Introduction
Colorectal cancer (CRC) is one of the most common
malignant tumors in the world, with its incidence steadily rising
in developing countries. The mortality rate associated with CRC has
risen to being the second highest worldwide, constituting ~10% of
all fatalities attributed to malignant tumors (1). This is a widespread situation, given
that 20–25% of patients present with distant metastases at the time
of their initial diagnosis (2–4). The
lung, ranked as the second most common site for CRC metastasis
following the liver, poses a serious threat to patient survival
(5,6). Surgery is considered the standard
treatment for patients with resectable CRC lung metastases. In
general, surgery is preferred for lung metastases from a single
lesion with low morbidity and mortality when the patient is able to
tolerate surgery and this treatment strategy has demonstrated
5-year survival rates of up to 70% (7). Surgery usually provides more complete
treatment results and a means of more effective local control.
However, surgery may be accompanied by a greater risk of trauma and
postoperative complications and therefore this treatment strategy
requires that the patient be both in relatively good health and
able to tolerate surgery. In clinical practice, only a minority of
patients with CRC with lung metastases are eligible for surgical
intervention. Numerous patients are unable to undergo surgery due
to the presence of double lung metastases or multiple lesions,
their having an advanced age, or because of comorbidities with
unmanageable underlying diseases. Therefore, various nonsurgical
approaches, including percutaneous ablation and stereotactic body
radiotherapy (SBRT), are increasingly explored as alternative
strategies for managing tumors in these patients.
Among the percutaneous ablation techniques, commonly
employed methods include radiofrequency ablation (RFA), microwave
ablation (MWA) and cryoablation. RFA is the most widely used and
well-validated approach (8,9). MWA has also been demonstrated to be a
safe and effective method for the treatment of CRC lung metastases,
yielding a median overall survival (OS) of 31–32.8 months. Notably,
it has exhibited distinct advantages in local tumor control
compared with other ablation methods (10,11).
Moreover, animal models have shown that MWA outperforms RFA in
terms of ablation zone size and expansion of the ablation border,
complete ablation and tumor control rates, sensitivity to the
‘heat-sink effect’ and reduced thermal conductivity of the
ventilated lungs. These factors are crucial for sufficiently large
lesions with a safe margin around the ablation zone (12–14).
In addition, MWA boasts a shorter ablation time and a larger
ablation range compared with RFA (14).
SBRT has emerged as a beneficial complement to
non-surgical treatment methods for lung metastases, including those
arising from CRC. SBRT can deliver high radiation doses to tumors
while minimizing radiation exposure to the surrounding normal
tissues, leading to a high rate of local tumor control and a
tolerable level of toxicity as far as normal tissues are concerned.
In comparison with surgery and ablative therapies, the key
advantages of SBRT lie in its non-invasiveness, low morbidity, good
tolerability and suitability for outpatient treatment (15). In terms of local control of CRC lung
metastases, previously published studies on the efficacy of SBRT
have shown considerable variability, with reported 2-year local
control rates ranging from 65.8–80% (16–19).
However, SBRT exhibits a distinct advantage over other methods for
larger tumors near blood vessels and both the number and location
of lung lesions and the presence of synchronous extrapulmonary
metastases and mediastinal lymph metastases are important
prognostic factors. Nevertheless, use of SBRT often leads to
radiation pneumonitis during the treatment of lung metastases while
the lesions shrink, causing irreversible damage to the lungs of
patients (20).
MWA and SBRT have become standard non-surgical
methods for lung metastases, demonstrating good efficacy in terms
of local control of lung metastases as well as patient survival,
similar to the level of efficacy observed with surgery (21). However, to date, to the best of our
knowledge, there is still no universally recognized standard for
selecting the most appropriate treatment approach. The literature
supporting such a standard is also limited, leaving the choice
between MWA and SBRT needing primarily to be made at the discretion
of individual clinicians. In the context of lung metastases from
CRC, there is currently a lack of studies that have directly
compared the efficacies of MWA and SBRT. The objectives of the
present study were therefore to compare the outcomes of MWA and
SBRT for treating CRC lung metastases and try to contribute to the
clarification of the selection standard.
Patients and methods
Patient population
Patients with CRC with lung metastases who were
treated between January 2015 and December 2022 at Sir Run Run Shaw
Hospital, Zhejiang University School of Medicine (Zhejiang, China)
were included in the present study who met the following inclusion
criteria: i) The patient was diagnosed with primary CRC based on
pathological evidence; ii) clinical or pathological diagnosis of
CRC lung metastases was made; iii) the first treatment the patient
received was MWA or SBRT; and iv) complete clinical and imaging
data were available. The exclusion criteria were as follows: i) The
patient was aged <18 years; ii) poor image quality or
significant artifacts were present; iii) the follow-up duration was
<6 months; and iv) the metastases were combined with other
primary tumors (Fig. 1). All
procedures performed in the present study involving medical record
information and data were approved by the Medical Ethics Committee
of Sir Run Run Shaw Hospital (Zhejiang, China; project number
20230430).
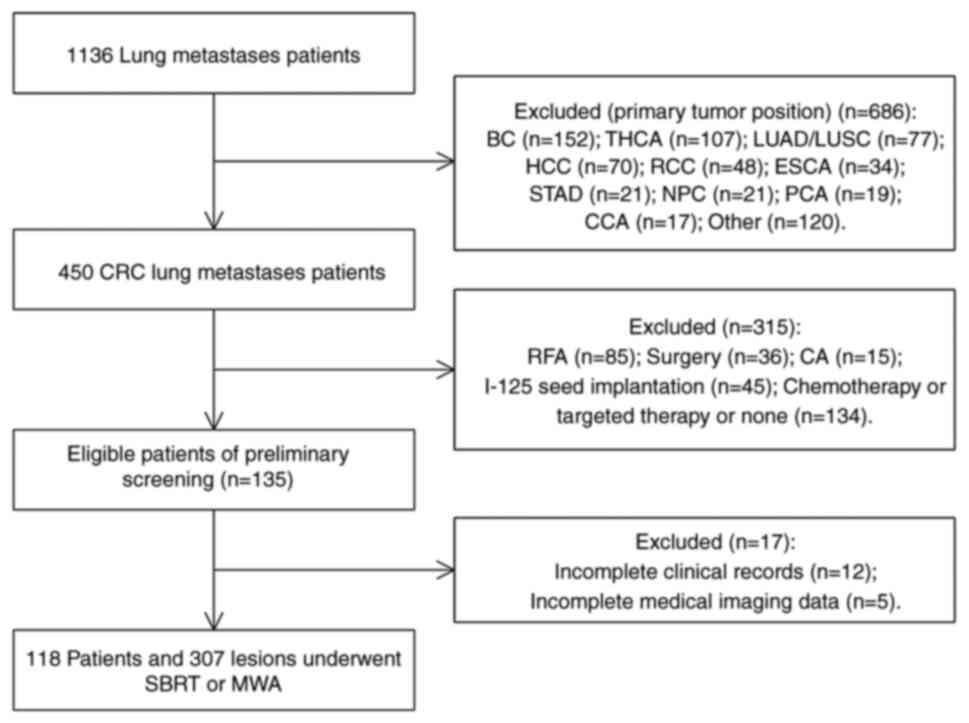 | Figure 1.Flow diagram of patient selection.
BC, breast cancer; THCA, thyroid carcinoma; LUAD, lung
adenocarcinoma; LUSC, lung squamous cell carcinoma; HCC,
hepatocellular carcinoma; RCC, renal cell carcinoma; ESCA,
esophageal carcinoma; STAD, stomach adenocarcinoma; NPC,
nasopharyngeal carcinoma; PCA, pancreatic cancer; CCA, cervical
cancer; RFA, radiofrequency ablation; CA, cryoablation; SBRT,
stereotactic body radiation therapy; MWA, microwave ablation. |
MWA or SBRT treatments
The MWA procedures were performed by interventional
radiologists with >8 years' experience in interventional
oncology at the Sir Run Run Shaw Hospital. The ECO-100A1 microwave
therapeutic instrument (ECO Medical Instrument Co., Ltd.), was used
to perform MWA. The appropriate microwave ablation needle was
selected according to the size and location of the tumor. Based on
the location of the target tumor, patients were positioned
accordingly (i.e., in the supine, lateral or prone position). The
skin-to-tumor distance was measured on computed tomography (CT)
images to determine the optimal puncture point and path for the
ablation needle, guided by CT imaging. Accurate puncture of the
lesion was performed under CT guidance and impedance monitoring and
temperature control were employed to regulate the treatment
process. Repeated CT scans were performed during ablation to
confirm the coverage of the ablated area. If no apparent signs of
bleeding were observed on the CT scans during the operation, the
ablation needle was removed and the patient was sent back to the
ward with local compression. In case of significant pneumothorax,
thoracic puncture drainage was performed during the procedure.
For the SBRT procedure, patients were placed in the
supine position to run chest CT localization scans. Radiation
oncologists subsequently delineated the target area on the
generated images. The gross target volume (GTV) was outlined on the
CT lung window image, including the short burr roots around the
lesion and the areas of pleural invasion. Lesions close to the
mediastinum were carefully observed on mediastinal window images in
order to assess their involvement in the mediastinum and
surrounding tissues and the target area was modified accordingly.
The planning target volume was generated by expanding the GTV
outlined on routine CT scans by 10 mm. The dose fractionation
varied, with the majority of the treatments comprising five
fractions or fewer and individual doses were typically of the order
of 10 Gy, with adjustments made based on each patient's specific
circumstances.
Follow-up and outcomes
All patients underwent follow-up using chest CT
following treatment. After treatment, patients underwent chest CT
scans monthly for the first three months, followed by scans every
2–3 months thereafter. The therapeutic outcomes between the MWA
group and the SBRT group were compared by evaluating the LTP-free
survival (LTPFS), disease-free survival (DFS) and OS rates. LTP was
defined as either the recurrence of the treated tumor itself, or
the emergence of a new local tumor within a 10-mm area around it on
the CT images following treatment, whereas LTPFS was defined as the
time interval from treatment to the occurrence of LTP, or to when
the patient succumbed, or to loss to follow-up (22). DFS was defined as the duration from
the initiation of MWA or SBRT to the detection of intra- or
extra-pulmonary metastasis during follow-up examinations. Finally,
OS values were calculated from the start of MWA or SBRT to either
the death of the patient or the last follow-up date. Follow-up
visits were conducted every three months, including recent patient
visits to the hospital. If the patients had not visited the
hospital recently, we called to inquire about their status,
including survival and disease progression. During follow-up, the
patients continued to receive treatments due to disease progression
to prolong the survival including ablation, SBRT and chemotherapy.
This was unavoidable in retrospective studies, although it affected
the OS of patients.
Statistical analysis
In comparing patient characteristics between the
SBRT and MWA groups, categorical variables were analyzed using the
Chi-square (χ2) test, whereas continuous variables that
did not pass the K-S normality test were analyzed using the
Mann-Whitney U-test. Univariate and multivariate analyses were
performed using the Cox proportional hazards regression model to
identify the potential factors affecting LTPFS, DFS and OS. To
determine the prognostic factors, multivariate analysis using
stepwise variable selection was performed. The Omnibus test was
used to evaluate the COX regression model. To mitigate treatment
selection bias and control for other potential confounding factors,
inverse probability of treatment weighting (IPTW) was used for
adjustment when comparing the OS, DFS and LTPFS between the MWA and
SBRT groups, as well as the 1- and 3-year LTP, OS, DFS and LTPFS
rates. For IPTW adjustments, patients in the MWA group were
weighted as the inverse of the propensity score (PS), while
patients in the SBRT group were weighted as the inverse of 1-PS.
The PS was calculated as the probability of receiving MWA,
determined through multivariate logistic regression analysis. The
Hosmer-Lemeshow goodness-of-fit test was performed to evaluate the
adequacy of the estimated PSs. Multivariate analysis included all
covariates used to calculate the PS. All statistical analyses were
conducted using SPSS v25 (IBM Corp.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Assessment of the patient
characteristics
The present study screened 1,136 patients with lung
metastases, of whom 450 were patients with CRC with lung metastases
and 332 patients who did not meet the criteria were excluded
(Fig. 1). A total of 118 patients
were included in the present study, of which 85 (72.0%) patients
with a total of 221 (out of an overall total of 307; 72.0%) lung
metastases were treated with SBRT, whereas 33 (28.0%) patients with
a total of 86 (out of an overall total of 307; 28.0%) lung
metastases were treated with MWA. Seventy-three (65.3%) patients
were male (see Table SI). Among
the 118 patients, the body mass index (BMI) ranged from 17.3–31.2
kg/m2 (mean, 23.67±3.04 kg/m2. The primary
site of the tumor was located in the rectum in a total of 76
(64.4%) cases. The degree of differentiation of the tumors was
found to be predominantly moderately differentiated (n=56; 47.5%).
Neoadjuvant therapy at the primary site was performed in 45 (38.1%)
patients. A total of 48 patients (40.7%) were found to have
solitary lung metastases. The liver was the most common
extrapulmonary organ. The essential characteristics of the lung
metastases are shown in Table SII.
The mean size of the metastatic lesions was 11.80±8.13 mm. Finally,
53 (17.3%) of the lesions exhibited internal features. The internal
features in this manuscript refer to cavity, vacuole sign or air
bronchogram sign. During follow-up, 63 patients in the SBRT group
received postoperative chemotherapy and 53 patients received
targeted therapy; seven patients received particle therapy because
of progression and four patients received ablative therapy. A total
of 22 patients in the MWA group received postoperative chemotherapy
and 19 patients received targeted therapy; one patient received
particle therapy because of progression and four patients received
ablative therapy.
Characteristics of the patients and their lesions
are shown in Table I. Statistically
significant differences were observed between the SBRT and MWA
groups regarding the diameter of the metastasis, preoperative
targeted therapy, concurrent extrapulmonary metastasis, proximity
to the diaphragm, pneumothorax, local progression and postoperative
adjuvant treatment (P<0.05). Subsequently, the effects of
different treatment modalities on the OS, DFS and LTPFS rates were
further analyzed. The Kaplan-Meier method was used to reveal that
the MWA group did not reach the median survival time. Furthermore,
the differences in DFS and LTPFS between the two treatment groups
were found to be statistically significant (P=0.022), although no
significant difference was observed in terms of the OS
(P=0.064).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Variable | SBRT (n=85) | MWA (n=33) | P-value |
|---|
| Age (mean,
IQR) | 61 (55–66) | 58 (55–67) | 0.824 |
| ≤70
years (%) | 71 (83.5) | 27 (81.8) |
|
| >70
years, n (%) | 14 (16.5) | 6 (18.2) |
|
| Sex, male (%) | 54 (63.5) | 23 (69.7) | 0.528 |
| Primary cancer
location (rectum) | 54 (63.5) | 23 (69.7) | 0.528 |
| Lung lobe
distribution |
|
| 0.169 |
| Left, n
(%) | 17 (20.0) | 10 (30.3) |
|
| Right,
n (%) | 32 (37.6) | 15 (45.5) |
|
| Both, n
(%) | 36 (42.4) | 8 (24.2) |
|
| Neoadjuvant
therapy, n (%) | 35 (41.2) | 10 (30.3) | 0.275 |
| Preoperative
targeted therapy, n (%) | 10 (11.8) | 9 (27.3) | 0.040a |
| Extrapulmonary
metastases, n (%) | 57 (67.1) | 13 (39.4) | 0.006a |
| Underlying
diseases, n (%) | 47 (55.3) | 16 (48.5) | 0.506 |
| BMI (<18.5 or
>23.9 kg/m2), n (%) | 40 (47.1) | 17 (51.2) | 0.664 |
| CEA (>5.0
ng/ml), n (%) | 30 (35.3) | 11 (33.3) | 0.841 |
| Preoperative
emphysema (%) | 17 (20.0) | 5 (15.2) | 0.544 |
| Preoperative lung
bullae, n (%) | 10 (11.8) | 7 (21.2) | 0.308 |
| Hilar
lymphadenopathy, n (%) | 2 (2.4) | 1 (3.0) | 1.000 |
| Mediastinal
lymphadenopathy, n (%) | 3 (3.5) | 1 (3.0) | 1.000 |
| Pneumothorax |
|
|
<0.001a |
| None, n
(%) | 85 (100) | 11 (33.3) |
|
| Little,
n (%) | 0 (0.0) | 14 (42.4) |
|
|
Moderate to large, n (%) | 0 (0.0) | 8 (24.2) |
|
| Adjuvant
(postoperative) therapy, n (%) | 69(81.2) | 24 (72.7) | 0.014a |
| Metastasis
diameter, mm. mean | 12.553±8.545 | 9.871±6.624 | 0.001a |
| Adjacent to vessels
>3 mm, n (%) | 37 (16.7) | 11 (12.8) | 0.392 |
| Adjacent to vessels
>5 mm, n (%) | 18 (8.1) | 5 (5.8) | 0.486 |
| Adjacent to the
bronchus >2 mm, n (%) | 39 (17.6) | 20 (23.2) | 0.263 |
| Near mediastinal
pleura 10 mm, n (%) | 33 (14.9) | 10 (11.6) | 0.454 |
| Near chest wall
pleura 10 mm, n (%) | 90 (40.7) | 31 (36.0) | 0.451 |
| Near pleura 0.5 mm,
n (%) | 53 (24.0) | 13 (15.1) | 0.090 |
| Near interlobar
pleura 10 mm, n (%) | 49 (22.2) | 17 (19.8) | 0.645 |
| Near the diaphragm
10 mm, n (%) | 28 (12.7) | 4 (4.7) | 0.039a |
| Internal features,
n (%) | 38 (17.2) | 15 (17.4) | 0.959 |
| Local progression,
n (%) | 96 (43.4) | 25 (29.1) | 0.021a |
Analysis of LTP values
The median follow-up duration for the SBRT group was
found to be 32 months [interquartile range (IQR), 20–44 months],
whereas for the MWA group, it was 26 months (IQR, 17–47 months).
Throughout the follow-up period, local progression was observed in
121 of the 307 lesions (39.4%). Specifically, 96 out of 221 (43.4%)
lesions in the SBRT group and 25 out of 86 (29.1%) lesions in the
MWA group exhibited local progression. The difference in 1-year LTP
between the SBRT and MWA groups was found to be insignificant (29.0
vs. 19.8%; P=0.101), although a significant difference was observed
for the 3-year LTP (43.0 vs. 29.1%; P=0.025) (Table II). However, following IPTW
adjustment, significant differences in the 1-year and 3-year LTP
rates were observed between the two groups (P<0.001).
 | Table II.Comparison of 1- and 3-year LTP, OS,
DFS and LTPFS for MWA and SBRT. |
Table II.
Comparison of 1- and 3-year LTP, OS,
DFS and LTPFS for MWA and SBRT.
| Variable | SBRT (%) | MWA (%) | P-value
(before) | P-value
(IPTW-adjusted) |
|---|
| LTP |
|
|
|
|
|
1-year | 64 (29.0) | 17 (19.8) | 0.101 |
<0.001a |
|
3-year | 95 (43.0) | 25 (29.1) | 0.025a |
<0.001a |
| OS |
|
|
|
|
|
1-year | 79 (92.9) | 31 (93.9) | 1.000 | 0.354 |
|
3-year | 38 (44.7) | 12 (36.4) | 0.410 |
<0.001a |
| DFS |
|
|
|
|
|
1-year | 38 (44.7) | 17 (51.5) | 0.506 |
<0.001a |
|
3-year | 6 (7.1) | 4 (12.1) | 0.604 | 0.016a |
| LTPFS |
|
|
|
|
|
1-year | 57 (67.1) | 23 (69.7) | 0.783 | 0.077 |
|
3-year | 12 (14.1) | 6 (18.2) | 0.582 | 0.204 |
The data from the univariate and multivariate
analyses for the predictors of LTPFS are summarized in Table III. According to the univariate
analysis, the potential predictors for LTPFS included
extrapulmonary metastases [hazard ratio (HR), 1.884; 95% confidence
intervals (95% CI), 1.066–3.327; P=0.029)], the level of
carbohydrate antigen 19-9 (CA19-9; HR, 2.204; 95% CI, 1.109–4.382;
P=0.024), metastasis diameter (HR, 1.564; 95% CI, 1.121–2.181;
P=0.009), internal features (HR, 1.760; 95% CI, 1.180–2.624;
P=0.006), local progression (HR, 3.649; 95% CI, 2.524–5.274;
P<0.001) and treatment modality (HR, 0.431; 95% CI, 0.203–0.913;
P=0.028). Subsequently, the multivariate analysis showed that the
CA199 level (HR, 2.487; 95% CI, 1.263–4.901; P=0.008), metastasis
diameter (HR, 1.485; 95% CI, 1.060–2.080; P=0.021), internal
features (HR, 1.642; 95% CI, 1.097–2.459; P=0.016), local
progression (HR, 3.649; 95% CI, 2.524–5.274; P<0.001) and
treatment modality (HR, 0.408; 95% CI, 0.192–0.867; P=0.020) were
significant predictors of LTPFS. In both the univariate and
IPTW-adjusted analyses, LTPFS was found to significantly differ
between the SBRT and MWA groups (Table
IV). However, no significant differences in the 1-year and
3-year LTPFS rates were observed between the two groups with or
without IPTW adjustment (67.1 vs. 69.7%; P=0.077; and 14.1 vs.
18.2%, P=0.204 for 1- and 3-year LTPFS, respectively; Table II). Subsequently, the associations
of metastasis diameter, local progression and treatment modality
were further explored, between the two treatment groups. The
results obtained showed that no significant difference in the
association of lesion diameter with local progression was observed
(P=0.099), although the difference between local progression and
treatment modality was significant (P=0.021). Stratifying the
metastasis diameter to explore the local control effect between the
two groups revealed that the difference between the two treatment
modalities with metastasis diameter >20 mm (71.4 vs. 51.9%;
P=0.426) was insignificant (Table
V). Moreover, when the metastasis diameter was ≤20 mm, no
statistically significant difference between the two groups in
terms of the metastasis diameter was noted (P=0.248), although MWA
was superior to SBRT in terms of local control (70.9 vs. 57.2%;
P=0.037). Similarly, when the metastasis diameter was ≤10 mm, no
statistically significant difference was observed in the metastasis
diameter between the two groups (P=0.528), although MWA was again
superior to SBRT in terms of local control (76.3 vs. 57.1%;
P=0.016). Therefore, the effect of the metastasis diameter on local
control in the two groups could be discounted (i.e. no significant
difference was found between the metastasis diameter and local
control). It could also be confirmed that MWA was superior to SBRT
in terms of local control.
 | Table III.Uni- and multivariate analyses for
local tumor progression-free survival. |
Table III.
Uni- and multivariate analyses for
local tumor progression-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, ≥70 years | 1.178
(0.593–2.339) | 0.639 |
|
|
| Sex, female | 0.771
(0.440–1.350) | 0.362 |
|
|
| BMI, <18.5 or
>23.9 kg/m2 | 0.709
(0.415–1.211) | 0.209 |
|
|
| Primary cancer
location, colon | 0.956
(0.532–1.716) | 0.880 |
|
|
| Extrapulmonary
metastases | 1.884
(1.066–3.327) | 0.029a |
|
|
| Underlying
diseases | 1.204
(0.701–2.069) | 0.502 |
|
|
| Preoperative
emphysema | 0.967
(0.499–1.874) | 0.920 |
|
|
| Preoperative lung
bullae | 1.450
(0.647–3.250) | 0.367 |
|
|
| Hilar
lymphadenopathy | 1.132
(0.274–4.669) | 0.864 |
|
|
| Mediastinal
lymphadenopathy | 1.829
(0.440–7.600) | 0.406 |
|
|
| Lung lobe
distribution |
|
|
|
|
|
Right | 0.565
(0.287–1.110) | 0.098 |
|
|
|
Both | 0.976
(0.499–1.911) | 0.945 |
|
|
| CEA, ≥5.0
ng/ml | 1.630
(0.951–2.791) | 0.075 |
|
|
| CA19-9, ≥37.0
IU/ml | 2.204
(1.109–4.382) | 0.024a | 2.487
(1.263–4.901) | 0.008a |
| CA125, ≥35.0
U/ml | 1.426
(0.440–4.623) | 0.544 |
|
|
| Treatment modality,
MWA | 0.431
(0.203–0.913) | 0.028a | 0.408
(0.192–0.867) | 0.020a |
| Neoadjuvant
therapy | 0.863
(0.499–1.491) | 0.597 |
|
|
| Preoperative
targeted therapy | 0.736
(0.332–1.631) | 0.450 |
|
|
| Preoperative I-125
seed implantation | 0.530
(0.129–2.182) | 0.379 |
|
|
| Metastasis diameter
>10 mm | 1.564
(1.121–2.181) | 0.009a | 1.485
(1.060–2.080) | 0.021a |
| Adjacent to vessels
>3 mm | 1.250
(0.783–1.994) | 0.350 |
|
|
| Adjacent to vessels
>5 mm | 1.029
(0.541–1.958) | 0.931 |
|
|
| Adjacent to the
bronchus >2 mm | 1.112
(0.715–1.730) | 0.638 |
|
|
| Near mediastinal
pleura 10 mm | 1.215
(0.756–1.953) | 0.422 |
|
|
| Near chest wall
pleura 10 mm | 1.228
(0.881–1.711) | 0.225 |
|
|
| Near pleura 0.5
mm | 1.165
(0.772–1.758) | 0.467 |
|
|
| Near interlobar
pleura 10 mm | 0.919
(0.627–1.347) | 0.666 |
|
|
| Near the diaphragm
10 mm | 1.028
(0.611–1.732) | 0.916 |
|
|
| Internal
features | 1.760
(1.180–2.624) | 0.006a | 1.642
(1.097–2.459) | 0.016a |
| Local
progression | 3.649
(2.524–5.274) |
<0.001a | 3.649
(2.524–5.274) |
<0.001a |
| Adjuvant
(postoperative) therapy | 1.564
(0.784–3.120) | 0.205 |
|
|
 | Table IV.HR for oncological outcomes according
to treatment modality. |
Table IV.
HR for oncological outcomes according
to treatment modality.
| Outcome | Method | HR (95% CI) | P-value |
|---|
| Overall
survival | Univariate | 0.501
(0.236–1.062) | 0.071 |
|
| IPTW-adjusted | 0.559
(0.464–0.674) |
<0.001a |
| Disease-free
survival | Univariate | 0.432
(0.204–0.916) | 0.029a |
|
| IPTW-adjusted | 0.495
(0.411–0.597) |
<0.001a |
| Local tumor
progression-free survival | Univariate | 0.431
(0.203–0.913) | 0.028a |
|
| IPTW-adjusted | 0.485
(0.441–0.533) |
<0.001a |
 | Table V.Stratified exploration of the
comparison between the two groups in terms of metastasis diameter
and local control. |
Table V.
Stratified exploration of the
comparison between the two groups in terms of metastasis diameter
and local control.
| Variable | SBRT | MWA | P-value |
|---|
| Metastasis diameter
>20 mm |
|
|
|
| Mean,
mm) | 30.759 | 27.971 | 0.456 |
| Local
control | 14 (51.9%) | 5 (71.4%) | 0.426 |
| Metastasis diameter
≤20 mm |
|
|
|
| Mean,
mm) | 10.019 | 8.267 | 0.248 |
| Local
control | 111 (57.2%) | 56 (70.9%) | 0.037a |
| Metastasis diameter
≤10 mm |
|
|
|
| Mean,
mm) | 6.962 | 6.764 | 0.528 |
| Local
control | 60 (57.1%) | 45 (76.3%) | 0.016a |
Analysis of OS rates
Data from the univariate and multivariate analyses
for the predictors of OS are summarized in Table VI. In the univariate analysis, the
potential predictors for OS included extrapulmonary metastases (HR,
1.850; 95% CI, 1.036–3.303; P=0.038), the carcinoembryonic antigen
(CEA) level (HR, 2.089; 95% CI, 1.212–3.598; P=0.008), metastasis
diameter >10 mm (HR, 1.600; 95% CI, 1.149–2.228; P=0.005),
internal features (HR, 1.618; 95% CI, 1.089–2.402; P=0.017) and
treatment modality (HR, 0.501; 95% CI, 0.236–1.062; P=0.071). After
having incorporated the aforementioned variables into the
multivariate Cox regression model, the results obtained showed that
the CEA level (HR, 2.089; 95% CI, 1.212–3.598; P=0.008), metastasis
diameter (HR, 1.534; 95% CI, 1.098–2.143; P=0.012) and internal
features (HR, 1.608; 95% CI, 1.078–2.400; P=0.020) served as
independent predictors of OS. The Omnibus test for model
coefficients indicated that there was a statistically significant
difference (χ2=7.355, P=0.007). The 1-year and 3-year
rates of OS were respectively found to be 92.9 and 44.7% in the
SBRT group and 93.9 and 36.4% in the MWA group (IPTW-adjusted:
1-year: P=0.354; 3-year: P<0.001; Table II). According to the univariate
analysis, the difference in OS between the SBRT and MWA groups was
not significantly significant (P=0.071), although, after IPTW
adjustment, a substantially significant difference was noted
between the two groups (P<0.001; Table IV).
 | Table VI.Univariate and multivariate analyses
for overall survival. |
Table VI.
Univariate and multivariate analyses
for overall survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, >70
years | 1.094
(0.550–2.175) | 0.797 |
|
|
| Sex, female | 0.809
(0.463–1.416) | 0.459 |
|
|
| BMI, <18.5 or
>23.9 kg/m2 | 0.797
(0.466–1.364) | 0.409 |
|
|
| Primary cancer
location, colon | 0.769
(0.429–1.381) | 0.380 |
|
|
| Extrapulmonary
metastases | 1.850
(1.036–3.303) | 0.038a |
|
|
| Underlying
diseases | 1.203
(0.703–2.061) | 0.500 |
|
|
| Preoperative
emphysema | 0.807
(0.405–1.605) | 0.541 |
|
|
| Preoperative lung
bullae | 0.958
(0.433–2.124) | 0.917 |
|
|
| Hilar
lymphadenopathy | 0.395
(0.054–2.891) | 0.361 |
|
|
| Mediastinal
lymphadenopathy | 0.609
(0.084–4.425) | 0.624 |
|
|
| Lung lobe
distribution |
|
|
|
|
|
Right | 0.622
(0.315–1.226) | 0.170 |
|
|
|
Both | 0.741
(0.379–1.449) | 0.380 |
|
|
| CEA, ≥5.0
ng/ml | 2.089
(1.212–3.598) | 0.008a | 2.089
(1.212–3.598) | 0.008a |
| CA19-9, ≥37.0
IU/ml | 1.745
(0.880–3.459) | 0.111 |
|
|
| CA125, ≥35.0
U/ml | 1.833
(0.364–9.216) | 0.430 |
|
|
| Treatment modality,
MWA | 0.501
(0.236–1.062) | 0.071 |
|
|
| Neoadjuvant
therapy | 1.050
(0.610–1.808) | 0.860 |
|
|
| Preoperative
targeted therapy | 1.043
(0.470–2.313) | 0.918 |
|
|
| Preoperative I-125
seed implantation | 0.650
(0.158–2.675) | 0.551 |
|
|
| Metastasis diameter
≥10 mm | 1.600
(1.149–2.228) | 0.005a | 1.534
(1.098–2.143) | 0.012a |
| Adjacent to vessels
over 3 mm | 1.317
(0.825–2.102) | 0.249 |
|
|
| Adjacent to vessels
over 5 mm | 0.973
(0.510–1.857) | 0.933 |
|
|
| Adjacent to the
bronchus over 2 mm | 1.136
(0.731–1.766) | 0.570 |
|
|
| Near mediastinal
pleura 10 mm | 0.884
(0.551–1.419) | 0.610 |
|
|
| Near chest wall
pleura 10 mm | 1.066
(0.764–1.488) | 0.705 |
|
|
| Near pleura 0.5
mm | 0.951
(0.632–1.430) | 0.808 |
|
|
| Near interlobar
pleura 10 mm | 1.202
(0.820–1.763) | 0.345 |
|
|
| Near the diaphragm
10 mm | 1.028
(0.611–1.732) | 0.916 |
|
|
| Internal
features | 1.618
(1.089–2.402) | 0.017a | 1.608
(1.078–2.400) | 0.020a |
| Local
progression | 1.035
(0.741–1.444) | 0.842 |
|
|
| Adjuvant
postoperative therapy | 1.413
(0.690–2.895) | 0.344 |
|
|
Kaplan-Meier method was subsequently used to analyze
the variables that showed significant differences according to the
multivariate Cox regression models. Although no significant
differences in treatment modality were noted for the OS values, the
OS data were still included in the study after IPTW adjustment,
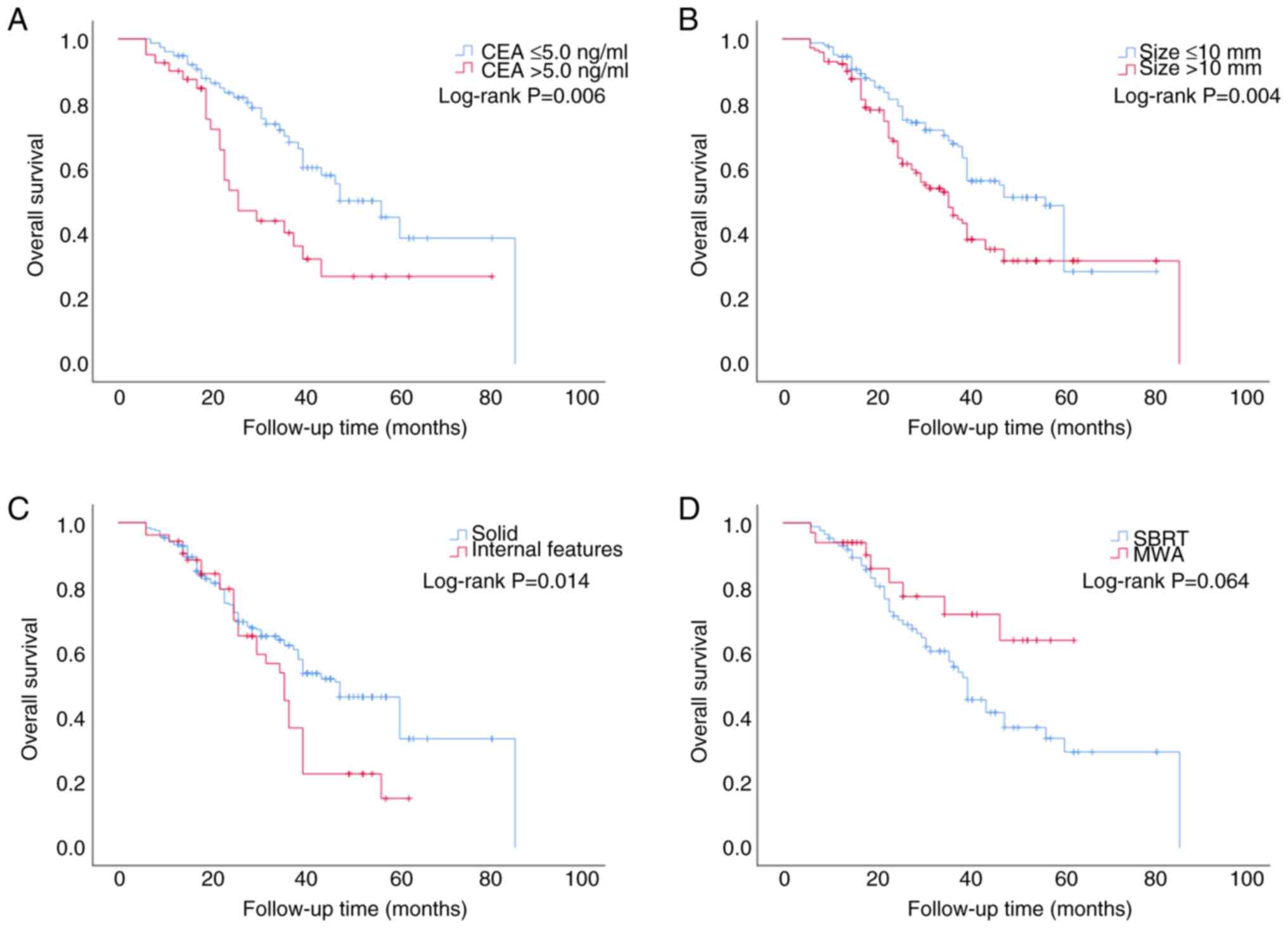
since the P-value was <0.05. The results obtained showed that
patients with regular CEA levels had a median OS of 57 months (95%
CI, 43.997–70.003), which was higher compared with that in patients
who had an abnormal CEA level (26 months; 95% CI, 16.482–35.518;
P=0.006; Fig. 2A). For CRC patients
with lung metastases with a metastasis diameter >10 mm, the
median OS was 36 months (95% CI, 29.422–42.578), which was a
shorter time compared with that in patients with lung metastases
with a metastasis diameter ≤10 mm (57 months; 95% CI,
51.757–62.243; P=0.004; Fig. 2B).
The median OS was determined to be 36 months for patients with
internal features (95% CI, 35.492–44.508), which was shorter than
that for patients with solid tumors (48 months; 95% CI,
41.100–54.900; P=0.014; Fig. 2C).
The median OS for patients in the SBRT group was 40 months; by
contrast, for the MWA group, the median observation duration was
not reached. Ultimately, for the two treatment modalities, the
difference in OS was not found to be statistically significant
(χ2=3.442, P=0.064; Fig.
2D).
Analysis of the DFS rates
During the follow-up period, tumor recurrence
occurred in 73 patients in the SBRT group (intrapulmonary
recurrence, n=55; extrapulmonary recurrence, n=18) and 27 patients
in the MWA group (intrapulmonary recurrence, n=18; extrapulmonary
recurrence, n=9). The 1-year and 3-year DFS rates were found to be
44.7 and 7.1% in the SBRT group and 51.5 and 12.1% in the MWA
group, respectively (IPTW-adjusted: 1-year: P<0.001; 3-year:
P=0.016; Table II). DFS refers to
more than local progression, but also includes recurrence of the
primary focus and distant metastases. LTPFS is more indicative of
local control than DFS. Data from the univariate and multivariate
analyses of the predictors of DFS are summarized in Table VII. The univariate analysis
revealed that extrapulmonary metastases (HR, 1.967; 95% CI,
1.112–3.481; P=0.020), the CEA level (HR, 1.725; 95% CI,
1.003–2.964; P=0.049), internal features (HR, 1.511; 95% CI,
1.018–2.242; P=0.040), local progression (HR, 2.041; 95% CI,
1.449–2.875; P<0.001) and the treatment modality (HR, 0.432; 95%
CI, 0.204–0.916; P=0.029) were potential predictors of DFS and the
differences were shown to be statistically significant (P<0.05).
Incorporating the aforementioned variables into the multivariate
Cox regression model, the results showed that internal features
(HR, 1.511; 95% CI, 1.018–2.242; P=0.040), local progression (HR,
2.041; 95% CI, 1.449–2.875; P<0.001) and the treatment modality
(HR, 0.413; 95% CI, 0.195–0.876; P=0.021) were independent
predictors of DFS. According to both the univariate and
IPTW-adjusted analyses, significant differences in DFS were
observed between the SBRT and MWA groups (Table IV).
 | Table VII.Univariate and multivariate analyses
for disease-free survival. |
Table VII.
Univariate and multivariate analyses
for disease-free survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, ≥70 years | 1.276
(0.642–2.536) | 0.486 |
|
|
| Sex, female | 0.805
(0.460–1.407) | 0.446 |
|
|
| BMI, <18.5 or
>23.9 kg/m2 | 0.751
(0.440–1.282) | 0.294 |
|
|
| Primary cancer
location, colon | 0.829
(0.462–1.485) | 0.528 |
|
|
| Extrapulmonary
metastases | 1.967
(1.112–3.481) | 0.020a |
|
|
| Underlying
diseases | 1.096
(0.644–1.864) | 0.736 |
|
|
| Preoperative
emphysema | 1.058
(0.546–2.050) | 0.868 |
|
|
| Preoperative lung
bullae | 1.263
(0.563–2.835) | 0.572 |
|
|
| Hilar
lymphadenopathy | 0.832
(0.201–3.441) | 0.800 |
|
|
| Mediastinal
lymphadenopathy | 1.262
(0.306–5.204) | 0.747 |
|
|
| Lung lobe
distribution |
|
|
|
|
|
Right | 0.652
(0.332–1.281) | 0.214 |
|
|
|
Both | 0.903
(0.461–1.769) | 0.767 |
|
|
| CEA, ≥5.0
ng/ml | 1.725
(1.003–2.964) | 0.049a |
|
|
| CA19-9, ≥37.0
IU/ml | 1.894
(0.957–3.749) | 0.067 |
|
|
| CA125, ≥35.0
U/ml | 1.365
(0.379–4.916) | 0.620 |
|
|
| Treatment modality,
MWA | 0.432
(0.204–0.916) | 0.029a | 0.413
(0.195–0.876) | 0.021a |
| Neoadjuvant
therapy | 0.990
(0.575–1.705) | 0.971 |
|
|
| Preoperative
targeted therapy | 0.788
(0.356–1.746) | 0.558 |
|
|
| Preoperative I-125
seed implantation | 0.426
(0.104–1.751) | 0.237 |
|
|
| Metastasis diameter
>10 mm | 1.195
(0.860–1.660) | 0.289 |
|
|
| Adjacent to vessels
>3 mm | 0.868
(0.546–1.381) | 0.551 |
|
|
| Adjacent to vessels
>5 mm | 0.907
(0.477–1.726) | 0.767 |
|
|
| Adjacent to the
bronchus >2 mm | 0.839
(0.541–1.302) | 0.434 |
|
|
| Near mediastinal
pleura 10 mm | 0.996
(0.621–1.598) | 0.986 |
|
|
| Near chest wall
pleura 10 mm | 1.202
(0.862–1.676) | 0.279 |
|
|
| Near pleura 0.5
mm | 1.065
(0.708–1.602) | 0.763 |
|
|
| Near interlobar
pleura 10 mm | 0.998
(0.681–1.462) | 0.992 |
|
|
| Near the diaphragm
10 mm | 0.857
(0.509–1.443) | 0.561 |
|
|
| Internal
features | 1.511
(1.018–2.242) | 0.040a | 1.511
(1.018–2.242) | 0.040a |
| Local
progression | 2.041
(1.449–2.875) |
<0.001a | 2.041
(1.449–2.875) |
<0.001a |
| Adjuvant
postoperative therapy | 1.749
(0.873–3.504) | 0.115 |
|
|
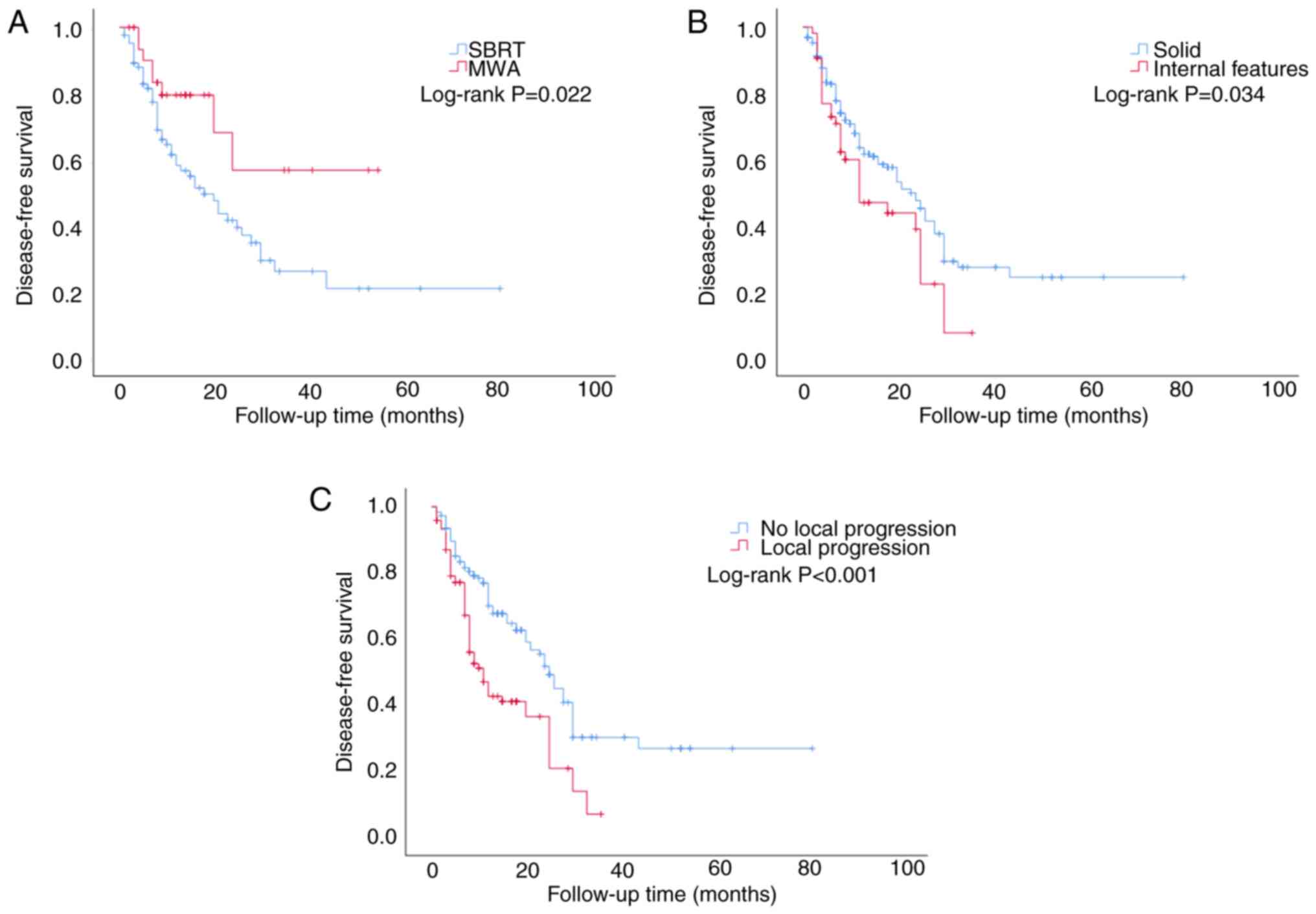
The Kaplan-Meier method was subsequently applied for
variables that showed significant differences according to the
multivariate Cox regression models. The results showed that the
median DFS for patients in the SBRT group was 18 months (95% CI,
10.611–25.389), whereas for the MWA group, the median observation
duration was not reached. A statistically significant difference in
the DFS values was identified between the two treatment modalities
(χ2=5.226, P=0.022; Fig.
3A). The median DFS was 12 months for patients with internal
features (95% CI, 3.778–20.222), which was shorter compared with
the median DFS for patients with solid tumors (23 months; 95% CI,
18.960–27.040; χ2=4.492; P=0.034; Fig. 3B). The median DFS for patients with
local progression of lung metastases was determined to be 11 months
(95% CI, 7.902–14.098), which was shorter than that for patients
who experienced no local progression of lung metastases (25 months;
95% CI, 22.274–27.726; χ2=18.220; P<0.001; Fig. 3C).
Analysis of treatment
complications
MWA and SBRT, as treatment modalities, were both
found to be well tolerated and no patients succumbed to
treatment-associated mortality. In the MWA group, pneumothorax
complications occurred in 22 (66.7%) patients, with eight (24.2%)
of the patients experiencing a moderate-to-large pneumothorax that
required thoracocentesis drainage (although this process resulted
in the issue being resolved satisfactorily for the majority of the
patients within a week). In addition, 14 patients (42.4%) developed
pleural effusions, although only one patient developed massive
pleural effusions. In the SBRT group, 54 (63.5%) of the patients
developed radiation pneumonitis within 1–3 months following
treatment, with 29 (34.1%) of the patients showing signs of
inflammation at ~3 months post-irradiation.
Discussion
For patients with CRC with lung metastases, in
addition to the traditional treatment of surgical resection,
emerging treatments, including percutaneous ablation therapy and
SBRT, have gradually attracted attention in recent years. The most
commonly used percutaneous ablation therapy is RFA; moreover, in
recent years, the application of MWA in the treatment of metastases
that are difficult to resect by surgery has also gradually
increased. In the present study, the MWA group had a total of 21
lung metastases with LTP; their maximum diameters ranged from
3.5–24.7 mm (median diameter: 10.1 mm). LTP was found not to exert
any significant impact on OS (P=0.842) and this finding was
consistent with the findings of the study published by Kurilova
et al (23).
Cheng et al (10) reported that 32 patients with CRC
with 48 lung metastases were treated with MWA and the 1-, 2- and
3-year OS rates were found to be 79.5, 63.1 and 44.4%,
respectively. In a study by Yang et al (24) on the treatment of non-small cell
lung cancer using MWA, the 1-, 2- and 3-year OS rates were reported
to be 89, 63 and 43%, respectively. In the present study, the 1-,
2- and 3-year OS rates following MWA treatment were found to be
93.9, 57.6 and 36.4%, respectively, which were similar to those
reported in the aforementioned studies.
Delpla et al (25) discussed the role of thermal ablation
in the treatment of CRC lung metastases and presented the main
results based on 12 relevant studies. They found that the incidence
of local control ranged from 62–91%, which broadly aligns with the
results of the MWA group in the present study (70.9%). Local
control has improved in recent studies, possibly due to
technological advances and patient/tumor selection and this has
been accomplished through an improved knowledge of the risk factors
for local recurrence of tumors.
In the present study, the multivariate analysis
showed that metastasis diameter was a prognostic factor for both OS
and DFS and these findings were consistent with those of previous
studies (26–28). It has been reported that independent
predictors of OS also include the location of the primary disease
(26), tumor stage (27), the number of metastases (26), metastasis diameter (26–28)
and extrapulmonary metastases (29). The findings of the present study
supported that extrapulmonary metastases is a potential predictor
of OS (P=0.038), but significant differences were not revealed
according to the multivariate analysis. Additionally, it found that
internal features of lung metastases significantly differed in
terms of the OS, LTPFS and DFS values (P<0.05), demonstrating
that these could also serve as prognostic factors for survival.
CEA, CA19-9 and CA125 were selected for this present
study. CEA is a serum glycoprotein and currently is the most widely
used marker for colon cancer (30).
CA19-9 is an antigen that elevated in numerous types of
gastrointestinal cancer including colorectal cancer, esophageal
cancer and hepatocellular carcinoma (31). CA125 is a glycoprotein antigen that
is associated with gastric, colon, lung, pancreatic and liver
cancers, as well as types of blood cancer (32). The present study found that CEA was
a prognostic factor for OS and CA19-9 was an independent predictor
of LTPFS. In colorectal cancers, other markers such as CA50, CA724
are also suitable markers. CA50 is an independent prognostic factor
for patients with CRC following radical resection and CA724 is a
glycoprotein, with higher levels in colorectal cancer (33). CSLEX and NCC-ST-439 are
tumor-associated carbohydrate antigens that can identify colon
cancer (34). The combination assay
of serum CEA, CA 19-9, STn and SLX will be beneficial for diagnosis
and follow-up of colorectal cancer (35). Unfortunately, the data for these
markers are poor and that is one of the limitations of the present
study.
The results of the present study emphasized the
potential of using MWA, especially for the treatment of surgically
unresectable CRC lung metastases. However, clinicians still need to
carefully select the most appropriate treatment modality and to
consider the characteristics of the lesion, the patient's overall
condition and the long-term outcome of the treatment in
patient-individualized treatment decisions.
On the other hand, in addition to thermal ablation
techniques, SBRT has attracted much attention as a useful addition
to the treatment of CRC lung metastases. Sharma et al
(36) reported on 118 patients with
CRC with a total of 202 lung metastases who were treated with SBRT
and the 2-, 3- and 5-year OS rates were reported to be 69, 55 and
36%, respectively. In the present study, the 2- and 3-year OS rates
following SBRT treatment were found to be 65.9 and 44.7%
respectively, which were a little lower than those reported in
their study. It was not possible to calculate the 5-year survival
period in Sharma et al (36), since the follow-up duration was
relatively short and there were few patients with a survival period
exceeding 5 years. In their meta-analysis, Zhang et al
(37), found that having a single
metastasis was a protective factor for OS, which aligned with the
results of the present study.
To the best of the authors' knowledge, no studies
have been published which have directly compared the treatment
methods of SBRT and MWA in patients with CRC with lung metastases.
Therefore, the present study may represent the first retrospective
analysis that has been focused on this particular topic. In the
present study, 118 patients with CRC who had a total of 307 lung
metastases underwent SBRT or MWA treatment. Multivariate COX
regression analysis revealed that the level of CA199, metastasis
diameter, internal features and the treatment modality were
significant predictors of LTPFS. Among these factors, lung
metastases with a normal level of CA199, metastases of diameter ≤10
mm, tumors without internal features and those treated with MWA
achieved improved local tumor control.
Ager et al (38) demonstrated the superiority of SBRT
over percutaneous local tumor ablation in terms of the OS rate in
their study on early-stage non-small cell lung cancer (1-year OS:
87.5 vs. 83.5%; 2-year OS: 68.0 vs. 63.0%; 3-year OS: 52.5 vs.
45.9%; P<0.001). The results of the present study indicated
that, following IPTW adjustment, no significant differences were
observed in the 1-year OS rates regarding the lung metastases of
patients with CRC having been treated with SBRT or MWA (92.9 vs.
93.9%, P=0.354). However, differences were observed in the 2- and
3-year OS rates (65.9 vs. 57.6%, P=0.001; and 44.7 vs. 36.4%,
respectively; both P<0.001).
Nieuwenhuizen et al (39) conducted a systematic review and
meta-analysis of multiple therapies, including MWA, RFA,
irreversible electroporation and stereotactic ablative body
radiotherapy, for the treatment of medium-sized (3–5 cm)
unresectable CRC liver metastases. However, despite the fact that
various studies have described long-term disease control, an
insufficient number of studies were identified that directly
compared these therapies and therefore no firm conclusions could be
drawn. Franzese et al (40),
in their study on CRC liver metastases, found that SBRT and MWA
were associated with similar disease control effects for small
lesions, whereas the use of SBRT led to an improvement in the
control of lesions >30 mm. In the present study, no significant
differences were identified between the two treatments when the
diameter was >20 mm (P=0.426). However, when the diameter was
≤20 mm or ≤10 mm, MWA was found to be superior to SBRT in terms of
local control (≤20 mm, P=0.037; and ≤10 mm, P=0.016,
respectively).
For patients with lung metastases, there are clear
benefits associated with the implementation of local therapy, which
can be provided in a variety of modalities. Markedly higher local
control of smaller lesions in RFA treatment and MWA is effective
for local control on the large lesions (21). This was confirmed in the present
study. When the diameter was >20 mm, the local control rate in
the MWA group was 71.4%; when the diameter was ≤20 mm, the local
control rate was 70.9%; when the diameter was ≤10 mm, the local
control rate was 76.3%. Despite the slightly lower rate of local
control of large lesions, MWA still has an objective effect.
Irrespective of the approach taken, localized treatment is capable
of providing patients with prolonged DFS rates. Surgical resection
with adequate margins provides the greatest long-term local control
for operable patients, whereas patients unable to obtain surgical
treatment may be treated with other modalities, such as SBRT,
ablation or other modalities that provide local control. Clearly,
OS for patients cannot be attributed to pulmonary ablation alone,
but rather to the comprehensive management of oligometastatic
disease. The majority of patients with CRC with lung metastases
receive systemic adjuvant therapy following the procedure,
including chemotherapy and targeted therapy. Compared with patients
receiving only chemotherapy for lung metastases, those undergoing
adjuvant radiotherapy or ablation were found to have significantly
prolonged 3-year survival rates (87.5 vs. 33.3%) (41). In the present study, however, no
significant differences were identified in terms of the effect of
postoperative adjuvant therapy on the OS rates (P=0.344).
MWA, as an interventional treatment, intrinsically
involves invasiveness. Pneumothorax is one of the common
complications. Yang et al (24) reported a very high incidence of
pneumothorax (63.8%), a finding that was very similar to the
present study (66.7%), although this was higher than that reported
in the majority of studies (10,42).
However, only 13.5% of the patients required chest drainage in the
aforementioned study, a finding that was lower than that identified
in our results (24.2%). In addition, pleural effusion is a
prevalent complication associated with MWA, with reported incidence
rates ranging from 15–45% (43). In
the present study, the incidence of pleural effusion was 42.4% and
the majority of cases involved small effusions that were capable of
self-absorption. For SBRT, a common adverse reaction is radiation
pneumonitis. Kobayashi et al (18), in their study on CRC lung
metastases, observed grade 1 radiation pneumonitis in 22 patients
(84.6%) and no patients developed pulmonary toxicity of grade ≥2.
In the present study, 54 patients (63.5%) were found to have
developed radiation pneumonitis (grade ≤2) following treatment,
representing a lower incidence of radiation pneumonitis compared
with their study.
The current study does, however, have certain
limitations. First, it was a retrospective and single-center study
and despite attempts to mitigate selection bias using IPTW
adjustment, the results may still be influenced by such bias.
Secondly, the sample size was relatively small, especially for
patients undergoing MWA. Thirdly, some patients were lost to
follow-up, a phenomenon that could have had an effect on the
assessment of the LTPFS, RFS and OS rates. Larger-scale randomized
controlled clinical trials are necessary to directly compare the
efficacy of SBRT and MWA in patients with CRC with lung metastases.
In addition, certain patients in the study continued to receive
additional treatments, such as chemotherapy, after having been
treated with SBRT or MWA. Although no significant differences were
found in OS with postoperative adjuvant therapy, this cannot
completely exclude the possibility of there being confounding
effects on the efficacy of using SBRT or MWA alone. In addition, as
the disease progresses, patients receive additional treatments to
control the progression of the disease and prolong the survival,
including ablation, radiotherapy, chemotherapy and so on. Although
LTPFS and DFS were not affected in this study, OS was affected.
When the disease progresses, patients who are treated tend to have
improved OS.
In conclusion, non-surgical treatments, including
thermal ablation and stereotactic body radiotherapy, are assuming
an increasingly crucial role in the management of CRC lung
metastases. The present study has preliminarily demonstrated that
SBRT and MWA have comparable efficacy in terms of treating CRC lung
metastases. However, it is worth emphasizing that MWA exhibits
greater advantages in local tumor control compared with SBRT,
especially when the tumor is <10 mm. Taken together, the
findings of the present study may be used to provide personalized
guidance for the treatment of unresectable CRC lung metastases in
clinical practice.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XW, JS, HF and TD contributed to the conception and
design of the study. TD, JL, PH and YS was responsible for data
collection and collation and statistical analysis. TD, JL and PH
contributed to manuscript drafting and critical revisions on the
intellectual content. All authors agreed to be accountable for all
aspects of the work. XW, HF and TD confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All procedures performed in the present study
involving medical record information and data were approved by the
Medical Ethics Committee of Sir Run Run Shaw Hospital (Zhejiang,
China; project number 20230430). The requirement for informed
consent was waived by the ethics committee. All methods were
performed in accordance with the relevant guidelines and
regulations (Declaration of Helsinki).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MWA
|
microwave ablation
|
|
SBRT
|
stereotactic body radiotherapy
|
|
CRC
|
colorectal cancer
|
|
CEA
|
carcinoembryonic antigen
|
|
CA125
|
glycocalyx antigen 125
|
|
CA19-9
|
glycocalyx antigen 19-9
|
|
BMI
|
body mass index
|
|
IPTW
|
inverse probability of treatment
weighting
|
|
LTP
|
local tumor progression
|
|
DFS
|
disease-free survival
|
|
OS
|
overall survival
|
|
RFA
|
radiofrequency ablation
|
|
LTPFS
|
local tumor progression-free
survival
|
|
LTA
|
local tumor ablation
|
|
IQR
|
interquartile range
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
Globocan estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Van Cutsem E, Nordlinger B, Adam R, Köhne
CH, Pozzo C, Poston G, Ychou M and Rougier P; European Colorectal
Metastases Treatment Group, : Towards a pan-European consensus on
the treatment of patients with colorectal liver metastases. Eur J
Cancer. 42:2212–2221. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Riihimäki M, Hemminki A, Sundquist J and
Hemminki K: Patterns of metastasis in colon and rectal cancer. Sci
Rep. 6:297652016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD, Sauer AG, Fedewa SA,
Butterly LF, Anderson JC, Cercek A, Smith RA and Jemal A:
Colorectal cancer statistics, 2020. CA Cancer J Clin. 70:145–164.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watanabe K, Saito N, Sugito M, Ito M,
Kobayashi A and Nishizawa Y: Incidence and predictive factors for
pulmonary metastases after curative resection of colon cancer. Ann
Surg Oncol. 20:1374–1380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang GQ, Taylor JP, Stem M, Almaazmi H,
Efron JE, Atallah C and Safar B: Aggressive multimodal treatment
and metastatic colorectal cancer survival. J Am Coll Surg.
230:689–698. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho JH, Kim S, Namgung M, Choi YS, Kim HK,
Zo JI, Shim YM and Kim J: The prognostic importance of the number
of metastases in pulmonary metastasectomy of colorectal cancer.
World J Surg Oncol. 13:2222015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong SL, Mangu PB, Choti MA, Crocenzi TS,
Dodd GD III, Dorfman GS, Eng C, Fong Y, Giusti AF, Lu D, et al:
American society of clinical oncology 2009 clinical evidence review
on radiofrequency ablation of hepatic metastases from colorectal
cancer. J Clin Oncol. 28:493–508. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ruers T, Van Coevorden F, Punt CJA, Pierie
JPEN, Borel-Rinkes I, Ledermann JA, Poston G, Bechstein W, Lentz
MA, Mauer M, et al: Local treatment of unresectable colorectal
liver metastases: Results of a randomized phase II trial. J Natl
Cancer Inst. 109:djx1502017. View Article : Google Scholar
|
|
10
|
Cheng G, Shi L, Qiang W, Wu J, Ji M, Lu Q,
Li X, Xu B, Jiang J and Wu C: The safety and efficacy of microwave
ablation for the treatment of CRC pulmonary metastases. Int J
Hyperthermia. 34:486–491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vogl TJ, Eckert R, Naguib NNN, Beeres M,
Gruber-Rouh T and Nour-Eldin NEA: Thermal ablation of colorectal
lung metastases: Retrospective comparison among laser-induced
thermotherapy, radiofrequency ablation, and microwave ablation. AJR
Am J Roentgenol. 207:1340–1349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lyons NJR, Pathak S, Daniels IR, Spiers A
and Smart NJ: Percutaneous management of pulmonary metastases
arising from colorectal cancer; a systematic review. Eur J Surg
Oncol. 41:1447–1455. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yuan Z, Wang Y, Zhang J, Zheng J and Li W:
A meta-analysis of clinical outcomes after radiofrequency ablation
and microwave ablation for lung cancer and pulmonary metastases. J
Am Coll Radiol. 16:302–314. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ferguson CD, Luis CR and Steinke K: Safety
and efficacy of microwave ablation for medically inoperable
colorectal pulmonary metastases: Single-centre experience. J Med
Imaging Radiat Oncol. 61:243–249. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Franzese C, Comito T, Toska E, Tozzi A,
Clerici E, De Rose F, Franceschini D, Navarria P, Reggiori G,
Tomatis S and Scorsetti M: Predictive factors for survival of
oligometastatic colorectal cancer treated with stereotactic body
radiation therapy. Radiother Oncol. 133:220–226. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi HS, Jeong BK, Kang KM, Jeong H, Song
JH, Ha IB and Kwon OY: Tumor control and overall survival after
stereotactic body radiotherapy a pulmonary oligometastases from
colorectal cancer: A meta-analysis. Cancer Res Treat. 52:1188–1198.
2020.PubMed/NCBI
|
|
17
|
Cao C, Wang D, Tian DH, Wilson-Smith A,
Huang J and Rimner A: A systematic review and meta-analysis of
stereotactic body radiation therapy for colorectal pulmonary
metastases. J Thorac Dis. 11:5187–5198. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kobayashi N, Abe T, Noda S-E, Kumazaki Y,
Hirai R, Igari M, Aoshika T, Saito S, Ryuno Y and Kato S:
Stereotactic body radiotherapy for pulmonary oligometastasis from
colorectal cancer. In Vivo. 34:2991–2996. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nicosia L, Cuccia F, Mazzola R, Ricchetti
F, Figlia V, Giaj-Levra N, Rigo M, Tomasini D, Pasinetti N,
Corradini S, et al: Disease course of lung oligometastatic
colorectal cancer treated with stereotactic body radiotherapy.
Strahlenther Onkol. 196:813–820. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kraus KM, Bauer C, Feuerecker B, Fischer
JC, Borm KJ, Bernhardt D and Combs SE: Pneumonitis after
stereotactic thoracic radioimmunotherapy with checkpoint
inhibitors: Exploration of the dose-volume-effect correlation.
Cancers (Basel). 14:29482022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Antonoff MB, Sofocleous CT, Callstrom MR
and Nguyen QN: The roles of surgery, stereotactic radiation, and
ablation for treatment of pulmonary metastases. J Thorac Cardiovasc
Surg. 163:495–502. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hasegawa T, Takaki H, Kodama H, Yamanaka
T, Nakatsuka A, Sato Y, Takao M, Katayama Y, Fukai I, Kato T, et
al: Three-year survival rate after radiofrequency ablation for
surgically resectable colorectal lung metastases: A prospective
multicenter study. Radiology. 294:686–695. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kurilova I, Gonzalez-Aguirre A, Beets-Tan
RG, Erinjeri J, Petre EN, Gonen M, Bains M, Kemeny NE, Solomon SB
and Sofocleous CT: Microwave ablation in the management of
colorectal cancer pulmonary metastases. Cardiovasc Intervent
Radiol. 41:1530–1544. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang X, Ye X, Zheng A, Huang G, Ni X, Wang
J, Han X, Li W and Wei Z: Percutaneous microwave ablation of stage
I medically inoperable non-small cell lung cancer: Clinical
evaluation of 47 cases. J Surg Oncol. 110:758–763. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Delpla A, de Baere T, Varin E, Deschamps
F, Roux C and Tselikas L: Role of thermal ablation in colorectal
cancer lung metastases. Cancers (Basel). 13:9082021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
de Baère T, Auperin A, Deschamps F,
Chevallier P, Gaubert Y, Boige V, Fonck M, Escudier B and
Palussiére J: Radiofrequency ablation is a valid treatment option
for lung metastases: Experience in 566 patients with 1037
metastases. Ann Oncol. 26:987–991. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zheng A, Ye X, Yang X, Huang G and Gai Y:
Local efficacy and survival after microwave ablation of lung
tumors: A retrospective study in 183 patients. J Vasc Interv
Radiol. 27:1806–1814. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wei Z, Ye X, Yang X, Huang G, Li W, Wang J
and Han X: Microwave ablation plus chemotherapy improved
progression-free survival of advanced non-small cell lung cancer
compared to chemotherapy alone. Med Oncol. 32:4642015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Matsui Y, Hiraki T, Gobara H, Iguchi T,
Fujiwara H, Nagasaka T, Toyooka S and Kanazawa S: Long-term
survival following percutaneous radiofrequency ablation of
colorectal lung metastases. J Vasc Interv Radiol. 26:303–311. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McKeown E, Nelson DW, Johnson EK, Maykel
JA, Stojadinovic A, Nissan A, Avital I, Brücher BL and Steele SR:
Current approaches and challenges for monitoring treatment response
in colon and rectal cancer. J Cancer. 5:31–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Perkins GL, Slater ED, Sanders GK and
Prichard JG: Serum tumor markers. Am Fam Physician. 68:1075–1082.
2003.PubMed/NCBI
|
|
32
|
Zhong W, Yu Z, Zhan J, Yu T, Lin Y, Xia
ZS, Yuan YH and Chen QK: Association of serum levels of CEA, CA199,
CA125, CYFRA21-1 and CA72-4 and disease characteristics in
colorectal cancer. Pathol Oncol Res. 21:83–95. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li Q, Dai W, Li Y, Xu Y, Li X and Cai S:
Nomograms for predicting the prognostic value of serological tumor
biomarkers in colorectal cancer patients after radical resection.
Sci Rep. 7:463452017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Nakagoe T, Sawai T, Tsujia T, Jibiki M,
Ohbatake M, Nanashima A, Yamaguchi H, Yasutake T, Ayabe H and
Arisawa K: Prognostic value of serum sialyl Lewis(a), sialyl
Lewis(x) and sialyl Tn antigens in blood from the tumor drainage
vein of colorectal cancer patients. Tumour Biol. 22:115–122. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sato T, Nishimura G, Nonomura A, Miwa K
and Miyazaki I: Serological studies on CEA, CA 19-9, STn and SLX in
colorectal cancer. Hepatogastroenterology. 46:914–919.
1999.PubMed/NCBI
|
|
36
|
Sharma A, Baker S, Duijm M, Oomen-de Hoop
E, Cornelissen R, Verhoef C, Hoogeman M and Nuyttens JJ: Prognostic
factors for local control and survival for inoperable pulmonary
colorectal oligometastases treated with stereotactic body
radiotherapy. Radiother Oncol. 144:23–29. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Yang F, Li B, Li H, Liu J, Huang
W, Wang D, Yi Y and Wang J: Which is the optimal biologically
effective dose of stereotactic body radiotherapy for Stage I
non-small-cell lung cancer? A meta-analysis. Int J Radiat Oncol
Biol Phys. 81:e305–e316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ager BJ, Wells SM, Gruhl JD, Stoddard GJ,
Tao R, Kokeny KE and Hitchcock YJ: Stereotactic body radiotherapy
versus percutaneous local tumor ablation for early-stage non-small
cell lung cancer. Lung Cancer. 138:6–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nieuwenhuizen S, Dijkstra M, Puijk RS,
Geboers B, Ruarus AH, Schouten EA, Nielsen K, de Vries JJJ,
Bruynzeel AME, Scheffer HJ, et al: Microwave ablation,
radiofrequency ablation, irreversible electroporation, and
stereotactic ablative body radiotherapy for intermediate size (3–5
cm) unresectable colorectal liver metastases: A systematic review
and meta-analysis. Curr Oncol Rep. 24:793–808. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Franzese C, Comito T, Clerici E, Di Brina
L, Tomatis S, Navarria P, Reggiori G, Viganò L, Poretti D, Pedicini
V, et al: Liver metastases from colorectal cancer: Propensity
score-based comparison of stereotactic body radiation therapy vs
microwave ablation. J Cancer Res Clin Oncol. 144:1777–1783. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Inoue Y, Miki C, Hiro J, Ojima E, Yamakado
K, Takeda K and Kusunoki M: Improved survival using multi-modality
therapy in patients with lung metastases from colorectal cancer: A
preliminary study. Oncol Rep. 14:1571–1576. 2005.PubMed/NCBI
|
|
42
|
Fan H, Xie X, Pang Z, Zhang L, Ding R, Wan
C, Li X, Yang Z, Sun J, Kan X, et al: Risk assessment of
pneumothorax in colorectal lung metastases treated by percutaneous
thermal ablation: A multicenter retrospective cohort study. Int J
Surg. 110:261–269. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu Q, Cao W, Huang L, Wan Y, Liu T, Cheng
Q, Han Y and Li X: CT-guided percutaneous microwave ablation of
pulmonary malignancies: Results in 69 cases. World J Surg Oncol.
10:802012. View Article : Google Scholar : PubMed/NCBI
|