Introduction
Among all cancer types, breast cancer, along with
prostate cancer, has the highest frequency of bone metastasis. Bone
metastases are observed in about half of the patients with
metastatic breast cancer (1). Bone
metastasis can cause severe pain, pathologic fractures, or nerve
compression symptoms, which are detrimental to patients' quality of
life. Bone metastases can now be manageable with radiation,
bisphosphonate preparations, and anti-RANKL antibody (denosumab)
administration, leading to easier control of the above symptoms
without opioids. However, these treatments do not always benefit
patients; due to limited radiation dose, the same lesion is only
given one or two opportunities of palliative radiation.
Bisphosphonates and denosumab have serious side effects, such as
osteonecrosis of the jaw and hypocalcemia. Because of these unmet
needs of patients with bone metastases, it is crucial to avoid bone
metastases in patients with breast cancer.
To date, it is unclear whether the prevention of
bone metastasis prolongs the prognosis of patients with cancer.
However, weak but positive results are being obtained in breast
cancer. A meta-analysis of 26 trials in which adjuvant
bisphosphonates were administered in patients with early-stage
breast cancer revealed that the risks of bone metastasis and breast
cancer mortality were slightly but significantly reduced in
postmenopausal patients (2). On the
contrary, the risk of recurrence of cancer in organs other than the
bone did not decrease. Similarly, adjuvant denosumab in addition to
aromatase inhibitors not only decreases clinical fractures but also
significantly prolongs disease-free survival in postmenopausal
women with hormone receptor-positive early-stage breast cancer
(3). In metastatic breast cancer,
patients who developed bone metastasis at any time point have
significantly shorter overall survival than patients who did not
(4). Based on these findings, the
identification of high-risk groups for bone metastasis may enable a
more effective prevention of bone metastasis and eventual reduction
in breast cancer mortality. To identify such a population, a
prediction tool for bone metastasis is essential. Nevertheless, no
simple method for predicting bone metastasis in breast cancer has
yet been developed.
Bone turnover markers reflect the status of bone
metabolism and thus are in routine clinical use as biomarkers. For
example, they are used as auxiliary diagnostics for osteoporosis
and are used to monitor the effects of bone-modifying agents. They
are classified as bone resorption markers and bone formation
markers. Bone resorption markers, including tartrate-resistant acid
phosphatase 5b (TRACP-5b), type I collagen-C-telopeptide (I-CTP),
and type I collagen cross-linked N-telopeptide (NTX), are useful
for the detection of bone metastasis in various cancer types,
including breast cancer (5).
Contrary to the aforementioned usage, bone turnover markers are not
generally used as prognosticators of bone metastasis because the
evidence on the predictive value of bone turnover markers is
limited (6).
Among bone turnover markers, TRACP-5b has several
notable properties as a reliable marker. TRACP-5b is specifically
secreted by osteoclasts in high amounts in bone tissue, thereby
correlating well with the degree of bone resorption (7). While TRACP-5b directly represents the
osteoclast number, other bone resorption markers, such as I-CTP and
NTX, only indirectly represent osteoclastic activity because they
are by-products of bone remodeling. In addition, TRACP-5b is more
sensitive than other bone turnover markers (8–10).
Moreover, in patients with bone metastasis from breast cancer, the
serum concentration of TRACP-5b reflects the degree of bone
metastasis (11,12). TRACP-5b also has advantages in
measurements, such as biochemical stability, less diurnal
variation, and no perturbation by renal function (13,14).
Thus, in this study, we investigated whether the serum
concentration of TRACP-5b can be a prognosticator of bone
metastasis.
Patients and methods
Patients
This retrospective cohort study investigated the
association between preoperative serum TRACP-5b levels and breast
cancer recurrence with a focus on bone metastasis. Patients who
underwent resectable breast surgery between May 2002 and August
2006 were consecutively investigated. Eligible patients were as
follows: Female patients diagnosed with stage I, II, or III breast
cancer; aged ≥20 years; underwent surgery without any presurgical
treatments; and had serum samples obtained before surgery. Patients
who had been prescribed bone-modifying agents at the time of blood
sampling were excluded.
Measurement of TRACP-5b
Sera were prepared from patients' blood drawn before
surgery according to the standard procedure containing a
centrifugation step. Serum samples were preserved at −80°C until
use. To measure the concentration of TRACP-5b in the patients'
sera, the enzyme-linked immunosorbent assay for TRACP-5b
(Osteolinks® TRAP-5b; SB Bioscience, Tokyo, Japan) was
employed. The measurements of TRACP-5b were conducted at Nittobo's
laboratory (Koriyama, Fukushima, Japan) without informing any
patients' information. Quality control was done by drawing standard
curves for each set of measurement. Reproducibility was checked by
measuring serum samples with known TRACP-5b concentrations.
TRACP-5b levels in 320 serum samples were measured successfully.
Sixteen samples were excluded from the subsequent analysis because
they were derived from patients with noninvasive breast cancer (9
samples) or patients taking bone-modifying agents for osteoporosis
(7 samples).
Data collection and statistics
Patients' age at the time of surgery, menopausal
status, tumor size, nodal status, estrogen receptor (ER) status,
progesterone receptor (PR) status, human epithelial growth factor
receptor-2 (HER2) status, tumor histological grade (HG), and
preoperative serum concentrations of carcinoembryonic antigen (CEA)
and cancer antigen 15-3 (CA15-3) were collected from each patient's
medical record. The cutoff CEA, CA15-3, and TRACP-5b levels were
set at the median values of all 304 cases (2 ng/ml, 11 U/ml and 347
mU/dl, respectively). Survival data, including date of surgery,
date of diagnosis of breast cancer recurrence, date of diagnosis of
bone metastasis, and date of death were collected from each
patient's medical record. Bone metastasis-free interval (BMFI) was
defined as duration between the surgery and diagnosis of bone
metastasis at any time point. Events, including the diagnosis of
the second malignancy, contralateral breast cancer, or death from
causes other than breast cancer, were censored. Recurrence-free
interval (RFI) was defined as described previously (15). To eliminate bone-only events, a
modified RFI (mRFI) was applied, wherein bone-only recurrences were
censored. Statistical testing was performed using JMP Pro 17 (JMP
Statistical Discovery; Cary, NC, USA). Data in contingency tables
were analyzed using Fisher's exact test. Kaplan-Meier curves among
groups were compared with a univariate log-rank test and a
multivariate Cox proportional hazard model. The correlation between
TRACP-5b and CEA or CA15-3 was examined using Pearson's test.
Survival curves were created using GraphPad Prism 6 (GraphPad
Software; San Diego, CA, USA). P<0.05 was considered to indicate
a statistically significant difference.
Results
Nodal status and HG are associated
with bone metastasis in all patient population
Initially, we aimed to determine the
clinicopathological factors which are associated with bone
metastasis in all patients with breast cancer included in the
present study. The age at the time of surgery was 26–81 years old.
The median follow-up was 3,722 days. Among the 304 patients with
operable breast cancer, 46 developed bone metastases. The imaging
modalities used to diagnose bone metastases were as follows: bone
scintigram (n=27; 58.7%), positron emission tomography-computed
tomography (CT) (n=7; 15.2%), magnetic resonance imaging (n=3;
6.5%), CT (n=6; 13.0%), X-ray (n=2; 4.3%), and unknown (n=1; 2.2%).
Bone metastases noted on CT were confirmed on subsequent bone
scintigrams in all but one patient. The baseline characteristics of
patients who developed or did not develop bone metastases are shown
in Table I. The ER-negative and
HER2-positive populations were relatively small because patients
receiving neoadjuvant chemotherapy, which is generally performed
for patients with triple-negative breast cancer and HER2-positive
breast cancer, were not included. Fisher's exact tests for 2 by 2
contingency tables showed that bone metastases were associated with
positive nodal status, low serum CEA levels, and adjuvant
chemotherapy (Table I). The serum
calcium level is sometimes elevated in patients with bone
metastasis (16). However, the
baseline serum calcium levels were not increased in patients who
developed bone metastasis as compared to those without bone
metastasis (4.55±0.40 vs. 4.58±0.37 mEq/l; P=0.45), suggesting that
patients who developed bone metastasis had no obvious bone
metastasis at the time of blood sampling. Univariate log-rank
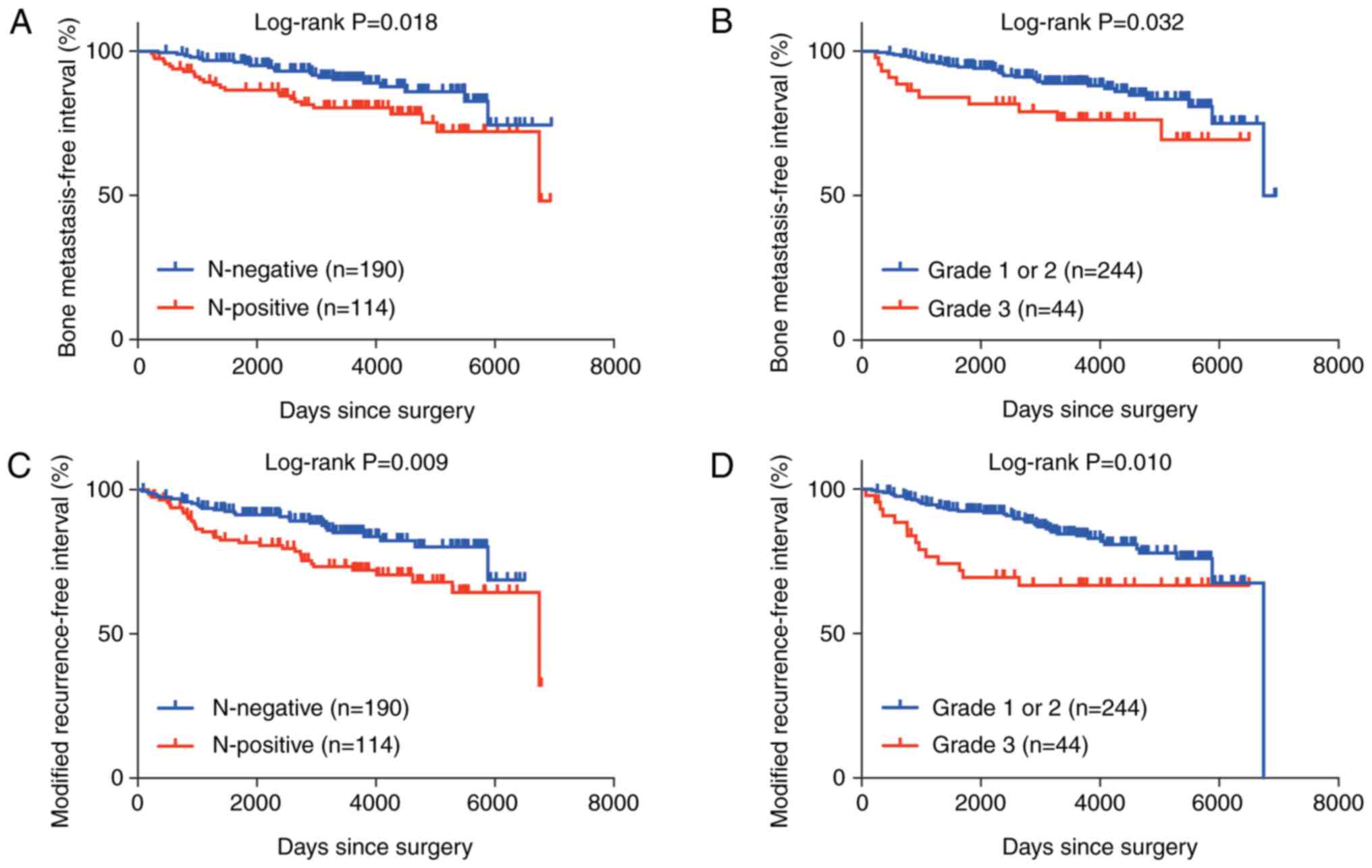
testing revealed that positive nodal status (P=0.018) and a higher
HG (P=0.032) were associated with worse outcomes of BMFI (Fig. 1A and B). In the multivariate
analysis, both positive nodal status [P=0.017; hazard ratio 2.09
(95% confidence interval, 1.14–3.81)] and a higher HG [P=0.030;
hazard ratio 2.15 (95% confidence interval, 1.08–4.28)] were
independently associated with worse outcomes of BMFI. To elucidate
whether nodal status and HG were associated with metastasis
specifically to the bones, mRFI (intervals free of any recurrence
except bone-only events) was analyzed. Both positive nodal status
and a higher HG were associated with worse outcomes of mRFI
(Fig. 1C and D), suggesting that
these factors were prognostic not only for bone metastasis but also
for metastasis in other organs. Log-rank testing of BMFI stratified
by TRACP-5b level showed that the baseline TRACP-5b level was not
associated with BMFI outcomes (P=0.684). TRACP-5b was weakly
correlated with CEA (P=0.027) but was not correlated with CA15-3
(P=0.094) (Fig. S1).
 | Table I.Baseline characteristics of all
patients who did or did not develop BM. |
Table I.
Baseline characteristics of all
patients who did or did not develop BM.
| Characteristic | BM developed, n
(%) | No BM developed, n
(%) | Fisher's P-value |
|---|
| Menopausal
status |
|
| 0.147 |
|
Premenopausal | 25 (8.2) | 109 (35.9) |
|
|
Postmenopausal | 21 (6.9) | 149 (49.0) |
|
| Tumor size |
|
| 0.076 |
| ≤2
cm | 15 (4.9) | 123 (40.5) |
|
| >2
cm | 31 (10.2) | 135 (44.4) |
|
| Lymph node
metastasis |
|
| 0.013 |
|
Negative | 21 (6.9) | 169 (55.6) |
|
|
Positive | 25 (8.2) | 89 (29.3) |
|
| ER |
|
| 0.566 |
|
Negative | 12 (3.9) | 56 (18.4) |
|
|
Positive | 34 (11.2) | 200 (65.8) |
|
|
Unknown | 0 (0.0) | 2 (0.7) |
|
| PR |
|
| 0.104 |
|
Negative | 25 (8.2) | 103 (33.9) |
|
|
Positive | 21 (6.9) | 152 (50.0) |
|
|
Unknown | 0 (0.0) | 3 (1.0) |
|
| HER2 |
|
| 0.101 |
|
Negative | 33 (10.9) | 200 (65.8) |
|
|
Positive | 13 (4.3) | 42 (13.8) |
|
|
Unknown | 0 (0.0) | 16 (5.3) |
|
| Pathological
type |
|
| 0.745 |
|
IDC | 40 (13.2) | 237 (78.0) |
|
|
Others | 3 (1.0) | 16 (5.3) |
|
|
Unknown | 3 (1.0) | 5 (1.6) |
|
| Histological
grade |
|
| 0.062 |
| Grade 1
or 2 | 32 (10.5) | 212 (69.7) |
|
| Grade
3 | 11 (3.6) | 33 (10.9) |
|
|
Unknown | 3 (1.0) | 13 (4.3) |
|
| CEA |
|
| <0.001 |
| Low (≤2
ng/ml) | 32 (10.5) | 89 (29.3) |
|
| High
(>2 ng/ml) | 14 (4.6) | 154 (50.7) |
|
|
Unknown | 0 (0.0) | 15 (4.9) |
|
| CA15-3 |
|
| 0.107 |
| Low
(≤11 U/ml) | 18 (5.9) | 128 (42.1) |
|
| High
(>11 U/ml) | 28 (9.2) | 114 (37.5) |
|
|
Unknown | 0 (0.0) | 16 (5.3) |
|
| TRACP-5b |
|
| 1.000 |
| Low
(≤347 mU/dl) | 23 (7.6) | 131 (43.1) |
|
| High
(>347 mU/dl) | 23 (7.6) | 127 (41.8) |
|
| Chemotherapy |
|
| 0.010 |
| No | 17 (5.6) | 148 (48.7) |
|
|
Yes | 29 (9.5) | 109 (35.9) |
|
| Unknown | 0 (0.0) | 1 (0.3) |
|
TRACP-5b independently correlates with
BMFI in patients with node-positive breast cancer
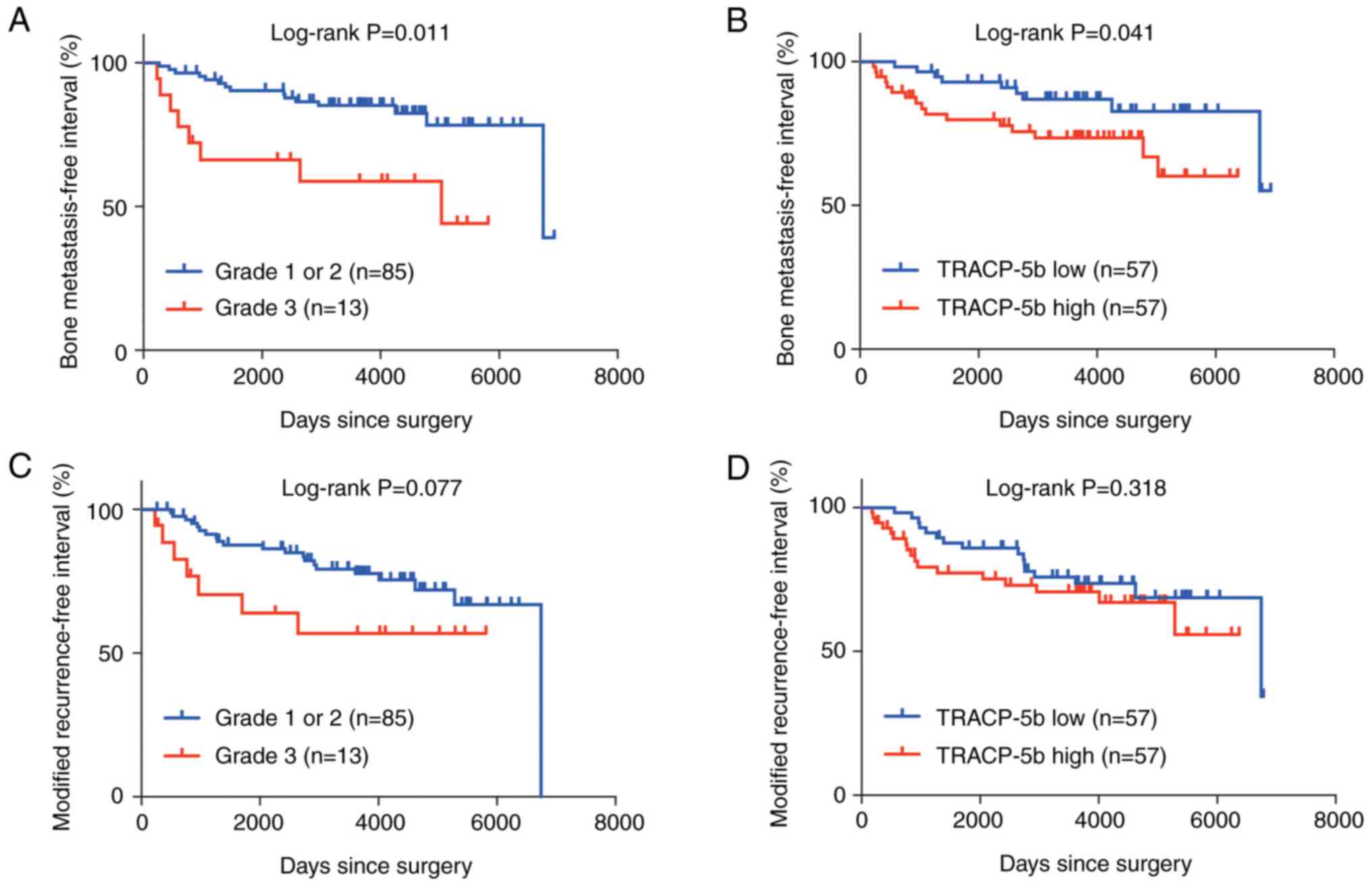
Given the previous findings, we aimed to determine
whether TRACP-5b was associated with bone metastasis in patients
with node-positive or high-grade breast cancer. The univariate
log-rank testing demonstrated that a higher HG and a higher
TRACP-5b level were associated with worse outcomes of BMFI
(Table II; Fig. 2A and B). In the multivariate
analysis, both a higher HG and a higher TRACP-5b level were
independently associated with worse outcomes of BMFI (Table II). The mRFIs of node-positive
patients were marginally different between the high-grade breast
cancer and low-grade breast cancer groups (Fig. 2C). The mRFIs of node-positive
patients were not significantly different between the high TRACP-5b
and low TRACP-5b groups (Fig. 2D),
suggesting that the effect of preoperative TRACP-5b levels on
recurrence was specific to the bone. TRACP-5b was not associated
with BMFI in patients with high-grade breast cancer; positive lymph
node status [multivariate P=0.046; hazard ratio, 3.67 (95%
confidence interval 0.94–14.31)] was the only factor independently
associated with worse BMFI outcomes. These results suggest that a
serum TRACP-5b level is a prognostic factor specific for bone
metastasis in patients with node-positive breast cancer. The
baseline characteristics of patients with node-positive breast
cancer stratified with TRACP-5b demonstrated that the high TRACP-5b
group includes more postmenopausal women, patients with a larger
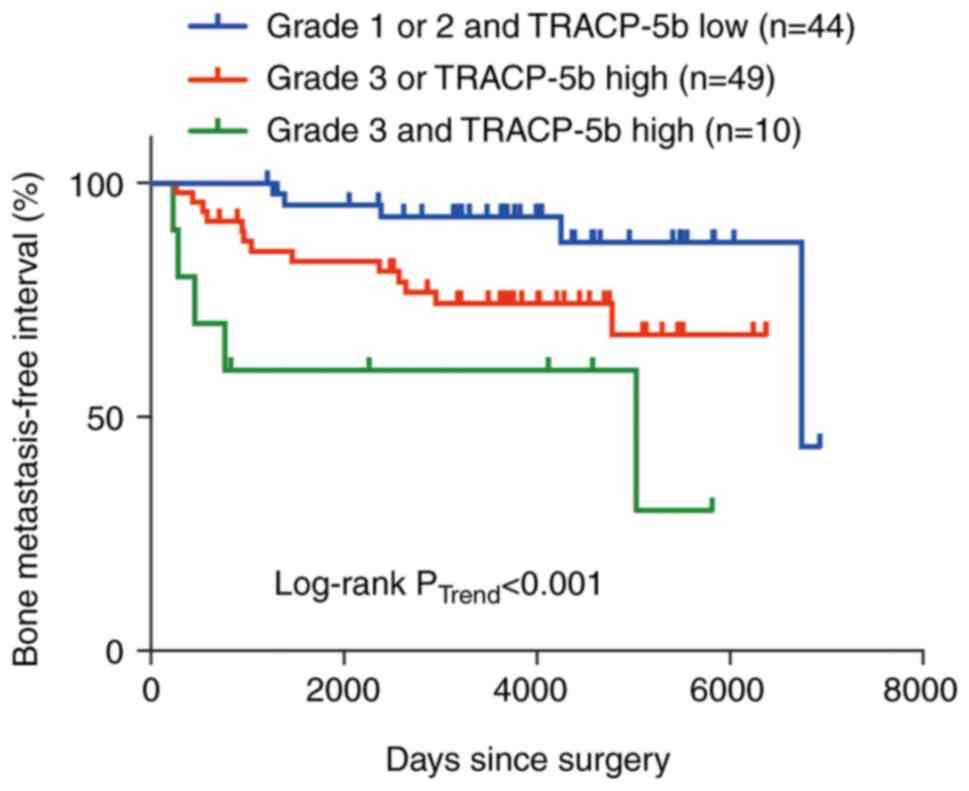
tumor size, negative ER and negative PR (Table III). Finally, prognostic value of
the combination of HG and TRACP-5b was estimated. The BMFIs
declined stepwise with increasing HG and increasing TRACP-5b level
(Fig. 3), implying that the
combination of HG and TRACP-5b offers improved predictive
capability for bone metastasis in node-positive patients, as
compared to utilizing either factor. No correlation was observed
between bone metastasis and HG or TRACP-5b (Table SI). Accordingly, we next assessed
the correlation between bone metastasis and the combination of HG
and TRACP-5b. There were two ways to separate these combinations;
one was to compare HG 3 or TRACP-5b high or both with HG 1/2 and
TRACP-5b low, and the other was to compare HG 3 and TRACP-5b high
with HG 1/2 or TRACP-5b low or both. No correlation was also
observed between bone metastasis and the combination of HG and
TRACP-5b in two possible ways of their separation (Table SI). The lack of correlation between
them may be because such an analysis cannot take into account the
time dependence of bone metastasis.
 | Table II.Univariate and multivariate analyses
of BMFI of patients with node-positive breast cancer. |
Table II.
Univariate and multivariate analyses
of BMFI of patients with node-positive breast cancer.
|
| BMFI |
|---|
|
|
|
|---|
| Characteristic | Univariate
P-valuea | Multivariate
P-valueb | Hazard ratio
(Wald's 95% CI) |
|---|
| Menopausal
status | 0.886 |
|
|
| Tumor size | 0.130 |
|
|
| ER | 0.622 |
|
|
| PR | 0.266 |
|
|
| HER2 | 0.508 |
|
|
| Histological
type | 0.773 |
|
|
| Histological
grade | 0.003 | 0.011 | 3.40
(1.42–8.15) |
| CEA | 0.165 |
|
|
| CA15-3 | 0.452 |
|
|
| TRACP-5b | 0.038 | 0.041 | 2.46
(1.00–6.03) |
 | Table III.Baseline characteristics of lymph
node-positive patients stratified by TRACP-5b levels. |
Table III.
Baseline characteristics of lymph
node-positive patients stratified by TRACP-5b levels.
| Characteristic | Low TRACP-5b, n
(%) | High TRACP-5b, n
(%) | P-value |
|---|
| Menopausal
status |
|
| 0.002 |
|
Premenopausal | 40 (35.1) | 23 (20.2) |
|
|
Postmenopausal | 17 (14.9) | 34 (29.8) |
|
| Tumor size |
|
| 0.024 |
| ≤2
cm | 23 (20.2) | 11 (9.6) |
|
| >2
cm | 34 (29.8) | 46 (40.4) |
|
| ER |
|
| 0.037 |
|
Negative | 7 (6.1) | 17 (14.9) |
|
|
Positive | 50 (43.9) | 40 (35.1) |
|
| PR |
|
| <0.001 |
|
Negative | 13 (11.4) | 35 (30.7) |
|
|
Positive | 44 (38.6) | 21 (18.4) |
|
|
Unknown | 0 (0.0) | 1 (0.9) |
|
| HER2 |
|
| 0.082 |
|
Negative | 49 (43.0) | 41 (36.0) |
|
|
Positive | 6 (5.3) | 13 (11.4) |
|
|
Unknown | 2 (1.8) | 3 (2.6) |
|
| Histological
type |
|
| 0.437 |
|
IDC | 49 (43.0) | 52 (45.6) |
|
|
Others | 4 (3.5) | 2 (1.8) |
|
|
Unknown | 4 (3.5) | 3 (2.6) |
|
| Histological
grade |
|
| 0.613 |
| Grade 1
or 2 | 44 (38.6) | 41 (36.0) |
|
| Grade
3 | 8 (7.0) | 10 (8.8) |
|
|
Unknown | 5 (4.4) | 6 (5.3) |
|
| CEA |
|
| 1.000 |
|
Low | 23 (20.2) | 22 (19.3) |
|
|
High | 34 (29.8) | 35 (30.7) |
|
| CA15-3 |
|
| 0.567 |
|
Low | 25 (21.9) | 21 (18.4) |
|
|
High | 32 (28.1) | 36 (31.6) |
|
| Chemotherapy |
|
| 0.070 |
| No | 13 (11.4) | 5 (4.4) |
|
|
Yes | 44 (38.6) | 52 (45.6) |
|
Discussion
Herein, we found that lymph node metastasis and HG
are independent prognostic factors for metastasis to the bone and
other organs. In patients with node-positive breast cancer, serum
TRACP-5b level is associated specifically with bone metastasis.
Thus, serum TRACP-5b level has a prognostic value in bone
metastasis in patients with early-stage node-positive breast
cancer.
The identification of risk factors for bone
metastasis has been extensively pursued. In our cohort, lymph node
metastasis and HG are independent risk factors for bone metastasis.
Consistent with this finding, lymph node metastasis and/or HG of
breast cancer have been identified as risk factors for bone
recurrence (17–19). Notably, these studies and ours have
included metastasis to the bone-only or bone and other site(s) as
bone metastasis. Luminal subtypes have the strongest association
with bone-only metastasis, whereas lymph node involvement and
histological grading appears to have only a minor influence on
bone-only metastasis (20). This
suggests that lymph node metastasis and HG are not specific risk
factors for bone metastasis. In line with these findings, our
observations also showed that lymph node status and HG are
associated not only with BMFI but also with mRFI. Although not
specific to the bone, lymph node metastasis and HG remain important
bone metastasis risk factors that should be considered. Applying
the Fisher's exact test, a low CEA level was associated with the
development of bone metastases. However, the log-rank test showed
no significant difference in BMFI between the low and high CEA
groups. Bone metastasis is a time-dependent event. The number of
bone metastasis-negative cases may be overestimated because the
current cohort included many cases with a short follow-up duration.
Thus, the log-rank test results should take precedence over the 2
by 2 contingency test results.
In patients with node-positive breast cancer, we
showed that serum TRACP-5b concentration is associated with BMFI,
suggesting that TRACP-5b predicts bone metastasis in such patients.
To date, there have been attempts to identify a bone turnover
marker as a prognosticator for bone metastasis, although reports of
successful identification are scarce. Type I collagen β C-terminal
telopeptide (B-CTx) is released into the bloodstream during bone
resorption. Hence, it is used as a bone resorption marker. In the
NCIC CTG MA.14 trial, which included 621 patients with hormone
receptor-positive early-stage breast cancer, patients with high
serum B-CTx concentration experienced bone-only metastasis with a
significantly higher incidence rate than patients with low B-CTx
(hazard ratio 2.80) (21). This
study did not include patients with breast cancer that metastasized
to the bone and other sites. However, in clinical settings, it is
not necessary to distinguish bone-only metastasis with metastasis
to the bone and other sites because the therapeutic approach to
bone metastasis is basically the same in both situations. In a
seminal study by Fujii et al (4), patients with metastatic breast cancer
were dichotomized in accordance with the emergence of bone
metastasis; patients who experienced bone metastasis at any time
point had worse overall survival than those who did not experience
bone metastasis during the follow-up periods, indicating that
breast cancers ever metastasizing to the bones are a subgroup
distinct from breast cancers that never metastasize to the bones.
Thus, this study, in which BMFI was defined irrespective of the
metastatic sites other than the bones, is reasonable and even more
practical to use as a prognosticator.
Previous studies have provided a rationale for
TRACP-5b as a prognosticator of bone metastasis. TRACP-5b is
secreted specifically from the osteoclasts, thereby reflecting
precise osteoclast number (22).
Osteoclasts play a vital role in the development of bone
metastasis; breast cancer cells colonizing the bones become dormant
in a niche for several years. Then, osteoclasts initiate a vicious
cycle by remodeling the bone niche to reactivate dormant tumor
cells. Proliferating breast cancer cells and osteoclasts stimulate
each other to modify the bone niche, enabling breast cancer cells
to form an overt metastasis (23).
This mechanistic insight into the role of osteoclasts in the
development of bone metastasis has been confirmed with a clinical
observation; a cohort study investigating the relationship between
bone metastasis and osteoporosis, in which osteoclastic resorption
is increased, demonstrated that osteoporosis accelerates the
progression of bone metastasis (24). These findings indicate that
TRACP-5b, osteoclasts, and bone metastasis are closely related.
Our study did not restrict eligible patients to
postmenopausal women with breast cancer. In postmenopausal women,
the number of osteoclasts increases because the estrogen-dependent
apoptosis of osteoclasts is suppressed, thereby increasing the
concentration of TRACP-5b (25).
However, menopausal status does not correlate with BMFI of all
patients or lymph node-positive patients, suggesting that
menopausal status has minimal effects on bone metastasis. In
accordance with this finding, a meta-analysis has shown that the
association of menopausal status with bone metastasis is not
significant (26). Given the
intimate association among TRACP-5b, osteoclasts, and bone
metastasis, we consider that excluding premenopausal women from the
analysis may lead to biased results.
This study has some limitations. First, this was a
retrospective, intermediate-sized cohort study; thus, the patient
background may have been biased. Nevertheless, to minimize the
bias, consecutive patients who had undergone resectable breast
surgery over a period were included. Some associations between
factors and outcomes may not have been obvious because of the
underpowered cohort size. Second, serum uric acid concentration,
which may influence TRACP-5b measurement, was not considered
(27). The factor could not be
retrieved from medical records in a majority of the cohort. Thus,
some TRACP-5b levels in patients with high uric acid may have been
underestimated.
In conclusion, TRACP-5b may predict ever bone
metastasis in patients with resectable node-positive breast cancer.
To determine whether TRACP-5b can be a prognosticator of bone
metastasis, a prospective large-sized cohort study is
warranted.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors thank Dr Shinzaburo Noguchi (Hyogo
Prefectural Nishinomiya Hospital) and Dr Hideki Ishihara (Nitto
Boseki Co., Ltd.) for their helpful discussion.
Funding
This study was funded in part by a Grant-in-Aid for Scientific
Research (C) from the Japan Society for the Promotion of Science
(grant no. 17K10547).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MS conceived and designed the analysis, collected
the data, provided serum samples, performed the analysis and wrote
the paper. YaS collected the data and performed the analysis. MS
and YaS confirm the authenticity of all the raw data. KA, NM, MT,
TY, YoS, TM and TT provided serum samples. KS provided serum
samples and supervised the study. All authors contributed to data
interpretation, and read and approved the final manuscript.
Ethics approval and consent to
participate
Collection and analyses of serum samples were done
in accordance with the protocol approved by the institutional
review board of Osaka University (approval no. 332). This study was
approved by the institutional review board of the Osaka University
Hospital (approval no. 14293). Written informed consent to
participate was obtained from each patient before collecting a
blood sample.
Patient consent for publication
Written informed consent for publication was
obtained from each patient before collecting a blood sample.
Competing interests
Nittobo Co., Ltd. funded TRACP-5b measurements for
111 out of 320 samples. MS and KS are conducting joint research
unrelated to this study with Nittobo Co., Ltd. The other authors
have no potential conflicts of interest.
Glossary
Abbreviations
Abbreviations:
|
TRACP-5b
|
tartrate-resistant acid phosphatase
5b
|
|
I-CTP
|
type I collagen-C-telopeptide
|
|
NTX
|
type I collagen cross-linked
N-telopeptide
|
|
ER
|
estrogen receptor
|
|
PR
|
progesterone receptor
|
|
HER2
|
human epithelial growth factor
receptor-2
|
|
HG
|
histological grade
|
|
CEA
|
carcinoembryonic antigen
|
|
CA15-3
|
cancer antigen 15-3
|
|
BMFI
|
bone metastasis-free interval
|
|
RFI
|
recurrence-free interval
|
|
mRFI
|
modified recurrence-free interval
|
|
B-CTx
|
type I collagen β, C-terminal
telopeptide
|
References
|
1
|
Huang JF, Shen J, Li X, Rengan R,
Silvestris N, Wang M, Derosa L, Zheng X, Belli A, Zhang XL, et al:
Incidence of patients with bone metastases at diagnosis of solid
tumors in adults: A large population-based study. Ann Transl Med.
8:4822020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Early Breast Cancer Trialists'
Collaborative Group (EBCTCG), . Adjuvant bisphosphonate treatment
in early breast cancer: Meta-analyses of individual patient data
from randomised trials. Lancet. 386:1353–1361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gnant M, Pfeiler G, Steger GG, Egle D,
Greil R, Fitzal F, Wette V, Balic M, Haslbauer F,
Melbinger-Zeinitzer E, et al: Adjuvant denosumab in postmenopausal
patients with hormone receptor-positive breast cancer (ABCSG-18):
Disease-free survival results from a randomised, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 20:339–351. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fujii T, Mason J, Chen A, Kuhn P, Woodward
WA, Tripathy D, Newton PK and Ueno NT: Prediction of bone
metastasis in inflammatory breast cancer using a markov chain
model. Oncologist. 24:1322–1330. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
D'Oronzo S, Brown J and Coleman R: The
role of biomarkers in the management of bone-homing malignancies. J
Bone Oncol. 9:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Coleman R, Hadji P, Body JJ, Santini D,
Chow E, Terpos E, Oudard S, Bruland Ø, Flamen P, Kurth A, et al:
Bone health in cancer: ESMO clinical practice guidelines. Ann
Oncol. 31:1650–1663. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Halleen JM, Tiitinen SL, Ylipahkala H,
Fagerlund KM and Väänänen HK: Tartrate-resistant acid phosphatase
5b (TRACP 5b) as a marker of bone resorption. Clin Lab. 52:499–509.
2006.PubMed/NCBI
|
|
8
|
Nenonen A, Cheng S, Ivaska KK, Alatalo SL,
Lehtimäki T, Schmidt-Gayk H, Uusi-Rasi K, Heinonen A, Kannus P,
Sievänen H, et al: Serum TRACP 5b is a useful marker for monitoring
alendronate treatment: Comparison with other markers of bone
turnover. J Bone Miner Res. 20:1804–1812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lumachi F, Basso SMM, Camozzi V, Tozzoli
R, Spaziante R and Ermani M: Bone turnover markers in women with
early stage breast cancer who developed bone metastases. A
prospective study with multivariate logistic regression analysis of
accuracy. Clin Chim Acta. 460:227–230. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishimukai A, Higuchi T, Ozawa H, Yanai A,
Miyagawa Y, Murase K, Imamura M, Takatsuka Y and Miyoshi Y:
Different patterns of change in bone turnover markers during
treatment with bone-modifying agents for breast cancer patients
with bone metastases. Breast Cancer. 24:245–253. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wada N, Fujisaki M, Ishii S, Ikeda T and
Kitajima M: Evaluation of bone metabolic markers in breast cancer
with bone metastasis. Breast Cancer. 8:131–137. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chao TY, Yu JC, Ku CH, Chen MM, Lee SH,
Janckila AJ and Yam LT: Tartrate-resistant acid phosphatase 5b is a
useful serum marker for extensive bone metastasis in breast cancer
patients. Clin Cancer Res. 11:544–550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Clowes JA, Hannon RA, Yap TS, Hoyle NR,
Blumsohn A and Eastell R: Effect of feeding on bone turnover
markers and its impact on biological variability of measurements.
Bone. 30:886–890. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yamada S, Inaba M, Kurajoh M, Shidara K,
Imanishi Y, Ishimura E and Nishizawa Y: Utility of serum
tartrate-resistant acid phosphatase (TRACP5b) as a bone resorption
marker in patients with chronic kidney disease: Independence from
renal dysfunction. Clin Endocrinol (Oxf). 69:189–196. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gourgou-Bourgade S, Cameron D, Poortmans
P, Asselain B, Azria D, Cardoso F, A'Hern R, Bliss J, Bogaerts J,
Bonnefoi H, et al: Guidelines for time-to-event end point
definitions in breast cancer trials: Results of the DATECAN
initiative (definition for the assessment of time-to-event
endpoints in CANcer trials). Ann Oncol. 26:873–879. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Goldner W: Cancer-related hypercalcemia. J
Oncol Pract. 12:426–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liede A, Jerzak KJ, Hernandez RK, Wade SW,
Sun P and Narod SA: The incidence of bone metastasis after
early-stage breast cancer in Canada. Breast Cancer Res Treat.
156:587–595. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamashiro H, Takada M, Nakatani E, Imai S,
Yamauchi A, Tsuyuki S, Matsutani Y, Sakata S, Wada Y, Okamura R, et
al: Prevalence and risk factors of bone metastasis and skeletal
related events in patients with primary breast cancer in Japan. Int
J Clin Oncol. 19:852–862. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang Z and Hu C, Liu K, Yuan L, Li Y,
Zhao C and Hu C: Risk factors, prognostic factors, and nomograms
for bone metastasis in patients with newly diagnosed infiltrating
duct carcinoma of the breast: A population-based study. BMC Cancer.
20:11452020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Diessner J, Wischnewsky M, Stüber T, Stein
R, Krockenberger M, Häusler S, Janni W, Kreienberg R, Blettner M,
Schwentner L, et al: Evaluation of clinical parameters influencing
the development of bone metastasis in breast cancer. BMC Cancer.
16:3072016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lipton A, Chapman JA, Demers L, Shepherd
LE, Han L, Wilson CF, Pritchard KI, Leitzel KE, Ali SM and Pollak
M: Elevated bone turnover predicts for bone metastasis in
postmenopausal breast cancer: Results of NCIC CTG MA.14. J Clin
Oncol. 29:3605–3610. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rissanen JP, Suominen MI, Peng Z and
Halleen JM: Secreted tartrate-resistant acid phosphatase 5b is a
marker of osteoclast number in human osteoclast cultures and the
rat ovariectomy model. Calcif Tissue Int. 82:108–115. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Croucher PI, McDonald MM and Martin TJ:
Bone metastasis: The importance of the neighbourhood. Nat Rev
Cancer. 16:373–386. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen HM, Chen FP, Yang KC and Yuan SS:
Association of bone metastasis with early-stage breast cancer in
women with and without precancer osteoporosis according to
osteoporosis therapy status. JAMA Netw Open. 2:e1904292019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Halleen JM, Ylipahkala H, Alatalo SL,
Janckila AJ, Heikkinen JE, Suominen H, Cheng S and Väänänen HK:
Serum tartrate-resistant acid phosphatase 5b, but not 5a,
correlates with other markers of bone turnover and bone mineral
density. Calcif Tissue Int. 71:20–25. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu H, Zhang X, Zhang S, Wang X and Yu S:
Factors associated with bone metastasis in breast cancer: A
systematic review and meta-analysis. Ann Palliat Med. 10:4435–4452.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu ZQ, Zhang Y, Xie E, Song WJ, Yang RX,
Yan CJ, Zhang BF and Xu HG: High uric acid (UA) negatively affects
serum tartrate-resistant acid phosphatase 5b (TRACP 5b)
immunoassay. PLoS One. 11:e01475542016. View Article : Google Scholar : PubMed/NCBI
|