Introduction
Ovarian cancer is the fifth most common cause of
cancer-associated mortality in females and has the lowest 5-year
survival rate among all types of gynecological cancers (1,2). In
2018, 295,414 new cases of ovarian cancer and 184,799 deaths from
ovarian cancer were reported worldwide (3).
The preliminary stages of ovarian cancer are
typically asymptomatic and difficult to detect (4,5),
resulting in diagnosis at later stages with a worse prognosis due
to lack of essential screening tools (1,4,5).
Tissue factor pathway inhibitor-2 (TFPI2), which
serves as a tumor suppressor gene in various types of cancer such
as gastric (6), colorectal
(7) and hepatocellular cancer
(8), has been investigated as a
diagnostic marker of ovarian clear cell carcinoma (OCCC) in Japan
(9–11). TFPI2 can serve as a serum tumor
marker for discriminating ovarian cancer from other types of
ovarian tumors (12). Accordingly,
TFPI2 has been covered by insurance providers in Japan since April
2021 and is gaining popularity nationwide (13,14).
TFPI2 cut-off value is ≥191 for ovarian cancer and ≥270 pg/ml for
OCCC. Unlike cancer antigen 125 (CA125), TFPI2 levels are not
elevated in ovarian endometrial cysts (10,11);
therefore, TFPI2 is the optimal single tumor marker for diagnosing
ovarian cancer (12). A recent
study confirmed that TFPI2 can diagnose venous thromboembolism
(VTE) in patients with epithelial ovarian cancer who have
positive-D-dimer results (13).
Moreover, the combination of D-dimer and TFPI2 levels can be used
to rule out VTE and identify patients at high risk of VTE (14).
Despite accumulating evidence regarding the
diagnostic accuracy of this tumor marker (10–12),
the association between preoperative serum TFPI2 levels and
outcomes in patients with ovarian cancer remains unclear. Our
previous study demonstrated that TFPI2 levels are associated with
survival outcome of patients with endometrial cancer (15) and clarified the potential link
between TFPI2 and cancer prognosis. The present study aimed to
determine whether serum TFPI2 could be a prognostic marker for
overall survival (OS) and progression-free survival (PFS) in
patients with ovarian cancer.
Materials and methods
Patient population
In the present retrospective study, 256 patients
(age range, 22–88 years) with a confirmed diagnosis of ovarian
cancer at Nara Medical University Hospital (Kashihara, Japan) were
recruited between January 2008 and January 2022. The inclusion
criteria were as follows: i) Confirmed pathological diagnosis of
ovarian cancer and ii) received treatment, not only supportive
care. The exclusion criteria were as follows: i) Combined with
other malignancies; ii) pregnant women; and iii) patients with
concomitant serious comorbidities. The ovarian cancer staging was
determined using the International Federation of Gynecology and
Obstetrics (FIGO) classification 2014 (16). Patients were diagnosed with ovarian
cancer based on histopathology, pelvic magnetic resonance imaging
and chest and abdominal computed tomography (Definition Flash,
Siemens AG; Definition AS, Siemens AG; and Aquilion ONE, Canon,
Inc.). Histopathology involved primary staining with
hematoxylin-eosin (room temperature, hematoxylin for 5 min and
eosin for 2 min), with additional p53 staining [primary antibody at
4°C for 12 h (rabbit polyclonal anti-p53; dilution, 1:200; cat no.
NCL-L-p53-CM5p; Leica Biosystems, Ltd.); secondary antibody at room
temperature for 1 h (mouse anti-rabbit IgG-HRP; dilution, 1:25; cat
no. sc-2357; Santa Cruz Biotechnology, Inc.)] for high-grade serous
carcinoma and HNF-1β staining [primary antibody at 4°C for 12 h
(rabbit polyclonal anti-HNF1β; dilution, 1:200; cat no. 12533-1-AP,
Proteintech Group, Inc.); secondary antibody at room temperature
for 1 h (mouse anti-rabbit IgG-HRP; dilution, 1:25; cat no.
sc-2357; Santa Cruz Biotechnology, Inc.)] for OCCC as an auxiliary
diagnosis. CT images were evaluated using 5 mm thick axial images.
Patient clinical data were collected, including age, body mass
index (BMI), parity, menopausal status, histological type and FIGO
stage. Proteins of interest were quantified using a fluorescence or
chemiluminescence immunoassay, including TFPI2 [E-Test TOSOH II
(TFPI2); Tosoh Corporation; cat. no. #0025245], CA125 (CL
AIA-PACK® OVCA; Tosoh Corporation, cat. no. #0029114;
ARCHITECT CA125 II; Abbott Japan LLC, cat. no. #2K45-28),
carbohydrate antigen (CA) 19-9 (CL AIA-PACK® Sla; Tosoh
Corporation, cat. no. #0029112) and carcinoembryonic antigen (CEA)
(CL AIA-PACK® CEA; Tosoh Corporation, #0029108)
according to the manufacturer's instructions. TFPI2, CA125 (CL
AIAPACK OVCA), CA19-9, and CEA concentrations were determined by
Tosoh Corporation using serum obtained before the surgery. Tumor
marker concentrations were measured by clinical laboratory
technologists blinded to the study. The present study adhered to
the guidelines of the Declaration of Helsinki. This was a
single-center retrospective study based on medical records and all
patient information was anonymized. The research project was
announced on an opt-out basis.
Only variables that could be assessed preoperatively
were included in uni- and multivariate analyses, including age,
BMI, menopausal status and tumor marker levels. The analysis was
performed in patients with OCCC and non-OCCC separately because
diagnostic cut-off value differs between OCCC and ovarian cancer
(11). The cut-off value for OS was
applied to analyze PFS and OS. OS was defined as the period from
treatment initiation until death or the last follow-up examination.
PFS was defined as the period from treatment initiation until date
of diagnosis as a progressive disease or the last follow-up
examination.
Treatment
Patients diagnosed with ovarian cancer underwent
primary debulking surgery (PDS) if optimal surgery was possible. If
the surgery was more extensive than bilateral oophorectomy and no
second surgery was performed, it was considered PDS. If surgery was
performed following chemotherapy, it was considered as an interval
debulking surgery (IDS). If surgery was minor relative to bilateral
oophorectomy, it was considered as no surgery.
The completion of surgery was considered optimal if
the diameter of the remaining tumor was <1 cm. The surgery was
considered suboptimal if the diameter of the remaining mass was ≥1
cm. Furthermore, information regarding lymphadenectomy was
collected. Unless the patient had a poor performance status and was
considered incapable of enduring a high invasive surgery, both
paraaortic and pelvic lymphadenectomy were performed. Adjuvant
chemotherapy, mostly comprised of taxane and carboplatin (TC)
therapy, was generally performed upon obtaining consent from the
patient.
Statistical analysis
The receiver operating characteristic (ROC) curve
was used to determine optimal cut-off points of TFPI2, CA125,
CA19-9, and CEA levels to predict OS for OCCC and non-OCCC. The
optimal cut-off value was determined using the Youden index to
predict OS. The outcome on the ROC curve was defined as survival or
death. The Kaplan-Meier life table analysis and the log-rank tests
were used to assess survival rates and differences based on
prognostic factors. Multivariate analysis of prognostic factors for
PFS and OS was performed using the Cox proportional hazard
regression model where univariate analysis revealed significant
differences. All statistical analyses were performed using SPSS
software (version 29.0, IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patients' clinical
characteristics
The present study included 256 patients with ovarian
cancer with a median age of 60 years (range, 22–88 years). The
median follow-up period was 52.4 months (range, 0.8–190.8
months).
A total of 121 cases (47.3%) were FIGO stage I or II
while 135 (52.7%) were stage III or IV (Table I). There were 109 cases (42.6%) of
serous carcinoma, 40 cases of mucinous carcinoma (15.6%), 15 cases
of endometrial carcinoma (5.9%), 66 cases of OCCC (25.8%) and 26
cases of others (10.2%; Table SI).
Other histological types included carcinosarcoma, malignant Brenner
tumor, seromucinous carcinoma, mixed epithelial tumor,
neuroendocrine carcinoma and undifferentiated carcinoma (data not
shown). The median preoperative serum levels of TFPI2, CA125,
CA19-9 and CEA were 219.0 (82.5–5,824.2) pg/ml, 278.6
(0.5–43,170.9) U/ml, 21.5 (0.0–217,474.9) U/ml and 2.1 (0.4–142.9)
ng/ml, respectively (Table I).
 | Table I.Clinicopathological characteristics
of patients. |
Table I.
Clinicopathological characteristics
of patients.
| Characteristic | All patients
(n=256) | Patients with OCCC
(n=66) | Patients with
non-OCCC (n=190) | P-value |
|---|
| Age,
yearsa | 60 (22–88) | 56 (35–79) | 61 (22–88) | 0.032 |
| BMI,
kg/m2a | 21.8
(15.2–40.8) | 21.8
(16.2–40.8) | 21.8
(15.2–34.3) | 0.547 |
| Parityb |
|
|
| 0.011 |
| 0 | 64 (29.0) | 23 (34.8) | 41 (26.5) |
|
| 1 | 38 (17.2) | 12 (18.2) | 26 (16.8) |
|
| ≥2 | 119 (53.8) | 31 (47.0) | 88 (56.8) |
|
| Menopausal
statusb |
|
|
| 0.825 |
|
Pre-menopause | 71 (27.7) | 19 (28.8) | 52 (27.4) |
|
|
Post-menopause | 185 (72.3) | 47 (71.2) | 138 (72.6) |
|
| Tumor
markera |
|
|
|
|
| TFPI2,
pg/ml | 219.0
(82.5–5,824.2) | 255.0
(82.5–5,824.2) | 214.5
(88.9–1,336.9 | 0.029 |
| CA125,
U/ml | 278.6
(0.5–43,170.9) | 55.4
(0.5–5,727.1) | 413.1
(5.9–43,170.9) | <0.001 |
| CA19-9,
U/ml | 21.5
(0.0–217,474.9) | 22.85
(0.0–11,588.4) | 20.3
(0.5–217,474.9) | 0.448 |
| CEA,
ng/ml | 2.1
(0.4–142.9) | 2.1 (0.7–11.5) | 2.1
(0.4–142.9) | 0.007 |
| FIGO
stageb |
|
|
| <0.001 |
|
I/II | 121 (47.3) | 53 (80.3) | 68 (35.8) |
|
|
III/IV | 135 (52.7) | 13 (19.7) | 122 (64.2) |
|
Treatment
A total of 163 (63.7%) patients underwent PDS, 75
(29.3%) underwent IDS and 18 (7.0%) underwent biopsy (Table II). Moreover, surgery was optimal
and suboptimal in 184 (71.9%) and 72 (28.1%) patients,
respectively.
 | Table II.Type of surgical treatment. |
Table II.
Type of surgical treatment.
| Characteristic | n (%) |
|---|
| Surgery |
|
| Primary
debulking | 163 (63.7) |
|
Interval debulking | 75 (29.3) |
|
None | 18 (7.0) |
| Completion |
|
|
Optimal | 184 (71.9) |
|
Suboptimal | 72 (28.1) |
|
Lymphadenectomy |
|
|
Yes | 106 (41.4) |
| No | 150 (58.6) |
Among the 256 patients, 106 (41.4%) underwent
lymphadenectomy while 150 (58.6%) did not. The adjuvant first-line
chemotherapy regimen mostly comprised TC therapy (n=198, 77.3%). Of
the remaining patients, most cases underwent platinum-based
chemotherapy, including docetaxel and carboplatin (n=5, 2.0%), TC
and bevacizumab (n=5, 2.0%), dose-dense TC (n=5, 2.0%), weekly TC
(n=2, 0.8%) and irinotecan and cisplatin therapy (n=2, 0.8%).
Weekly paclitaxel therapy was performed in two patients (0.8%),
while docetaxel and gemcitabine therapy was performed in one
patient (0.4%). However, 36 patients (14.1%) did not receive
adjuvant chemotherapy (Table
SII).
Analysis of non-OCCC
For non-OCCC, the cut-off value of TFPI2 for
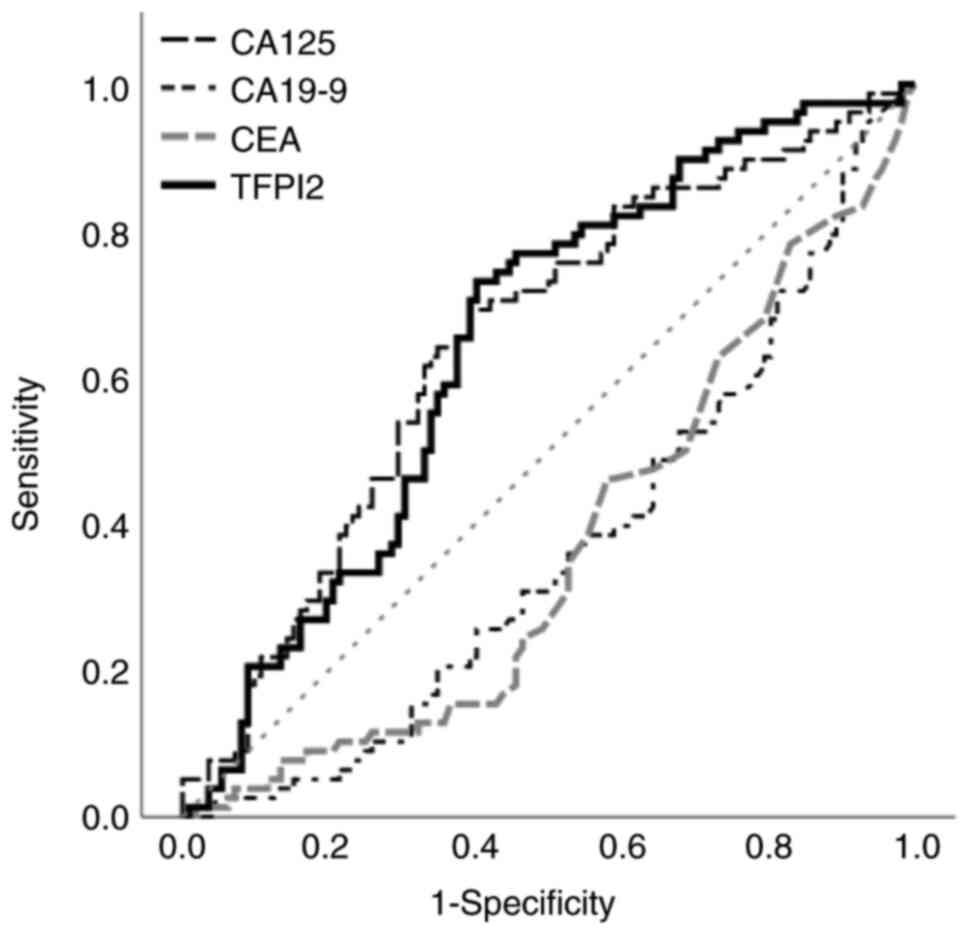
predicting OS was 201 pg/ml based on the Youden index [area under
the curve (AUC), 0.646; sensitivity, 73.1%; specificity, 59.8%; 95%
confidential interval (CI), 0.568–0.724], while that for CA125 was
394 U/ml (AUC, 0.648; sensitivity, 69.2%; specificity, 60.7%; 95%
CI, 0.569–0.727; Fig. 1). CA19-9
and CEA were excluded because they showed negative associations
with OS based on ROC curve results. TFPI2 values <201 and ≥201
pg/ml were defined as negative and positive, respectively.
Similarly, CA125 values <394 and ≥394 U/ml were defined as
negative and positive, respectively.
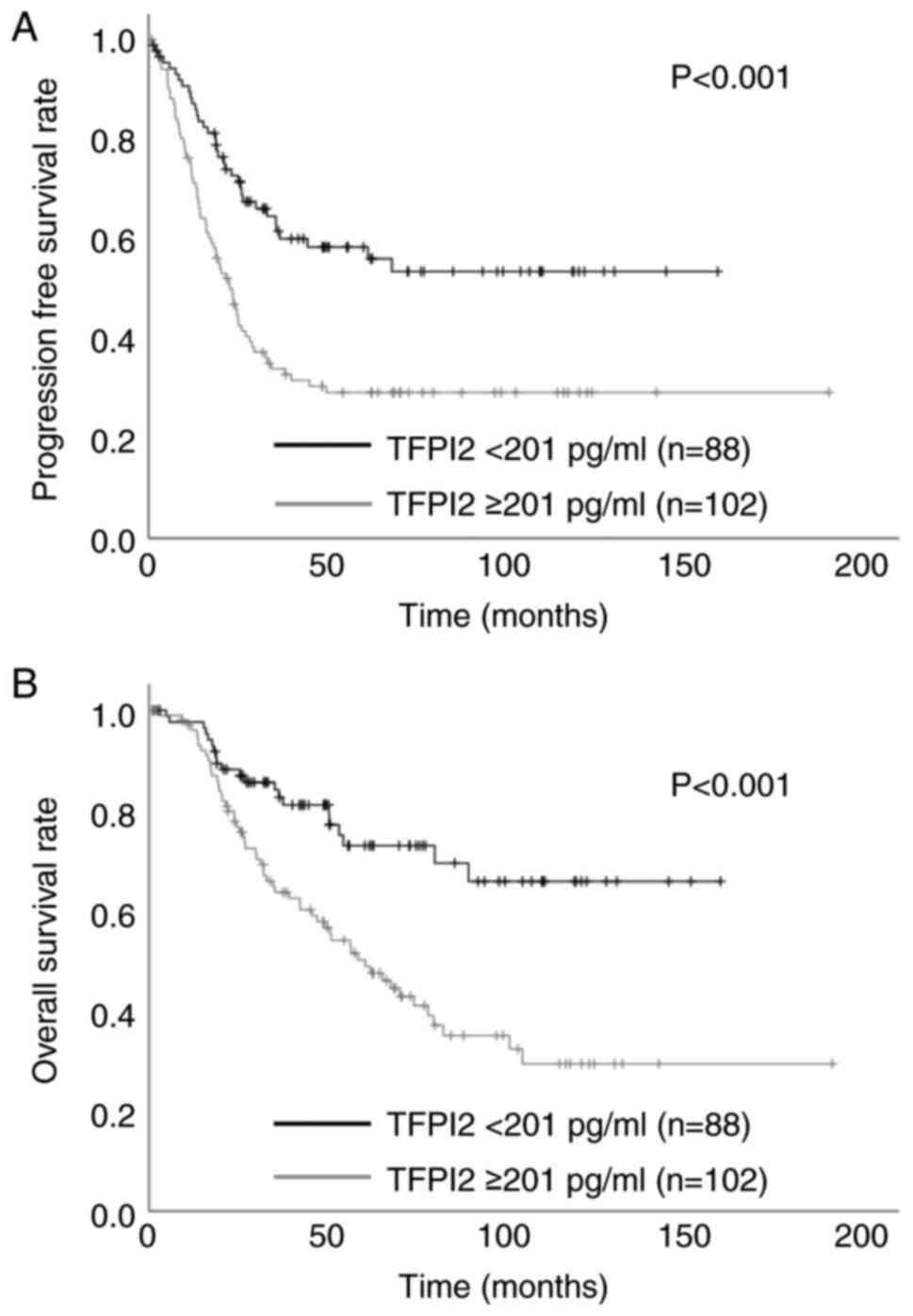
In the univariate analysis, TFPI2 ≥201 pg/ml was
significantly associated with PFS (Fig.
2A) and OS (Fig. 2B). Table III shows uni- and multivariate
analyses of prognostic factors for PFS and OS. For PFS. Univariate
analysis showed significant differences in age ≥60 years,
post-menopausal status, TFPI2 ≥201 pg/ml and CA125 ≥394 U/ml. For
OS, univariate analysis showed significant differences in age ≥60
years, post-menopausal status, TFPI2 ≥201 pg/ml and CA125 ≥394
U/ml. Cox multivariate analysis revealed that TFPI2 was a
significant independent prognostic factor affecting OS.
 | Table III.Univariate and multivariate analysis
of prognostic factors for progression-free survival and overall
survival in patients with non-ovarian clear cell carcinoma. |
Table III.
Univariate and multivariate analysis
of prognostic factors for progression-free survival and overall
survival in patients with non-ovarian clear cell carcinoma.
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Cox multivariate
analysis | Univariate
analysis | Cox multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
| <60
(n=87) | 0.011 | 1.285 | 0.807–2.046 | 0.290 | 0.021 | 1.306 | 0.763–2.236 | 0.331 |
| ≥60
(n=103) |
|
|
|
|
|
|
|
|
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
| <25
(n=144) | 0.125 | - |
|
| 0.176 |
|
|
|
| ≥25
(n=46) |
|
|
|
|
|
|
|
|
| Menopausal
status |
|
|
|
|
|
|
|
|
|
Pre-menopausal (n=52) | 0.049 | 1.238 | 0.720–2.128 | 0.440 | 0.043 | 1.300 | 0.682–2.475 | 0.425 |
|
Post-menopausal (n=138) |
|
|
|
|
|
|
|
|
| TFPI2, pg/ml |
|
|
|
|
|
|
|
|
| <201
(n=88) | <0.001 | 1.513 | 0.966–2.370 | 0.071 | <0.001 | 1.890 | 1.100–3.247 | 0.021 |
| ≥201
(n=102) |
|
|
|
|
|
|
|
|
| CA125, U/ml |
|
|
|
|
|
|
|
|
| <394
(n=92) | <0.001 | 2.093 | 1.332–3.288 | 0.001 | <0.001 | 1.772 | 1.052–2.983 | 0.031 |
| ≥394
(n=98) |
|
|
|
|
|
|
|
|
Analysis of OCCC
Next, analysis was conducted for patients with OCCC.
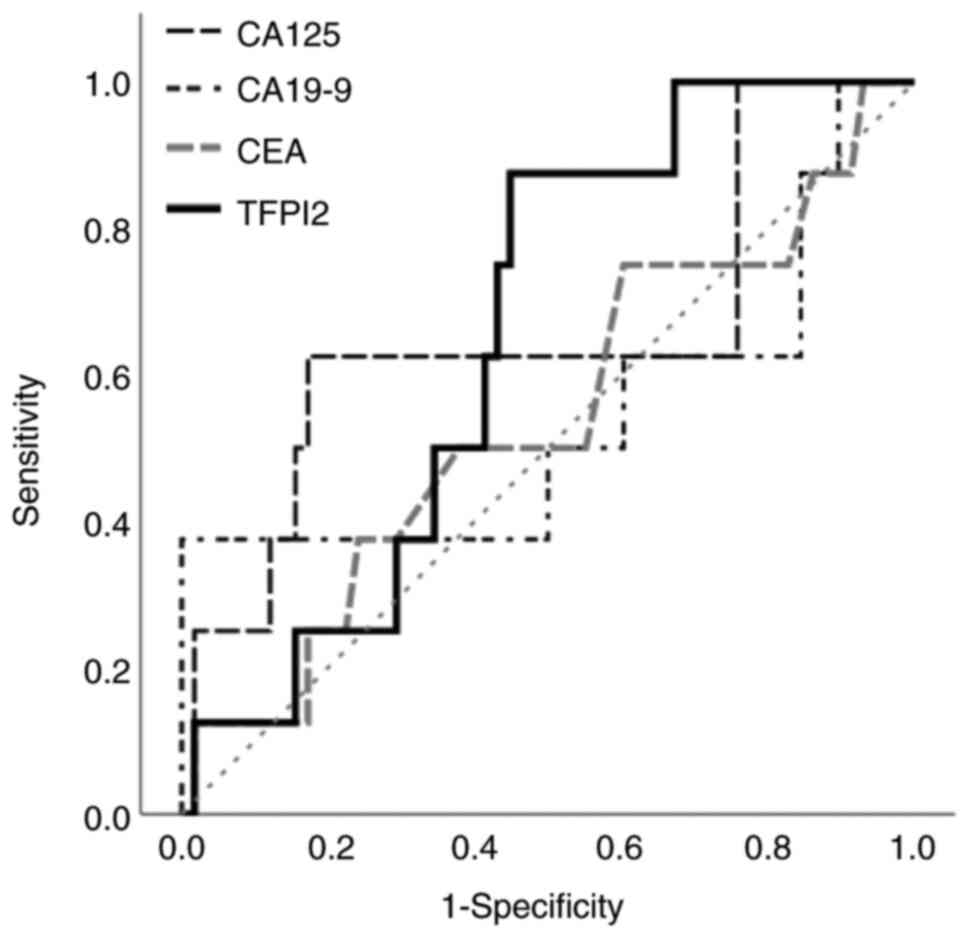
The Youden index was used to calculate a cut-off value of 255 pg/ml
for TFPI2 for predicting OS (AUC, 0.653; sensitivity, 87.5%;
specificity, 55.2%; 95% CI, 0.494–0.812) and 363 U/ml for CA125
(AUC; 0.655, sensitivity; 62.5%, specificity; 82.8%, 95% CI;
0.421–0.890; Fig. 3). TFPI2 levels
<255 and ≥255 pg/ml were defined as negative and positive,
respectively. Similarly, CA125 values <363 and ≥363 U/ml were
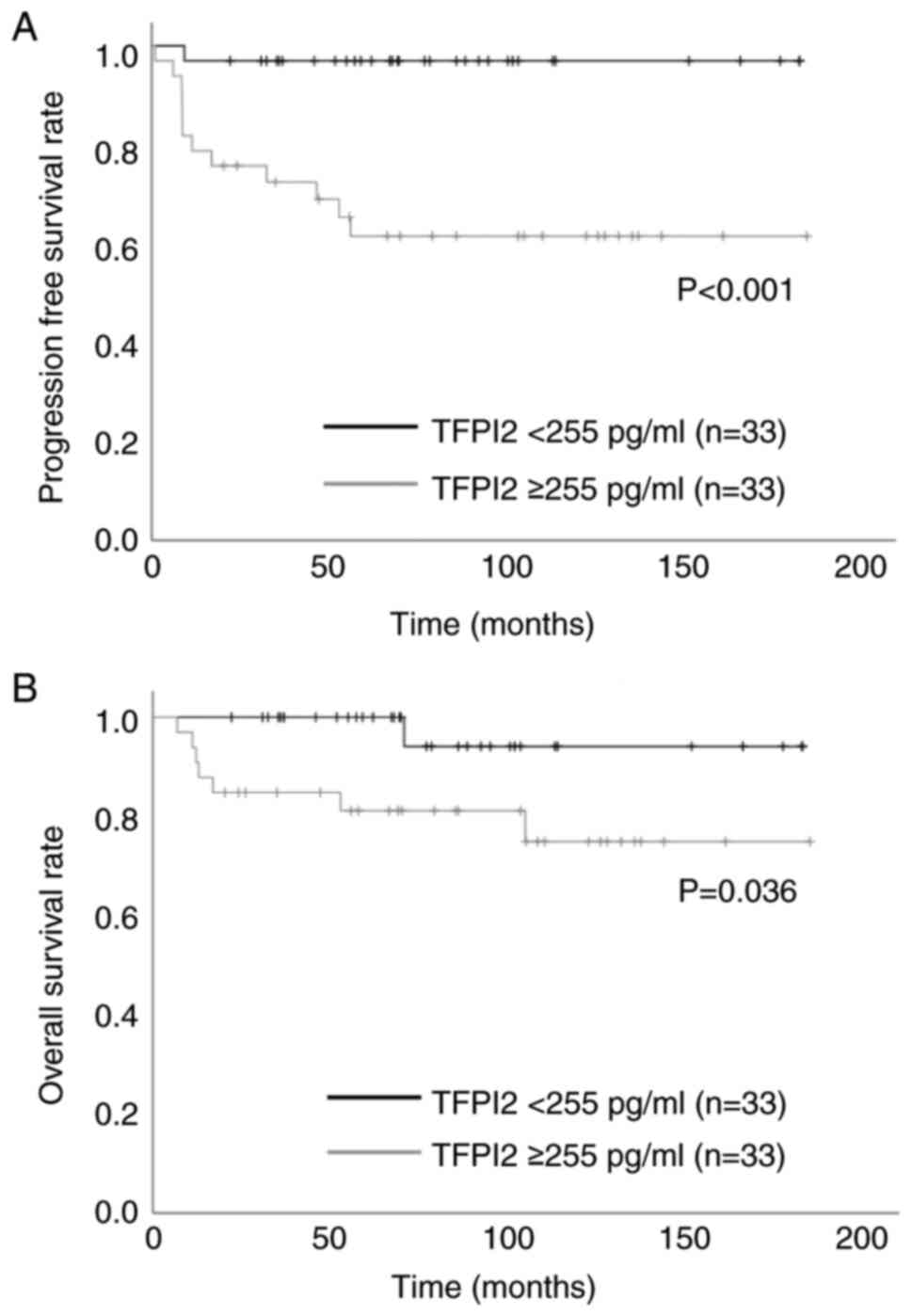
defined as negative and positive, respectively. In univariate
analysis, TFPI2 ≥255 pg/ml was significantly associated with PFS
(Fig. 4A) and OS (Fig. 4B). Table IV shows univariate and multivariate
analyses of prognostic factors for PFS and OS. For PFS, univariate
analysis showed significant differences in TFPI2 ≥255 pg/ml and
CA125 ≥363 U/ml. Contrastingly, for OS, univariate analysis showed
significant differences in BMI ≥25, TFPI2 ≥255 pg/ml and CA125 ≥363
U/ml. When Cox multivariate analysis was applied, only TFPI2 was a
significant independent prognostic factor affecting PFS in patients
with OCCC.
 | Table IV.Univariate and multivariate analysis
of prognostic factors for progression free survival and overall
survival in ovarian clear cell carcinoma. |
Table IV.
Univariate and multivariate analysis
of prognostic factors for progression free survival and overall
survival in ovarian clear cell carcinoma.
|
| Progression-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Cox multivariate
analysis | Univariate
analysis | Cox multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value |
|---|
| Age, years |
|
|
|
|
|
|
|
|
| <60
(n=38) | 0.316 |
|
|
| 0.541 |
|
|
|
| ≥60
(n=28) |
|
|
|
|
|
|
|
|
| BMI,
kg/m2 |
|
|
|
|
|
|
|
|
| <25
(n=50) | 0.157 |
|
|
| 0.049 | 4.171 | 1.008–17.264 | 0.049 |
| ≥25
(n=16) |
|
|
|
|
|
|
|
|
| Menopausal
status |
|
|
|
|
|
|
|
|
|
Pre-menopausal (n=19) | 0.534 |
|
|
| 0.702 |
|
|
|
|
Post-menopausal (n=47) |
|
|
|
|
|
|
|
|
| TFPI2, pg/ml |
|
|
|
|
|
|
|
|
| <255
(n=33) | <0.001 | 11.627 | 1.476–91.597 | 0.020 | 0.036 | 5.280 | 0.611–45.616 | 0.130 |
| ≥255
(n=33) |
|
|
|
|
|
|
|
|
| CA125, U/ml |
|
|
|
|
|
|
|
|
| <363
(n=51) | 0.014 | 2.223 | 0.728–6.786 | 0.161 | <0.001 | 6.320 | 1.317–30.325 | 0.021 |
| ≥363
(n=15) |
|
|
|
|
|
|
|
|
Discussion
Epithelial ovarian cancer is divided into two
classes based on the criteria of Kurman and Shih (17). Type I includes low-grade serous,
endometrial and mucinous carcinoma and OCCC, which are low-grade
and relatively slow-growing (18,19).
Type II includes high-grade serous, endometrial and
undifferentiated carcinoma as well as carcinosarcoma, which are
high-grade and relatively fast-growing (18,19).
Type I ovarian cancer is genetically stable and is often detected
in the initial stages.
Compared with type I, type II ovarian cancer has a
high frequency of TP53 mutations and is usually genetically
unstable (17,20). High-grade serous carcinoma, which is
a type II ovarian cancer, accounts for >70% of epithelial
ovarian cancers worldwide (21–23).
However, because type I ovarian cancer is relatively rare, there
may be a less urgent need for research into its mechanism and
treatment in non-East Asian countries (22,24).
OCCC is a type I ovarian cancer that is more prevalent in Japan
(11.7–26.9%) than in North American and Western countries
(4.6–12.0%) (22,24–26).
Moreover, because the initial stages of OCCC are more
prognostically favorable compared with other histological types of
ovarian cancer (25,27), there is a need to determine a method
for diagnosing these cancer types in the initial stages (26).
Arakawa et al (9) identified TFPI2 as a diagnostic marker
for OCCC. TFPI2 is produced in vascular endothelial cells,
platelets and macrophages (26).
Moreover, an immunohistochemical study revealed that TFPI2 is
localized in normal muscle, skeletal, breast, liver, kidney,
pancreas, stomach and colon tissue (28). It can also be detected in both OCCC
and endometrial clear cell carcinoma cells using
immunohistochemical staining (26,28,29).
Serum TFPI2 has a high specificity for OCCC and is often negative
in patients with endometriosis (9–12,26).
All histological types of ovarian cancer are associated with TFPI2
as a tumor marker (12). TFPI2
levels are elevated in other histological types, although not as
high as in OCCC (10,12). In Japan, measuring TFPI2 serum
levels is already covered by insurance (13,14),
and the official cut-off values for diagnosing ovarian cancer and
OCCC are 191 and 270 pg/ml, respectively. Therefore, TFPI2 shows
different features between OCCC and non-OCCC.
Jacobs and Oram suggested that CA125 is an important
tumor marker for distinguishing benign from malignant tumors
(30). Other than CA125, various
tumor markers are used in gynecology, including CA19-9, CEA and
human epididymis protein (HE) 4 (12,19,25,31).
However, CA125 is affected by various factors, such as menopausal
status, pregnancy, infection and endometriosis (10,11,32).
Furthermore, there have been studies on the association between
tumor markers and cancer prognosis: CA125 is a prognostic tool for
predicting relapse and progression of ovarian cancer; however,
since CA125 is also known to be influenced by tumor histology and
clinical stage, it remains controversial (33,34).
Other studies have shown that pretreatment serum CA125 level is
associated with disease progression of ovarian cancer (33–35).
According to a previous study, serum CA19-9 levels >70.3 U/ml
decrease the odds of survival in OCCC, whereas CA125 and HE4 levels
do not (25). Our previous study
found that preoperative serum TFPI2 levels serve as a prognostic
marker for endometrial cancer (15). TFPI2 levels ≥177 pg/ml significantly
increase the risk of recurrence and death (15). The present study investigated the
utility of TFPI2 as a prognostic marker of OS and PFS in patients
with ovarian cancer and showed that elevated serum TFPI2 levels
were linked to cancer progression and indicated poor prognosis.
Although results of the univariate analysis showed
significant differences in both OS and PFS in OCCC and non-OCCC,
those of the multivariate analysis only showed significant
differences in PFS in OCCC and OS in non-OCCC. In a previous study,
early-stage detection is more often achieved in OCCC than in
non-OCCC; (36). Likewise, in our
study, most patients with OCCC were in the early stages and did not
die during the study period. This may explain why OS did not show
significant differences in OCCC. Including a higher number of cases
may yield better multivariate analysis results regarding OS.
To the best of our knowledge, the present study is
the first to demonstrate that high preoperative serum levels of
TFPI2 are associated with ovarian cancer progression. TFPI2 levels
≥201 pg/ml for predicting OS for non-OCCC and ≥255 pg/ml for
predicting PFS were the cut-off values. The present results
highlighted the effectiveness of TFPI2 as a prognostic marker for
ovarian cancer.
A strength of the present study is that the
prognostic factors included in the analysis could be preoperatively
measured. Accordingly, determining the pre-treatment prognosis may
help patients decide on treatment plans.
The present study has certain limitations. First,
this was a single-center, small-scale retrospective study. Second,
the present study did not measure serum HE4 levels and thus could
not employ the risk of ovarian malignancy algorithm. Compared with
CA125 and HE4, TFPI2 is less sensitive in detecting serous
carcinoma (12) and the present
results may be different, especially in the non-OCCC group. Third,
the present study did not consider tumor size in the multivariate
analysis because it only included items that could be assessed
preoperatively. Although it remains controversial, preoperative
serum levels of CA125 are positively associated with tumor size
(37). Therefore, TFPI2 may also be
related to tumor size and affect the results regarding OS and
PFS.
In conclusion, TFPI2 is a potential reliable
biomarker for predicting the prognosis of ovarian cancer. With
insurance coverage, more cases can be assessed, which will
facilitate elucidation of the utility of TFPI2 as a prognostic
marker.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
TM, RK and YY conceived and designed the study. TM
and YY collected data and produced the tables and figures. TM and
YY confirmed the authenticity of all the raw data. TM wrote the
manuscript. TM, RK, KN, NK, YY and FK analyzed and interpreted data
and revised the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of Nara Medical University, Kashihara, Japan
(approval no. 3115) and conducted in accordance with the guidelines
of the Declaration of Helsinki. This was a single-center
retrospective study based on medical records and histopathological
findings. All patient information was anonymized; thus, the need
for informed consent was waived and information regarding the
implementation of the study was disclosed by the opt-out
method.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dinkelspiel HE, Champer M, Hou J, Tergas
A, Burke WM, Huang Y, Neught AI, Ananth CV, Hershman DL and Wright
JD: Long-term mortality among women with epithelial ovarian cancer.
Gynecol Oncol. 138:421–428. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liberto JM, Chen SY, Shih IM, Wang TH,
Wang TL and Pisanic TR II: Current and emerging methods for ovarian
cancer screening and diagnostics: A comprehensive review. Cancers
(Basel). 14:28852022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Smith LH, Morris CR, Yasmeen S,
Parikh-Patel A, Cress RD and Romano PS: Ovarian cancer: Can we make
the clinical diagnosis earlier? Cancer. 104:1398–1407. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takada H, Wakabayashi N, Dohi O, Yasui K,
Sakakura C, Mitsufuji S, Taniwaki M and Yoshizawa T: Tissue factor
pathway inhibitor 2 (TFPI2) is frequently silenced by aberrant
promoter hypermethylation in gastric cancer. Cancer Genet
Cytogenet. 197:16–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hibi K, Goto T, Kitamura YH, Yokomizo K,
Sakuraba K, Shirahata A, Mizukami H, Saito M, Ishibashi K, Kigawa
G, et al: Methylation of TFPI2 gene is frequently detected in
advanced well-differentiated colorectal cancer. Anticancer Res.
30:1205–1207. 2010.PubMed/NCBI
|
|
8
|
Sun FK, Fan YC, Zhao J, Zhang F, Gao S,
Zhao ZH, Sun Q and Wang K: Detection of TFPI2 methylation in the
serum of hepatocellular carcinoma patients. Dig Dis Sci.
58:1010–1015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arakawa N, Miyagi E, Nomura A, Morita E,
Ino Y, Ohtake N, Miyagi Y, Hirahara F and Hirano H: Secretome-based
identification of TFPI2, a novel serum biomarker for detection of
ovarian clear cell adenocarcinoma. J Proteome Res. 12:4340–4050.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Miyagi E, Arakawa N, Sakamaki K, Yokota
NR, Yamanaka T, Yamada Y, Yamaguchi S, Nagao S, Hirashima Y,
Kasamatsu Y, et al: Validation of tissue factor pathway inhibitor 2
as a specific biomarker for preoperative prediction of clear cell
carcinoma of the ovary. Int J Clin Oncol. 26:1336–1344. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Arakawa N, Kobayashi H, Yonemoto N,
Masuishi Y, Ino Y, Shigetomi H, Furukawa N, Ohtake N, Miyagi Y,
Hirahara F, et al: Clinical significance of tissue factor pathway
inhibitor 2, a serum biomarker candidate for ovarian clear cell
carcinoma. PLoS One. 11:e01656092016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kobayashi H, Yamada Y, Kawaguchi R, Ootake
N, Myoba S and Kimura F: Tissue factor pathway inhibitor 2: A
potential diagnostic marker for discriminating benign from
malignant ovarian tumors. J Obstet Gynaecol Res. 48:2442–2451.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miyake R, Yamada Y, Yamanaka S, Kawaguchi
R, Ootake N, Myoba S and Kobayashi H: Tissue factor pathway
inhibitor 2 as a serum marker for diagnosing asymptomatic venous
thromboembolism in patients with epithelial ovarian cancer and
positive D-dimer results. Mol Clin Oncol. 16:1–5. 2022.PubMed/NCBI
|
|
14
|
Yamanaka S, Miyake R, Yamada Y, Kawaguchi
R, Ootake N, Myoba S and Kobayashi H: Tissue factor pathway
inhibitor 2: A novel biomarker for predicting asymptomatic venous
thromboembolism in patients with epithelial ovarian cancer. Gynecol
Obstet Invest. 87:133–140. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kawaguchi R, Maehana T, Yamanaka S, Miyake
R, Kawahara N, Iwai K, Yamada Y and Kimura F: Preoperative serum
tissue factor pathway inhibitor-2 level as a prognostic marker for
endometrial cancer: A single-center retrospective study. Oncol
Lett. 26:4632023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Prat J; FIGO Committee on Gynecologic
Oncology, : Staging classification for cancer of the ovary,
fallopian tube, and peritoneum. Int J Gynaecol Obstet. 124:1–5.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kurman RJ and Shin IM: Molecular
pathogenesis and extraovarian origin of epithelial ovarian cancer.
Shifting the paradigm. Hum Pathol. 42:918–931. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nagell JRV, Burgess BT, Miller RW, Baldwin
L, DeSimone CP, Ueland FR, Huang B, Chen Q, Kryscio RJ and Pavlik
EJ: Survival of women with type I and II epithelial ovarian cancer
detected by ultrasound screening. Obstet Gynecol. 132:1091–1100.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawahara N, Kawaguchi R, Maehana T,
Yamanaka S, Yamada Y, Kobayashi H and Kimura F: The endometriotic
neoplasm algorithm for risk assessment (e-NARA) index sheds light
on the discrimination of endometriosis-associated ovarian cancer
from ovarian endometrioma. Biomedicines. 10:26832022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura M, Obata T, Daikoku T and
Fujiwara H: The association and significance of p53 in gynecologic
cancers: The potential of targeted therapy. Int J Mol Sci.
20:54822019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Throwba HPK, Unnikrishnan L, Pangath M,
Vasudevan K, Jayaraman S, Li M, Iyaswamy A, Palaniyandi K and
Gnanasampanthapandian G: The epigenetic correlation among ovarian
cancer, endometriosis and PCOS: A review. Crit Rev Oncol Hematol.
180:1038522022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kurman RJ and Shih IM: The dualistic model
of ovarian cancer. Am J Pathol. 186:733–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Charkhchi P, Cybulski C, Gronwald J, Wong
FO, Narod SA and Akbari MR: CA125 and ovarian cancer: A
comprehensive review. Cancers (Basel). 12:37302020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang H, Liu Y, Wang X, Guan L, Chen W,
Jiang H and Lu Y: Clear cell carcinoma of the ovary
Clinicopathologic features and outcomes in a Chinese cohort.
Medicine (Baltimor). 97:e108812018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu C, Zhu J, Qian L, Liu H, Shen Z, Wu D,
Zhao W, Xiao W and Zhou Y: Clinical characteristics and prognosis
of ovarian clear cell carcinoma: A 10-year retrospective study. BMC
Cancer. 21:3222021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ota Y, Koizumi S, Nakamura Y, Yoshihara M,
Takahashi T, Sato S, Myoba S, Ohtake N, Kato H, Yokose T, et al:
Tissue factor pathway inhibitor-2 is specifically expressed in
ovarian clear cell carcinoma tissues in the nucleus, cytoplasm and
extracellular matrix. Oncol Rep. 45:1023–1032. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matsuzaki S, Yoshino K, Ueda Y, Matsuzaki
S, Kakuda M, Okazawa A, Egawa-Takata T, Kobayashi E and Kimura T:
Potential targets for ovarian clear cell carcinoma: A review of
updates and future perspectives. Cancer Cell Int. 15:1172015.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wojtukiewicz MZ, Sierko E, Zimnoch L,
Kozlowski L and Kisiel W: Immunohistochemical localization of
tissue factor pathway inhibitor-2 in human tumor tissue. Thromb
Haemost. 90:140–146. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawaguchi R, Maehana T, Sugimoto S,
Kawahara N, Iwai K, Yamada Y and Kimura F: Immunohistochemical
analysis of the tissue factor pathway inhibitor-2 in endometrial
clear cell carcinoma: A single-center retrospective study. Int J
Gynecol Pathol. 43:25–32. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jacobs I and Oram D: Screening for ovarian
cancer. Biomed Pharmacother. 42:589–596. 1988.PubMed/NCBI
|
|
31
|
Chen SY, Chang TC, Lin CY, Lai CH, Wu RC,
Yang LY, Chang WY, Lee YS, Yang WV and Chao A: Serum levels of
alpha1-antitrypsin isoforms in patients with ovarian clear cell
carcinoma: An exploratory study. J Chin Med Assoc. 84:1048–1053.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Muyldermans M, Cornillie FJ and Koninckx
PR: CA125 and endometriosis. Hum Reprod Update. 1:173–187. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Morales-Vásquez F, Pedernera E,
Reynaga-Obregón J, López-Basave HN, Gómora MJ, Carlón E, Cárdenas
S, Silva-Ayala R, Almaraz M and Méndez C: High levels of
pretreatment CA125 are associated to improved survival in high
grade serous ovarian carcinoma. J Ovarian Res. 9:412016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Colaković S, Lukiç V, Mitroviç L, Jeliç S,
Susnjar S and Marinkoviç J: Prognostic value of CA125 kinetics and
half-life in advanced ovarian cancer. Int J Biol Markers.
15:147–152. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kawahara N, Kawaguchi R, Waki K, Maehana
T, Yamanaka S, Yamada Y and Kimura F: The prognosis predictive
score around primary debulking surgery (PPSP) improves diagnostic
efficacy in predicting the prognosis of ovarian cancer. Sci Rep.
12:226362022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gadducci A, Multinu F, Cosio S, Carinelli
S, Ghioni M and Aletti GD: Clear cell carcinoma of the ovary:
Epidemiology, pathological and biological features, treatment
options and clinical outcomes. Gynecol Oncol. 162:741–750. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin JX, Huang YQ, Wang ZK, Xie JW, Wang
JB, Lu J, Chen QY, Cao LL, Lin M, Tu RH, et al: Prognostic
importance of dynamic changes in systemic inflammatory markers for
patients with gastric cancer. J Surg Oncol. 124:282–292. 2021.
View Article : Google Scholar : PubMed/NCBI
|