counts during chemotherapy
appears to have a significant effect on PFS but not OS with P=0.015
and P=0.119 for PFS and OS, respectively (Fig. 3C and D). Median OS was not reached
for the entire group after a median follow-up of 29.8 months
(range, 0.4 to 49.9 months) until the cut-off date of October
2023.
CTC count represents an independent
negative prognostic factor in the multivariate analysis
Univariate and multivariate Cox regression analyses
were used to elucidate the prognostic role of CTCs, considering all
known potential prognostic factors. In the univariate analysis, age
at diagnosis (P=0.023), FIGO staging (P=0.018), baseline CTC counts
(P=0.030) and CTC decline within the first three months (P=0.002)
were identified as prognostic factors for disease progression. In
the multivariate analysis assessing the risk of cancer progression,
CTC decline (P=0.024) and baseline CTC counts (P=0.011) remained
independent prognostic factors. Regarding cancer mortality, FIGO
staging (P=0.05) and baseline CTC counts (P<0.0001) showed
prognostic significance. In the multivariate analysis for the risk
of death, the baseline CTC count was the sole independent
prognostic factor (P=0.005) (Table
III).
 | Table III.Univariate and multivariate analysis
of progression-free and overall survival. |
Table III.
Univariate and multivariate analysis
of progression-free and overall survival.
| A, Progression-free
survival |
|---|
|
|---|
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|---|
| Age
(continuous) | 1.052 | (1.007–1.100) | 0.023 |
|
|
|
| FIGO stage (IV vs.
III vs. II vs. I) | 2.173 | (1.140–4.141) | 0.018 |
|
|
|
| Pathology (serous
vs. non-serous) | 1.530 | (0.809–2.893) | 0.191 |
|
|
|
| pN1 or M1 vs.
pN0M0 | 2.459 | (0.883–6.845) | 0.085 |
|
|
|
| Baseline CA125
level (continuous) | 1.000 | (1.000–1.000) | 0.929 |
|
|
|
| CTC decline in the
first 3rd month (continuous) | 0.178 | (0.037–0.849) | 0.030 | 0.154 | (0.030–0.784) | 0.024 |
| Baseline CTC counts
(continuous) | 1.182 | (1.063–1.315) | 0.002 | 1.188 | (1.040–1.357) | 0.011 |
|
| B, Overall
survival |
|
|
|
Univariate |
Multivariate |
|
|
|
|
|
Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
|
| Age
(continuous) | 1.029 | (0.978–1.083) | 0.269 |
|
|
|
| FIGO stage (IV vs.
III vs. II vs. I) | 2.059 | (1.000–42.54) | 0.050 |
|
|
|
| Pathology (serous
vs. non-serous) | 1.626 | (0.890–2.972) | 0.114 |
|
|
|
| pN1 or M1 vs.
pN0M0 | 2.351 | (0.678–8.144) | 0.178 |
|
|
|
| Baseline CA125
level (continuous) | 1.000 | (0.999–1.001) | 0.715 |
|
|
|
| CTC decline in the
first 3rd month (continuous) | 0.206 | (0.023–1.851) | 0.159 |
|
|
|
| Baseline CTC counts
(continuous) | 1.291 | (1.120–1.489) | <0.0001 | 1.480 | (1.129–1.941) | 0.005 |
Discussion
A review and summation of previous studies on CTCs
in ovarian cancer as performed (Table
IV). PCR-based methodologies have been previously used to
identify the presence of CTCs (37–39),
these studies provided molecular proof of the existence of CTCs,
though they did not capture CTCs directly. Other studies have
reported the use of physical isolation/capture methods, such as
filtration systems like MetaCell (40), polydimethylsiloxane microchannels
(41), tapered-slit membrane
filters with immunocytochemistry staining (42), optimized tapered-slit filter
platforms (43) and fluid-assisted
separation technology discs (44).
The major concerns with these methods stem from the variety of
devices and the lack of sufficient external validation, which casts
doubt on their clinical applicability. The most prevalent CTC
enumeration/isolation methodologies are immunomagnetic beads with
staining, exemplified by the CellSearch platform (45,46),
and other widely used devices or technologies, such as flow
cytometry (47,48) or immunocytochemistry staining
(49). The present study advocates
for the use of a commonly available platform over specific CTC
testing innovations and provides evidence of its clinical value. It
is crucial to emphasize that the goal was not to replace standard
diagnostic and treatment methods but to complement them, offering a
less invasive yet discriminative avenue for understanding and
managing tumor behavior.
 | Table IV.Literature review for CTCs addressing
clinical correlation. |
Table IV.
Literature review for CTCs addressing
clinical correlation.
| A, PCR based |
|---|
|
|---|
| First author,
year | Country | n | CTC platform | Healthy
control | Times/time points
of CTC collection | CTC positivity
threshold/detection rate (%) | Main findings | (Refs.) |
|---|
| Zuo et al,
2021 | China | 30 | EpCAM liposome
magnetic | Yes (n=30) | NA/NA | ≥1 CTCs/7.5
ml/80.0% | miR-181a detection
in CTCs can help in CTCs can help cancerdiagnosis and
prognosis. | (37) |
| Obermayr et
al, 2021 | Austria | 215 | qPCR and
immuno-fluorescent staining | No | 2/At baseline and
six months after adjuvant treatment | ≥1 CTCs/9 ml/50.5%
(baseline) | CTCs were
associated with elevated risk of recurrence and death. | (38) |
| Obermayr et
al, 2021 | Austria | 185 | qPCR | No | 1/Before
treatment | ≥1 CTCs/25
ml/19.6% | PPIC-positive CTCs
were significantly associated with a high CCES. | (39) |
|
| B, Microchannel
or filter systems |
|
| Kolostova et
al, 2016 | Czech Republic | 40 |
MetaCell®, MetaCell s.r.o.,
Ostrava, Czech Republic | No | NA/NA | ≥1 CTCs/8
ml/58.0% | KRT7, WT1, EPCAM,
MUC16, MUC1, KRT18, and KRT19 detection can indicate CTC
presence. | (40) |
| Lee et al,
2017 | South Korea | 54 |
Polydimethylsiloxane microchannels | No | 1/Before surgery or
adjuvant therapy | ≥1 CTCs/10
ml/98.1% | PFS decrement and
platinum resistance are correlated with CTCs ≥3 cells, and positive
CTC-cluster, respectively. | (41) |
| Suh et al,
2017 | South Korea | 31 | Tapered-slit
membrane filters + ICC | Yes (n=22) | 1/Before
surgery | ≥1 CTCs/5
ml/77.4% | CTCs before surgery
could discriminate early ovarian cancer from benign ovarian
tumors. | (42) |
| Kim et al,
2019 | South Korea | 30 | Optimized
taperedslit filter platform | No | 2/Before and after
surgery | ≥1 CTCs/5
ml/76.7% | No significant
correlation was noted between CTCs and clinical outcomes. | (43) |
| Kim et al,
2020 | South Korea | 13 | Fluid-assisted
separation technology disc | No | >3 (varies)/At
diagnosis, before and after treatment | ≥1 CTCs/3
ml/84.6% | CTC counts was
better associated with treatment response and recurrence than CA125
levels. Change in CTCs correlates to clinical disease status. | (44) |
|
| C,
Immune-fluorescent detection |
|
| Pearl et al,
2015 | USA | 31 | CAM uptake-cell
enrichment + flow cytometry | Yes (n=64) | 9/Before treatment,
follow-up at 1,3,6,9, 12,18, and 24 months after treatment | ≥5 CTCs/ml/
100.0% | Continuous invasive
CTC measurements could be a predictor of chemotherapy
efficacy. | (47) |
| Lou et al,
2018 | USA | 29 | CellSearch | Yes (n=14) | 1/Before
treatment | ≥1 CTCs/7.5
ml/17.0% | CTCs are more
abundant in ovarian meta-stasis from other cancer (vs. primary
ovarian cancer). | (45) |
| Guo et al,
2018 | China | 30 | Size based
microfluidic technique + ICC | Yes (n=25) | 1/Before
surgery | ≥0.5 CTCs/1
ml/73.3% | Higher
DAPI+/E&M+/CD45-/HE4+ CTC counts were found in EOC (vs. benign
tumors). | (49) |
| Banys-Paluchowski
et al, 2020 | Germany | 34 | CellSearch | No | 3/Prior to
chemotherapy, after 3 and 6 cycles. | ≥2 CTCs/7.5
ml/26.0% | Patients with ≥1
CTCs at baseline had significantly shorter OS and PFS than those
with CTC-negative patients. | (46) |
| Gening et
al, 2021 | Russia | 38 | Negative selection
+ flow cytometry (Cytoflex S) | No | 2/Before treatment
and during first-line chemotherapy | NA/NA | CD133 + ALDH + CTCs
have the greatest prognostic potential in ovarian cancer. | (48) |
| Kou et al,
2024 | Taiwan | 20 | Negative selection
+ flow cytometry | Yes (n=38) | 4/Baseline, at 6,
9, 12 months after treatment | ≥5 CTCs/1 ml/100.0%
(for CTC >0 cells/ml) | Post-treatment CTC
decline rather than baseline CTC counts could serve as an
independent prognostic factor. | Present study |
Criteria for positive CTC presence, including
cut-off values, varied across the studies reviewed (Table IV). These differences primarily
stemmed from the varying detection limits of different CTC
isolation platforms (30,40). In EOC, detection limits ranged from
1 CTC/25 ml to 5 CTCs/ml. Using flow cytometry technology, the
present study identified positive CTC presence as 4.75 cells/ml,
nearing the upper limit of 5 cells/ml. Efforts were made to avoid
incorrectly labeling cells in human circulation obtained under
predefined conditions (i.e., EpCAM+CD45-) from healthy individuals
as CTCs, it would be inappropriate to call them CTCs in subjects
without cancer. However, a consensus within the academic community
is lacking, as these numbers may merely signify the background
values of a detection tool, not necessarily indicating the presence
of cancer. This scenario is similar to tumor markers, such as CEA
and AFP, where distinctions exist between reference (or background)
and abnormal values, and the mere presence of these markers does
not definitively signify cancer (50). Furthermore, cell-free (cf)DNA can
sometimes harbor clonal hematopoiesis of indeterminate potential in
individuals without cancer. Extensive research is required to
identify DNA abnormalities that are not cancer-related, similar to
those observed in healthy individuals (51). In the future, extensive studies may
help differentiate these cells in cancer patients or assign
alternative names, such as the historical term-circulating
epithelial cells (52).
Furthermore, the presence of false positives, where certain cells
expressing EpCAM are detected in healthy subjects, does not support
a cancer diagnosis. Conversely, false negatives, where cells do not
express typical epithelial markers but instead express vimentin
markers, may introduce a potential bias in the utilization of CTCs.
In the present proof-of-concept study, a negative selection and
immunofluorescence identification platform was used to enumerate
CTCs. It was demonstrated that baseline CTC counts could be used to
differentiate between patients with ovarian cancer and those with
benign ovarian diseases, achieving an AUC of 0.900 (P<0.001).
While an age imbalance was observed during case enrollment between
the cancer group and healthy donors (P=0.013), no difference was
noted between the EOC and benign lesion groups (P=0.107),
suggesting that the ability to differentiate EOC from benign
lesions is reliable. The results indicated that a decline in CTCs
during the first three months of first-line treatment (HR, 0.154;
P=0.024) and low baseline CTC counts (<4.75 cells/ml; HR, 1.188;
P=0.011) were both significantly associated with longer PFS.
Additionally, patients with low baseline CTC counts might
experience prolonged OS (HR, 1.480; 95% CI, 1.129–1.941; Table III). However, due to the limited
number of events (deaths) in this cohort, a model using CTCs to
predict OS remains unreliable. While numerous studies have reported
CTCs to be closely related to OS and PFS (36,37,39,42,44),
this result is not universal (43).
To the best of our knowledge, the present study is the first to
suggest an independent prognostic role for baseline CTC counts and
the decline in CTCs within the first three months after treatment,
in predicting clinical outcomes for patients with EOC.
Few previous studies have addressed the value of
changes in CTC counts through serial measurements (44,47).
Pearl et al (47) conducted
nine serial CTC measurements in 31 patients with EOC and reported
that continuous invasive CTC measurements more accurately predicted
chemotherapy efficacy than CA125 levels. In a small-scale study,
Kim et al (44) reported
positive predictive ability for clinical survival in 47 serial CTC
measurements across 13 patients with EOC. Banys-Paluchowski et
al (46) suggested that
chemotherapy rapidly reduced CTC counts within the first three
months following cancer therapy, with CTCs correlating with
clinical scenarios. While the present study demonstrated that
changes in CTC counts were associated with survival outcomes
(Fig. 3).
In academic research on liquid biopsy, ctDNA is
often compared with CTCs, both being important and rapidly evolving
tools (53). Although considered to
be liquid biopsies, they differ markedly in their biology,
applications (i.e. finding targeted drugs or xenografts for ex
vivo testing), and respective advantages and disadvantages.
Detecting or capturing CTCs typically involves analyzing living
cancer cells, while ctDNA reflects cancer-specific genes regardless
of the cancer cells' viability. Consequently, CTCs are beneficial
for studies that require living cells, such as CTC culture,
CTC-derived xenografts and ex-vivo CTC drug testing
(54). However, the advantage of
CTCs is offset by the challenge of capturing cells, as the unstable
expression of surface markers can lead to difficulties in
identifying a small subset of cells. These issues include atypical
CTCs that lack EpCAM expression and CTC subgroup heterogeneity
(55). When choosing between CTCs
and ctDNA as a liquid biopsy tool, it is crucial to carefully
consider the research characteristics, acknowledging the
coexistence of both benefits and challenges associated with
CTCs.
The present study had certain limitations. Firstly,
as a pilot and proof-of-concept study, only a small number of cases
were considered. In future experiments, it is advisable to compare
patients with different types of malignancies or peritoneal
metastases, this approach would support assessment of the
specificity of the CTC enumeration method specifically for ovarian
cancer rather than malignancy in general. Secondly, the FDA has not
approved the CTC enumeration methodology. Nevertheless, the flow
cytometer, a device commonly used for the quantification of
labelled cell populations, has been employed in similar
applications to detect minimal evidence of malignancy in
circulation, particularly in hematologic malignancies such as
leukemia (56). Consequently, we
suggest that this methodology could be broadly applicable in
clinical settings, particularly for patients with EOC. Thirdly, it
is recommended that future experiments incorporate the tracking of
long-term survival rates to comprehensively elucidate the
correlation between the initial decline in CTC and overall
survival. The absence of extended survival rate data is a
limitation of the current study. In addition, the definition of
CTCs in the present study does not consider interstitial CTC, which
are EpCAM negative. The prospect has been extensively discussed in
the literature (57,58). It is commonly held that
incorporating more cancer-specific surface markers, such as Her2,
may enhance the detection rate of particular cancers. It was found
that augmenting the panel with markers such as CSV antibodies could
reveal the stemness of CTCs. However, the challenge of tumor
heterogeneity was also encountered, as not all cancers exhibit
differentiation towards the same surface marker (58). Therefore, while the present study
refrained from employing additional surface markers, their
utilization to aid in the identification of EpCAM-positive CTCs
with greater accuracy should be considered.
In conclusion, this proof-of-concept study utilized
a negative selection and immunofluorescence identification platform
to enumerate CTCs. The results demonstrated that baseline CTC
counts could differentiate between patients with ovarian cancer and
those with benign disease. Furthermore, longitudinal follow-up of
CTC changes independently predicted PFS with a greater significance
than baseline CTC counts. Furthermore, a decline in CTC counts may
contribute to prolonged OS. While these results are promising for
predicting survival in patients with EOC, further research with a
larger sample size is necessary to independently validate the
findings in this study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The study was partially funded by Chang Gung Memorial Hospital
grants (grant nos. CMRPVVK0093, CMRPVVL0262 and CMRPG3M0931) and a
National Science and Technology Council, R.O.C. grant (grant no.
NSC 112-2314-B-182A-028-).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CHC was responsible for conception and design,
analysis and drafting the manuscript. YCK was responsible for
conception and design, analysis and drafting the manuscript. HCK
was responsible for the collection of data from medical records.
CTL, AC, HJH and HMW were responsible for conception, patient
enrollment and supervision of the protocol and study. JCHH and HHC
were responsible for conception, design, acquisition of funding,
patient enrollment, data collection and analysis, writing the
manuscript and they confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
Chang Gung Memorial Hospital institutional and national research
committee (approval nos. 201802203B0C502 and 201601461B0) and with
the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. Written informed consent was obtained
from all individual participants involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer. 149:778–789. 2021. View Article : Google Scholar
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cancer registry annual report, 2021
Taiwan, Department of Health, Executive Yuan. https://www.hpa.gov.tw/File/Attach/17639/File_23506.pdfFebruary
25–2024
|
|
4
|
NCCN, . The NCCN Clinical Practice
Guidelines in Oncology (NCCN Guidelines®). 2024.version
1.0.https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1453Febuary
25–2024
|
|
5
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Incorporation of bevacizumab in the primary treatment of
ovarian cancer. N Engl J Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colombo N, Van Gorp T, Parma G, Amant F,
Gatta G, Sessa C and Vergote I: Ovarian cancer. Crit Rev Oncol
Hematol. 60:159–179. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cancer Stat Facts. Ovarian Cancer.
2020.Available at. https://seer.cancer.gov/statfacts/html/ovary.htmlOctober
10–2023
|
|
9
|
Yeung TL, Leung CS, Yip KP, Au Yeung CL,
Wong ST and Mok SC: Cellular and molecular processes in ovarian
cancer metastasis. A Review in the Theme: Cell and molecular
processes in cancer metastasis. Am J Physiol Cell Physiol.
309:C444–C456. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tuxen MK, Sölétormos G and Dombernowsky P:
Tumor markers in the management of patients with ovarian cancer.
Cancer Treat Rev. 21:215–245. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qing X, Liu L and Mao X: A Clinical
diagnostic value analysis of serum CA125, CA199, and HE4 in Women
with early ovarian cancer: Systematic review and meta-analysis.
Comput Math Methods Med. 2022:93393252022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moore LE, Fung ET, McGuire M, Rabkin CC,
Molinaro A, Wang Z, Zhang F, Wang J, Yip C, Meng XY and Pfeiffer
RM: Evaluation of apolipoprotein A1 and posttranslationally
modified forms of transthyretin as biomarkers for ovarian cancer
detection in an independent study population. Cancer Epidemiol
Biomarkers Prev. 15:1641–1646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schweigert FJ and Sehouli J:
Transthyretin, a biomarker for nutritional status and ovarian
cancer. Cancer Res. 65:11142005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Macuks R, Baidekalna I, Gritcina J,
Avdejeva A and Donina S: Apolipoprotein A1 and transferrin as
biomarkers in ovarian cancer diagnostics. Acta Chirurgica
Latviensis. 10:16–20. 2010. View Article : Google Scholar
|
|
15
|
Giampaolino P, Foreste V, Della Corte L,
Di Filippo C, Iorio G and Bifulco G: Role of biomarkers for early
detection of ovarian cancer recurrence. Gland Surg. 9:1102–1111.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang WL, Lu Z and Bast RC Jr: The role of
biomarkers in the management of epithelial ovarian cancer. Expert
Rev Mol Diagn. 17:577–591. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muinao T, Deka Boruah HP and Pal M:
Diagnostic and Prognostic Biomarkers in ovarian cancer and the
potential roles of cancer stem cells-An updated review. Exp Cell
Res. 362:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang F, Zhang Y, Ke C, Li A, Wang W, Yang
K, Liu H, Xie H, Deng K, Zhao W, et al: Predicting ovarian cancer
recurrence by plasma metabolic profiles before and after surgery.
Metabolomics. 14:652018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashworth T: A case of cancer in which
cells similar to those in the tumours were seen in the blood after
death. Aust Med J. 14:1461869.
|
|
20
|
Yousefi M, Dehghani S, Nosrati R, Ghanei
M, Salmaninejad A, Rajaie S, Hasanzadeh M and Pasdar A: Current
insights into the metastasis of epithelial ovarian cancer-hopes and
hurdles. Cell Oncol. 43:515–538. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coffman LG, Burgos-Ojeda D, Wu R, Cho K,
Bai S and Buckanovich RJ: New models of hematogenous ovarian cancer
metastasis demonstrate preferential spread to the ovary and a
requirement for the ovary for abdominal dissemination. Transl Res.
175:92–102.e2. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Joosse SA, Gorges TM and Pantel K:
Biology, detection, and clinical implications of circulating tumor
cells. EMBO Mol Med. 7:1–11. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giannopoulou L, Kasimir-Bauer S and
Lianidou ES: Liquid biopsy in ovarian cancer: Recent advances on
circulating tumor cells and circulating tumor DNA. Clin Chem Lab
Med. 56:186–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu M, Bardia A, Wittner BS, Stott SL, Smas
ME, Ting DT, Isakoff SJ, Ciciliano JC, Wells MN, Shah AM, et al:
Circulating breast tumor cells exhibit dynamic changes in
epithelial and mesenchymal composition. Science. 339:580–584. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fan T, Zhao Q, Chen JJ, Chen WT and Pearl
ML: Clinical significance of circulating tumor cells detected by an
invasion assay in peripheral blood of patients with ovarian cancer.
Gynecol Oncol. 112:185–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van der Auwera I, Peeters D, Benoy IH,
Elst HJ, Van Laere SJ, Prové A, Maes H, Huget P, van Dam P,
Vermeulen PB and Dirix LY: Circulating tumour cell detection: A
direct comparison between the CellSearch System, the AdnaTest and
CK-19/mammaglobin RT-PCR in patients with metastatic breast cancer.
Br J Cancer. 102:276–284. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Su PJ, Wu MH, Wang HM, Lee CL, Huang WK,
Wu CE, Chang HK, Chao YK, Tseng CK, Chiu TK, et al: Circulating
tumour cells as an independent prognostic factor in patients with
advanced oesophageal squamous cell carcinoma undergoing
chemoradiotherapy. Sci Rep. 6:314232016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bankó P, Lee SY, Nagygyörgy V, Zrínyi M,
Chae CH, Cho DH and Telekes A: Technologies for circulating tumor
cell separation from whole blood. J Hematol Oncol. 12:482019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chu PY, Hsieh CH and Wu MH: The
Combination of immunomagnetic bead-based cell isolation and
optically induced dielectrophoresis (ODEP)-based microfluidic
device for the negative selection-based isolation of circulating
tumor cells (CTCs). Front Bioeng Biotechnol. 8:9212020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hsieh JCH and Wu TMH: The selection
strategy for circulating tumor cells (CTCs) isolation and
enumeration: Technical features methods, and clinical applications.
IntechOpen London. 2016.
|
|
31
|
Li SH, Wu MH, Wang HM, Hsu PC, Fang YF,
Wang CL, Chu HC, Lin HC, Lee LY, Wu CY, et al: Circulating EGFR
mutations in patients with lung adenocarcinoma by circulating tumor
cell isolation systems: A concordance study. Int J Mol Sci.
23:106612022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sauerbrei W, Taube SE, McShane LM,
Cavenagh MM and Altman DG: Reporting recommendations for tumor
marker prognostic studies (REMARK): An abridged explanation and
elaboration. J Natl Cancer Inst. 110:803–811. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wu CY, Fu JY, Wu CF, Hsieh MJ, Liu YH, Liu
HP, Hsieh JC and Peng YT: Malignancy prediction capacity and
possible prediction model of circulating tumor cells for suspicious
pulmonary lesions. J Pers Med. 11:4442021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao X, Leow OQY, Chiu CH, Hou MM, Hsieh
JCH and Chao YK: Clinical utility of circulating tumor cells for
predicting major histopathological response after neoadjuvant
chemoradiotherapy in patients with esophageal cancer. J Pers Med.
12:14402022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Berek JS, Renz M, Kehoe S, Kumar L and
Friedlander M: Cancer of the ovary, fallopian tube, and peritoneum:
2021 update. Int J Gynaecol Obstet. 155 (Suppl 1):S61–S85. 2021.
View Article : Google Scholar
|
|
37
|
Zuo L, Li X, Zhu H, Li A and Wang Y:
Expression of mir-181a in circulating tumor cells of ovarian cancer
and its clinical application. ACS Omega. 6:22011–22019. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Obermayr E, Reiner A, Brandt B, Braicu EI,
Reinthaller A, Loverix L, Concin N, Woelber L, Mahner S, Sehouli J,
et al: The long-term prognostic significance of circulating tumor
cells in ovarian cancer-A study of the OVCAD consortium. Cancers.
13:26132021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Obermayr E, Braicu EI, Polterauer S,
Loverix L, Concin N, Woelber L, Mahner S, Sehouli J, Van Gorp T,
Vergote I, et al: Association of a combined cancer exhaustion score
with circulating tumor cells and outcome in ovarian cancer-a study
of the OVCAD consortium. Cancers. 13:58652021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kolostova K, Pinkas M, Jakabova A,
Pospisilova E, Svobodova P, Spicka J, Cegan M, Matkowski R and
Bobek V: Molecular characterization of circulating tumor cells in
ovarian cancer. Am J Cancer Res. 6:9732016.PubMed/NCBI
|
|
41
|
Lee M, Kim EJ, Cho Y, Kim S, Chung HH,
Park NH and Song YS: Predictive value of circulating tumor cells
(CTCs) captured by microfluidic device in patients with epithelial
ovarian cancer. Gynecol Oncol. 145:361–365. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suh DH, Kim M, Choi JY, Bu J, Kang YT,
Kwon BS, Lee B, Kim K, No JH, Kim YB and Cho YH: Circulating tumor
cells in the differential diagnosis of adnexal masses. Oncotarget.
8:771952017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim M, Suh DH, Choi JY, Bu J, Kang YT, Kim
K, No JH, Kim YB and Cho YH: Post-debulking circulating tumor cell
as a poor prognostic marker in advanced stage ovarian cancer: A
prospective observational study. Medicine (Baltimore).
98:e153542019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim H, Lim M, Kim JY, Shin SJ, Cho YK and
Cho CH: Circulating tumor cells enumerated by a centrifugal
microfluidic device as a predictive marker for monitoring ovarian
cancer treatment: A pilot study. Diagnostics. 10:2492020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lou E, Vogel RI, Teoh D, Hoostal S, Grad
A, Gerber M, Monu M, Lukaszewski T, Deshpande J, Linden MA and
Geller MA: Assessment of circulating tumor cells as a predictive
biomarker of histology in women with suspected ovarian cancer. Lab
Med. 49:134–139. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Banys-Paluchowski M, Fehm T, Neubauer H,
Paluchowski P, Krawczyk N, Meier-Stiegen F, Wallach C, Kaczerowsky
A and Gebauer G: Clinical relevance of circulating tumor cells in
ovarian, fallopian tube and peritoneal cancer. Arch Gynecol Obstet.
301:1027–1035. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pearl ML, Dong H, Tulley S, Zhao Q,
Golightly M, Zucker S and Chen WT: Treatment monitoring of patients
with epithelial ovarian cancer using invasive circulating tumor
cells (iCTCs). Gynecol Oncol. 137:229–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gening SO, Abakumova TV, Gafurbaeva DU,
Rizvanov AA, Antoneeva II, Miftakhova RR, Peskov AB and Gening TP:
The detection of stem-like circulating tumor cells could increase
the clinical applicability of liquid biopsy in ovarian cancer. Life
(Basel). 11:8152021.PubMed/NCBI
|
|
49
|
Guo YX, Neoh KH, Chang XH, Sun Y, Cheng
HY, Ye X, Ma RQ, Han RPS and Cui H: Diagnostic value of HE4+
circulating tumor cells in patients with suspicious ovarian cancer.
Oncotarget. 9:75222018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Luo HJ, Hu ZD, Cui M, Zhang XF, Tian WY,
Ma CQ, Ren YN and Dong ZL: Diagnostic performance of CA125, HE4,
ROMA, and CPH-I in identifying primary ovarian cancer. J Obstet
Gynaecol Res. 49:998–1006. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chan HT, Chin YM, Nakamura Y and Low SK:
Clonal hematopoiesis in liquid biopsy: From biological noise to
valuable clinical implications. Cancers (Basel). 12:22772020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Alix-Panabières C and Pantel K:
Circulating tumor cells: Liquid biopsy of cancer. Clin Chem.
59:110–118. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Asante DB, Calapre L, Ziman M, Meniawy TM
and Gray ES: Liquid biopsy in ovarian cancer using circulating
tumor DNA and cells: Ready for prime time? Cancer Lett. 468:59–71.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Diamantopoulou Z, Castro-Giner F and Aceto
N: Circulating tumor cells: Ready for translation? J Exp Med.
217:e202003562020. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lin D, Shen L, Luo M, Zhang K, Li J, Yang
Q, Zhu F, Zhou D, Zheng S, Chen Y and Zhou J: Circulating tumor
cells: Biology and clinical significance. Signal Transduct Target
Ther. 6:4042021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Palladini G, Paiva B, Wechalekar A, Massa
M, Milani P, Lasa M, Ravichandran S, Krsnik I, Basset M, Burgos L,
et al: Minimal residual disease negativity by next-generation flow
cytometry is associated with improved organ response in AL
amyloidosis. Blood Cancer J. 11:342021. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nguyen TNA, Huang PS, Chu PY, Hsieh CH and
Wu MH: Recent progress in enhanced cancer diagnosis, prognosis, and
monitoring using a combined analysis of the number of circulating
tumor cells (CTCs) and other clinical parameters. Cancers (Basel).
15:53722023. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Asante DB, Mohan G, Acheampong E, Ziman M,
Calapre L, Meniawy TM, Gray ES and Beasley AB: Genetic analysis of
heterogeneous subsets of circulating tumour cells from high grade
serous ovarian carcinoma patients. Sci Rep. 13:25522023. View Article : Google Scholar : PubMed/NCBI
|