Introduction
As of 2020, gastric cancer (GC) is among the most
prevalent malignancies globally, accounting for approximately one
million newly diagnosed cases and an estimated 770,000
cancer-related deaths annually (1).
This staggering prevalence makes GC the third leading cause of
cancer-related mortality worldwide. Unfortunately, GC diagnosis
often occurs at advanced or metastatic stages, making the tumor
unresectable through surgery (2).
Among these cases, distant metastases markedly diminish overall
patient survival. The most common metastatic site is the liver,
accounting for 17.4% of all metastatic occurrences (3).
The primary treatment option for patients with
advanced GC is systemic chemotherapy. The conventional treatment
regimens typically include fluorinated pyrimidines and
platinum-based agents (4,5). However, patients with liver failure
cannot tolerate cytotoxic agents because they are primarily
metabolized by the liver. Consequently, case reports in the
literature detailing chemotherapy administration in patients with
severe liver failure are limited.
Recently, immune checkpoint inhibitors (ICIs) have
gained prominence for treating various cancers, including GC. ICIs
relieve the inhibition of T cell activation by binding to immune
checkpoint molecules such as programmed cell death protein 1 (PD-1)
and its ligand (PD-L1), as well as cytotoxic
T-lymphocyte-associated protein 4 (CTLA-4), thus triggering strong
antitumor effects. Importantly, their use is not contraindicated,
even in the presence of severe liver failure (6). Nevertheless, documented cases of
treating patients with cancer and severe liver failure using ICIs
are limited. Only two instances have been reported: One in 2017
involving the use of nivolumab for a patient with Hodgkin lymphoma
(7), and another in 2021 where
pembrolizumab was administered to a patient with non-small cell
lung cancer (8). Therefore, their
application in advanced GC with severe liver failure remains
relatively uncharted territory.
We herein report a case in which, for the first time
in GC cases, long-term survival was achieved using ICIs in a
patient with cancer and concomitant severe liver failure.
Case report
A 57-year-old man presented to Shimane Prefectural
Central Hospital (Izumo, Japan) with a 2-week history of appetite
loss and jaundice in April 2020 to be admitted on the same day. An
upper gastrointestinal endoscopy revealed advanced gastric cancer
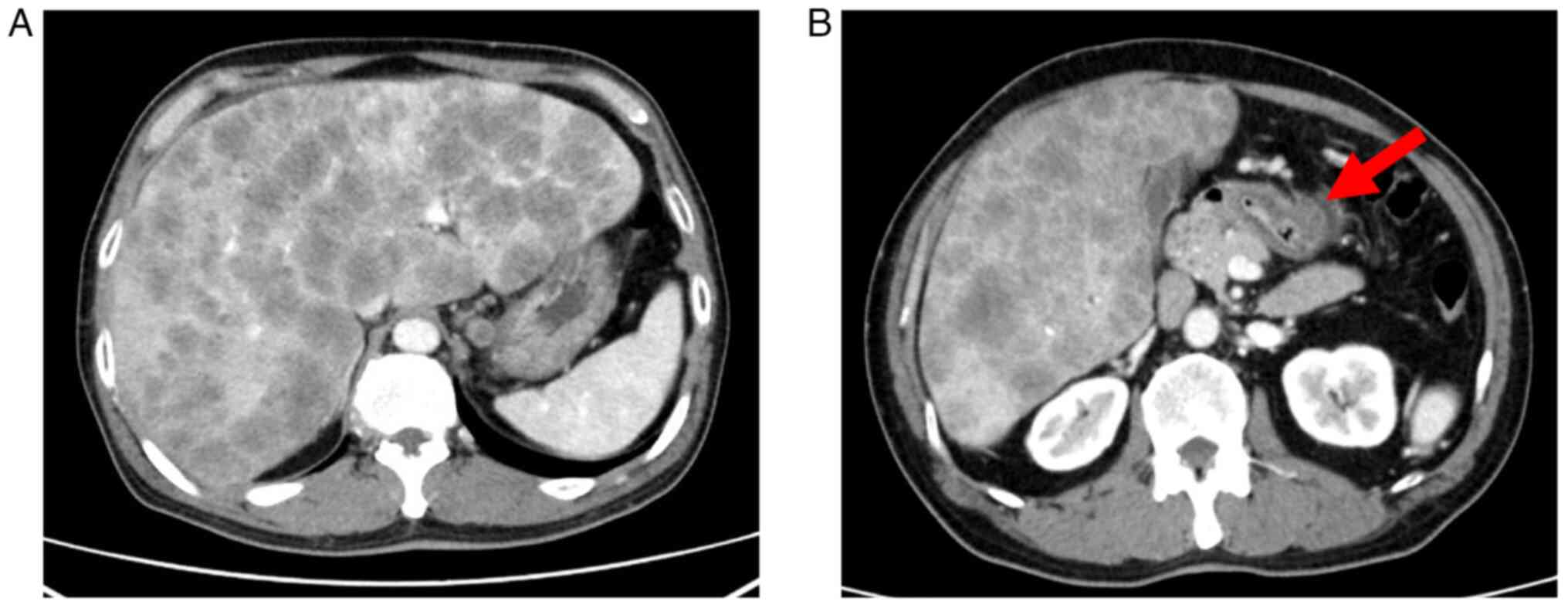
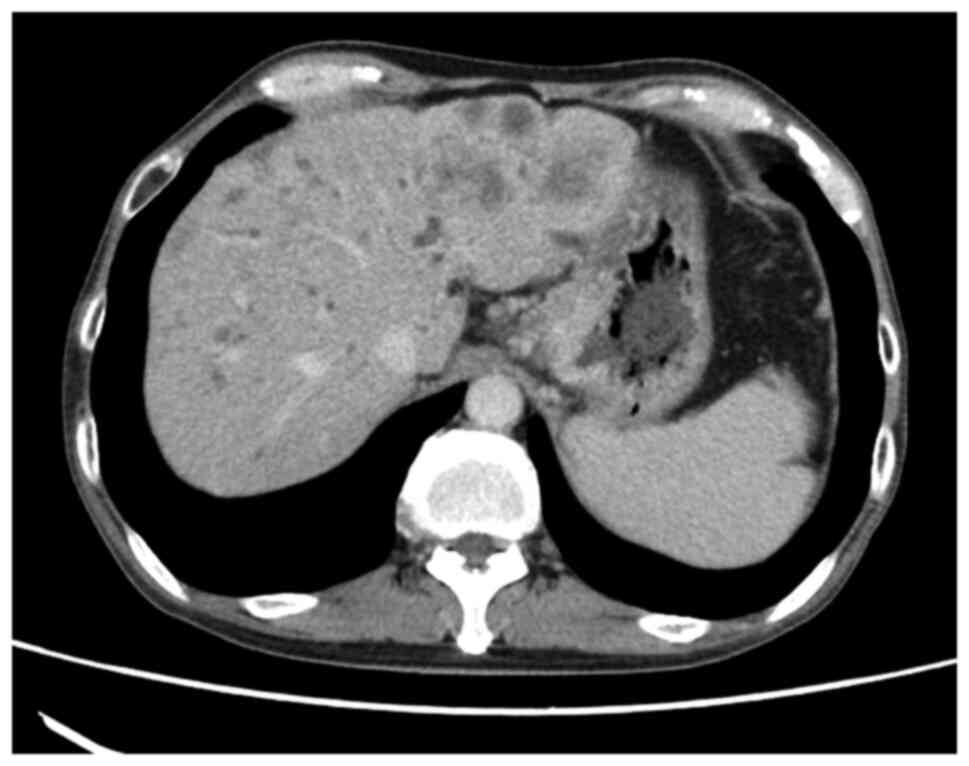
(type IV). Computed tomography (CT) examination confirmed wall
thickening of the gastric pylorus and multiple liver metastases
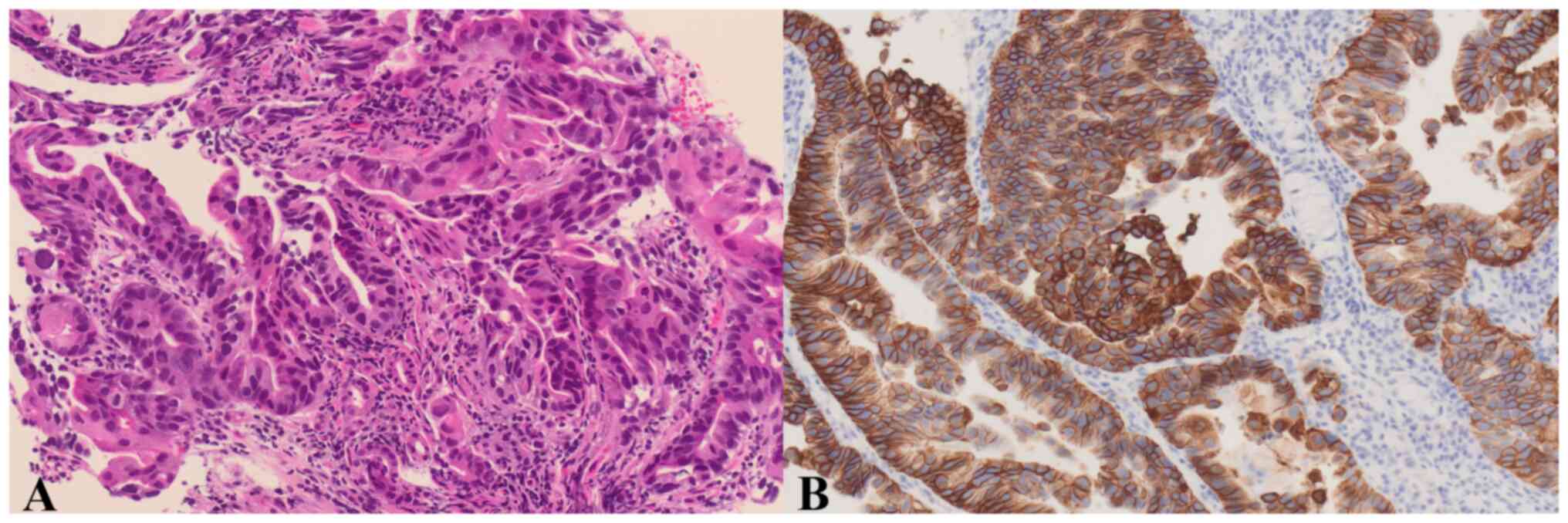
(Fig. 1). A gastric mucosal biopsy
confirmed the diagnosis of HER2-positive gastric adenocarcinoma
(Fig. 2). Chemotherapy with S-1 +
cisplatin was initiated promptly; however, hepatic impairment
worsened during the first course. The patient was deemed unsuitable
for continuing chemotherapy owing to rapidly elevated bilirubin
levels, acute liver failure, and deteriorating performance status.
He was discharged for palliative care.
After seeking a second opinion, he was referred to
Shimane University Hospital (Izumo, Japan) the following month. On
admission, the patient presented a performance status (ECOG) score
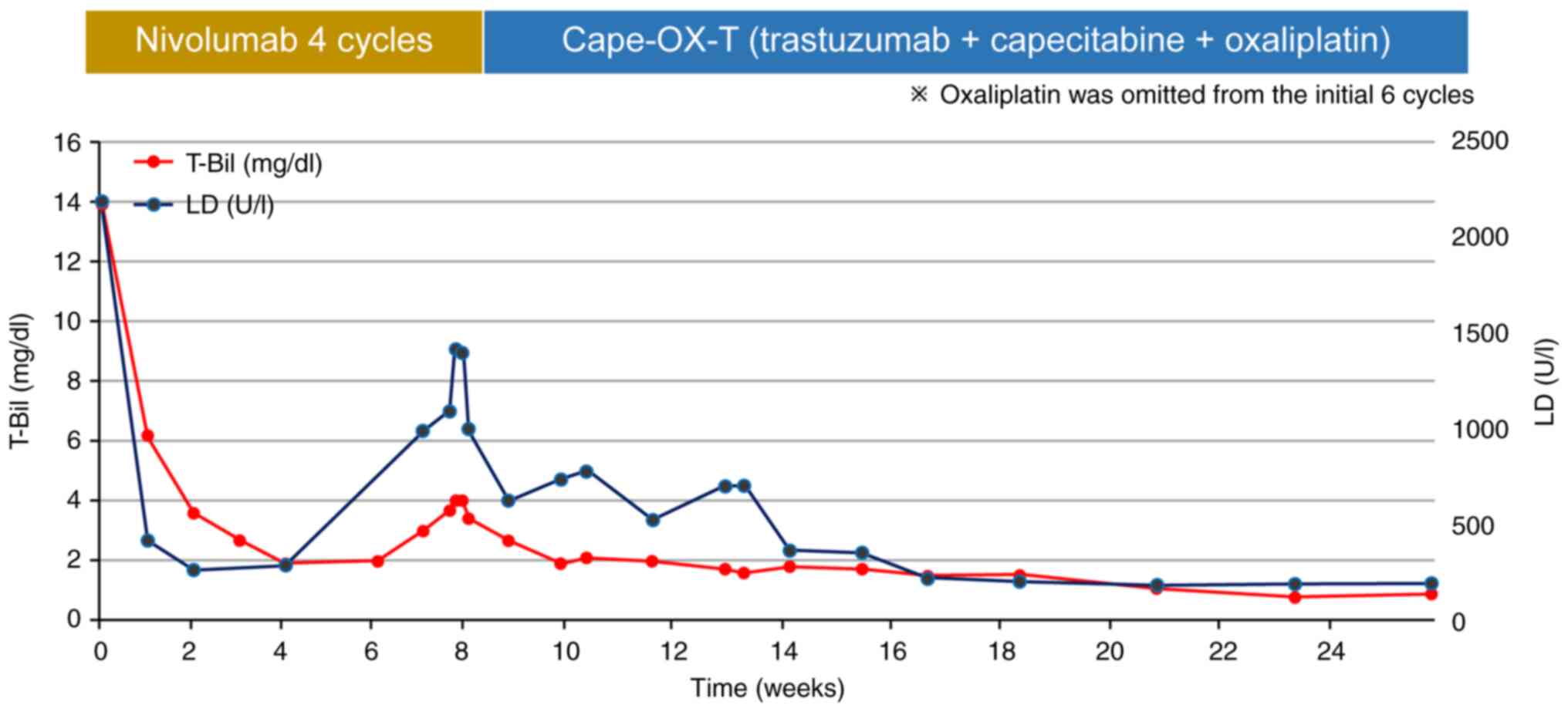
of 3. Laboratory results indicated severe liver failure with a
total bilirubin level of 13.9 mg/dl (normal range: <1.8 mg/dl)
and a lactate dehydrogenase (LDH) level of 2,182 U/l (normal range:
135–214 U/l), alongside elevated tumor markers (CA19-9: 992 U/ml,
CEA: 2,700 ng/ml) (Fig. 3). CT
images indicated no cholestasis. The patient's Child-Pugh score was
10 (Class C). Based on these findings, the patient was diagnosed
with HER2-positive unresectable gastric cancer (cStage IV, TNM) and
severe liver failure.
Following admission, we promptly commenced nivolumab
treatment (240 mg every 2 weeks) upon obtaining informed consent
from the patient. After one cycle of chemotherapy, the total
bilirubin level decreased from 13.9 to 3.6 mg/dl, and LDH decreased
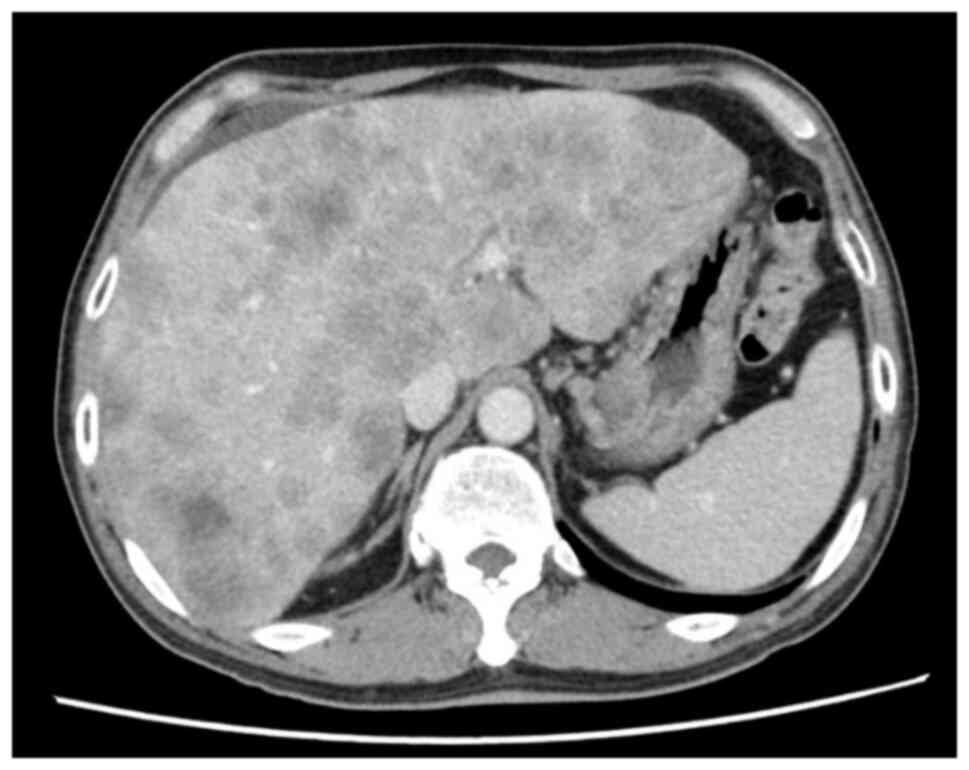
from 2,182 to 268 U/l. Furthermore, hepatomegaly and performance
status improved, resulting in a partial response as observed in CT
images (Fig. 4). The side effects
were mild, characterized by a grade 1 immune-related skin rash
(CTCAE, ver. 5.0). The typical side effects associated with
conventional chemotherapy, such as nausea, vomiting, and bone
marrow suppression, were completely absent. Instead, the patient's
quality of life improved noticeably, marked by increased food
intake and improved activity levels. After four treatment cycles,
he presented with progressive disease; however, his performance
status and liver function had improved notably. We switched to a
chemotherapy regimen involving trastuzumab, oxaliplatin, and
capecitabine [referred to as Cape-OX-T treatment; trastuzumab 6
mg/kg (initially 8 mg/kg) on day 1 every 3 weeks, oxaliplatin 130
mg/m2 on day 1 every 3 weeks, capecitabine 2,000
mg/m2 on days 1–14 every 3 weeks]. Oxaliplatin was
omitted for the initial six cycles owing to renal and liver
impairment. This combination therapy achieved normal levels of
total bilirubin, LDH, and tumor markers, leading to size reduction
of the metastatic liver tumor (Figs.
3 and 5). The patient died 2
years and 6 months after the treatment initiation, representing a
notable long-term survival.
Discussion
The introduction of ICIs has unequivocally reshaped
the therapeutic landscape of solid tumors. Notably, in the
ATTRACTION-2 trial, aimed at investigating the efficacy of
nivolumab in patients with recurrent and refractory GC, ICIs
demonstrated a markedly prolonged overall survival compared to
placebo (9). Nevertheless, prior
crucial clinical trials have consistently excluded patients with
severe organ dysfunction, particularly advanced liver failure.
Regarding the application of chemotherapy in clinical settings,
such as in patients with cancer and severe liver failure, a lack of
substantial evidence has forced clinicians to rely mainly on
anecdotal case reports. Furthermore, these case reports are
scarce.
Quidde et al reported a literature review of
26 cases of secondary hyperbilirubinemia due to liver metastasis in
patients with gastrointestinal cancer in 2016 (10). Among the 26 cases included, only one
case of GC was documented, and the remaining 25 cases were of
colorectal cancer. Of patients other than those with GC, 18
received chemotherapy, including oxaliplatin, and seven were
treated with cetuximab monotherapy. In the report by Shitara et
al, seven patients treated with cetuximab monotherapy exhibited
good tolerance to the treatment, but the median overall survival
was unsatisfactory at 2.8 months (11). Hwang et al reported a single
case of GC treated with CapeOX, which exhibited hyperbilirubinemia
due to multiple liver metastases. The total bilirubin decreased
from 10.9 to 2.1 mg/dl after two cycles of CapeOX therapy, with no
grade 3 or higher adverse effects during the first four cycles
(12). For most of the other
reported colorectal cancer cases, FOLFOX therapy was administered,
with 13 out of 17 (one case with missing data) exhibiting a
decrease in total bilirubin levels by over 50% compared with
pre-treatment levels. No Grade 3 or higher adverse effects were
reported. Based on these findings, Quidde et al concluded
that regimens including oxaliplatin and 5-FU might be promising for
patients with gastrointestinal cancer and liver failure.
Reports on the use of ICIs in patients with cancer
and severe liver failure are scarce. Only two published cases
exist: One involving the treatment of Hodgkin lymphoma with severe
liver failure and encephalopathy using nivolumab monotherapy and
another using pembrolizumab, cisplatin, and pemetrexed combination
therapy to treat non-small cell lung cancer with multiple bile duct
metastases, where bilirubin levels increased to 383 µmol/l
(<20.5). In both cases, the ICIs were safely administered.
Kanz et al reported a multicenter
retrospective analysis of treatment with nivolumab or pembrolizumab
in patients with advanced cancer with impaired cardiac, renal, or
hepatic function. Hepatic impairment in that study was defined as
aspartate aminotransferase, alanine transaminase, and/or total
bilirubin ≥3 times the upper limit of normal. Among the 27 analyzed
patients, seven had hepatic function impairment, and four had liver
cirrhosis. Notably, none of the patients had life-threatening
encephalopathy or acute liver failure (6).
Zhao et al summarized the pharmacokinetics of
ICIs administered to patients with liver and kidney impairment.
They suggested that mild-to-moderate liver impairment requires no
ICI dosage adjustments. However, in cases of severe liver
impairment, ICIs have been administered only in two reported cases
(13).
Herein, we present a case in which ICIs were safely
administered to a patient classified as Child-Pugh class C with
severe liver failure, resulting in prolonged survival. The
prognosis for patients with gastrointestinal cancer and liver
failure is typically speculated to be less than 3 months, as
reported by Shitara et al (11). However, in this instance, the
patient achieved a survival of 2 years and 6 months, significantly
surpassing the 17.1-month median overall survival reported in the
ToGA trial for the standard treatment of HER2-positive advanced GC,
which includes trastuzumab, cisplatin, and capecitabine or
fluorouracil therapy. Furthermore, the ToGA trial targeted patients
with normal liver function and preserved organ capacity (14). To the best of our knowledge, this is
the first study reporting a survival of 2 years and 6 months
following targeted treatment in a patient with gastric cancer and
severe liver failure. This observation suggests the possibility of
therapeutic options for cases previously considered untreatable
because of severe liver failure. We are aware of only one study
(12) on the successful treatment
of gastric cancer in patients with severe liver failure. However,
this report only provides information up to the fifth session of
chemotherapy (spanning a period of 2 and a half months). Most other
studies have primarily focused on reports of liver metastases from
colon cancer, with the most extensive case study being the report
by Shitara et al (11). We
believe this report is the most representative in this context.
Additionally, to illustrate the general prognosis of inoperable
HER2-positive gastric cancer patients without liver failure, we
referenced literature (14). Our
report shows an overall survival exceeding that of inoperable
gastric cancer patients without liver failure documented in this
literature.
In conclusion, documented cases involving ICI
administration in patients with cancer and severe liver failure
remain scarce. By accumulating additional insights, including the
findings presented in this case, ICIs may solidify their position
as a secure and efficacious therapeutic option even for instances
of liver failure that complicate cancers previously considered
incompatible for treatment with conventional chemotherapy.
Acknowledgements
The authors would like to thank Dr Hideyuki Ohnuma
(Department of Pathology, Shimane Prefectural Central Hospital) for
providing the pathological images used in this study.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
FI, KY and KT participated in the conception, design
and data acquisition of the study. FI drafted the manuscript. KY
and KT revised the manuscript. FI and KT confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient's wife, as the patient had passed away, for the case
information and images to be published in this case report.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript, and
subsequently, the authors revised and edited the content produced
by the AI tools as necessary, taking full responsibility for the
ultimate content of the present manuscript.
Glossary
Abbreviations
Abbreviations:
|
GC
|
gastric cancer
|
|
CT
|
computed tomography
|
|
ICIs
|
immune checkpoint inhibitors
|
|
LDH
|
lactate dehydrogenase
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wagner AD, Grothe W, Haerting J, Kleber G,
Grothey A and Fleig WE: Chemotherapy in advanced gastric cancer: A
systematic review and meta-analysis based on aggregate data. J Clin
Oncol. 24:2903–2909. 2006. View Article : Google Scholar
|
|
3
|
Wang L, Liang B, Jiang Y, Huang G, Tang A,
Liu Z, Wang Y, Zhou R, Yang N, Wu J, et al: Subsite-specific
metastatic organotropism and risk in gastric cancer: A
population-based cohort study of the US SEER database and a Chinese
single-institutional registry. Cancer Med. 12:19595–19606. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar
|
|
5
|
Cunningham D, Starling N, Rao S, Iveson T,
Nicolson M, Coxon F, Middleton G, Daniel F, Oates J and Norman AR;
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom, : Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kanz BA, Pollack MH, Johnpulle R, Puzanov
I, Horn L, Morgans A, Sosman JA, Rapisuwon S, Conry RM, Eroglu Z
and Johnson DB: Safety and efficacy of anti-PD-1 in patients with
baseline cardiac, renal, or hepatic dysfunction. J Immunother
Cancer. 4:602016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sandoval-Sus JD, Mogollon-Duffo F, Patel
A, Visweshwar N, Laber DA, Kim R and Jagal MV: Nivolumab as salvage
treatment in a patient with HIV-related relapsed/refractory Hodgkin
lymphoma and liver failure with encephalopathy. J Immunother
Cancer. 5:492017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shaforostova I, Huss S, Gerwing M, Evers G
and Bleckmann A: To treat or not to treat: A rare case of response
to pembrolizumab-based immunotherapy-chemotherapy in non-small cell
lung cancer with acute liver failure due to multiple bile duct
metastases. Thorac Cancer. 12:553–556. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang Y-K, Boku N, Satoh T, Ryu MH, Chao Y,
Kato K, Chung HC, Chen JS, Muro K, Kang WK, et al: Nivolumab in
patients with advanced gastric or gastro-oesophageal junction
cancer refractory to, or intolerant of, at least two previous
chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet.
390:2461–2471. 2017. View Article : Google Scholar
|
|
10
|
Quidde J, Azémar M, Bokemeyer C, Arnold D
and Stein A: Treatment approach in patients with hyperbilirubinemia
secondary to liver metastases in gastrointestinal malignancies: a
case series and review of literature. Ther Adv Med Oncol.
8:144–152. 2016. View Article : Google Scholar
|
|
11
|
Shitara K, Takahari D, Yokota T, Shibata
T, Ura T, Muro K, Inaba Y, Yamaura H, Sato Y, Najima M and
Utsunomiya S: Case series of cetuximab monotherapy for patients
with pre-treated colorectal cancer complicated with
hyperbilirubinemia due to severe liver metastasis. Jpn J Clin
Oncol. 40:275–277. 2010. View Article : Google Scholar
|
|
12
|
Hwang SJ, Park JW, Lee SD, Kim GJ, Sin CH,
Nam S-H and Kim B-S: Capecitabine and oxaliplatin (XELOX) for the
treatment of patients with metastatic gastric cancer and severe
liver dysfunction. Korean J Intern Med. 21:252–255. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao D, Long X, Fan H and Wang J: Effects
of hepatic or renal impairment on the pharmacokinetics of immune
checkpoint inhibitors. Am J Cancer Res. 12:4892–4903.
2022.PubMed/NCBI
|
|
14
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: Trastuzumab in combination with chemotherapy versus
chemotherapy alone for treatment of HER2-positive advanced gastric
or gastro-oesophageal junction cancer (ToGA): a phase 3,
open-label, randomised controlled trial. Lancet. 376:687–697. 2010.
View Article : Google Scholar
|