Introduction
Sacral schwannomas are infrequent tumors accounting
for 1–5% of all spinal schwannomas (1–3). The
tumors occur most commonly at 50–60 years of age (median, 56 years)
(4). In 1932, Masson revealed that
Schwann cells were derived from the nerve sheaths of myelinated
nerves (5). Schwannomas can occur
in any Schwann cell-containing body part, such as the head, neck
and extremities, but retroperitoneal schwannomas are rare.
Retroperitoneal schwannomas are solid or cystic benign tumors
encapsulated within a membrane; they are often located in the
paraspinal and presacral areas. Latent symptomatic retroperitoneal
schwannomas account for 47.7–66.6% of all schwannomas (6). In addition, 5–8% of schwannomas are
accompanied by neurofibromatosis (7). Sacral schwannomas have a relatively
slow growth rate, along with a large retroperitoneal space,
excellent ductility and lack of pain transmission. Consequently,
when detected, sacral schwannomas have a large volume and present
with numerous degenerative pathological changes, such as cystic
degeneration, calcification and hemorrhage, with cystic
degeneration and calcification occurring in 60 and 23% of
individuals, respectively (8).
Computed tomography (CT) or magnetic resonance imaging (MRI)
results exhibiting these features allow clinicians to consider the
possibility of sacral schwannomas.
The incidence of malignant schwannomas, in which the
nerve sheath membrane tends to undergo malignant differentiation,
is 0.001% and accounts for 3–10% of soft-tissue sarcomas (9). Malignant schwannomas are considered
high-grade sarcomas that are highly malignant, strongly invasive,
and prone to recurrence and metastasis, as well as having a low
long-term survival rate; therefore, an early diagnosis is
particularly critical. According to the literature, 15–70% of
patients with malignant schwannomas have combined neurofibromatosis
type 1, which may be associated with the malignancy development of
peripheral neurofibromas (10,11).
The World Health Organization (WHO) has used the terms ‘malignant
Schwann cell tumor’, ‘neurofibrosarcoma’ and ‘malignant schwannoma’
to indicate the origin and malignant behavior of schwannomas. In
2000, schwannomas were formally defined by the WHO as any malignant
tumor originating in the peripheral nerves or showing nerve sheath
differentiation, except for tumors originating in the nerve
periphery or peripheral neurovascular system. Malignant
schwannomas, including two specific subtypes, epithelioid malignant
schwannomas and malignant salamander tumors, were classified as
soft-tissue tumors in 2013 (12).
As sacral schwannomas usually exhibit slow growth
and unspecific symptoms, diagnosis and treatment are often delayed
until the tumor enlarges (1). Most
patients do not show specific clinical manifestations and
characteristic imaging features; thus, the preoperative diagnosis
of sacral schwannomas is usually difficult (13). Benign schwannomas are differentiated
from malignant ones based on the duration of the disease, clinical
neuroerosive corrosive symptoms, capsule integrity on CT or MRI,
and pathological examination results. The present study describes
the surgical management of a giant sacral schwannoma and reviews
the relevant literature, particularly associated case reports.
Case report
A 54-year-old man complaining of paraesthesia and
radiating pain in the lower left extremity visited Jiaozhou Branch
of Shanghai East Hospital, Tongji University (Qingdao, China) in
February 2022. Radiating pain in the sole of the foot had increased
in intensity gradually during the last 3 years, and the stool had
become thin in size over the last 6 months. The patient also had a
history of mild back pain and sciatica over the last 3 years and
had been diagnosed previously as having L3,4,
L4,5, L5 and S1 lumbar disc
herniation on the basis of CT images. Left foot CT and MRI revealed
osteochondral injury with degenerative cysts in the inner side of
the trochlea in the left talus and a small effusion in the left
ankle. The patient had undergone some massage therapy and
physiotherapy in a number of the larger hospitals in Qingdao. The
diagnosis was delayed, as an abdominal CT had not been performed. A
history of trauma was not observed, and the patient was otherwise
healthy. The physical examination revealed mild limitation in the
movement of the left lower extremity, atrophy in the left buttock,
thigh and calf muscles, and skin hypoesthesia. The circumference of
the left lower extremity was 34 cm at 10 cm above the knee, 30 cm
at 10 cm below the knee and 19.4 cm at 5 cm above the ankle. The
circumference of the right lower extremity was 35.5 cm at 10 cm
above the knee, 31.8 cm at 10 cm below the knee and 20.3 cm at 5 cm
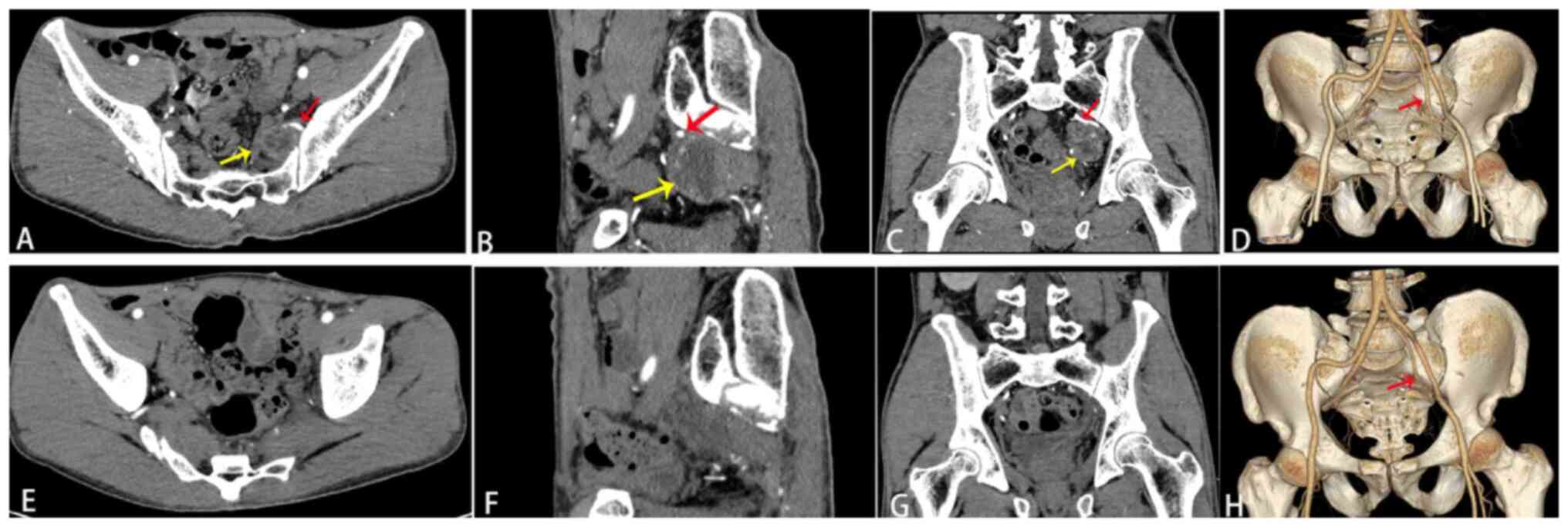
above the ankle. Contrast-enhanced CT of the pelvis demonstrated a
cystic-solid mass on the left side of the pelvis. Three-dimensional
CT reconstruction technology revealed that the feeding artery that
originated from the internal iliac artery flowed into the tumor.
There were more branches of the left internal iliac artery than
usual, and tortuous branches were observed along the tumor margin
(Fig. 1A-C). The diagnosis at the
time of hospital admission was of a pelvic tumor with nerve
compression syndrome in the left lower extremity. After the patient
underwent blood routine, coagulation function, liver and kidney
function, blood glucose, blood fat, electrolyte, carcinoembryonic
antigen, α-fetoprotein, cancer antigen (CA)19-9, CA125 and CA153
tests, abdominal ultrasound and lower extremity electromyogram
examinations, and was administered preoperative multi-disciplinary
treatment, the pelvic tumor was resected using the transabdominal
anterior approach in March 2022. After opening the abdomen, a large
encapsulated retroperitoneal mass of ~5 cm in diameter was detected
by the anterior approach in the left side of the pelvis region,
displacing the urinary bladder upwards and rectosigmoid colon to
the right side. The anterior approach has the advantage of not
causing spinal instability. The lesion was immobile, without
evidence of local invasion. The excision of the mass was
challenging. Due to its size, the posterior peritoneum was opened
over the mass. The sigmoid colon was mobilized from the presacral
space to expose the tumor mass. The ureters were identified
bilaterally and protected. A plane was then established between the
tumor mass and the presacral alar tissue. As the tumor's blood
supply originated from the left internal iliac artery, and thick,
tortuous veins were observed on the tumor surface, the left
internal iliac artery was first ligated and severed. The tumor was
firmly attached by fibrous tissue to the presacral fascia and left
iliac vein. A tissue block was collected from the tumor surface and
subjected to rapid frozen pathology. The pathology revealed a
benign schwannoma. Using an ultrasonic knife, in circumferential
fashion, the tumor was completely mobilized from the sigmoid colon
and upper rectum, the left iliac vein and the other surrounding
anatomical structures with great care for exact hemostasis. The
tumor envelope was then incised along the longitudinal axis of the
nerve, and using sharp dissection. Finally, the remaining
attachment of the tumor tissue to the presacral nerve root(s) was
identified and carefully dissected free with ligasure device. The
tumor was then completely resected by an intralesional resection
(the indications of intralesional resection included the large size
of the tumor and the clinical manifestations of nerve bundle
erosion). For giant schwannomas, we believe it is not possible to
lift the peritoneal sac as the tumor mass is so large. In addition,
this mass had to be broken up to safely dissect the iliac vessels
and ureters, Moreover, it was a multidisciplinary event, especially
for the digestive surgeon, who had a perfect knowledge about this
type of surgical approach while being an expert in laparoscopic
surgery. The digestive surgeon performed the first operating phase
with the surgical approach and the identification of vessels and
ureters, and the neurosurgeon, by virtue of having knowledge of the
lumbosacral plexus, helped with the identification of the sacral
roots and the excision of the lesion. This allowed the association
of two complementary specialists and reduced both operating time
and surgical risks.
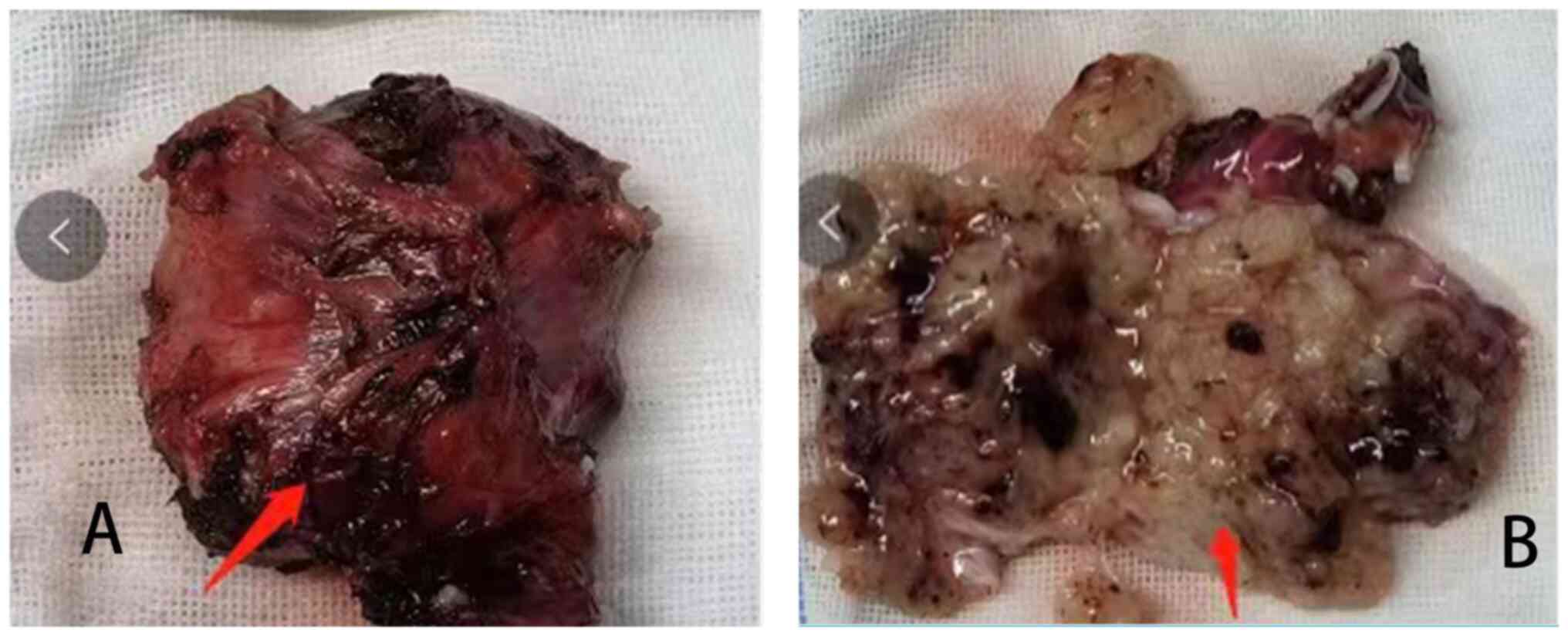
The central region of the tumor was cystic in
appearance and the tumor measured 5×4×4 cm3 (Fig. 2A and B). A schwannoma was diagnosed
on the basis of the postoperative pathology. Specimens were fixed
in 10% neutral formalin solution at room temperature for 24 h,
dehydrated, embedded and serially sliced into 4-µm sections. One
slice of the sample was used for H&E staining. The sections
were baked at 60°C for 30 min, and the sections were viewed under
an optical microscope. For the immunohistochemical analysis,
specimens were fixed in 10% neutral formalin solution at room
temperature for 24 h, dehydrated, embedded and serially sliced into
4-µm sections. Antigen retrieval was performed using endogenous
peroxidase blocker at room temperature for 10 min, and the
non-specific antigens were blocked. The immunohistochemical SP
method was performed. Sections were then incubated overnight at 4°C
with primary monoclonal antibodies (ready-to-use; rabbit anti-human
S-100, cat. no. ZM-0224; Vimentin, cat. no. ZM-0260; and Ki-67,
cat. no. ZM-0224; OriGene Technologies, Inc.), followed by
incubation at room temperature for 30 min with enzyme-labeled sheep
anti-mouse/rabbit IgG polymer secondary antibody (ready-to-use;
cat. nos. PV-6000D and IB000084; OriGene Technologies, Inc.) with
diaminobenzidine (DAB) substrate buffer solution and DAB
concentrated colour reagent, and counterstained with hematoxylin at
room temperature for 5 min. The negative control group was studied
using the same steps, but phosphate-buffered saline was used
instead of primary antibody. The sections were viewed under a BX51
optical microscope (Olympus Corporation) at ×200 and ×400
magnification.
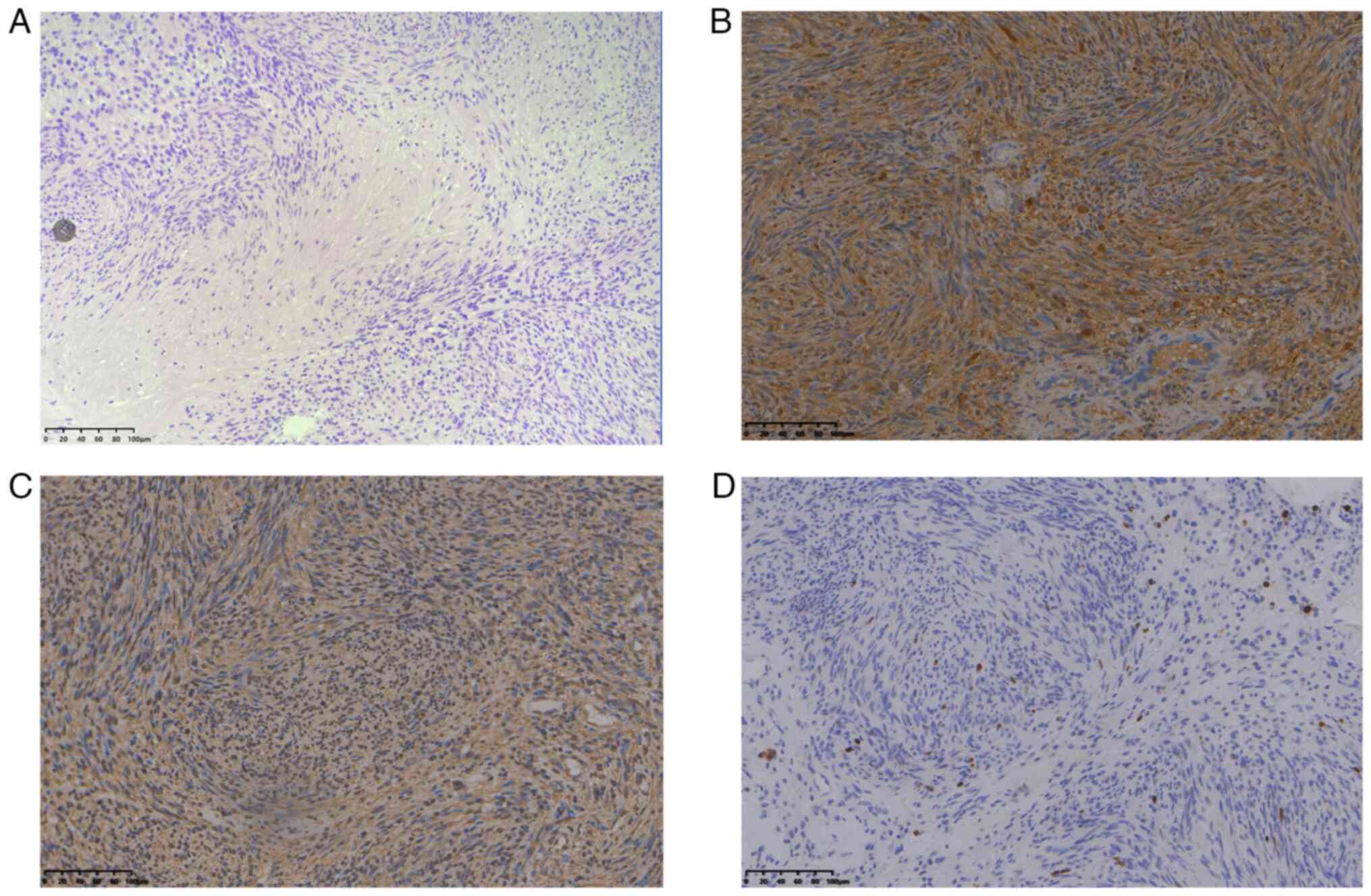
Immunohistochemical observations were as follows:
S-100-positive, vimentin-positive and Ki-67-positive diffuse tumor
cells were observed, and the Ki-67 index of the original tumor was
1% (Fig. 3A-D). Postoperative
three-dimensional CT reconstruction technology revealed that the
tumor was completely resected, with a significant reduction in
blood vessels in the surgical area. The left internal iliac artery
was severed and only a stump of the artery remained (Fig. 1D-F). Follow-up of the patient was
performed every 6 months, including EMG of the left lower
extremity, enhanced CT and MRI. No recurrence was observed during
the 21-month follow-up, and the patient did not receive any further
treatment.
Discussion
Although the pathogenesis of schwannomas is unclear,
their occurrence may be related to relevant gene mutations.
Peripheral nerve schwannomas are well demarcated, encapsulated
tumors, typically round and attached to the nerve; they arise from
the Schwann cells and are eccentric in location, involving one or
two fascicles, and sparing and displacing the other neural
fascicles of the involved nerve. The tumors occur within the
endoneurium and are surrounded by perineurium and fibrous
epineurium that encapsulate the tumor (14). In retroperitoneal schwannomas, the
mean tumor growth rate is 1.9 mm/year. The majority of peripheral
tumors are solitary (96%), while only 4% are plexiform (15). According to their anatomical
locations, schwannomas are divided into intraosseous, dumbbell and
retroperitoneal types. The intraosseous type refers to tumors
located in or invading the spinal canal. Dumbbell-type schwannomas
are tumors extending outside the spinal canal through the
intervertebral foramen. Retroperitoneal-type schwannoma refers to a
tumor located in the posterior peritoneum outside the spinal canal
(16). The tumor size is generally
based on length, but the size of the dumbbell type is based on the
largest part of the tumor. Sacral schwannomas have a slow growth
rate. Moreover, schwannomas display strong concealment abilities
due to the large pelvic space. Various clinical symptoms, including
pelvic organ or nerve tissue compression, bone destruction,
sciatica, lower back pain, bladder and rectum compression due to
difficultyin urination and defecation, and weakness in lower limbs,
are associated with sacral schwannomas (17–19).
Feldenzer et al (20) found
that 67% of patients with sacral schwannomas exhibited rectal
irritation symptoms due to tumor compression. Therefore, a delayed
diagnosis is a clinical feature of sacral schwannomas. In the
present case, the patient had suffered from numbness in the left
buttock and lower extremity, with persistent radiating pain in the
foot sole for 3 years. The patient had visited several hospitals in
Qingdao previously, but only the presence of lumbar and foot
lesions was determined by the treating physicians, who ignored the
results of the abdominal CT examination. The tumor remained
undetected until the patient developed rectal compression symptoms
and underwent an abdominal CT, which is an informative lesson for
clinicians. Clinically, the numbness of the skin of the lower limbs
on the affected side and the presence of muscle atrophy suggested
that the tumor had eroded nerve bundles. According to one previous
study, the mean time for a sacral schwannoma diagnosis was 5.2
years, and the longest time was 11 years (21). In general, sacral malignant tumors
are common in chordoma and chondrosarcoma, and are metastatic,
whereas sacral benign tumors are common in giant osteocytoma,
aneurysmal bone cysts and osteoblastoma (22). However, sacral schwannomas are rare
and need to be differentiated from fibrosarcomas, liposarcomas,
astrocytomas, hydatid cysts, hematomas and connective tissue
diseases (23).
On enhanced CT scans, the cystic wall presents with
delayed slight enhancement, and parenchymal components exhibit
plaque-like progressive but irregular enhancement. MRI T2-weighted
imaging (T2WI) sequencing displays the following pathological
characteristics of the internal tumor: The Antoni A area with dense
cells showing an equal or slightly high signal, and the Antoni B
area rich in stroma and mucus showing a higher signal (24). Verstraete et al (13) and Cuesta et al (4) summarized the imaging data of
schwannomas and found tumors with low-density changes on enhanced
CT, whereas unique changes were present in the schwannomas on MRI
(a low or equal signal on T1WI and a high or heterogeneous signal
on T2WI). Pan et al (25)
reported the following diversified CT and MRI changes: Hemorrhage,
degeneration, necrosis and liquefaction. On MRI, the low- and
high-density signals on T1WI and T2WI, respectively, helped
surgeons to provide a reference for a schwannoma diagnosis before
the operation. Makni et al (26) suggested that the CT density helped
to evaluate fluid and adipose tissues, determine how tumors and the
sacrum or the coccyx and rectum are anatomically related, and
determine the presence or absence of calcification (excluding
chordoma). MRI can help estimate the tumor size, exact location and
relationship of the tumor with adjacent nerves. Preoperative
CT-based 3D reconstruction technology can more clearly reveal the
relationship between tumors and adjacent blood vessels and nerves
(27). Large volumes are
characteristic of retroperitoneal schwannomas. In a previous study,
CT revealed that degeneration and hemorrhage often occur in the
tumor body, and calcification was observed (28). Additionally, a single benign
schwannoma can be detected using fluorodeoxyglucose positron
emission tomography/CT (29). The
standard agreed diagnostic criterion for giant schwannomas is a
tumor size of ≥5 cm on CT or MRI (1,30). In
the present case, enhanced CT and three-dimensional reconstruction
of the pelvis unveiled that the tumor was round, cystic-solid, with
a thin cystic wall and smooth inner and outer walls. The tumor
exhibited uniform enhancement, predominantly hypointense, with
delayed and mild enhancement of the cystic wall. The tumor was
partially adjacent to the sacrum, and the branches of the left
internal iliac artery were increased in number and traveled along
the tumor margin. The venous phase exhibited further mild
progressive enhancement, with cloudy flocculent. The demarcation
between high and low densities was more clearly defined. The
aforementioned imaging changes were consistent with the imaging
features of schwannomas. Enhanced CT and three-dimensional
reconstruction of the pelvis revealed that the tumor was completely
resected, and the blood vessels in the surgical area were reduced
in number. The left internal iliac artery was severed and only a
stump of the artery remained. With sacral schwannoma, accurate
preoperative imaging evaluation is important for choosing a
rational surgical approach (both to completely resect the tumor and
to avoid nerve trunk damage). In the case, preoperative
three-dimensional CT reconstruction revealed that the tumor
originated from the left internal iliac artery, suggesting that
surgeons should make clear the relationship between tumor and blood
vessels, and choose a reasonable surgical approach, so as to avoid
accidental massive haemorrhage during the operation. Moreover, a
MRI view that shows the continuity to a nerve root may help the
diagnosis. In the present case, MRI was not performed before the
operation, which is a limitation of the study.
In the present study, based on the gross
pathological examination, the schwannoma was white/yellow with a
hard texture. Under the microscope, several densely arranged
spindle cells were observed in the Antoni A area, and fewer tumor
cells with the mucinous matrix were observed in the Antoni B area.
Xu et al (23) and Yu et
al (29) reported that
schwannoma pathology is based on degenerative changes and nuclear
atypia. Immunohistochemical analyses, such as analysis of levels of
S-100, neuron-specific enolase or vimentin, are crucial for the
pathological diagnosis. Ki-67 refers to the nuclear proliferation
index. This index reflects cell proliferation and simultaneously
indicates the probability of tumor recurrence after operation.
Ogose et al (30) reported
that the Ki-67 index of presacral schwannomas was generally <1%.
In the present case, the gross specimen view revealed that the
tumor was quasi-circular, cystic-solid, and thick veins were
observed on the surface. On dissecting the tumor, cystic
degeneration and intracapsular hemorrhagic changes were found.
Under the microscope, numerous densely arranged spindle cells were
noted in the Antoni A area, and sparse tumor cells rich in mucus
stroma were observed in the Antoni B area. Positive expression of
S-100, vimentin and Ki-67 was also observed. Schwannoma is a tumor
originating from the mesenchymal tissues. The positive expression
of S-100, a neuroendocrine marker, indicates that the tumor
originates from neural tissues (31,32).
Vimentin is a fibroblast component that belongs to the
cytoskeleton; it is mainly distributed among cytoskeletal elements,
as well as in tumors originating from mesenchymal cells, such as
schwannomas or neurofibromas. Vimentin-positive expression also
suggests a tumor originating from neural tissues (33). Ki-67 is a nuclear antigen that
reflects cell proliferative activity (34,35).
In the present case, the Ki-67 index of the original tumor was 1%,
which indicated a benign tumor, and there was a low recurrence
rate. Ozdemir et al (36)
found that 50% of patients with sacral schwannomas exhibited nerve
erosion, and emphasized that invasion and erosion are two different
concepts. Invasion is tissue destruction by the tumor, whereas
erosion is tissue replacement by the tumor. These concepts suggest
that surgeons should consider both the thoroughness of tumor
resection and the preservation of the nerve function when opting
for surgical procedures. Considering the pathological
characteristics of giant sacral schwannomas, surgeons must perform
a reasonable operation rather than a perfect operation.
The envelopes of schwannomas are normal nerve sheath
tissues that are not a part of the tumor; thus, the encapsulated
tumor should be completely removed. A potential risk of nerve
injury may arise when approaches such as extracapsular tumor
resection are used to deliberately reduce the recurrence rate.
Therefore, the complete resection of the enveloped tumor is
sufficient for preventing its recurrence, protecting nerve
functions, reducing nerve damage and reducing the probability of
surgical complications. Studies have highlighted the use of 3D
imaging technology-based preoperative needle biopsy for designing
surgical protocols, as well as the need for multidisciplinary team
collaboration and careful discussion, to avoid any possible
complications (3,37). The use of a preoperative needle
biopsy remains controversial. The advocators of this technique
believe that a biopsy can help in making a clear diagnosis of the
tumor, excluding lymphoma, and in the selection of reasonable
surgical procedures. The opponents argue that a risk of infection
and bleeding exists; nonetheless, most scholars have advocated for
a preoperative hollow-needle biopsy (2,22,38).
No consensus exists on the surgical management of sacral
schwannomas. Surgical procedures may include a total or subtotal
resection or a partial resection of the schwannoma. In the
so-called subtotal resection, the tumor is resected to ≥90%, while
<90% of the tumor is resected in a partial resection (1,18).
Handa et al (16) reported
that giant sacral schwannomas were surgically removed in 11
patients who were followed up for a mean time of 50 months, and
that no recurrence was observed in 8 patients who underwent a total
or partial resection, whereas recurrence was observed in 2 out of
the 3 patients who underwent a partial resection. Using curettage
and radiotherapy approaches for giant sacral schwannomas in 4
patients maximally protect the sacral nerve bundle in a study by
Chandhanayingyong et al (39); however, the recurrence rate was 54%.
Some scholars have proposed that nerve monitoring should be
performed during the removal of giant sacral schwannomas to achieve
maximal tumor resection while avoiding nerve injury and reducing
tumor recurrence (19,40). The diagnosis must be reliable before
surgical treatment for giant sacral schwannoma, avoiding the
intralesional resection of retroperitoneal malignancies (41). In this present case, the abdomen was
explored using the anterior approach, and the posterior peritoneum
was opened. The tumor's blood supply originated from the left
internal iliac artery, and thick, tortuous veins were observed on
the tumor surface. The left internal iliac artery was first ligated
and severed, and the nerve trunk was completely exposed at the
tumor's base. Using an ultrasonic knife, the tumor envelope was
separated, and the nerve trunk at the tumor's base was completely
exposed to determine the tumor-nerve association. A tissue block
was collected from the tumor surface and subjected to rapid frozen
pathology. The pathology revealed a benign schwannoma. The tumor
envelope was then incised along the longitudinal axis of the nerve,
and using sharp dissection, the tumor was completely resected
(intralesional resection indications include the large size of the
tumor and the clinical manifestations of nerve bundle erosion).
While resecting the schwannoma, nerve bundle damage was avoided as
much as possible. Most studies have recommended careful
intracapsular excision of the tumor to minimize postoperative
neural deficits, since most schwannomas are encapsulated. If nerve
fibers surround the tumor surface, intracapsular enucleation can be
carried out while preserving nerve fibers by making a small
longitudinal incision in the capsule (14,23,42,43). A
number of studies have used laparoscopy or robotic assistance to
clearly observe and enlarge the characteristics of tissue
structures in a narrow space, remove tumors completely and protect
nerve structure integrity, in alignment with the concept of
precision and minimally invasive surgeries (2,44).
Adjuvant therapies for sacral schwannomas are
embolization, chemotherapy, hyperthermia, Gamma Knife®
therapy and cryosurgery (30,45).
Different postoperative complications and prognoses of sacral
schwannomas have been reported. Based on a review of the
literature, Paulo et al (21) reported that of the 68 patients with
sacral schwannomas, 10% had movement disorders, 6% had
incontinence, 18% developed recurrence and 9% underwent reoperation
after the initial surgery. Mualem et al (46) reported that, in 27 patients in whom
sacral schwannomas were resected, 59.3% experienced a complete
recovery from symptoms, 33.3% displayed a partial recovery from
symptoms and 11.1% showed no symptom recovery. Yu et al
(29) reported 12 cases of sacral
schwannoma, 6 of which exhibited Ki-67 levels of >2% and 4 of
which displayed postoperative recurrence. Thus, the postoperative
recurrence of schwannomas is associated with the surgical procedure
and biological tumor characteristics. The recurrence of schwannoma
is affected by tumor size and the degree of resection. Therefore,
laparoscopic or robotic surgery is recommended for giant sacral
schwannomas, as the tumor location is deep and microscopic surgery
cannot be performed. The magnification and clarity of image
technology can facilitate minimally invasive and complete removal
of the tumor, and prevent nerve damage while reducing the
recurrence rate. In the present case, no recurrence was observed
during the 21-month follow-up period and the patient was
asymptomatic.
In conclusion, sacral schwannomas are rare, and
their clinical diagnosis is frequently delayed. Furthermore,
surgical procedures for removing sacral schwannomas are diverse.
Problems such as postoperative nerve injuries and tumor recurrence
may be a challenge for surgeons when choosing appropriate treatment
strategies for sacral schwannomas. Therefore, future studies should
concentrate on measures for avoiding nerve injuries and reducing
the postoperative recurrence rate.
Acknowledgements
The authors would like to thank Professor Yang Qing
(Department of Pathology, Jiaozhou Branch of Shanghai East
Hospital, Tongji University, Qingdao, Shandong, China) for guidance
and help with the pathology in this study.
Funding
This study was funded by the Military Logistic Project of China
(grant no. AWS17J008).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SZ and SW conceived the report and participated in
data acquisition and the drafting of the manuscript. HC and YZ
participated in the conception and design of the report, and
critically revised the manuscript. LL and WD collected imaging data
and performed study revisions. XM was responsible for the
pathological analysis. SZ and HC confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The reporting of this study conforms to the CARE
guidelines. This study was exempt from ethical approval by the
ethics committee of the Jiaozhou Branch of Shanghai East Hospital,
Tongji University.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and the accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID: Professor Haibo Chu, https://orcid.org/0000-0002-3568-6431.
References
|
1
|
Macciò A, Kotsonis P, Aste L, Voicu MA,
Madeddu C, Conti C and Camparini S: An interdisciplinary approach
for laparoscopic removal of a large retroperitoneal pelvic
schwannoma attached to vital vessels: A case report. Medicine
(Baltimore). 98:e1814920202019. View Article : Google Scholar
|
|
2
|
Ohsawa M, Miguchi M, Yoshimitsu M, Oishi
K, Kohashi T, Hihara J, Mukaida H, Kaneko M, Egi H, Ohdan H and
Hirabayashi N: Laparoscopic excision of a retroperitoneal
schwannoma: A case report. Asian J Endosc Surg. 12:192–196. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Korduke O, Omar A, Viner W and Kodeda K:
Pre-sacral schwannoma. ANZ J Surg. 90:1805–1807. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cuesta JP, Rodríguez LC, Bastidas N and
Hernández M: Sacral schwannoma a case report and review of the
literature. Neuroradiol J. 36:371–374. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masson P: Experimantal and spontaneous
schwannomas (peripheral glinomas): IL spontaneous schwannomas. Am J
Pathal. 8:389–416. 1932.
|
|
6
|
Brian KPG, Tan YM, Chuang YFA, Pierce KHC,
London LPJO and Wong WK: Retroperitoneal schwannoma. Am J Surg.
192:14–18. 2006. View Article : Google Scholar
|
|
7
|
Liu QY, Lin XF, Zhang WD, Li HG and Gao M:
Retroperitoneal schwannomas in the anterior pararenal space:
Dynamic enhanced multi-slice CT and MR findings. Abdom Imaging.
38:201–210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kudo T, Kawakami H, Kuwatani M, Ehira N,
Yamato H, Eto K, Kubota K and Asaka M: Three cases of
retroperitoneal schwannoma diagnosed by EUS-FNA. World J
Gastroenterol. 17:3459–3464. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yun JS, Lee MH, Lee SM, Lee JS, Kim HJ,
Lee SJ, Chung HW, Lee SH and Shin MJ: Peripheral nerve sheath
tumor: differentiation of malignant from benign tumors with
conventional and diffusion-weighted MRI. Eur Radio. 31:1548–57.
2020. View Article : Google Scholar
|
|
10
|
Stramare R, Beltrame V, Gazzola M, Gerardi
M, Scattolin G, Coran A, Faccinetto A, Rastrelli M and Rossi CR:
Imaging of soft-tissue tumors. J Magn Reson Imaging. 37:791–804.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baheti AD, O'Malley RB, Kim S, Keraliya
AR, Tirumani SH, Ramaiya NH and Wang CL: Soft-tissue sarcomas: An
update for radiologists based on the revised 2013 world health
organization classification. AJR. 206:924–932. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Doyle LA: Sarcoma classification: an
update based on the 2013 world health organization classification
of tumors of soft tissue and bone. Cancer. 120:1763–1774. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Verstraete KL, Achten E, De Schepper A,
Ramon F, Parizel P, Degryse H and Dierick AM: Nerve sheath tumors:
Evaluation with CT and MR imaging. J Belge Radiol. 75:311–320.
1992.PubMed/NCBI
|
|
14
|
Suárez C, López F, Rodrigo JP, Mendenhall
WM, de Bree R, Mäkitie AA, Vander Poorten V, Takes RP, Bondi S,
Kowalski LP, et al: Benign peripheral non-cranial nerve sheath
tumors of the neck. Adv Ther. 39:3449–3471. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
El Sayed L, Masmejean EH, Parfait B,
Kalamarides M, Biau D and Peyre M: Natural history of peripheral
nerve schwannomas. Acta Neurochir (Wien). 162:1883–1889. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Handa K, Ozawa H, Aizawa T, Hashimoto K,
Kanno H, Tateda S and Itoi E: Surgical management of giant sacral
schwannoma: A case series and literature review. World Neurosurg.
129:e216–e223. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khan UA, Ismayl G and Malik I: Giant
sacral schwannoma treated with a 360 approach: A rare case and
systematic review of the literature. World Neurosurg. 115:65–72.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tahta A, Dinc C, Ozdenkaya Y and Cakir A:
Giant sacral schwannoma causing bilateral hydronephrosis: Case
report and review of the literature. World Neurosurg. 142:184–187.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ragurajaprakash K, Hanakita J, Takahashi
T, Ueno M, Minami M, Tomita Y, Tsujimoto Y and Kanematsu R: Giant
invasive sacral schwannoma with aortic bifurcation compressionand
hydronephrosis. World Neurosurg. 135:267–272. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feldenzer JA, McGauley JL and McGillicuddy
JE: Sacraland presacral tumors: Problems in diagnosis and
management. Neurosurgery. 25:884–891. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Paulo D, Semonche A and Tyagi R: Surgical
management of lumbosacral giant invasivespinal schwannoma: Acase
report and literature review. World Neurosurg. 114:13–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Turner ML, Mulhern CB and Dalinka MK:
Lesions of the sacrum differential diagnosis and radiological
evaluation. JAMA. 245:275–277. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu H, Sha N, Li HW, Bai M, Chen X, Hu HL
and Wu CL: A giant pelvic malignant schwannoma: A case report
andliterature review. Int J Clin Exp Pathol. 8:15363–15368.
2015.PubMed/NCBI
|
|
24
|
Leclerc A, Lebreton G, Huet A, Alves A and
Emery E: Management of giant presacral schwannoma. Clinical series
and literature review. Clin Neurol Neurosurg. 200:1064092021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pan W, Wang Z, Lin N, Huang X, Liu M, Yan
X and Ye Z: Clinical features and surgical treatment of sacral
schwannomas. Oncotarget. 8:38061–38068. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Makni A, Fetirich F, Mbarek M and Ben
Safta Z: Presacral schwannoma. J Visc Surg. 149:426–427. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kinoshita T, Naganuma H, Ishii K and Itoh
H: CT features of retroperitoneal neurilemmoma. Eur J Radiol.
27:67–71. 2015. View Article : Google Scholar
|
|
28
|
Bai X and Wang XM: Solitary benign
schwannoma mimics residual malignancy on FDG PET/CT. Clin Nucl Med.
43:782–784. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu NH, Lee SE, Jahng TA and Chung CK:
Giant invasive spinal schwannoma: Its clinical features and
surgical management. Neurosurgery. 71:58–66. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ogose A, Hotta T, Hatano H, Kawashima H,
Umezu H, Higuchi T and Endo N: Presacral multiple cellular
schwannomas. Spine (Phila Pa 1976). 28:E426–E429. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mokhtari M, Iranpour P, Golbahar Haghighi
A and Ghahramani L: Schwannoma of the rectosigmoid colon. Adv
Biomed Res. 11:52022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Doan L and Cassarino DS: A rare case of
cutaneous pseudoglandular schwannoma. J Cutan Pathol. 50:798–800.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bohlok A, El Khoury M, Bormans A, Galdon
MG, Vouche M, El Nakadi I, Donckier V and Liberale G: Schwannoma of
the colon and rectum: A systematic literature review. World J Surg
Oncol. 16:1252018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prueter J, Norvell D and Backous D: Ki-67
index as a predictor of vestibular schwannoma regrowth or
recurrence. J Laryngol Otol. 133:205–207. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Singh A, Aggarwal M, Chadalavada P,
Siddiqui MT, Garg R, Lai K and Chahal P: Natural history of
gastrointestinal schwannomas. Endosc Int Open. 10:E801–E808. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ozdemir N, Bezircioğlu H and Akar O: Giant
erosive spinal schwannomas: Surgical management. Br J Neurosurg.
24:526–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin CL, Fang JJ and Lin RM: Resection of
giant invasive sacral schwannoma using image-based customized
osteotomy tools. Eur Spine J. 25:4103–4107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Çağlı S, Işık HS, Yıldırım U, Akıntürk N
and Zileli M: Giant sacral schwannomas. J Neurooncol. 110:105–110.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chandhanayingyong C, Asavamongkolkul A,
Lektrakul N and Muangsomboon S: The management of sacral
schwannoma: Report of four cases and review of literature. Sarcoma.
2008:8451322008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Colonna MR, Costa AL, Mastrojeni C, Rizzo
V, Nirta G, Angileri FF, Ieni A, Milone E and Macrì A: Giant sacral
schwannoma excised under intraoperative neuromonitoring in an
elderly patient: case report. J Surg Case Rep. 2021:rjab4602021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Berner EA, Hung YP, Nielsen GP and
Lozano-Calderón SA: Malignant peripheral nerve sheath tumors
arising from schwannomas: Case series and literature review. APMIS.
129:524–532. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ram S, Vivek V, Shekhar R, Gabbita AC and
Ganesh K: Giant Cervicodorsal Schwannoma. J Exp Ther Oncol.
13:155–158. 2019.PubMed/NCBI
|
|
43
|
Ozturk C, Mirzanli C, Karatoprak O, Tezer
M, Aydogan M and Hamzaoglu A: Giant sacral schwannoma: A case
report and review of the literature. Acta Orthop Belg. 75:705–710.
2009.PubMed/NCBI
|
|
44
|
Yin J, Wu H, Tu J, Zou C, Huang G, Xie X,
He Y and Shen J: Robot-assisted sacral tumor resection: A
preliminary study. BMC Musculoskelet Disord. 19:1862018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Yang CC, Chen HC and Chen CM:
Endoscopicresection of a presacral schwannoma: Case report. J
Neurosurg Spine. 7:86–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Mualem W, Ghaith AK, Rush D, Jarrah R,
Alexander Y, Zamanian C, Atkinson JLD, Yaszemski MJ, Krauss WE,
Spinner RJ and Bydon M: Surgical management of sacral schwannomas:
A 21-year mayo clinic experience and comparative literature
analysis. J Neurooncol. 159:1–14. 2022. View Article : Google Scholar : PubMed/NCBI
|