Introduction
Leptomeningeal disease (LMD) is a serious
complication of non-small cell lung cancer (NSCLC). A total of 3–5%
of patients with NSCLC will eventually develop LMD, which has a
poor prognosis and life expectancy of 3–6 months after the
diagnosis of LMD (1). Clinical
manifestations, such as headaches, nausea, vomiting, altered mental
status, or focal neurological deficits, magnetic resonance imaging
(MRI) and cerebrospinal fluid (CSF) biopsy are key factors in
diagnosing NSCLC with LMD. CSF cytology alone is insufficient and
should be combined with liquid biopsy (2).
Although extended survival has been achieved with
advances in lung cancer treatment, the longer course of disease has
also resulted in an increase in the incidence of LMD, especially
for patients with actionable mutations, such as EGFR T790M and ALK
rearrangements. Furthermore, there is still no standard treatment
for LMD (3). The efficacy of
personalized therapies like tyrosine kinase inhibitors (TKIs) in
patients with LMD remains unknown as these patients are often
excluded from clinical trials (4).
BRAF mutations occur in 1.5–3.5% of patients with
NSCLC, and >50% of these mutations were V600E (5). A case reported that vemurafenib
demonstrated efficacy in the improvement of neurologic symptoms and
disease control for a patient with BRAF V600E and lung
adenocarcinoma with LMD (6).
However, resistance is inevitable in most patients with BRAF/MEK
inhibition (7). The application of
radiotherapy, intrathecal therapy, targeted therapy and
immunotherapy provides more options for patients with LMD. Each
treatment method has a certain efficacy and can be used alone or in
combination for individual patients. However, there is currently no
clear consensus on the optimal management of LMD (8).
Previous studies have demonstrated that CSF
collected from patients with LMD contains tumor cell-free (cf)DNA,
which can be used to detect LMD and monitor disease progression
(9–11). The present study reports the results
of a case where longitudinal liquid biopsies of blood and CSF were
used to inform the selection of a series of targeted drugs to treat
a patient with LMD from lung adenocarcinoma.
Case report
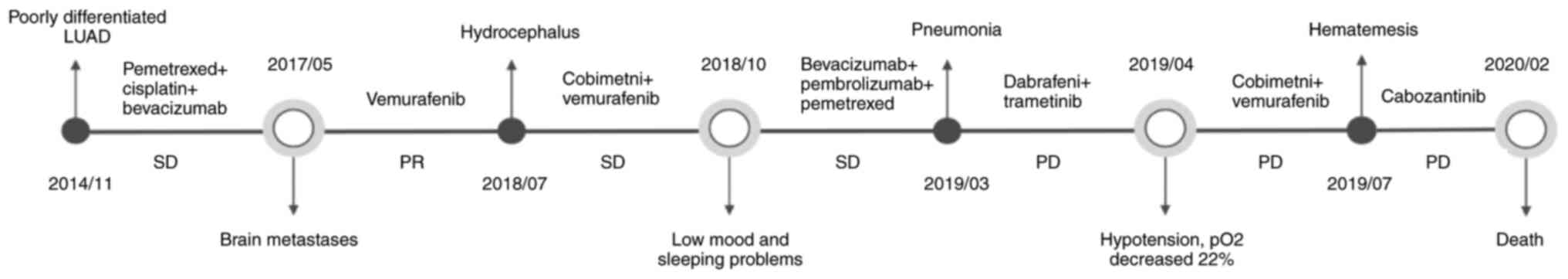
In November 2014, a 42-year-old female patient was
admitted to Sun Yat-sen University Cancer Center (Guangzhou,
China). The patient was diagnosed with poorly differentiated lung
adenocarcinoma with lymph node and bone metastases. The full
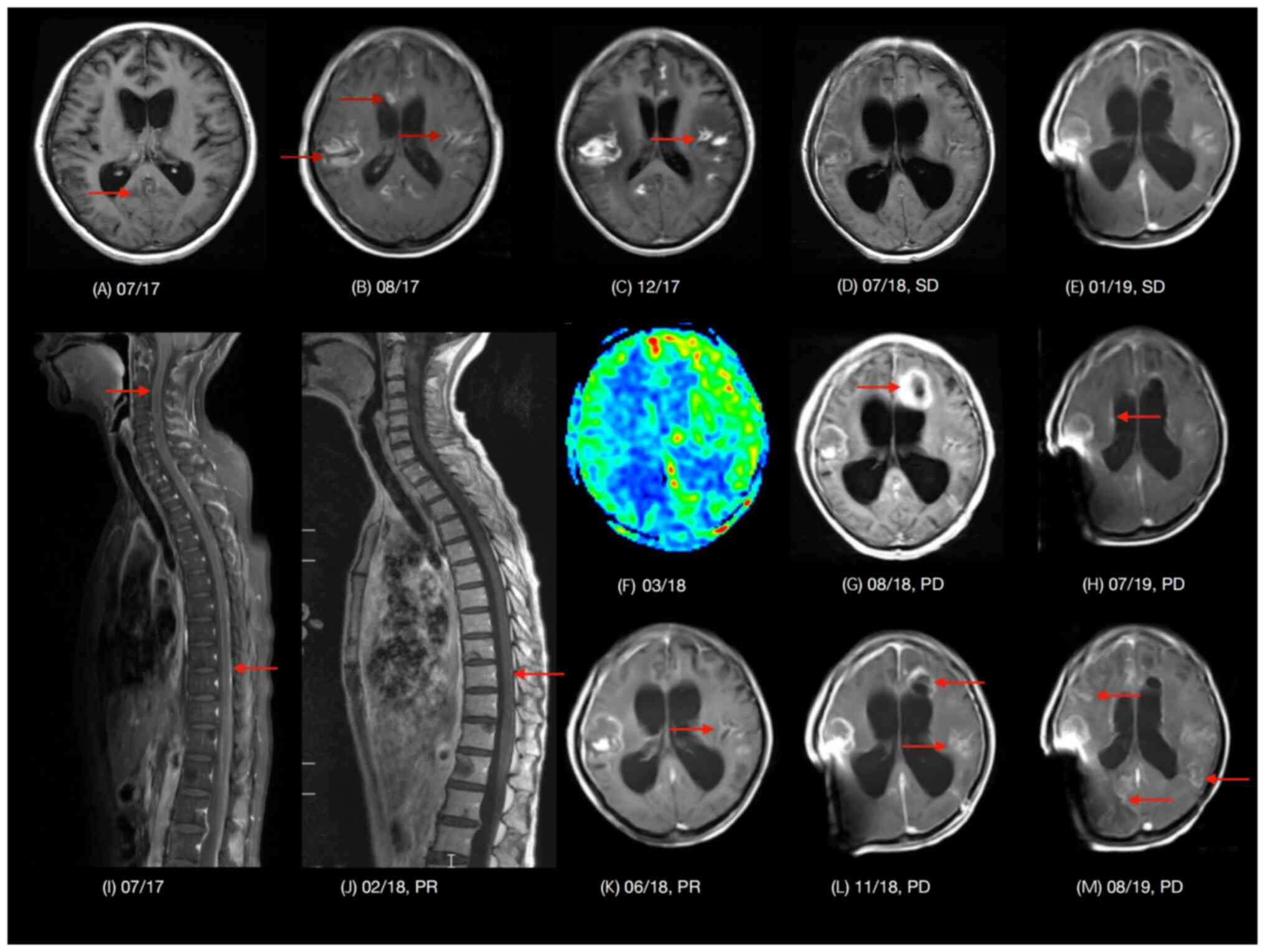
clinical course of the patient is presented in Fig. 1 and representative MRI images are
shown in Fig. 2.
Clinical sample processing and bioinformatic
analysis pipelines are described in our previous study (12). Clinical samples were obtained
through an approved informed consent process by Guangdong Sanjiu
Brain Hospital Institutional Review Board (Guangdong, China), and
only excess CSF that was not required for pathological diagnosis
was utilized in the present study. Briefly, blood and CSF were
collected in BCT® tubes (Streck LLC). cfDNA from plasma and CSF was
isolated using the QIAamp® Circulating Nucleic Acid Kit (cat. no.
55114; Qiagen GmbH). following the manufacturer's instructions.
Extracted DNA was then quantified by Qubit 2.0 (Thermo Fisher
Scientific, USA) in accordance with manufacturer's protocols.
Sequencing libraries were prepared using the KAPA Hyper Prep Kit
(KK8504l; Kapa Biosystems). Targeted enrichment was performed with
a 464-gene panel (HaploX Biotechnology). A full gene list is
presented in Table SI. Library
concentration was assessed by the Qubit dsDNA HS Assay kit.
Fragment length was determined on the 4200 Bioanalyzer using the
DNA 1000 Kit (Agilent). Then the libraries were sequenced using 150
bp paired-end runs on the Illumina NovaSeq 6000 system (Illumina,
Inc.). Raw sequencing reads were filtered by fastp v0.18.0
(13) and aligned to the hg19
genome (GRch37) using the Burrows-Wheeler Aligner v0.7.15-r1140
(14). Duplicated reads were
removed using Gencore v0.12.0 (15). SAMtools v0.1.19 was used to generate
pileup files of properly paired reads with a mapping quality ≥60
(16). Somatic variants were
identified using VarScan2 v2.3.8 (17) with the following parameters: Minimum
read depth at a position=20; variant allele frequency (VAF)
threshold ≥0.001; strand-filter ≥1; otherwise by default. VarScan 2
requires minimum phred base quality of 20 and a P-value of
<0.05. A full mutation list is presented in Table SII.
Genetic testing of the patient's lung biopsy sample
identified no mutations in EGFR, anaplastic lymphoma kinase, ROS
proto-oncogene 1, receptor tyrosine kinase and c-MET. The patient
received two cycles of chemotherapy with on day 1 of each 21-day
cycle comprising pemetrexed (500 mg/m2) and cisplatin
(75 mg/m2) through intravenous infusion and four cycles
of chemotherapy with on day 1 of each 21-day cycle comprising
pemetrexed (500 mg/m2) d1, cisplatin (75
mg/m2) d1 and bevacizumab (15 mg/kg) through intravenous
infusion. Serial computed tomography (CT) scans showed stable
disease status (Fig. S1).
In May 2017, the patient presented with nausea,
vomiting and dizziness and was admitted to our hospital and
underwent a lumbar puncture. For the purpose of cytological
examination, cells were collected from the patient's cerebrospinal
fluid samples. These cells were obtained by centrifuging 0.5 ml of
the sample at 140 × g for 5 min at 4°C. The post-centrifugation
samples were collected onto slides. After collection, the samples
were air-dried and then fixed in pure ethanol for 5 min at room
temperature. Subsequently, these specimens were stained using the
May-Grünwald-Giemsa (MGG) method. Briefly, the slide was fully
covered with May-Grünwald-phosphate mixture and incubate for 10 min
at room temperature. Then, the May Grunwald-phosphate mixture was
decanted from the slide and the slide fully covered with
Giemsa-phosphate mixture and incubated for 15 min at room
temperature. Next, the mixture was decanted and the slide rinsed
with slow running tap water. Air dry the slide. The morphological
details of the MGG-stained specimens were examined under an optical
microscope at 40× and 100× magnification. CSF cytological
assessment demonstrated malignant cells (Fig. S2) and LMD was confirmed by MRI
(Fig. 2A, I, B). A blood sample
collected at this time underwent circulating tumor DNA sequencing
and a BRAF p.V600E mutation was identified. The patient was
subsequently treated with vemurafenib orally 960 mg twice per day
continuously until disease progression, but the symptoms persisted
and an external ventricular drain (EVD) was placed 5 days later.
The patient then underwent whole brain radiation therapy (WBRT)
with a radiation dose of 30 Gy in 10 fractions in 2 weeks, and the
EVD was removed after 2 weeks of its placement.
MRI of the spine in February 2018 (Fig. 2J) identified a shrunk lesion in the
spinal cord. Surveillance imaging by conventional MRI or arterial
spin labeling MRI perfusion (Fig. 2C, F
and K) demonstrated visible brain lesions, which was the
radiation induced brain injury. Stable disease remained until the
patient's symptoms relapsed in July 2018 (Fig. 2D). A head CT was performed which
demonstrated new hydrocephalus (Fig.
S3). From June 2018, CSF was regularly sampled and sent for
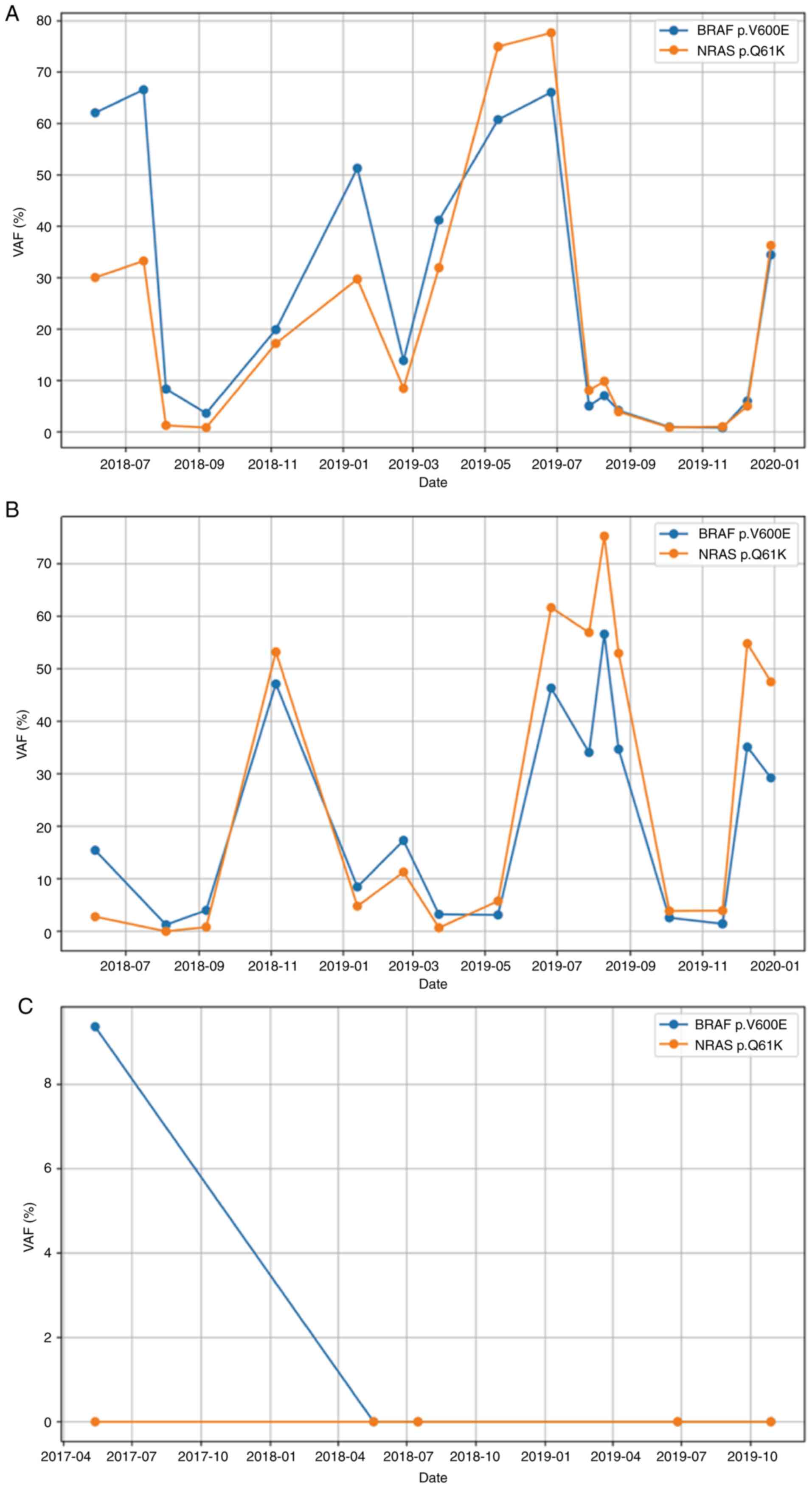
clinical and genetic testing. Fig.
3 presents the molecular change over time. CfDNA in CSF was
sequenced with the targeted 464-gene panel (HaploX Biotechnology)
for 19 months, and BRAF p.V600E and NRAS p.Q61K mutations were
found. NRAS p.Q61K has been reported to be resistant to
single-agent BRAF inhibitors (5).
Subsequently, the patient received cobimetinib orally at a dose of
60 mg once daily for the first 21 days of each 28-day cycle plus
vemurafenib orally at a dose of 960 mg twice daily. The patient
presented with transient remission of disease symptoms.
In August 2018, a new lesion was found using MRI
(Fig. 2G). The patient underwent
ventriculoperitoneal shunt (VPS) placement with an adjustable valve
(Sophysa Polaris®; Sophysa SA; Tokibo Co., Ltd.). In November 2018,
MRI (Fig. 2L) showed evidence of
disease progression. In response, treatment was switched from TKIs
to intravenous bevacizumab (300 mg) plus pembrolizumab (100 mg) and
pemetrexed (700 mg) every 3 weeks for three cycles. Subsequently,
though intracranial SD indicated on the CT scan (Fig. 2E), the patient began to experience
low mood and insomnia after the pemetrexed infusions. Therefore,
pemetrexed was removed from the treatment regimen.
In February 2019, the VAF of BRAF p.V600E and NRAS
p.Q61K were found to have increased in the CSF. In response,
therapy was adjusted to oral dabrafenib 75 mg twice daily and
trametinib 2 mg once daily. A month later, the patient presented
with severe shortness of breath secondary to pneumonia and was
hospitalized for a week. In April 2019, the patient developed
hypotension and an increased oxygen requirement. Cobimetnib plus
vemurafenib was administered again in clinic. The patient received
cobimetinib orally at a dose of 60 mg once daily for the first 21
days of each 28-day cycle plus vemurafenib orally at a dose of 960
mg twice daily. In July 2019, the patient developed hematemesis,
and a new lesion was found in right lateral ventricle wall
(Fig. 2H). TKIs were stopped and
intravenous pemetrexed at a dose of 700 mg for a cycle of 3 weeks
was administered. In August 2019, new lesions were found on the MRI
image (Fig. 2M). The patient was
started on a new TKI, cabozantinib taken orally at a dose of 20 mg
once daily and also temozolomide, an oral active DNA alkylating
agent that can cross the blood-brain barrier to treat brain tumors
(18), taken at a dose of 100 mg
once daily for 5 days. Due to severe constipation, temozolomide was
discontinued after 5 days. In September 2019, the patient presented
to the hospital with acute respiratory failure and underwent
endotracheal intubation. Cabozantinib was increased to a dose of 60
mg once daily. The patient remained hospitalized for 4 months and
subsequently died in February 2020.
Discussion
Although CSF cytology can help in the diagnosis of
LMD, it does not provide a quantitative assessment of the severity
of disease (19). In the present
case, CSF was regularly and frequently sampled from the reservoir
of the patient's VPS, which allowed the longitudinal tracking of
CSF cfDNA and cellular DNA (Fig. 3A and
B). A total of 17 time points of CSF supernatant and 16 time
points of CSF cell pellets were prepared for targeted sequencing.
Unfortunately, CSF from June 2017 (time of diagnosis) to June 2018
was not sent for sequencing due to lack of CSF samples, so genetic
information was not obtained in this period. The patient's symptoms
were generally consistent with the trend in VAF changes of driver
mutations in cfDNA. When the patient presented with severe symptoms
or when there was evidence of disease progression, the VAF of
driver mutations was high. During periods of time when the patient
had evidence of stable disease, the VAF in cfDNA was low. The
association of CSF mutation burden in LMD has been reported
previously (11), demonstrating its
potential as a clinical marker for managing this disease.
In the present case, no actionable mutations were
identified in the primary tumor. After presenting with neurologic
symptoms in June 2017, the patient was found to have a BRAF p.V600E
mutation in blood cfDNA. The patient was started on targeted
therapy (vemurafenib), and later also received four rounds of WBRT.
Notably, radiation therapy was only applied to the brain, not other
sites. Lesions in the spinal cord shrunk after vemurafenib
treatment (Fig. 2I and J),
indicating the efficacy of this TKI in the central nervous system
(CNS). Altogether, the patient had 13 months of progression-free
survival from undergoing this regimen in June 2017 to the
progression of the disease in July 2018. Subsequent genetic tests
using blood samples (May and July 2018) did not identify the BRAF
mutation or any other cancer-related mutations (Fig. 3C), indicating that the systemic
disease was well controlled after treatment. However, the disease
ultimately relapsed in CNS.
As the disease relapsed in June 2018, CSF was
regularly sampled and sequenced, and genetic test results were used
to inform treatment plans. Notably, sequencing data for CSF pellets
from a time point in July 2018 were missing due to inadequate
cellular DNA for library preparation. Consolidation of cobimetnib
plus vemurafenib was given based on the high mutation frequency of
BRAF p.V600E identified in CSF cfDNA sampled in July 2018. After ~8
months following the second disease relapse, the patient's targeted
drugs were switched to dabrafenib plus trametinib. The genetic
testing of CSF from the previous 7 months identified increasing
mutation frequency, indicating possible drug resistance. In August
2019, high mutation frequencies of BRAF p.V600E and NRAS p.Q61K
were found in CSF cellular DNA with corresponding lower VAF in
cfDNA. This was likely due to a high quantity of malignant cells in
CSF. The patient was subsequently started on cabozantinib. Notably,
from the longitudinal tracking of CSF DNA over 19 months, the BRAF
mutation was initially more abundant than the NRAS mutation both in
cellular and cfDNA (Fig. 3A and B).
Subsequently, the NRAS mutation became dominant in the cellular
DNA, and VAF of these two mutations in cfDNA were similar. We
hypothesize that the TKIs placed selection pressure on different
tumor clones and resulted in the change of VAF of different
mutations.
In summary, the present study reported the case of a
patient with an unusual case of LMD from primary lung
adenocarcinoma who underwent >20 time points of genetic sampling
and testing whilst receiving a combinational treatment of
chemotherapy, radiation therapy, immunotherapy and targeted
therapy. The patient survived for 33 months after the LMD
diagnosis. Although further research is needed to validate the
efficacy of targeted therapy in LMD, the present case presents a
potential strategy using reliable genetic testing of CSF to help
clinical monitoring and treatment of LMD.
Supplementary Material
Supporting Data
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Mr. Siyang Xu and
Ms. Manhu Shi (HaploX Biotechnology) for handling the image
formats.
Funding
The present study was supported by the Natural Science
Foundation of Guangdong Province (grant no. 2019A1515011943) and
the Science and Technology Innovation Committee of Shenzhen (grant.
no. KQTD20161129103502213).
Availability of data and materials
The data generated in the present study may be found
in the Genome Sequence Archive under accession number HRA004612 or
at the following URL: https://bigd.big.ac.cn/gsa-human/browse/HRA004612.
Access to the data may be requested from the corresponding
author.
Authors' contributions
SC, LC, MLa, TM and MLi conceived and designed the
work. QH and JL collected clinical sample, patient information and
medical records YL and TH contributed to analysis of the patient's
data. YL, QH, MLa, TM and MLi drafted the manuscript. All authors
have read and approved the final manuscript. SC and LC confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The present research was approved by the Guangdong
Sanjiu Brain Hospital Institutional Review (Guangzhou, China;
approval no. 2020-010-089). All procedures in the present study
were performed according to the guidelines put forth in the
Declaration of Helsinki.
Patient consent for publication
The patient provided written informed consent for
the publication of any associated data.
Competing interests
TM, MLi, YL, TH and SC are employed by Shenzhen
HaploX Biotechnology, and SC is a stakeholder of HaploX
Biotechnology. HaploX Biotechnology performed the sequencing for
the study. All other authors declare that they have no competing
interests.
References
|
1
|
Cheng H and Perez-Soler R: Leptomeningeal
metastases in non-small-cell lung cancer. Lancet Oncol. 19:e43–e55.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nakasu Y, Deguchi S, Nakasu S, Yamazaki M,
Notsu A, Mitsuya K and Hayashi N: Diagnostic accuracy of
cerebrospinal fluid liquid biopsy and MRI for leptomeningeal
metastases in solid cancers: A systematic review and meta-analysis.
Neurooncol Adv. 5:vdad0022023.PubMed/NCBI
|
|
3
|
Li D, Song Z, Dong B, Song W, Cheng C,
Zhang Y and Zhang W: Advances in targeted therapy in non-small cell
lung cancer with actionable mutations and leptomeningeal
metastasis. J Clin Pharm Ther. 47:24–32. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Remon J, Le Rhun E and Besse B:
Leptomeningeal carcinomatosis in non-small cell lung cancer
patients: A continuing challenge in the personalized treatment era.
Cancer Treat Rev. 53:128–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sforza V, Palumbo G, Cascetta P, Carillio
G, Manzo A, Montanino A, Sandomenico C, Costanzo R, Esposito G,
Laudato F, et al: BRAF inhibitors in non-small cell lung cancer.
Cancers (Basel). 14:48632022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fernandes MG, Costa J, Reis J, Jacob M,
Moura C, Machado J and Hespanhol V: OA08. 07 BRAF-V600E Advanced
lung adenocarcinoma with leptomeningeal (LM) disease treated with
vemurafenib. J Thor Oncol. 12:S274–S275. 2017. View Article : Google Scholar
|
|
7
|
Tabbò F, Pisano C, Mazieres J, Mezquita L,
Nadal E, Planchard D, Pradines A, Santamaria D, Swalduz A, Ambrogio
C, et al: How far we have come targeting BRAF-mutant non-small cell
lung cancer (NSCLC). Cancer Treat Rev. 103:1023352022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Le Rhun E, Preusser M, van den Bent M,
Andratschke N and Weller M: How we treat patients with
leptomeningeal metastases. ESMO Open. 4 (Suppl 2):e0005072019.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pan W, Gu W, Nagpal S, Gephart MH and
Quake SR: Brain tumor mutations detected in cerebral spinal fluid.
Clin Chem. 61:514–522. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Springer S, Zhang M, McMahon KW,
Kinde I, Dobbyn L, Ptak J, Brem H, Chaichana K, Gallia GL, et al:
Detection of tumor-derived DNA in cerebrospinal fluid of patients
with primary tumors of the brain and spinal cord. Proc Natl Acad
Sci USA. 112:9704–9709. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li Y, Pan W, Connolly ID, Reddy S, Nagpal
S, Quake S and Gephart MH: Tumor DNA in cerebral spinal fluid
reflects clinical course in a patient with melanoma leptomeningeal
brain metastases. J Neurooncol. 128:93–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu J, Mao G, Li Y, Tao L, Wang W, Peng X,
Wang J, Li X, Luan X, Luo R, et al: Targeted deep sequencing helps
distinguish independent primary tumors from intrapulmonary
metastasis for lung cancer diagnosis. J Cancer Res Clin Oncol.
146:2359–2367. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen S, Zhou Y, Chen Y and Gu J: Fastp: An
ultra-fast all-in-one FASTQ preprocessor. Bioinformatics.
34:i884–i890. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li H and Durbin R: Fast and accurate
long-read alignment with Burrows-Wheeler transform. Bioinformatics.
26:589–595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Zhou Y, Chen Y, Huang T, Liao W,
Xu Y, Li Z and Gu J: Gencore: An efficient tool to generate
consensus reads for error suppressing and duplicate removing of NGS
data. BMC Bioinformatics. 20 (Suppl 23):S6062019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li H, Handsaker B, Wysoker A, Fennell T,
Ruan J, Homer N, Marth G, Abecasis G and Durbin R; 1000 Genome
Project Data Processing Subgroup, : The Sequence Alignment/Map
format and SAMtools. Bioinformatics. 25:2078–2079. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koboldt DC, Zhang Q, Larson DE, Shen D,
McLellan MD, Lin L, Miller CA, Mardis ER, Ding L and Wilson RK:
VarScan 2: Somatic mutation and copy number alteration discovery in
cancer by exome sequencing. Genome Res. 22:568–576. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lajous H, Lelièvre B, Vauléon E, Lecomte P
and Garcion E: Rethinking alkylating (−like) agents for solid tumor
management. Trends Pharmacol Sci. 40:342–357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nguyen A, Nguyen A, Dada OT, Desai PD,
Ricci JC, Godbole NB, Pierre K and Lucke-Wold B: Leptomeningeal
metastasis: A review of the pathophysiology, diagnostic
methodology, and therapeutic landscape. Curr Oncol. 30:5906–5931.
2023. View Article : Google Scholar : PubMed/NCBI
|