Introduction
According to American Cancer Society estimates of
cancer data, primary lung cancer ranked second in incidence and
first in mortality in 2023, with distant metastasis being a major
contributor to the high fatality rate (1). Lung cancer commonly metastasizes to
the brain, bones, adrenal glands and liver; however, metastasis in
the breast is relatively uncommon. According to clinical and
autopsy findings (2), only 0.5–1.2
and 1.7–6.6% of malignant tumors metastasize to the breast,
respectively. Given that breast cancer is the most common cancer
type in women and considering the lack of specific features in
imaging examinations that can be used to distinguish primary from
metastatic tumors (3), patients
with lung cancer breast metastasis are often misdiagnosed as having
primary breast cancer, leading to erroneous treatment strategies.
Immunohistochemistry (IHC) combined with next-generation sequencing
(NGS) can increase diagnostic accuracy and provide crucial insight
into disease treatment and etiology. Furthermore, NGS has become a
powerful tool for diagnosis in challenging cases (4).
The rate of anaplastic lymphoma kinase (ALK)
gene mutation in primary lung cancer is ~3–5%, with the EMAP like 4
(EML4)-ALK fusion being the most common anomaly.
ALK is an important target for targeted lung cancer therapy.
With ongoing research into the molecular mechanisms of tumors and
advancements in clinical trials of targeted drugs, various
ALK tyrosine kinase inhibitors (TKIs) have proven to be
effective treatments for patients with ALK fusion-positive
lung cancer; these treatments have provided significant clinical
benefits (5–7).
The present study reported on a rare case in which a
patient was diagnosed with primary lung adenocarcinoma with breast
metastasis through IHC and genomic analysis. The EML4-ALK
fusion was confirmed, and ALK TKI treatment prolonged
patient survival. With this case, challenges in accurate diagnosis
and treatment were encountered and showcased. Due to the low
frequency of breast metastasis in patients with lung cancer and the
potential for misdiagnosis, imaging and IHC have certain
limitations. Comprehensive genomic testing holds significant
importance for identifying effective therapeutic strategies for
patients, which can extend progression-free survival, alleviate
symptoms and improve the overall quality of life. Furthermore, the
present findings provide evidence that shows the effectiveness of
ALK TKIs in the treatment of patients with EML4-ALK
fusion-positive lung cancer. The outcome for this patient suggests
the need for more targeted and personalized therapeutic approaches
in similar cases.
Case report
A 42-year-old non-smoking female sought medical
attention at the Affiliated Hospital of Zunyi Medical University
(Zunyi, China) in March 2020 due to a palpable and painful lump in
the left breast. Breast MRI revealed multiple nodules in the left
breast and axilla. Pathologists described the microscopic
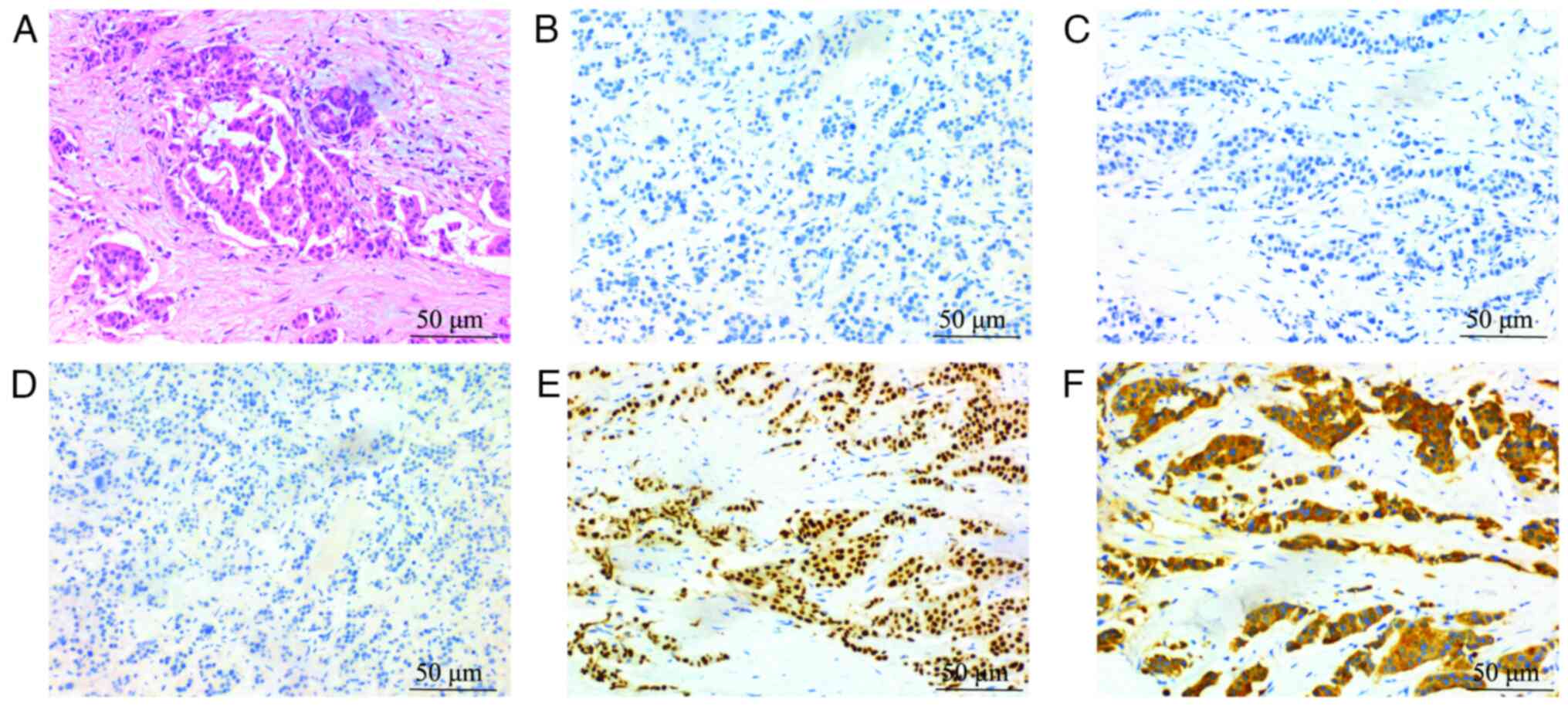
characteristics of the H&E-stained sections as follows: Tubular
structures were observed; the nuclei of the cancer cells were
generally enlarged, with variability in size and shape, including
the formation of small nucleoli; and mitotic figures were present.
According to the Nottingham grading system (8), the evaluation indicated 3 points for
tubular formation, 2 points for nuclear grade and 1 point for
mitotic count, resulting in a total of 6 points, indicating grade
II (moderately differentiated). IHC (9) was performed on a BOND-MAX Fully
Automated IHC and ISH Staining System (Leica Microsystems, GmbH).
The ready-to-use primary antibodies, including CK5/6 (cat. no.
GT243802), P120 (cat. no. GT209902), CK7 (cat. no. GT244602),
E-cadherin (cat. no. GT210702), TTF-1 (cat. no. GT218002), Ki-67
(cat. no. GM724002) and p63 (cat. no. GT253202), were all purchased
from Gene Tech Co., Ltd. The secondary antibody and the chromogenic
system were included as part of the instrument's kit. IHC revealed
negative expression of estrogen receptor (ER), progesterone
receptor (PR), human epidermal growth factor receptor (HER)2 and
cytokeratin (CK)5/6, but positive expression of P120, CK7,
E-cadherin, thyroid transcription factor (TTF)-1 and Ki-67; there
was also no p63 expression in tumor-associated myoepithelial cells
(Fig. 1A-F). No evidence of
metastasis was found in the lymph nodes. The pathologist was
initially unaware of the lung nodules, the histological features of
the breast specimens were consistent with those of primary breast
cancer and TTF-1 positivity was not a concern among the
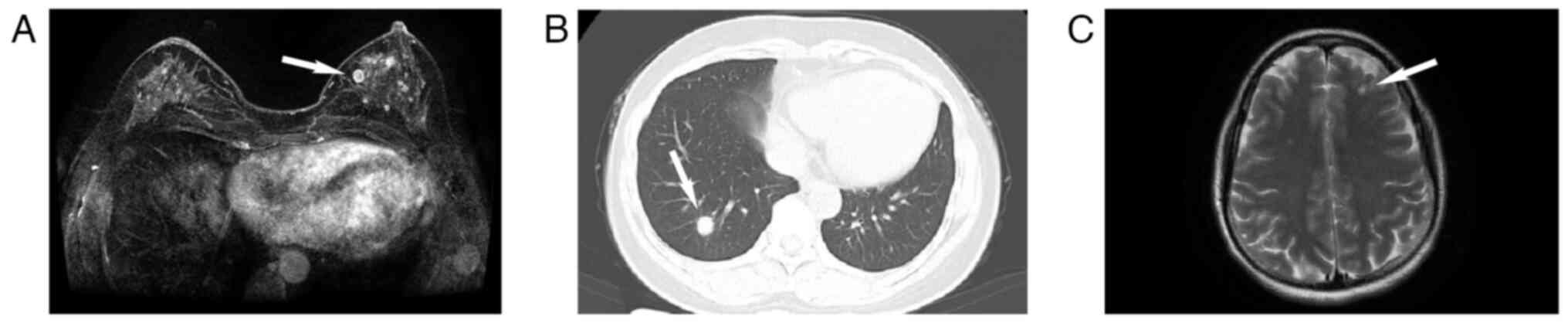
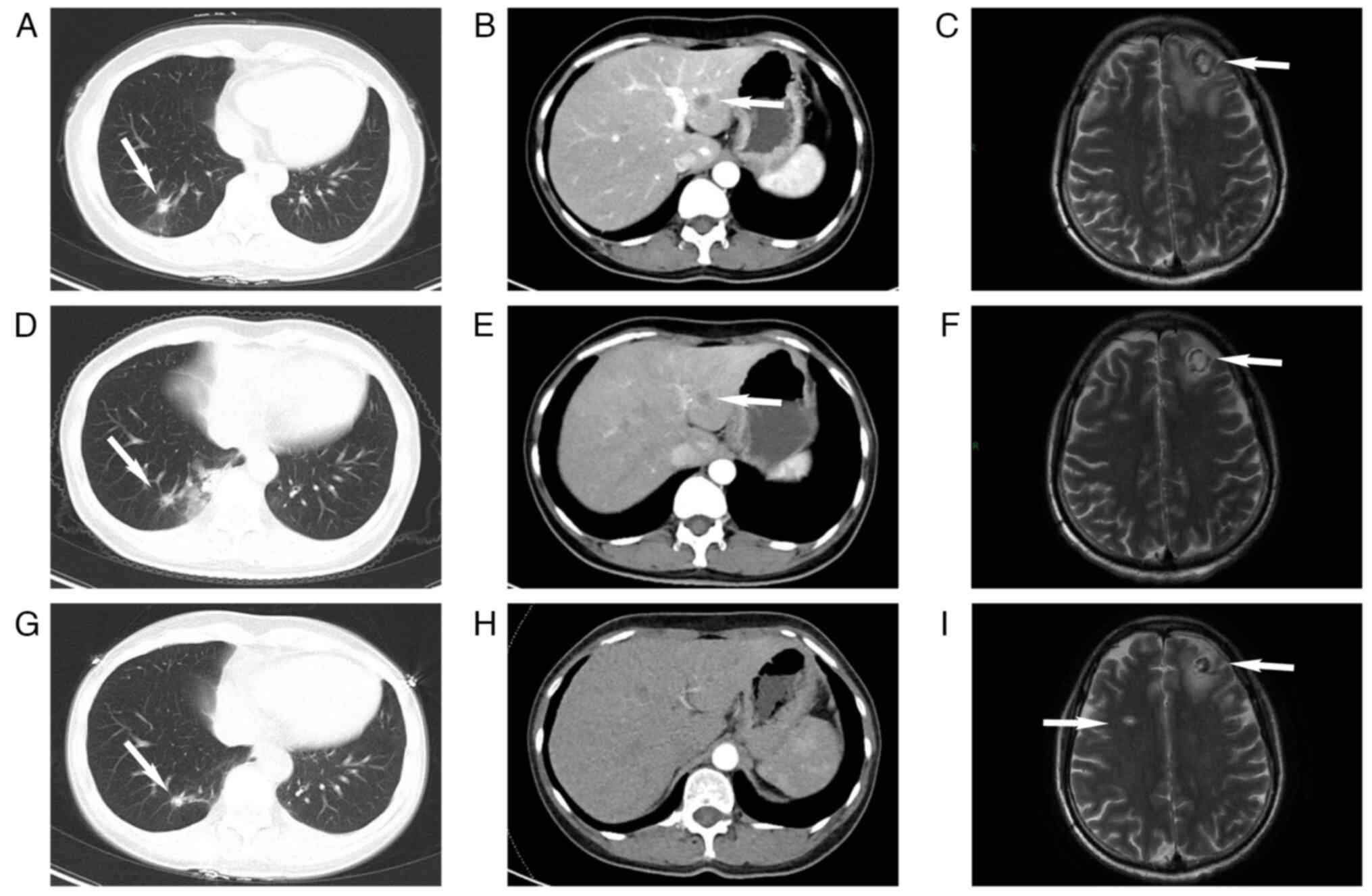
pathologists. Subsequent imaging studies, including chest CT and
brain MRI, revealed nodules in the lungs and intracranial regions
(Fig. 2A-C). However, the
pathologists were not able to distinguish between metastatic breast
carcinoma and primary breast carcinoma morphologically. Then,
considering the absence of respiratory symptoms in the patient, the
greater number and size of breast nodules compared to lung nodules
and the 40% occurrence rate of lung metastases in triple-negative
breast cancer patients (10),
clinicians ultimately diagnosed the patient with triple-negative
infiltrating breast cancer (T1N0M1, stage IV) with pulmonary and
intracranial metastases following discussions among pathologists
and clinicians. Systemic chemotherapy was recommended, but the
patient declined and opted for an alternative Traditional Chinese
Medicine-based antitumor treatment, which the patient
self-administered (details not disclosed).
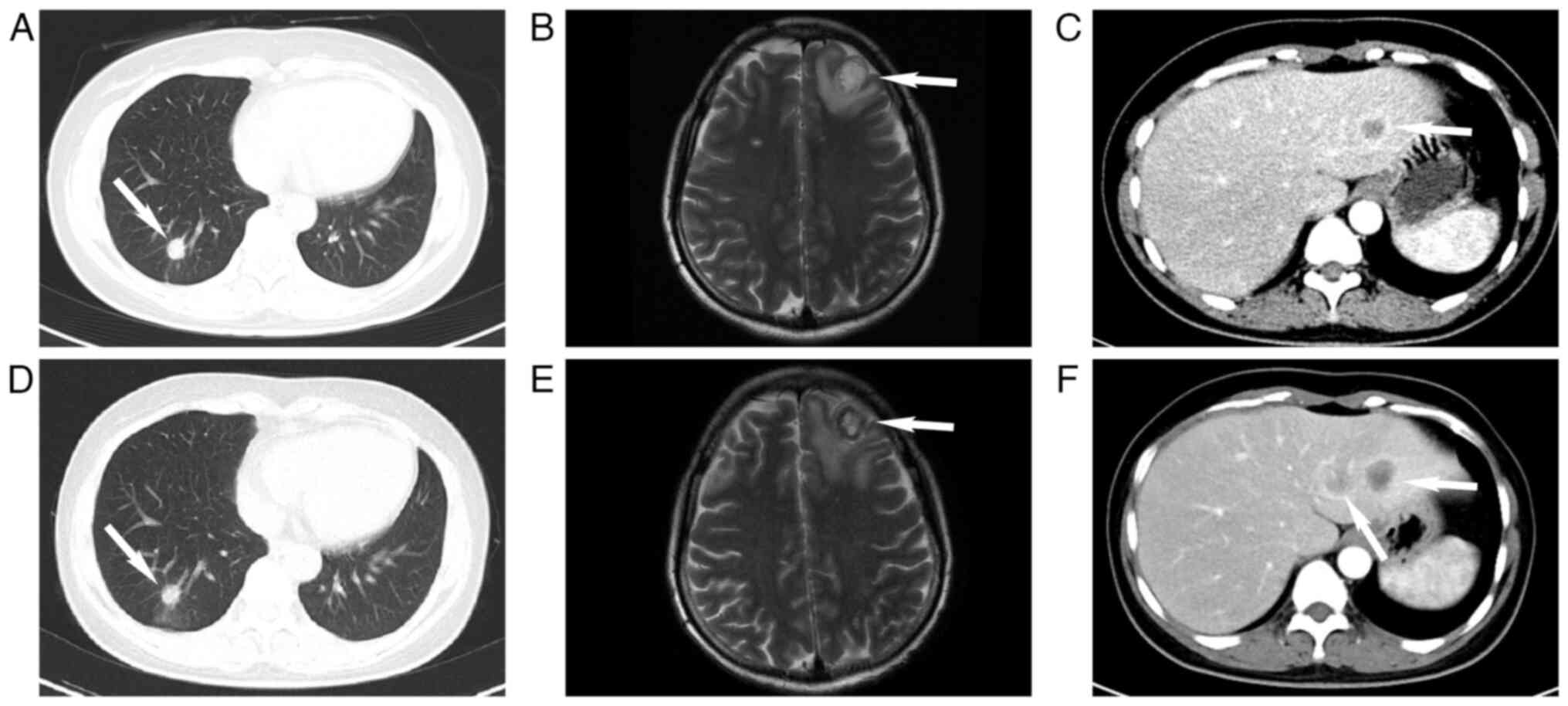
In August 2020, the patient experienced aggravated
symptoms, including lower back pain, without cough. Chest and
abdominal CT scans, along with cranial MRI, indicated enlargement
of the lesions when compared to previous assessments (Fig. 3A and B). The newly identified
metastatic sites included the 8th thoracic vertebra and the 4th
lumbar vertebra (Fig. S1A and B),
as well as liver metastases (Fig.
3C). Breast ultrasound revealed enlargement of the left breast
nodule and emergence of a new nodule in the right breast.
Considering the progression of the disease, the patient was
referred to the Second Affiliated Hospital of Zunyi Medical
University (Zunyi, China). Based on the breast pathology results,
following the guidelines for stage IV breast cancer, a salvage
chemotherapy regimen comprising taxanes and anthracyclines was
initiated [intravenous paclitaxel (albumin-bound) 400 mg +
epirubicin 120 mg]. Palliative radiotherapy was administered to
alleviate symptoms at the metastatic lesion in the 4th lumbar
vertebra.
After the second round of chemotherapy in October
2020, the patient was reevaluated using imaging. The breast and
pulmonary lesions showed no significant changes, but increases in
the size and number of metastatic tumors were observed in the
intracranial and hepatic regions (Fig.
3D-F). A new metastatic lesion in the left scapula was also
identified (Fig. S2). Treatment
response was assessed and determined as progressive disease
according to the Response Evaluation Criteria in Solid Tumors
criteria 1.1 (11). Concurrently,
the patient experienced severe headaches, prompting palliative
whole-brain radiotherapy. Due to the ineffectiveness of breast
cancer treatment regimens, the possibility of primary lung cancer
with breast metastasis was suspected, and local anesthesia-assisted
biopsy of the right lower lung nodule was performed. After
examination of the histological features of the specimen, the
pathologists were inclined to diagnose the patient with primary
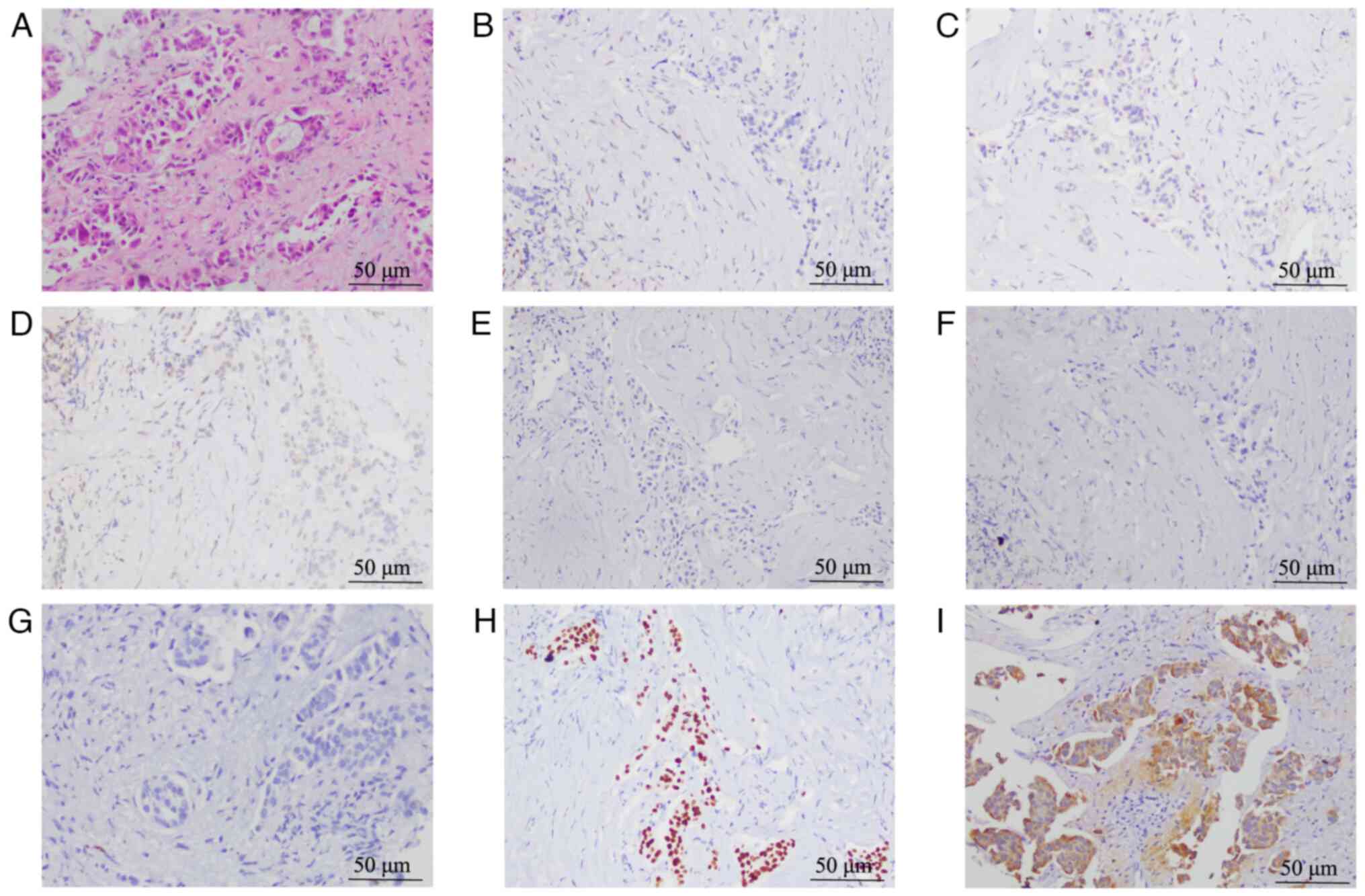
lung adenocarcinoma. The ready-to-use antibody reagents for
GCDFP-15 (cat. no. GT204902), GATA3 (cat. no. GT218702) and
Napsin-A (cat. no. GT218502) were also purchased from Gene Tech
Co., Ltd. IHC results indicated positivity for CK7, TTF-1 and Ki-67
but negativity for Napsin-A, ER, PR, HER2, gross cystic disease
fluid protein 15 (GCDFP-15) and GATA binding protein 3 (GATA3)
(Fig. 4A-G). Further lung tissue
detection assays were performed using the Multi-Mutation Gene
Diagnostic Kit (Amoy Diagnostics, Co., Ltd.). Using
ADx-ARMS® technology, lung tissue DNA was analyzed for
EGFR mutations, and ALK and ROS1 gene fusions
were evaluated in RNA samples via reverse transcription PCR
(12). The results revealed an
EML4-ALK mutation, but the specific fusion type could not be
determined. This evidence suggested that the lung was the primary
site, and clarification was needed to determine whether the breast
lesion was primary or metastatic. Previous breast tissue samples
were sent to the College of American Pathologists and Clinical
Laboratory Improvements Amendments accredited central laboratory at
Nanjing Geneseeq Technology, Inc. for analysis. A customized xGen
lockdown probe panel (Integrated DNA Technologies) was used for
targeted enrichment of 425 predefined genes. The enriched libraries
were sequenced on HiSeq 4000 NGS platforms (Illumina, Inc.)
(13) to coverage depths of at
least ×100 and ×300 after removing PCR duplicates for tumor and
normal tissue, respectively. The results confirmed EML4-ALK
fusion (E18:A20). In addition, mutations in breast cancer
susceptibility genes, including GATA3, BRCA1, BRCA2, tumor
protein 53 and partner and localizer of BRCA2 (PALB2), were
negative. Furthermore, the pathologist observed no microacinar
structures or signet ring tumor cells in the histology images of
the breast and lung tissue specimens (Figs. 1A and 4A). The combined IHC and genetic test
results for both the lung and breast tissue samples were
comprehensively assessed and it was determined that the patient had
right lower lung adenocarcinoma T1bN3M1c-stage IVB (according to
the 8th edition of the American Joint Committee on Cancer staging
manual) (14) with the
EML4-ALK fusion. The subsequent treatment plan involved
ALK-TKI targeted therapy.
Oral treatment with crizotinib capsules [250 mg per
os (PO) twice daily] was initiated in November 2020, and
remarkably, this targeted therapy demonstrated significant
efficacy. The primary lung lesion and other metastatic lesions
consistently decreased in size at 6 months post-treatment
initiation (Fig. 5A-F). However,
after 9 months of targeted therapy, there was an indication of
increased lesions in the brain, although no clinical symptoms were
reported. The right lower lobe nodule continued to shrink and the
other lesions remained stable in size (Fig. 5G-I). During treatment, the patient's
quality of life was assessed using a scale developed by the
European Organisation for Research and Treatment of Cancer (EORTC),
the EORTC QLO-C30 (V3.0) (15).
This tool, known for its reliability and validity, covers the
essential aspects of health-related quality of life and is suitable
for all cancer patients. The results indicated that during the 9
months of crizotinib treatment, the patients' quality of life
improved compared to that during the radiotherapy and chemotherapy
periods. Specifically, improvements were observed in physical
functioning (daily activities, walking, etc.), role functioning,
emotional functioning (anxiety, worry, irritability, depression),
social functioning and overall health status. However, no
significant differences were observed in terms of fatigue, pain or
nausea/vomiting. After approximately one year of treatment, the
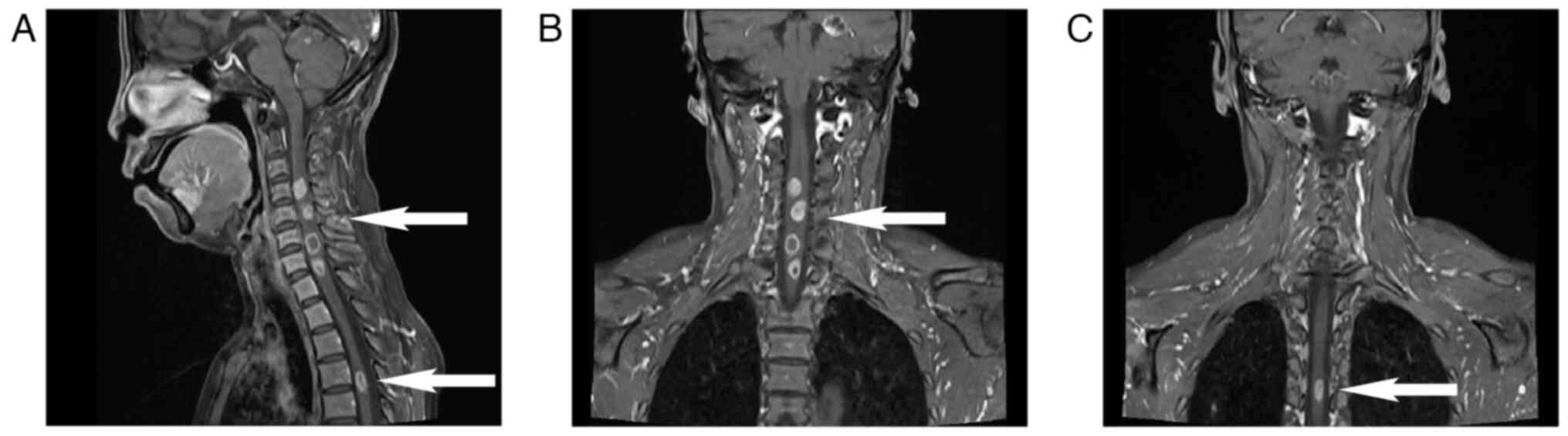
patient's condition deteriorated and she presented with bilateral
lower limb numbness and motor abnormalities. MRI revealed multiple
new metastases in the cervical and thoracic spinal cord (Fig. 6A-C). Despite no significant changes
in the primary lung lesion or other metastatic lesions, disease
progression was considered. In November 2021, the patient commenced
oral treatment with second-generation ALK-TKI ceritinib
capsules (450 mg PO once a day) for targeted therapy. Palliative
radiotherapy was administered to the cervical spine and lumbar
vertebrae. During radiotherapy, the patient's quality of life
gradually decreased, mainly manifested as decreases in physical
functioning (sensory disturbances in both lower limbs, urinary and
fecal incontinence) and overall health status (Grade III bone
marrow suppression, fatigue and severe pain). Consequently,
radiotherapy was temporarily stopped and the patient was discharged
to continue oral ceritinib treatment. Subsequent telephone
follow-ups were performed twice, at 1 and 2 months after discharge,
with a total survival period of 22 months and 26 days (the starting
point was the date of the patient's first visit to the
hospital).
Discussion
Primary lung adenocarcinoma is a common malignancy
with a high mortality rate, and the occurrence of distant
metastasis is the most critical factor that impacts clinical
treatment and prognosis. Breast metastasis is uncommon in lung
adenocarcinoma, and the incidence of metastasis in the breast from
non-mammary sources is estimated to not exceed 2% (16). Accurately distinguishing between
primary breast cancer and metastatic breast cancer poses a
significant challenge.
A clear distinction between primary and secondary
tumors is crucial for clinical treatment decision-making and
prognostic assessment. Differential diagnosis is challenging to
achieve through imaging methods alone, but IHC provides valuable
insight. In previous case reports (17–19),
IHC was commonly used to determine the tumor origin, which is
consistent with the initial diagnostic approach for the patient of
the present study. TTF-1 and Napsin-A are robust biological markers
for lung adenocarcinoma; they are expressed in ~80% of cases but
are rarely expressed in other cancers. The combined use of TTF-1
and Napsin-A promotes the accurate identification of metastatic
adenocarcinoma originating from the lungs. HER2, ER, PR, GATA3 and
GCDFP-15 are key and characteristic IHC markers for breast cancer.
ER and PR are expressed in ~80 and 60% of primary breast cancers,
respectively (20). GATA3 and
GCDFP-15 are recently confirmed markers with high sensitivity in
primary breast cancer and are expressed in 67–95 and 60% of cases,
respectively (21). These markers
are rarely expressed in lung adenocarcinoma and can, to a certain
extent, assist in making a diagnosis and differential
diagnosis.
However, IHC results lack 100% sensitivity and
specificity, and although rare, TTF-1 positivity can occur in
patients with primary breast cancer (22). Of note, there are certain
differences between the present case and previously reported cases.
First, Wang et al (23)
integrated data of 7 patients with primary lung cancer breast
metastasis and proposed that the combination of TTF-1 and Napsin-A
can provide the greatest benefit in clinical practice, suggesting
that metastatic breast nodules typically present unilaterally
without pain. Ji et al (24)
reported two cases of lung adenocarcinoma breast metastasis in
which the diagnosis primarily relied on medical history and IHC
results, and both patients had a history of lung cancer. In the
case of the current study, the patient presented with painful
breast nodules as the initial symptom, with breast nodules larger
than the pulmonary lesion and no history of lung cancer, thus
leading to clinicians' confusion. In addition, Ali et al
(25) analyzed 12 cases of
non-small cell lung cancer (NSCLC) breast metastasis, 5 of which
were initially misinterpreted by pathologists as primary breast
cancer (PBC). Distinguishing poorly differentiated lung
adenocarcinoma from triple-negative PBC is morphologically
challenging, as indicated by their study. Initially, the breast
specimen in the present case suggested moderately differentiated
adenocarcinoma, and upon learning about the patient's pulmonary
nodules, pathologists should have been alerted, prompting further
testing for GCDFP-15 or GATA3, as these markers may indeed be
expressed in PBC. Furthermore, relying solely on TTF-1 results
lacks a certain degree of reliability. In the present case, due to
the negative expression of Naspin-A in the lung specimen and the
positive expression of TTF-1, in combination with the fact that
triple-negative breast cancer is more prone to metastasis compared
to other types of breast cancer, it may be reasoned that relying
solely on TTF-1 positivity is insufficient to support the diagnosis
of primary lung cancer metastasizing to the breast. Initially,
chemotherapy with paclitaxel was employed, which is also a
frontline chemotherapy drug for lung adenocarcinoma. However, after
2 cycles of treatment, the pulmonary nodules did not shrink, adding
to the clinical confusion in terms of diagnosis and treatment.
Genetic testing can assist in addressing this
dilemma; it may be used to understand whether a patient carries
susceptibility gene mutations for cancer to help confirm diagnostic
suspicions and to determine whether the tumor may be sensitive to
certain drugs, thus further guiding precise treatment and extending
patient survival. As demonstrated in the present case, conducting
additional genetic testing on both breast and lung tissue samples
did not only provide useful information regarding the ALK
mutation, allowing the patient to benefit from targeted therapy,
but also demonstrated that the patient tested negative for
susceptibility gene mutations associated with breast cancer. This
method further strengthens our ability to accurately diagnose
patients and guide anticancer treatment. Of note, as NGS is rarely
used for primary breast cancer and previous reports seldom utilize
NGS to aid in diagnosis, the breast biopsy specimen was not
immediately sent for genetic testing at initial diagnosis. However,
in similar cases in the future, when the patient is unwilling to
clarify the nature of lung lesions, sending breast specimens for
NGS may provide more treatment options for patients, leading to
prolonged survival. In the future, if a patient presents with both
lung and breast lesions, particularly if the breast tumor is
triple-negative, regardless of the size of the lesion or the
presence of respiratory symptoms, further clarifying the nature of
the lung lesion is advisable. If the patient is unwilling to
clarify the nature of the lung lesions, breast specimens should be
sent for NGS. This approach may offer patients more treatment
options and reduce the risk of misdiagnosis.
In the present study, the ARMS-PCR method was
utilized to analyze lung specimens and the results revealed the
EML4-ALK fusion. Unfortunately, due to the use of PCR for
testing lung tissue specimens, it was not possible to determine the
type of EML4-ALK variant. With respect to the breast biopsy
specimens, 425-panel NGS was performed and the results indicated
that the EML4-ALK fusion gene was present (E18:A20). The
EML4-ALK fusion gene has been conclusively identified as a
characteristic gene in NSCLC. In a study by Fukuyoshi et al
(26), 90 patients with breast
cancer were assessed, although the EML4-ALK fusion was not
detected. Another study (27)
reported that in comprehensive genomic analyses, ALK
fusions/rearrangements were identified in ~0.5–0.8% of cancers
(28,29). Specifically, among patients with
NSCLC, the prevalence of ALK fusions/rearrangements exceeds
3%, while the frequency of ALK fusions/rearrangements in
non-NSCLC tumors is ~0.2%. This finding suggests that ALK
fusions/rearrangements are rare in breast cancer. The positive
ALK result in the present case strongly supported the
hypothesis that the primary tumor was NSCLC. Furthermore, genetic
analysis of the breast specimen revealed negativity for mutations
in breast cancer susceptibility genes such as BRCA1, BRCA2,
TP53 and PALB2, thus providing further evidence that the
breast lesion was a metastatic deposit from the primary lung
adenocarcinoma rather than a dual-origin tumor (30–32).
In terms of treatment, the ALK fusion protein
serves as a crucial target for molecular targeted therapy in lung
cancer, with the EML4-ALK fusion being the predominant
fusion type, constituting 90–95% of all ALK fusions. There
are eight variants of the EML4-ALK fusion protein that are
classified into ‘long’ and ‘short’ types, with protein stability
being the primary biological distinction; these variants ultimately
lead to varying responses to ALK TKIs (33,34).
Clinical trial results suggest that ALK TKIs are most
effective against tumors harboring the V2 variant fusion, while
tumors harboring the V3 variant fusion exhibit a shorter duration
of response to ALK TKIs. The E18:A20 variant identified in
the patient described herein was categorized as the V5 subtype,
representing 1.56% of all variants (35,36).
Currently, there is no established evidence regarding the potential
benefits of ALK-TKI treatment for tumors with the V5 variant
fusion. In the present case, the patient was treated with the
first-generation ALK-TKI crizotinib and exhibited one-year
progression-free survival. This outcome may provide insight into
clinical treatment and targeted drug selection for this specific
type of mutation.
In conclusion, breast metastatic carcinoma of
non-mammary origin is rare and is prone to misdiagnosis and
oversight in clinical settings. Vigilance should be maintained for
such patients, particularly when encountering situations similar to
those experienced by the patient described herein. The patient in
this case sought medical attention with a chief complaint of a
breast lump, posing a diagnostic challenge because there were no
apparent respiratory symptoms. Both the breast and lung lesions
exhibited multiple nodules of comparable size, complicating the
precision of the diagnosis. In the initial assessment, emphasis
should be placed on distinguishing between the primary lesion and
metastatic lesions. In addition, heightened attention should be
directed toward the application of genetic testing technologies,
which promote accurate diagnoses, personalized precision medicine
in clinical practice and ultimately prolong patient survival.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the Joint Zunyi City Science and
Technology Plan Project [grant no. Zunyi City Kehe HZ Zi (2020) No.
80].
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The present manuscript
contains original data generated using NGS, which may be found in
the Sequence Read Archive (SRA) under accession no. SRR28475470 or
at the following URL: https://www.ncbi.nlm.nih.gov/sra/SRR28475470.
Authors' contributions
SX and YB were primarily responsible for the study
and contributed to its conception and design. WZ and YZ obtained
and analyzed the patient information and contributed to manuscript
drafting and critical revision of the intellectual content. LZ
performed the analysis and interpretation of the CT and MRI data.
NT performed the histological examination of the tumor. WZ, YZ, LZ,
NT, YB and SX confirm the authenticity of all the raw data. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
This study was conducted at the Second Affiliated
Hospital of Zunyi Medical University and was approved by the
Institutional Ethics Review Board (Zunyi, China; approval no.
KYLL-2023-032). The patient voluntarily agreed to participate in
the study and provided written informed consent, which included the
publication of this case report.
Patient consent for publication
Written informed consent for publication of the
article, including clinical data and images, was obtained from the
patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei LY, Kong M, Zhang Z and Zhang XC:
Breast metastasis of gastric signet-ring cell carcinoma. J Zhejiang
Univ Sci B. 18:1026–1030. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Choudhery S, Xiao L and Zingula S: Review
of nonmammary metastases to the breast: Imaging and clinical
presentation. Curr Probl Diagn Radiol. 50:495–498. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu C, Ding L, Sun B and Wu S: Bilateral
breast adenocarcinomas with EML4-ALK fusion in a patient with
multiple metastases successfully treated with crizotinib: Is lung
the primary site? Onco Targets Ther. 9:3589–3593. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lei Y, Lei Y, Shi X and Wang J: EML4-ALK
fusion gene in non-small cell lung cancer. Oncol Lett. 24:2772022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Golding B, Luu A, Jones R and
Viloria-Petit AM: The function and therapeutic targeting of
anaplastic lymphoma kinase (ALK) in non-small cell lung cancer
(NSCLC). Mol Cancer. 17:522018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hirsch FR, Suda K, Wiens J and Bunn PA Jr:
New and emerging targeted treatments in advanced non-small-cell
lung cancer. Lancet. 388:1012–1024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rakha EA, Alsaleem M, ElSharawy KA, Toss
MS, Raafat S, Mihai R, Minhas FA, Green AR, Rajpoot NM, Dalton LW
and Mongan NP: Visual histological assessment of morphological
features reflects the underlying molecular profile in invasive
breast cancer: A morphomolecular study. Histopathology. 77:631–645.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hussaini HM, Seo B and Rich AM:
Immunohistochemistry and Immunofluorescence. Methods Mol Biol.
2588:439–450. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schneider C, Fehr MK, Steiner RA, Hagen D,
Haller U and Fink D: Frequency and distribution pattern of distant
metastases in breast cancer patients at the time of primary
presentation. Arch Gynecol Obstet. 269:9–12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bachman J: Reverse-transcription PCR
(RT-PCR). Methods Enzymol. 530:67–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang Z, Wang W, Hu Q, Zhou P, Zhang Y,
Tang Y, Wu Q, Fu Y, Li X, Shao Y and Jiang L: Pulmonary large cell
carcinoma with neuroendocrine morphology shows genetic similarity
to large cell neuroendocrine carcinoma. Diagn Pathol. 17:262022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sui X, Jiang W, Chen H, Yang F, Wang J and
Wang Q: Validation of the stage groupings in the eighth edition of
the TNM classification for lung cancer. J Thorac Oncol.
12:1679–1686. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cocks K, Wells JR, Johnson C, Schmidt H,
Koller M, Oerlemans S, Velikova G, Pinto M, Tomaszewski KA,
Aaronson NK, et al: Content validity of the EORTC quality of life
questionnaire QLQ-C30 for use in cancer. Eur J Cancer. 178:128–138.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee SK, Kim WW, Kim SH, Hur SM, Kim S,
Choi JH, Cho EY, Han SY, Hahn BK, Choe JH, et al: Characteristics
of metastasis in the breast from extramammary malignancies. J Surg
Oncol. 101:137–140. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Babu KS, Roberts F, Bryden F, McCafferty
A, Downer P, Hansell DT, Jones R and Milroy R: Metastases to breast
from primary lung cancer. J Thorac Oncol. 4:540–542. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao X, Chen P, Agyekum EA, Zhang Q, Qian
X, Wu T, Chambers KH and Yin L: Lung cancer with breast metastasis:
A case report and review of the literature. J Int Med Res.
51:30006052311882872023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li J, Liu YS, Liu DD and Li XL: Metastasis
to breast from primary lung cancer: A rare case report. Asian J
Surg. 45:2562–2563. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Z, Wei H, Li S, Wu P and Mao X: The
role of progesterone receptors in breast cancer. Drug Des Devel
Ther. 16:305–314. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ni YB, Tsang JYS, Shao MM, Chan SK, Cheung
SY, Tong J, To KF and Tse GM: GATA-3 is superior to GCDFP-15 and
mammaglobin to identify primary and metastatic breast cancer.
Breast Cancer Res Treat. 169:25–32. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Robens J, Goldstein L, Gown AM and Schnitt
SJ: Thyroid transcription factor-1 expression in breast carcinomas.
Am J Surg Pathol. 34:1881–1885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang B, Jiang Y, Li SY, Niu RL, Blasberg
JD, Kaifi JT, Liu G and Wang ZL: Breast metastases from primary
lung cancer: A retrospective case series on clinical,
ultrasonographic, and immunohistochemical features. Transl Lung
Cancer Res. 10:3226–3235. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ji FF, Gao P, Wang JG, Zhao J and Zhao P:
Contralateral breast metastasis from pulmonary adenocarcinoma: Two
cases report and literature review. J Thorac Dis. 4:384–389.
2012.PubMed/NCBI
|
|
25
|
Ali RH, Taraboanta C, Mohammad T, Hayes MM
and Ionescu DN: Metastatic non-small cell lung carcinoma a mimic of
primary breast carcinoma-case series and literature review.
Virchows Arch. 472:771–777. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fukuyoshi Y, Inoue H, Kita Y, Utsunomiya
T, Ishida T and Mori M: EML4-ALK fusion transcript is not found in
gastrointestinal and breast cancers. Br J Cancer. 98:1536–1539.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shreenivas A, Janku F, Gouda MA, Chen HZ,
George B, Kato S and Kurzrock R: ALK fusions in the pan-cancer
setting: Another tumor-agnostic target? NPJ Precis Oncol.
7:1012023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ross JS, Ali SM, Fasan O, Block J, Pal S,
Elvin JA, Schrock AB, Suh J, Nozad S, Kim S, et al: ALK fusions in
a wide variety of tumor types respond to anti-ALK targeted therapy.
Oncologist. 22:1444–1450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
AACR Project GENIE Consortium, . AACR
project GENIE: Powering precision medicine through an international
consortium. Cancer Discov. 7:818–831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Narod SA: Which genes for hereditary
breast cancer? N Engl J Med. 384:471–473. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hu C, Hart SN, Gnanaolivu R, Huang H, Lee
KY, Na J, Gao C, Lilyquist J, Yadav S, Boddicker NJ, et al: A
population-based study of genes previously implicated in breast
cancer. N Engl J Med. 384:440–451. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Breast Cancer Association Consortium, .
Dorling L, Carvalho S, Allen J, González-Neira A, Luccarini C,
Wahlström C, Pooley KA, Parsons MT, Fortuno C, et al: Breast cancer
risk genes-association analysis in more than 113,000 women. N Engl
J Med. 384:428–439. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maddalo D, Manchado E, Concepcion CP,
Bonetti C, Vidigal JA, Han YC, Ogrodowski P, Crippa A, Rekhtman N,
de Stanchina E, et al: In vivo engineering of oncogenic chromosomal
rearrangements with the CRISPR/Cas9 system. Nature. 516:423–427.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yoshida T, Oya Y, Tanaka K, Shimizu J,
Horio Y, Kuroda H, Sakao Y, Hida T and Yatabe Y: Differential
crizotinib response duration among ALK fusion variants in
ALK-positive non-small-cell lung cancer. J Clin Oncol.
34:3383–3389. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang SS, Nagasaka M, Zhu VW and Ou SHI:
Going beneath the tip of the iceberg. Identifying and understanding
EML4-ALK variants and TP53 mutations to optimize treatment of ALK
fusion positive (ALK+) NSCLC. Lung Cancer. 158:126–136. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tabbò F, Muscarella LA, Gobbini E,
Trombetta D, Castellana S, Rigutto A, Galetta D, Maiello E,
Martelli O, Tiseo M, et al: Detection of ALK fusion variants by
RNA-based NGS and clinical outcome correlation in NSCLC patients
treated with ALK-TKI sequences. Eur J Cancer. 174:200–211. 2022.
View Article : Google Scholar : PubMed/NCBI
|