Introduction
Cervical cancer is a common malignant tumour in
women worldwide that is associated with high morbidity and
mortality, threatening the life and health of female patients.
According to Global Cancer Statistics 2020 (GLOBOCAN 2020 data)
(1), the incidence and mortality of
cervical cancer rank fourth among all malignant tumours in women
worldwide, accounting for ~6.5 and 7.7%, respectively. The China
Cancer Registry data showed that the mortality-to-incidence ratio
was 0.30 in 2014. In 2014, ~102,000 new cases of cervical cancer
were estimated to have occurred in China, with a crude incidence
rate of 15.30/100,000 (2).
Currently, the conventional treatment methods for cervical cancer
include surgery, radiotherapy and chemotherapy (3,4), but
the toxic side effects of chemotherapy drugs and the occurrence of
drug resistance affect the treatment efficacy (5). Therefore, it is important to identify
low-toxicity and high-efficiency anticancer drugs.
Chinese herbal medicine has a history in the
treatment of cancer, and its low toxicity and side effects provide
it with unique characteristics in the treatment of cancer.
Artesunate (ART), derived from artemisinin, is an antimalarial
drug, used for severe and drug-resistant malaria (6–8). ART
has antioxidant, immunomodulatory and antitumour effects in
addition to its antimalarial effects (9–11). The
antitumour effects of ART have been previously studied and the
results showed that ART has growth-inhibitory effects on a variety
of tumour cells, with low toxicity and side effects (12–15).
The study by Våtsveen et al (16) demonstrated that ART has potent
apoptosis-inducing effects on a broad range of B-cell lymphoma cell
lines both in vitro and in vivo. Furthermore, the
study by Yin et al (17)
indicated that ART could be an effective antitumour agent through
modulating the oestrogen receptor (ER)-α-mediated liver kinase
B1/AMP-activated protein kinase/mTOR pathway in a heart- and neural
crest derivatives-expressed protein 2-dependent manner, and that
ART is an effective therapeutic agent for ER-α-positive endometrial
cancer. The study by Mancuso et al (18) reported that ART inhibits the growth
of leukaemia, multiple myeloma and lymphoma cells by inducing cell
apoptosis, autophagy and ferroptosis. Additionally, other studies
indicated that ART inhibits the growth of oesophageal cancer and
gastric cancer cells by inducing apoptosis (19,20).
However, there are only a small number of reports on the molecular
mechanism by which ART inhibits cervical cancer, such as the study
by Saeed et al (21).
Apoptosis is a type of cell death, in which the
orderly and autonomous death of cells controlled by genes can
eliminate abnormal cells in the body. This serves an important role
in maintaining the stability of the internal environment. Apoptosis
is an active form of cell death controlled by multiple genes,
including those of the Bcl2 and caspase families, and is regulated
by multiple pathways, including the membrane receptor pathway and
the mitochondrial pathway. The mitochondria-mediated apoptosis
pathway is associated with the antitumour effect of drugs, such as
epigallocatechin-3-gallate (22).
Mitochondria are unique and important organelles and are the ‘power
factories’ of the cell (23).
Changes in mitochondrial function are associated with apoptosis and
participate in the process of apoptosis by releasing proapoptotic
factors, increasing the generation of reactive oxygen species
(ROS), and increasing intracellular calcium (Ca2+) ion
levels (24–26). There are numerous members of the
Bcl2 family, including Bcl2, Bcl-xl, (myeloid cell leukaemia 1)
Mcl-1, Bcl2-like protein 11 (BIM), (Bcl2-related ovarian killer
protein) Bok, Bax and (Bcl2 homologous antagonist/killer) Bak.
These proteins have either antiapoptotic or proapoptotic effects.
Most members of the Bcl2 family have two structural homology
regions through which different members can form heterodimers, and
Bcl2 members are functional or functionally regulated through
dimerization. Bcl2 can localize to mitochondria, stabilize the
mitochondrial membrane potential, prevent apoptosis and protect
cells (27). Dysfunction of the
expression of Bcl2 family members can cause dysregulation of
apoptosis and lead to the occurrence of diseases, including cancer
and autoimmune diseases (27).
Tumour cells have the ability to avoid apoptosis and survive, thus,
apoptotic disorders can lead to malignant tumours (28).
In the present study, the anticancer effects of ART
in vitro and the associated molecular mechanisms of a
low-toxicity and high-efficiency dose of ART for the clinical
treatment of cervical cancer were investigated.
Materials and methods
Cancer cell line and culture
The SiHa cell line (cat. no. CL-0210) was obtained
from Procell Life Science & Technology Co., Ltd., and was
cultured in minimum essential medium (MEM) supplemented with 10%
FBS and 1% penicillin and streptomycin (cat. no. CM-0210; Procell
Life Science & Technology Co., Ltd.). The cells were maintained
in an incubator at 37°C with 5% CO2.
Chemicals and reagents
ART was purchased from Guilin Pharmaceutical
(Shanghai) Co., Ltd. An annexin V-phycoerythrin
(PE)/7-aminoactinomycin D (7-AAD) kit (cat. no. 559763) and
propidium iodide were purchased from BD Biosciences. The primers
used in the present study were purchased from Sangon Biotech Co.,
Ltd.
Cytotoxicity assay
The cells were seeded in 96-well plates at a density
of 1×104 cells/well. Once the cells were attached,
serially diluted ART solution was added at a final concentration of
0.5 1, 5, 10, 50, 100, 400 or 800 µg/ml in a final volume of 200
µl/well. Normal saline (NS) was used for the control group. After
drug treatment for 24 h at 37°C, the medium was replaced with an
equivalent volume of fresh MEM containing 20 µl Cell Counting Kit-8
(CCK-8; Wuhan Boster Biological Technology, Ltd.), followed by
incubation for an additional 2 h. The cytotoxic effects of ART were
determined by measuring the optical density at 450 nm using a
microplate reader. The growth inhibition rate was calculated as
follows: [(1-absorbance of the ART treated group)/absorbance of the
control group] ×100.
ART intervention experiments
SiHa cells were seeded in 6-well plates at a density
of 1×105 cells/well. Once the cells reached 80–85%
confluence, ART was added to each well at concentrations of 15, 30
and 100 µg/ml, and NS was added to the control group. ART
concentrations of 15, 30 and 100 µg/ml were selected that were
close to the half-maximal inhibitory concentration
(IC50) value of ART. After ART treatment for 24 and 48 h
at 37°C, SiHa cells were collected using centrifugation (200 × g,
25°C, 5 min). The cell concentration was adjusted to
1×106/ml. Each experiment was repeated three times. SiHa
cells treated with NS or 15, 30, or 100 µg/ml ART were referred to
as the control and 15, 30 or 100 µg/ml ART groups,
respectively.
Assessment of cell apoptosis using
flow cytometry (FCM)
A SiHa cell suspension (1 ml containing
1×106 cells/ml) was collected, washed once with cold PBS
(4°C), and resuspended in 100 µl 1X binding buffer. Subsequently, 5
µl annexin V-PE was added, and the mixture was placed on ice for 15
min in the dark. Next, 390 µl 1X binding buffer and 5 µl 7-AAD were
added, and the mixture was incubated for 15 min in the dark at
37°C. Cell apoptosis was measured using a FC500 flow cytometer
(Beckman Coulter, Inc.). The EXPO32 ADC software version 1.2
(Beckman Coulter, Inc.) was used to analyse the fluorescence data
and evaluate the apoptosis rate.
Assessment of cell cycle distribution
using FCM
SiHa cell suspension (1 ml) was fixed with 70%
ethanol at 4°C for 24 h. Subsequently, the cells were washed once
with cold PBS (4°C), and 1 ml propidium iodide (containing RNAse A)
was added before incubation at 4°C in the dark for 30 min. The
cells were then measured using a FC500 flow cytometer, and the cell
cycle data were analysed using MultiCycle AV software version 275
(Phoenix Flow Systems, Inc.). The proliferation index (PI) was
calculated using the following formula: PI=(S +
G2/M)/(G0/1 + S + G2/M) ×100%. The
PI represents the state of cell proliferation (29).
Flow cytometric analysis of the
generation of ROS in SiHa cells after ART treatment
SiHa cells were washed with cold PBS (4°C), and
stained with 1 ml dichlorodihydrofluorescein diacetate (5 µg/ml;
Cayman Chemical Company) for 30 min in the dark at 37°C. The
stained cells were then washed once with cold PBS (4°C),
resuspended in 1 ml PBS, and analysed using a FC500 flow cytometer.
Fluorescence data were analysed using EXPO32 ADC software version
1.2.
Analysis of Ca2+ levels in
SiHa cells after ART treatment using FCM
SiHa cells were washed with cold PBS (4°C), and
stained with 1 ml Fluo-3 AM (1 µM; Beyotime Institute of
Biotechnology) for 30 min in the dark at 37°C. The stained cells
were then washed once with cold PBS (4°C), resuspended in 1 ml PBS,
and analysed using a FC500 flow cytometer. Fluorescence data were
analysed using EXPO32 ADC software version 1.2.
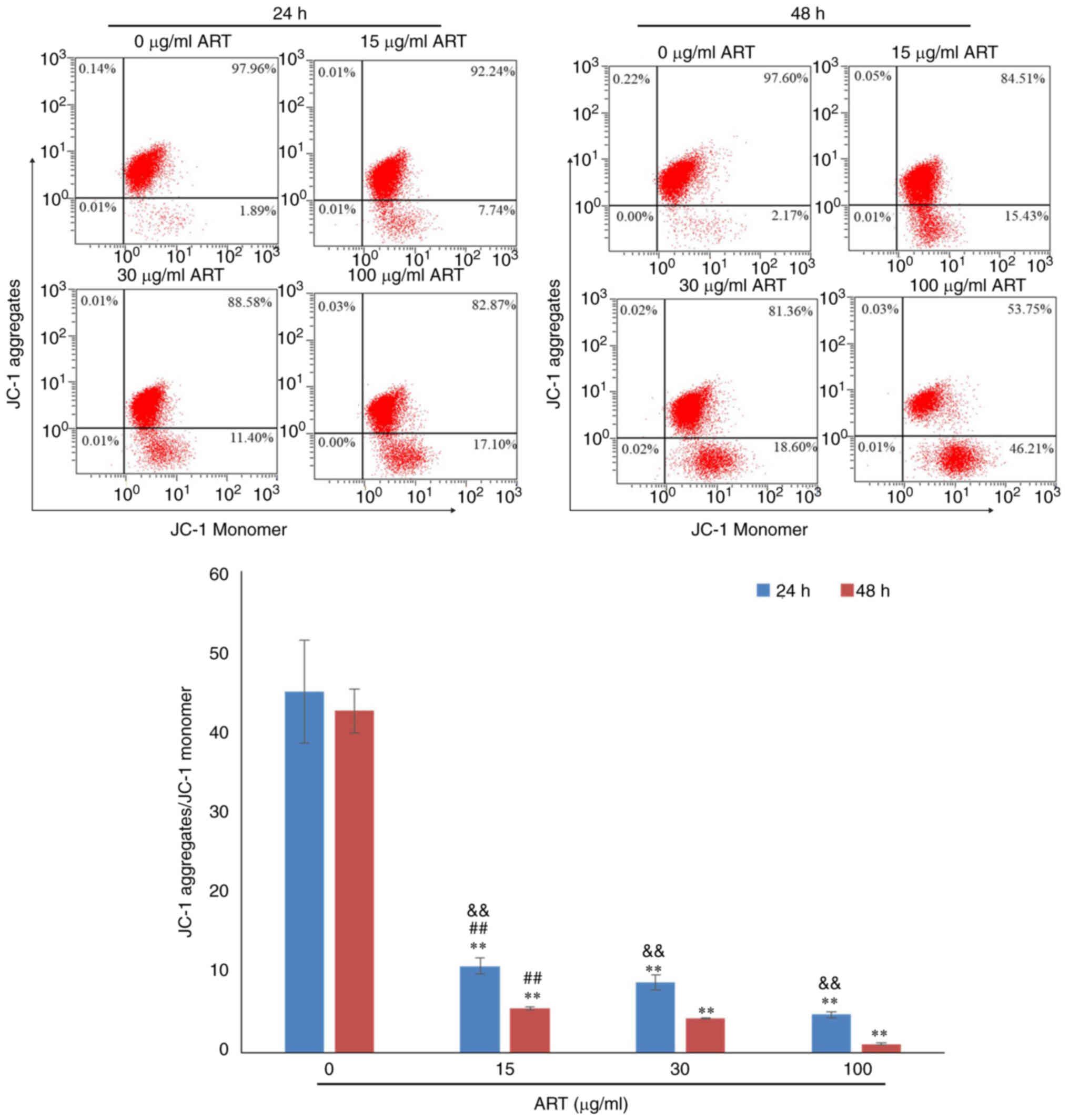
Flow cytometric analysis of the
mitochondrial membrane potential of SiHa cells after ART
treatment
SiHa cells were washed with cold PBS (4°C), and
stained with 0.5 ml JC-1 reagent (Beyotime Institute of
Biotechnology) for 20 min in the dark at 37°C. The stained cells
were then washed twice with 1X JC-1 staining buffer, resuspended in
1 ml 1X JC-1 staining buffer, and analysed using a FC500 flow
cytometer. Fluorescence data were analysed using EXPO32 ADC
software version 1.2 and presented as the ratio of JC-1
aggregates/JC-1 monomers.
Evaluation of mRNA expression levels
using reverse transcription-quantitative PCR (RT-qPCR)
SiHa cells were collected using centrifugation (200
× g, 25°C, 5 min) and washed once with PBS. Total RNA was extracted
from the cells using 1 ml RNA isolater (Vazyme Biotech Co., Ltd.).
As the template for PCR, cDNA was synthesized using the HiScript II
First Strand cDNA Synthesis kit (Vazyme Biotech Co., Ltd.)
according to the manufacturer's protocol. Based on the
manufacturer's protocol for the qPCR SYBR-Green Master Mix kit
(Vazyme Biotech Co., Ltd.), qPCR was performed. The primer
sequences used were: Bcl2 forward, 5′-ATCGCCCTGTGGATGACTGAGT-3′ and
reverse, 5′-GCCAGGAGAAATCAAACAGAGGC-3′; Bcl-xl forward,
5′-GCCACTTACCTGAATGACCACC-3′ and reverse,
5′-AACCAGCGGTTGAAGCGTTCCT-3′; BIM forward,
5′-CAAGAGTTGCGGCGTATTGGAG-3′ and reverse,
5′-ACACCAGGCGGACAATGTAACG-3′; Mcl-1 forward,
5′-CCAAGAAAGCTGCATCGAACCAT-3′ and reverse,
5′-CAGCACATTCCTGATGCCACCT-3′; Bax forward,
5′-TCAGGATGCGTCCACCAAGAAG-3′ and reverse,
5′-TGTGTCCACGGCGGCAATCATC-3′; Bak forward,
5′-TTACCGCCATCAGCAGGAACAG-3′ and reverse,
5′-GGAACTCTGAGTCATAGCGTCG-3′; Bok forward,
5′-ACGCCTGGCTGAGGTGTGCG-3′ and reverse,
5′-AGGAACGCATCGGTCACCACAG-3′; and GAPDH forward,
5′-GTCTCCTCTGACTTCAACAGCG-3′ and reverse,
5′-ACCACCCTGTTGCTGTAGCCAA-3′. The internal reference used was human
GAPDH. The thermocycling procedure used was as follows: Initial
denaturation at 95°C for 5 min; followed by 40 cycles of 95°C for
10 sec and 60°C for 30 sec. Dissociation was performed at 95°C for
15 sec, 60°C for 1 min and 95°C for 15 sec. For each sample, each
experiment was repeated three times. The relative mRNA expression
levels of Bcl2, Bcl-xl, Mcl-1, BIM, Bok, Bak and Bax were
calculated using the 2−ΔΔCq method (30).
Statistical analysis
All the data are presented as the mean ± SD (n=3).
Two-way ANOVA were performed to compare multiple groups, followed
by the Bonferroni post hoc test. The data were analysed using SPSS
software (version 21; IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Inhibitory effect of ART on SiHa
cells
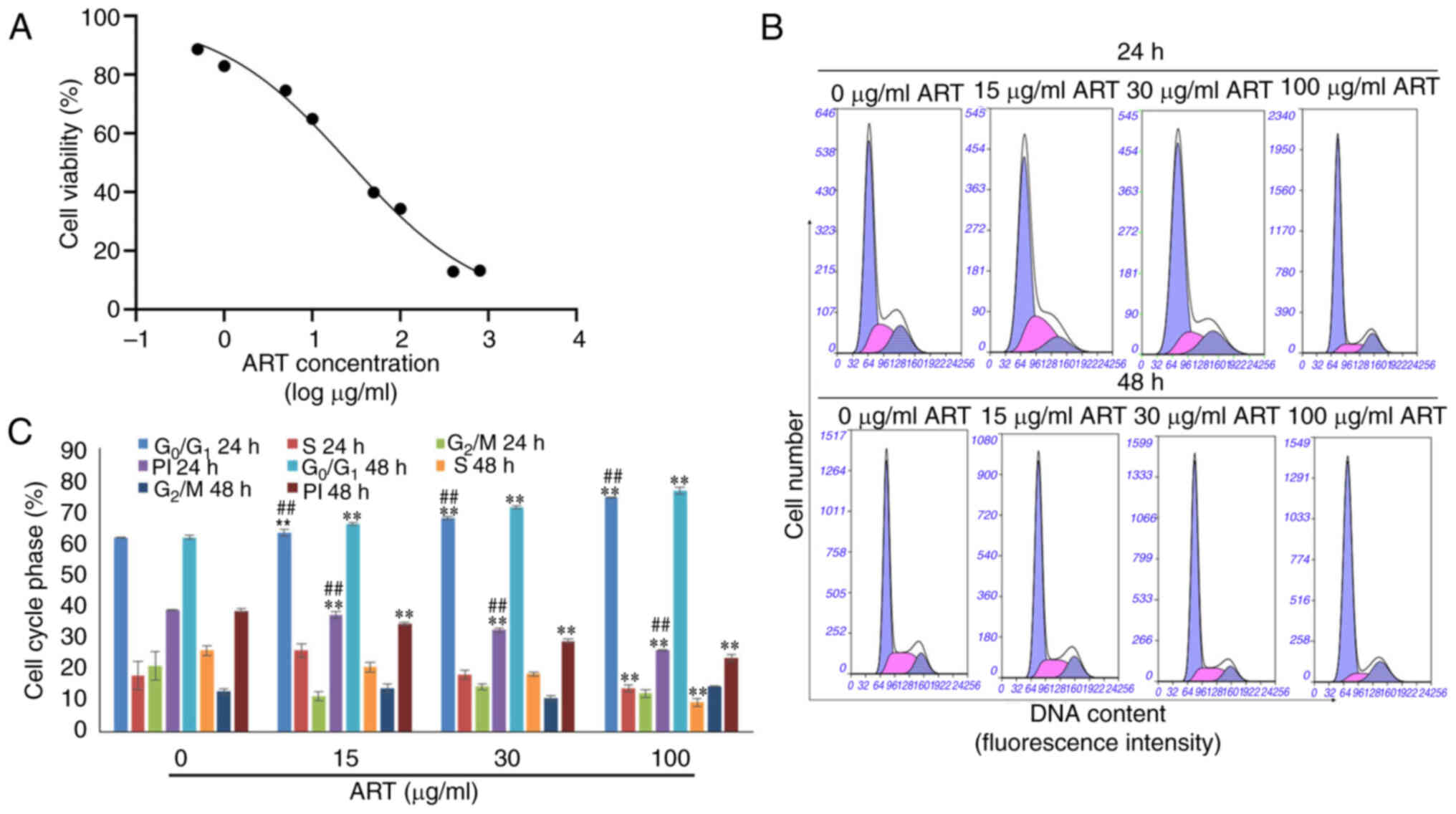
As shown in Fig. 1A,
SiHa cell survival decreased in a dose-dependent manner after
treatment with different concentrations of ART ranging from 0.5–800
µg/ml for 24 h. After treatment with ART for 24 h, the
IC50 was 26.32 µg/ml. Based on the IC50
value, three concentrations of ART (15, 30 and 100 µg/ml) were
selected for the subsequent experiments.
In addition, the proliferation indices of SiHa cells
were notably reduced in the ART-treated groups compared with that
in the control group in a dose- and time-dependent manner.
Furthermore, the proportion of cells in the G0/1 phase
was notably increased in the ART-treated groups compared with that
in the control group in a dose- and time-dependent manner (Fig. 1B and C).
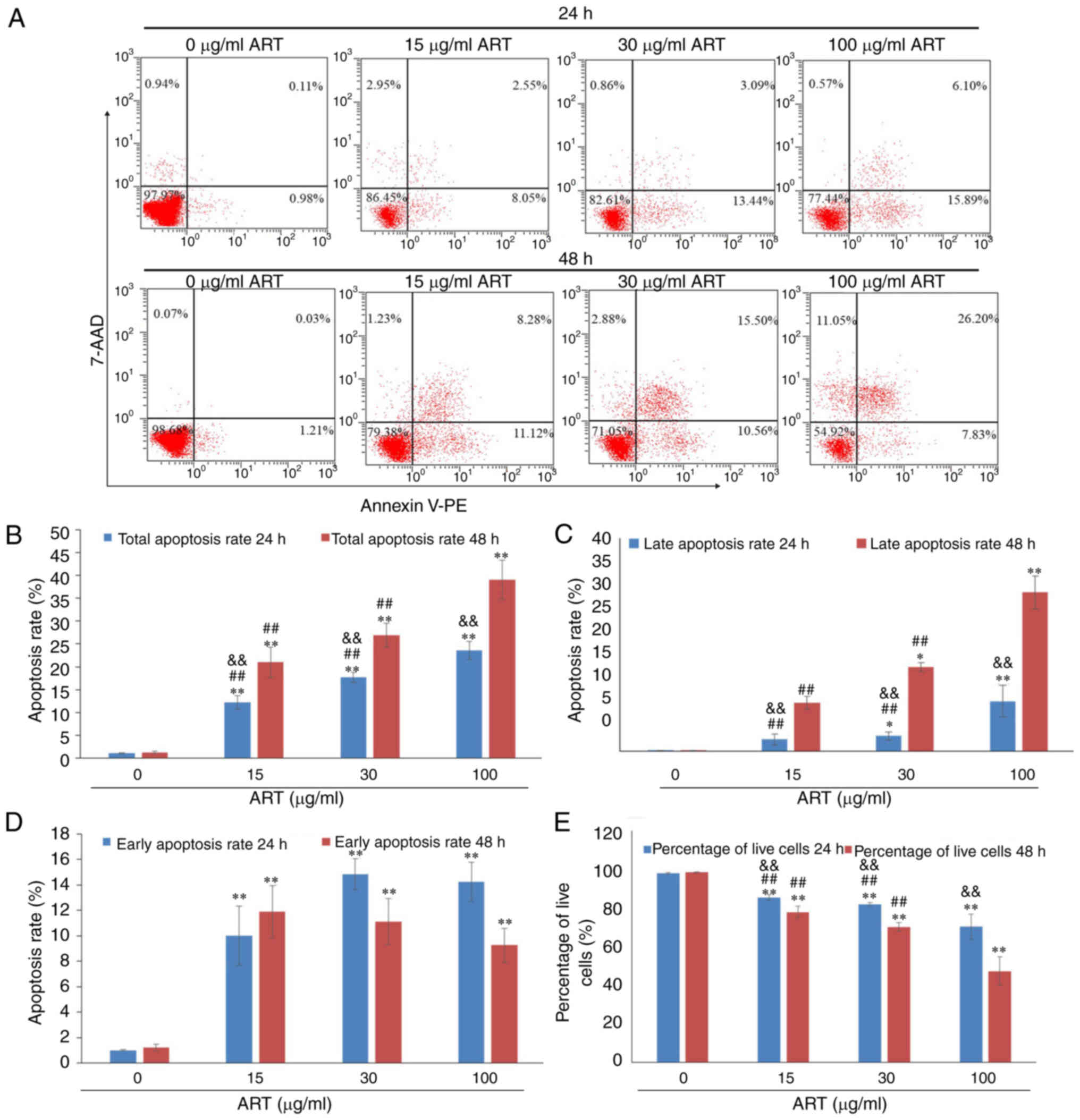
ART induces SiHa cell apoptosis
After exposure to various concentrations of ART (15,
30 or 100 µg/ml) for 24 or 48 h, with 0 µg/ml ART used as the
control, SiHa cells exhibited a dose- and time-dependent apoptosis
as demonstrated using Annexin V-PE/7-AAD staining and FCM (Fig. 2A).
The total apoptosis rate of SiHa cells, including
the early apoptosis rate and late apoptosis rate, was increased by
15, 30 or 100 µg/ml ART in a dose- and time-dependent manner
(Fig. 2B). The total apoptosis
rates of SiHa cells in the 15, 30 and 100 µg/ml ART groups were
significantly increased compared with that in the control group
(P<0.01). Furthermore, the total apoptosis rate of SiHa cells in
the 100 µg/ml ART group was significantly increased compared with
that in the 15 and 30 µg/ml ART groups (P<0.01). In addition,
with the increasing treatment duration and dose of ART, the late
apoptosis rate increased compared with that of the control group
(P<0.05; Fig. 2C). The early
apoptosis rates of SiHa cells in the 15, 30 and 100 µg/ml ART
groups were significantly increased compared with that in the
control group (P<0.01; Fig. 2D).
The percentages of live cells in the 15, 30 and 100 µg/ml ART
groups were significantly decreased compared with that in the
control group (P<0.01; Fig.
2E).
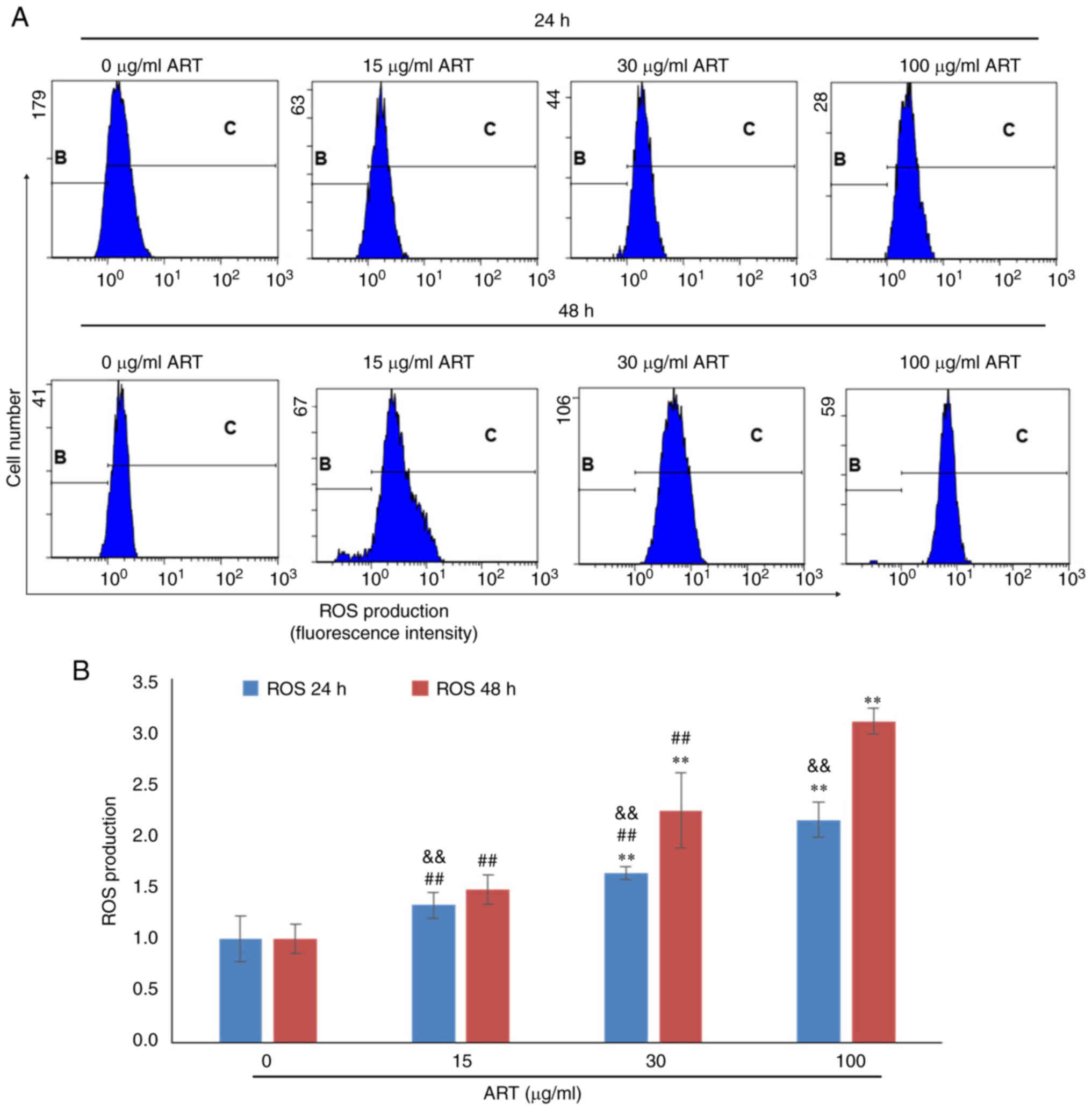
ART modulates ROS production,
Ca2+ levels and the mitochondrial membrane
potential
To further investigate the mechanism of SiHa cell
apoptosis induced by ART, the ROS production, Ca2+ level
and mitochondrial membrane potential of SiHa cells after ART
treatment were detected using FCM (Fig.
3, Fig. 4, Fig. 5). The results indicated that ROS
production in SiHa cells increased after 30 or 100 µg/ml ART
treatment in a dose- and time-dependent manner (Fig. 3A). ROS production in the 30 and 100
µg/ml ART groups was significantly increased compared with that in
the control group (P<0.01; Fig.
3B). Furthermore, ROS production in the 100 µg/ml ART group was
significantly increased compared with that in the 15 and 30 µg/ml
ART groups (P<0.01; Fig. 3B).
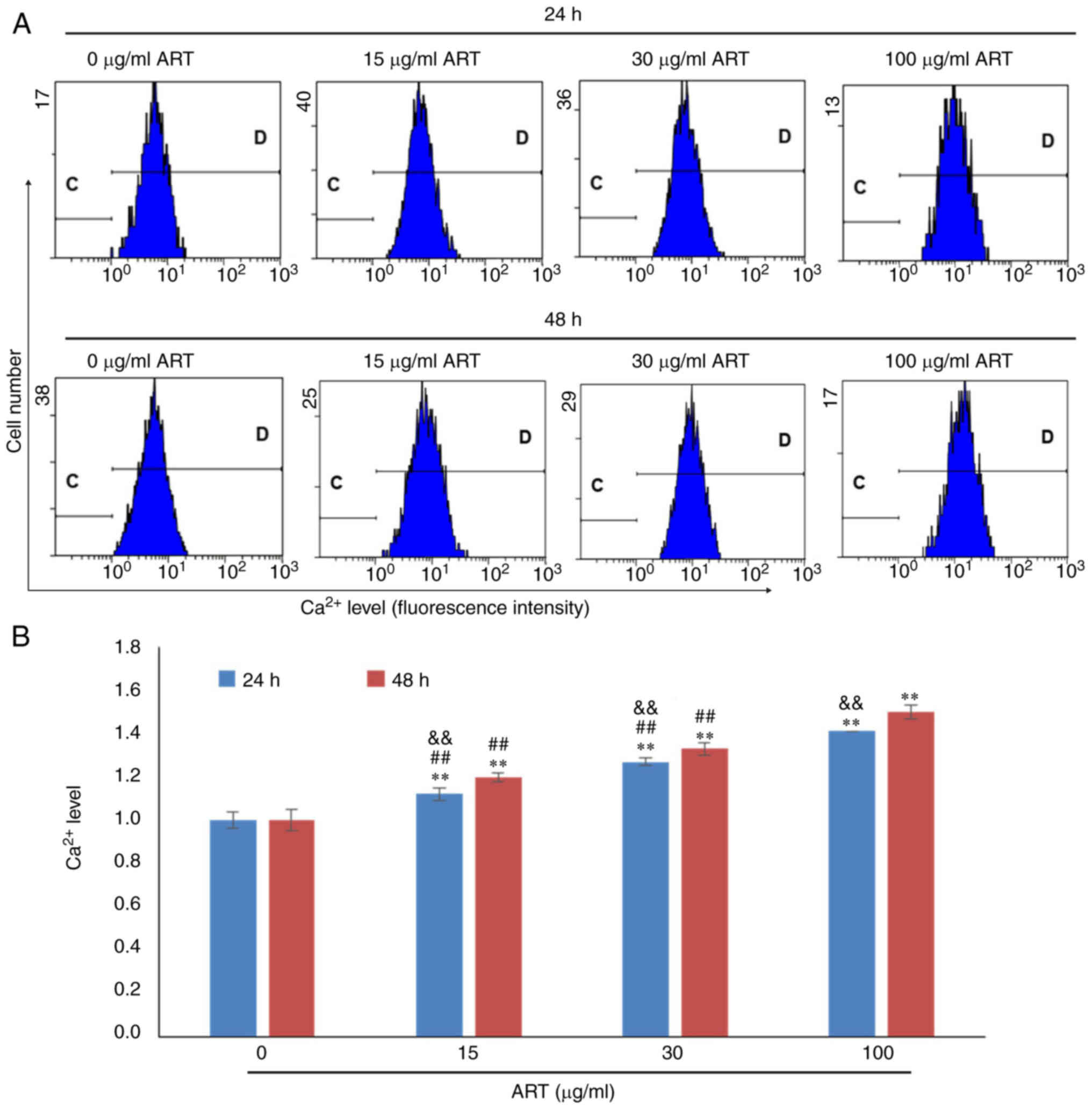
Additionally, the Ca2+ concentrations in SiHa cells in
the 15, 30 and 100 µg/ml ART groups were notably increased compared
with that in the control group in a dose- and time-dependent manner
(Fig. 4A and B). The
Ca2+ concentration in the 100 µg/ml ART group was also
significantly increased compared with that in the 15 and 30 µg/ml
ART groups (P<0.01; Fig.
4B).
In addition, the mitochondrial membrane potential in
the 15, 30 and 100 µg/ml ART groups was notably decreased compared
with that in the control group in a dose- and time-dependent manner
(Fig. 5).
Treatment with ART notably increased ROS production
and Ca2+ and decreased the mitochondrial membrane
potential, suggesting that ART triggered apoptosis in SiHa cells in
a dose- and time-dependent manner accompanied by the modulation of
ROS, Ca2+ and the mitochondrial membrane potential.
ART modulates Bcl2 family expression
levels in SiHa cells
Bcl2, Bcl-xl, Mcl-1, BIM, Bok, Bax and Bak regulate
cell apoptosis via pore formation in mitochondrial complexes, and
the balanced expression of Bcl2, Bcl-xl, Mcl-1, BIM, Bok, Bax and
Bak maintains the stability of the mitochondrial membrane.
Therefore, these molecules can serve as markers for the analysis of
the mechanism of action of the mitochondrial signalling
pathway-induced apoptosis (31).
RT-qPCR revealed notable dose- and time-dependent decreases in the
expression levels of Bcl2, Bcl-xl and Mcl-1 mRNAs compared with
those in the control group (Fig.
6). Furthermore, the expression levels of BIM, Bok, Bak and Bax
mRNAs, which are proapoptotic gene transcripts (32), were notably upregulated in a dose-
and time-dependent manner compared with that in the control group
following treatment with ART for 24 and 48 h (Fig. 6). These expression profiles were in
line with the increase in apoptotic activity and mitochondrial
membrane modulation observed in SiHa cells after ART treatment.
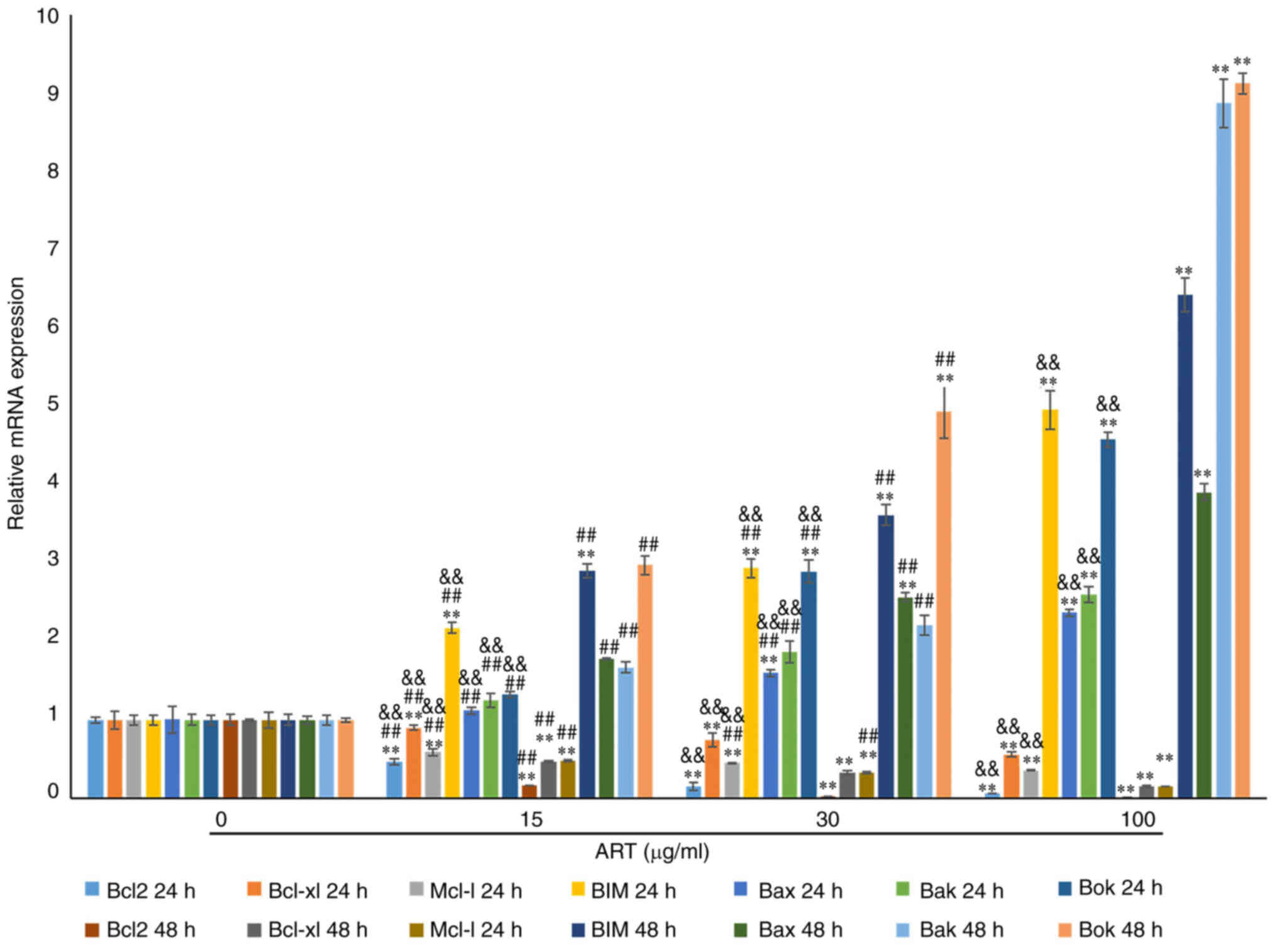 | Figure 6.ART modulates the expression of the
Bcl2 family in SiHa cells. RT-qPCR results showed that the mRNA
expression of Bcl2, Bcl-xl and Mcl-1 in the 15, 30 and 100 µg/ml
ART treated groups was notably reduced compared with that in the
control group in a dose- and time-dependent manner. The mRNA
expression of Bcl2, Bcl-xl and Mcl-1 in the 100 µg/ml ART group was
significantly reduced compared with that in the 15 µg/ml ART group.
RT-qPCR results also showed that the mRNA expression of Bax, Bak,
Bok and BIM in the 15, 30 and 100 µg/ml ART treated groups was
notably increased compared with that in the control group in a
dose- and time-dependent manner. The mRNA expression of Bax, Bak,
Bok and BIM in the 100 µg/ml ART group was significantly increased
compared with that in the 15 and 30 µg/ml ART groups (P<0.01).
**P<0.01 vs. 0 µg/ml ART group; ##P<0.01 vs. 100
µg/ml ART group; &&P<0.01 vs. 48 h group.
ART, artesunate; RT-qPCR, reverse transcription-quantitative
PCR. |
Discussion
In the present study, the inhibitory effect of ART
on the growth of SiHa cervical cancer cells was detected using a
CCK-8 assay. ART inhibited the growth of SiHa cells in the range of
0.5–800 µg/ml ART in a concentration-dependent manner. The
IC50 value for SiHa cells treated with ART for 24 h was
26.32 µg/ml. It was demonstrated that ART could inhibit the growth
of SiHa cells, which is consistent with the findings of previous
reports showing that ART inhibits the growth of tumour cells
(33,34). The inhibitory effect of ART on SiHa
cell growth and the underlying molecular mechanism were
investigated further. ART increased SiHa cell apoptosis and
decreased the PI of SiHa cells.
Mitochondria are at the core of the
mitochondria-mediated apoptosis pathway, and changes in the
mitochondrial membrane potential are associated with cell apoptosis
(35). A decrease in the
mitochondrial membrane potential leads to the release of cytochrome
c, the entry of Ca2+ into the cytosol and the
increase in the generation of ROS, resulting in irreversible
apoptosis (36).
Mitochondria-mediated apoptosis serves an important role in the
therapeutic efficacy of new antitumour drugs, such as
epigallocatechin-3-gallate and ropivacaine (22,37).
In the present study, compared with those in the control group, the
mitochondrial membrane potential of SiHa cells treated with 30 and
100 µg/ml ART for 24 and 48 h significantly decreased, and the
production of ROS and intracellular Ca2+ levels
significantly increased. These results are consistent with the
reported mechanism of drug-induced apoptosis in tumours (38,39).
Greenshields et al (40)
reported a dose- and time-dependent inhibitory effect of ART on the
growth of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3
breast cancer cells. ART inhibited breast cancer cell proliferation
via ROS-dependent G2/M arrest and ROS-independent
G1 arrest. ART-treated MDA-MB-468 and SK-BR-3 cells also
exhibited apoptotic cell death, which was both ROS- and
iron-dependent. ART-induced oxidative stress was indicated to
impair the mitochondrial outer membrane integrity and damage the
cellular DNA of MDA-MB-468 and SK-BR-3 cells (27). Huang et al (41) reported that ART serves as a
senescence and autophagy inducer to exert its inhibitory effect on
colorectal cancer in a ROS-dependent manner. The results of the
present study suggested that ART can induce apoptosis in cervical
cancer SiHa cells through a mitochondria-mediated apoptosis
pathway. The molecular mechanism through which ART may regulate the
mitochondrial membrane potential in SiHa cells was further
investigated.
The expression profile of the Bcl2 family on the
mitochondrial membrane is associated with the function of the
mitochondria. A number of Bcl2 family members, including Bcl2,
Bcl-xl, Mcl-1, BIM, Bax, Bok and Bak, are expressed on the outer
membrane of mitochondria. In the state of increasing the Bax
expression, Bax forms a heterodimer with the Bcl2 homology 3 (BH3)
domain of Bcl2 and Bcl-xl (31). An
increase in the expression of Bax or the expression of BIM, Bak or
Bok can cause Bax to dissociate from the heterodimer and
translocate from the cytosol to the mitochondrial outer membrane,
possibly leading to the release of proapoptotic factors (ROS and
Ca2+) (32). High
expression of Bcl2 in the mitochondrial outer membrane can result
in binding to BIM, Bax, Bak and Bok proteins to prevent their
function and reduce the transmembrane flow of Ca2+
(42). The results of the present
study revealed that the expression levels of Bcl2, Bcl-xl and
Mcl-1, which inhibit apoptosis, were significantly decreased in
SiHa cells treated with 15, 30 and 100 µg/ml ART for 24 and 48 h.
The expression levels of the Bcl2 family members BIM, Bax, Bak and
Bok were notably increased after ART treatment. Holien et al
(43) reported that ART treatment
efficiently inhibits cell growth and induces apoptosis in myeloma
and diffuse large B-cell lymphoma cell lines. Apoptosis is induced
concomitantly with the downregulation of Myc and antiapoptotic Bcl2
expression, as well as with the cleavage of caspase-3 (43). In this study, the role of Bcl2
family members in ART-induced apoptosis of cervical cancer SiHa
cells was comprehensively studied to provide an experimental basis
for the further study of the molecular mechanism through which ART
regulates the mitochondrial membrane potential of SiHa cells.
Furthermore, in the present study, the association between the
expression of the multimolecular Bcl2 family and mitochondrial
apoptosis was investigated. To the best of our knowledge, the
molecular mechanism via which ART regulates the Bcl2 family
molecules to induce apoptosis by modulating the mitochondrial
membrane potential has not been reported in the literature.
However, the lack of western blotting experiments was a limitation
of the present study that should be conducted in future
studies.
At present, a number of studies on the antitumour
effect of ART have been reported (41,44,45).
In the present study, ART-induced apoptosis in cervical cancer SiHa
cells through the induction of the mitochondria-mediated apoptosis
pathway was investigated. Additionally, the molecular mechanism
through which ART may regulate the mitochondrial membrane potential
was also explored. The results of the present study revealed that
ART had an anti-growth effect on SiHa cervical cancer cells, and
the mechanism was associated with the induction of SiHa cell
apoptosis and inhibition of cell proliferation. The mechanism of
apoptosis induction is associated with the regulation of the
expression levels of the Bcl2 family members Bcl2, Bcl-xl, Mcl-1,
BIM, Bax, Bak and Bok, which mediate the mitochondrial apoptosis
pathway (31,32,42).
The present study provides an experimental basis for the clinical
application of ART as an anticancer drug. Other potential
anticancer mechanisms of ART, such as the molecular mechanism
associated with inhibiting cell proliferation and inducing cell
apoptosis, will be investigated in future studies. In further
experiments, the toxic effects of ART on cervical cancer cells
using non-cancer cervical cells and positive drug groups will also
be investigated.
In conclusion, ART had an antiproliferative effect
on SiHa cervical cancer cells, and the mechanism was associated
with the induction of SiHa cell apoptosis and inhibition of cell
proliferation. The mechanism of apoptosis induction may involve the
regulation of the Bcl2 family members Bcl2, Bcl-xl, Mcl-1, BIM,
Bax, Bak and Bok, which mediate the mitochondrial apoptosis
pathway. The molecular mechanism of ART-induced SiHa cell apoptosis
should be studied further, and the molecular mechanism through
which ART inhibits cell proliferation should also be investigated
in future experiments.
Acknowledgements
Not applicable.
Funding
This study was supported by the Key Medical Science Project of
Hebei Province (grant no. 20211027).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QZ performed the experiments and wrote the
manuscript. XL and CH performed the experiments and the statistical
analysis. RZ and JW performed the experiments. LL designed and
performed the experiments, and revised the manuscript. All authors
read and approved the final manuscript. QZ and LL confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ART
|
artesunate
|
|
IC50
|
half-maximal inhibitory
concentration
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gu XY, Zheng RS, Sun KX, Zhang SW, Zeng
HM, Zou XN, Chen WQ and He J: Incidence and mortality of cervical
cancer in China, 2014. Zhonghua Zhong Liu Za Zhi. 40:241–246.
2018.(In Chinese). PubMed/NCBI
|
|
3
|
Kaidar-Person O, Bortnyak-Abdah R, Amit A,
Berniger A, Ben-Yosef R and Kuten A: Current principles for
radiotherapy in cervical cancer. Med Oncol. 29:2919–2922. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sharma S, Deep A and Sharma AK: Current
treatment for cervical cancer: An update. Anticancer Agents Med
Chem. 20:1768–1779. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Diaz-Padilla I, Monk BJ, Mackay HJ and
Oaknin A: Treatment of metastatic cervical cancer: Future
directions involving targeted agents. Crit Rev Oncol Hematol.
85:303–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lefèvre A and Léonard P: Artesunate and
severe malaria in paediatrics. Rev Med Liege. 74:503–507.
2019.PubMed/NCBI
|
|
7
|
Abanyie F, Acharya SD, Leavy I, Bowe M and
Tan KR: Safety and effectiveness of intravenous artesunate for
treatment of severe malaria in the united States-April 2019 through
december 2020. Clin Infect Dis. 73:1965–1972. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roussel C, Ndour PA, Kendjo E, Larréché S,
Taieb A, Henry B, Lebrun-Vignes B, Chambrion C, Argy N, Houzé S, et
al: Intravenous artesunate for the treatment of severe imported
malaria: Implementation, efficacy, and safety in 1391 patients.
Clin Infect Dis. 73:1795–1804. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsuda K, Miyamoto L, Hamano S, Morimoto Y,
Kangawa Y, Fukue C, Kagawa Y, Horinouchi Y, Xu W, Ikeda Y, et al:
Mechanisms of the pH- and Oxygen-dependent oxidation activities of
artesunate. Biol Pharmaceutical Bull. 41:555–563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li T, Chen H, Liu XG, Zhou YX and Bai SF:
Immunoregulatory effect of artesunate on allergic contact
dermatitis and its mechanism. Yao Xue Xue Bao. 47:884–889.
2012.PubMed/NCBI
|
|
11
|
Meng QF, Zhang XX, Zhang Z, Chen W, Li XL,
Wang YJ, Li FF and Li YB: Therapeutic potential of artesunate in
experimental autoimmune myasthenia gravis by upregulated T
regulatory cells and regulation of Th1/Th2 cytokines. Pharmazie.
73:526–532. 2018.PubMed/NCBI
|
|
12
|
Wang N, Zeng GZ, Yin JL and Bian ZX:
Artesunate activates the ATF4-CHOP-CHAC1 pathway and affects
ferroptosis in Burkitt's Lymphoma. Biochem Biophys Res Commun.
519:533–539. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang F, Zhou JY, Zhang D, Liu MH and Chen
YG: Artesunate induces apoptosis and autophagy in HCT116 colon
cancer cells, and autophagy inhibition enhances the
artesunate-induced apoptosis. Int J Mol Med. 42:1295–1304.
2018.PubMed/NCBI
|
|
14
|
Zhao F, Vakhrusheva O, Markowitsch SD,
Slade KS, Tsaur I, Cinatl J Jr, Michaelis M, Efferth T, Haferkamp A
and Juengel E: Artesunate impairs growth in cisplatin-resistant
bladder cancer cells by cell cycle arrest, apoptosis and autophagy
induction. Cells. 9:26432020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Gan S, Han L, Li X, Xie X, Zou D
and Sun H: Artesunate induces apoptosis and inhibits the
proliferation, stemness, and tumorigenesis of leukemia. Ann Transl
Med. 8:7672020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Våtsveen TK, Myhre MR, Steen CB, Wälchli
S, Lingjærde OC, Bai B, Dillard P, Theodossiou TA, Holien T, Sundan
A, et al: Artesunate shows potent anti-tumor activity in B-cell
lymphoma. J Hematol Oncol. 11:232018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yin X, Liu Y, Qin J, Wu Y, Huang J, Zhao
Q, Dang T, Tian Y, Yu P and Huang X: Artesunate suppresses the
proliferation and development of estrogen receptor-α-Positive
endometrial cancer in HAND2-Dependent pathway. Front Cell Dev Biol.
8:6069692020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mancuso RI, Foglio MA and Olalla Saad ST:
Artemisinin-type drugs for the treatment of hematological
malignancies. Cancer Chemother Pharmacol. 87:1–22. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu L, Zuo LF, Zuo J and Wang J:
Artesunate induces apoptosis and inhibits growth of Eca109 and
Ec9706 human esophageal cancer cell lines in vitro and in vivo. Mol
Med Rep. 12:1465–1472. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang L, Liu L, Wang J and Chen Y:
Inhibitory effect of artesunate on growth and apoptosis of gastric
cancer cells. Arch Med Res. 48:623–630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saeed MEM, Cives-Losada C and Efferth T:
Biomarker expression profiling in cervix carcinoma biopsies
unravels WT1 as a target of artesunate. Cancer Genomics Proteomics.
19:727–739. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Ju Y, Wang J and Zhou R:
Epigallocatechin-3-gallate promotes apoptosis and reversal of
multidrug resistance in esophageal cancer cells. Pathol Res Pract.
213:1242–1250. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kriváková P, Cervinková Z, Lotková H,
Kucera O and Rousar T: Mitochondria and their role in cell
metabolism. Acta Medica (Hradec Kralove) Suppl. 48:57–67. 2005.(In
Czech). PubMed/NCBI
|
|
24
|
Estaquier J, Vallette F, Vayssiere JL and
Mignotte B: The mitochondrial pathways of apoptosis. Adv Exp Med
Biol. 942:157–183. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lopez J and Tait SW: Mitochondrial
apoptosis: Killing cancer using the enemy within. Br J Cancer.
112:957–962. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jeong SY and Seol DW: The role of
mitochondria in apoptosis. BMB Rep. 41:11–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kaloni D, Diepstraten ST, Strasser A and
Kelly GL: BCL-2 protein family: Attractive targets for cancer
therapy. Apoptosis. 28:20–38. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kashyap D, Garg VK and Goel N: Intrinsic
and extrinsic pathways of apoptosis: Role in cancer development and
prognosis. Adv Protein Chem Struct Biol. 125:73–120. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Ju Y, Liu J, Chen Y, Huo X and Liu
L: Inhibition of proliferation and migration and induction of
apoptosis in glioma cells by silencing TLR4 expression levels via
RNA interference. Oncol Lett. 21:132021.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Warren CFA, Wong-Brown MW and Bowden NA:
BCL-2 family isoforms in apoptosis and cancer. Cell Death Dis.
10:1772019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hafezi S and Rahmani M: Targeting BCL-2 in
cancer: Advances, challenges, and perspectives. Cancers (Basel).
13:12922021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wen L, Lv G, Zhao J, Lu S, Gong Y, Li Y,
Zheng H, Chen B, Gao H, Tian C and Wang J: In vitro and in vivo
effects of artesunate on echinococcus granulosus protoscoleces and
metacestodes. Drug Des Devel Ther. 14:4685–4694. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Q, Ni W, Deng Z, Liu M, She L and Xie
Q: Targeting nasopharyngeal carcinoma by artesunate through
inhibiting Akt/mTOR and inducing oxidative stress. Fundam Clin
Pharmacol. 31:301–310. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Abate M, Festa A, Falco M, Lombardi A,
Luce A, Grimaldi A, Zappavigna S, Sperlongano P, Irace C, Caraglia
M and Misso G: Mitochondria as playmakers of apoptosis, autophagy
and senescence. Semin Cell Dev Bio. 98:139–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li J, Cui J, Li Z, Fu X, Li J, Li H, Wang
S and Zhang M: ORP8 induces apoptosis by releasing cytochrome c
from mitochondria in non-small cell lung cancer. Oncol Rep.
43:1516–1524. 2020.PubMed/NCBI
|
|
37
|
Wang W, Zhu M, Xu Z, Li W, Dong X, Chen Y,
Lin B and Li M: Ropivacaine promotes apoptosis of hepatocellular
carcinoma cells through damaging mitochondria and activating
caspase-3 activity. Biol Res. 52:362019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou X, Chen Y, Wang F, Wu H, Zhang Y, Liu
J, Cai Y, Huang S, He N, Hu Z and Jin X: Artesunate induces
autophagy dependent apoptosis through upregulating ROS and
activating AMPK-mTOR-ULK1 axis in human bladder cancer cells. Chem
Biol Interact. 331:1092732020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ji P, Huang H, Yuan S, Wang L, Wang S,
Chen Y, Feng N, Veroniaina H, Wu Z, Wu Z and Qi X: ROS-mediated
apoptosis and anticancer effect achieved by artesunate and
auxiliary fe(II) released from ferriferous Oxide-Containing
recombinant apoferritin. Adv Healthc Mater. 8:e19009112019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Greenshields AL, Fernando W and Hoskin DW:
The anti-malarial drug artesunate causes cell cycle arrest and
apoptosis of triple-negative MDA-MB-468 and HER2-enriched SK-BR-3
breast cancer cells. Exp Mol Pathol. 107:10–22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang Z, Gan S, Zhuang X, Chen Y, Lu L,
Wang Y, Qi X, Feng Q, Huang Q, Du B, et al: Artesunate Inhibits the
cell growth in colorectal cancer by promoting ROS-Dependent cell
senescence and autophagy. Cells. 11:24722022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Qian S, Wei Z, Yang W, Huang J, Yang Y and
Wang J: The role of BCL-2 family proteins in regulating apoptosis
and cancer therapy. Front Oncol. 12:9853632022. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Holien T, Olsen OE, Misund K, Hella H,
Waage A, Rø TB and Sundan A: Lymphoma and myeloma cells are highly
sensitive to growth arrest and apoptosis induced by artesunate. Eur
J Haematol. 91:339–346. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cao D, Chen D, Xia JN, Wang WY, Zhu GY,
Chen LW, Zhang C, Tan B, Li H and Li YW: Artesunate promoted
anti-tumor immunity and overcame EGFR-TKI resistance in
non-small-cell lung cancer by enhancing oncogenic TAZ degradation.
Biomed Pharmacothe. 155:1137052022. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li ZJ, Dai HQ, Huang XW, Feng J, Deng JH,
Wang ZX, Yang XM, Liu YJ, Wu Y, Chen PH, et al: Artesunate
synergizes with sorafenib to induce ferroptosis in hepatocellular
carcinoma. Acta Pharmacol Sin. 42:301–310. 2021. View Article : Google Scholar : PubMed/NCBI
|