Introduction
Myeloid sarcoma (MS) is a rare extramedullary tumor
mass composed of primitive or immature myeloid cells of myeloid
origin, and is also known as a green tumor or granulocytic sarcoma.
MS is classified as a type of acute myeloid leukemia (AML) by the
World Health Organization (1). The
tumor can manifest as primary bone marrow sarcoma with or without
bone marrow involvement, and can occur following the relapse of AML
or during the progression of myelodysplastic syndrome (MDS),
myeloproliferative neoplasms and chronic myelogenous leukemia. MS
is frequently associated with AML (2). Occasionally, MS develops as an
isolated lesion, with a wide variation in size and location,
accounting for 2 cases per million adults (3). MS is described in ~9% of patients with
AML as an early manifestation of the disease, or is in the relapsed
setting, which is observed frequently after allogeneic
hematopoietic stem cell transplantation (allo-HCT) (4). The diagnosis of MS is mainly based on
clinical manifestations and cytochemical and/or immunophenotypic
factors. Overall, 54–70% of patients with MS present with
abnormalities at the molecular level, including positivity for
myeloperoxidase (MPO), and nucleophosmin 1 and NRAS proto-oncogene
GTPase mutations (5–7). In a previous study, the positive rates
of MPO, CD43 and CD117 expression in 39 patients were 92.1%
(35/38), 91.3% (21/23) and 42.3% (11/26), respectively, with high
sensitivity (8). Previously,
mutations in tumor protein p53 (TP53) were considered to occur at a
relatively low frequency in sarcomas (9). This is mainly since mutations in TP53
were identified by sequencing only exonic regions in the DNA
binding domain or by performing immunohistochemistry (IHC) to
detect positive staining in p53-mutated tumors due to the long
half-life of the mutant protein (10). However, recent whole-genome
sequencing analyses have revealed more frequent alterations in
TP53, including structural alterations in TP53 intron 1 (11). TP53+-MS is often
accompanied by complex chromosome karyotypes (4).
Due to the lack of research surrounding MS, the most
effective modes of treatment remain unclear, and at present, the
AML chemotherapy regimen is the most commonly used (5). It has been reported that 70% of
osteosarcomas have structural variations or mutations of TP53
(10). In TP53-mutant AML,
decitabine-based hypomethylation chemotherapy has certain
advantages (4). One study showed a
100% response rate in TP53mut AML and high mutation
clearance with a 10-day regimen of decitabine (12). Allo-HCT treatment should be
considered for recurrent patients or patients with bone marrow
lesions (5). However, due to
various changes in TP53, TP53 mutations/null are often used as
therapeutic targets for patients with MS. TP53-null often uses
inhibitors for wee1 kinase, Chk1 and equine-like kinase 1, and TP53
mutations often use inhibitors for PRIMA-1 and PRIMA-1Met to
re-activate wild-type TP53 activity (10). This is often abandoned due to bone
marrow suppression and other side effects. If not treated in time,
almost all patients may develop systemic disease and experience
progression to acute leukemia (13). The present study describes, to the
best of our knowledge, the first case of an elderly patient with
lymph node MS combined with TP53 (V173G) mutation, who eventually
died of respiratory failure due to refractory pneumonia after
treatment with a variety of antibiotics.
Case report
In September 2016, a 65-year-old male patient with a
recurrent fever for >4 months was admitted to the First
Affiliated Hospital of Zhejiang Chinese Medical University
(Hangzhou, China). The patient presented with a fever of ~38°C,
accompanied by chills, fatigue and occasional abdominal pain. The
fever subsided without medical intervention, but did return at
irregular intervals. The physical examination revealed roughly
soybean-sized lymph nodes in the bilateral neck, armpit and groin,
with tough, clear boundaries. The patient had a >3-year history
of a gastric antrum ulcer and was being treated with pantoprazole.
The patient had suffered from malaria >30 years previously and
had recent weight loss of ~10 kg.
The laboratory examination results showed the
following levels: Platelets (PLTs), 39×109/l (normal
range, 125–350×109/l); white blood cells (WBCs),
3.2×109/l (normal range, 3.5–9.5×109/l),
hemoglobin (Hb), 62 g/l (normal range, 115–150 g/l); and
reticulocytes, 3.5% (0.5–1.5%). High-resolution computed tomography
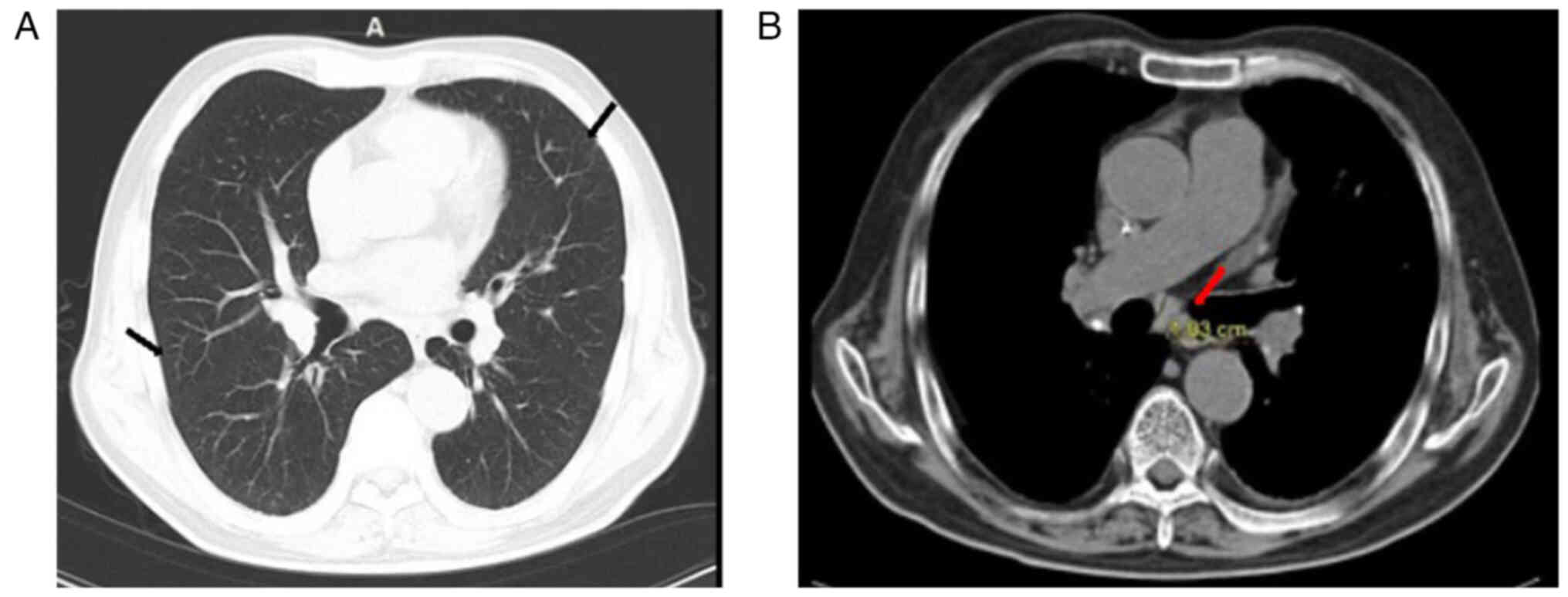
(HRCT) of the lungs shows scattered inflammation in both lungs,
with multiple enlarged lymph nodes in the mediastinum (Fig. 1). Primordial cells were recorded at
1% (normal, 0%) and Epstein-Barr virus DNA at
5.95×103/ml (normal range, 0–4×102/ml). A
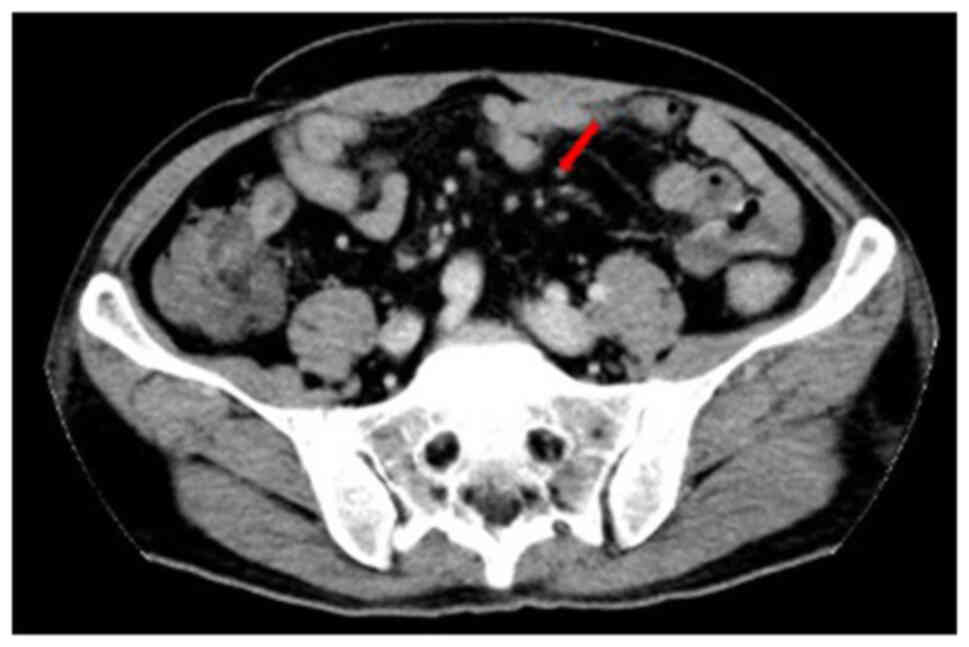
B-scan and CT showed multiple enlarged lymph nodes in the neck,
groin and mesentery (Fig. 2).
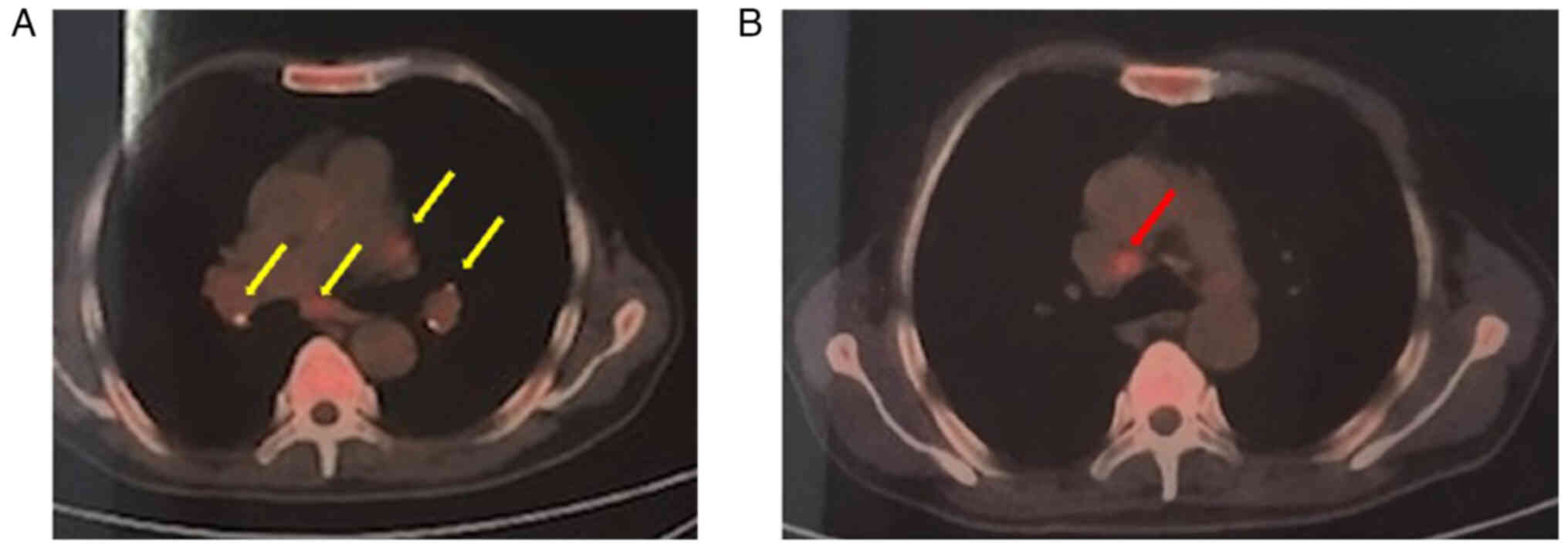
Positron emission tomography-CT results indicated a diagnosis of
lymphoma, and the maximum standardized uptake value of the lymph
nodes was 4.3 (normal, <2.0) (Fig.
3). Bone marrow routine examination showed a
granulocyte:erythroid cell ratio of 0.1:1 (normal range, 2–4:1) and
occasional erythroid dysplasia. In October 2016,
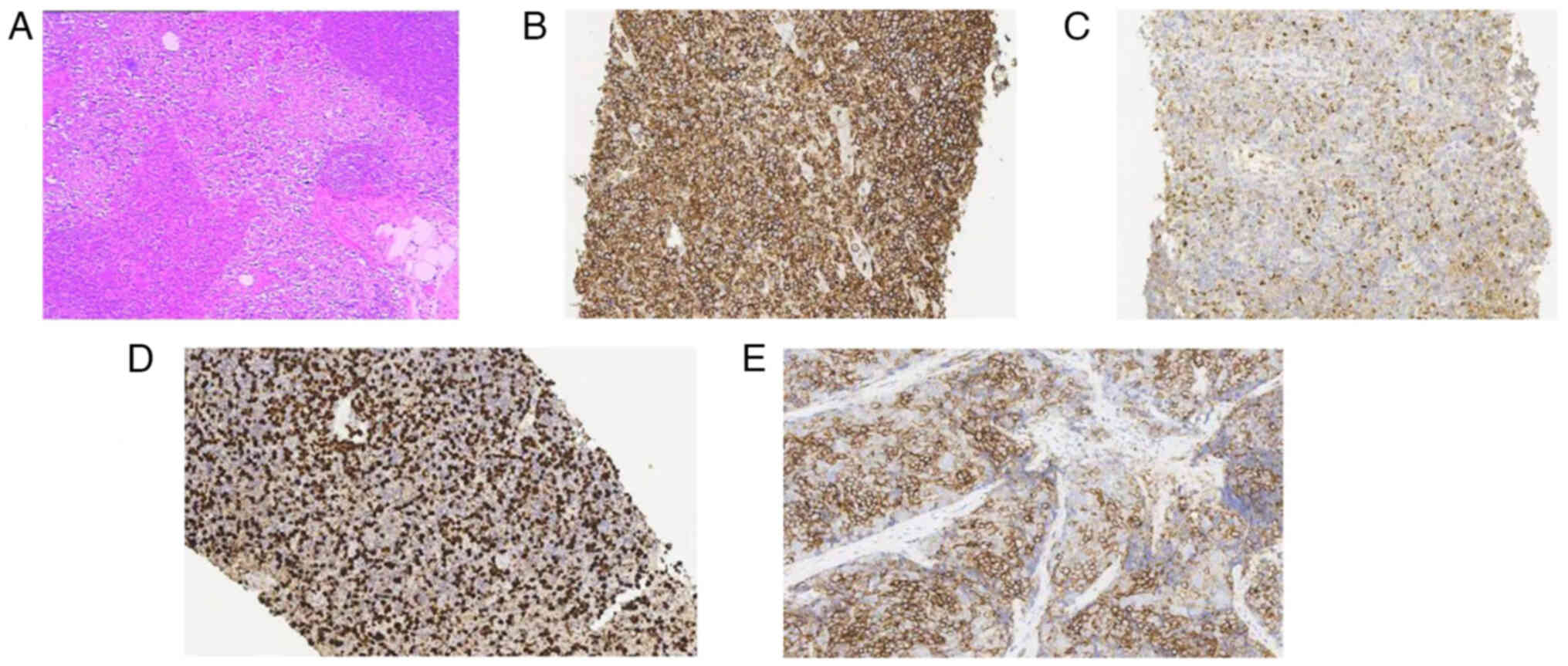
immunohistochemical analysis of the bone marrow and lymph nodes,
performed in the Zhejiang Provincial Hospital of Traditional
Chinese Medicine (Zhejiang, China), showed the following results:
MPO+, CD117+, CD20+,
CD79a+, PAX-5+, CD3+,
CD43+ and Ki-67 20% (Fig.
4). The immunohistochemical staining was performed as follows:
Fresh tissues were cut into small pieces with a diameter not
exceeding 5 mm, and fixed at 4°C overnight with 4% paraformaldehyde
(prepared with PBS and pre cooled at 4°C). Conventional
paraffin-embedded sections (2-µm thick) were created by slicing,
baking for 15 min and then embedding at 75°C for 15 min before
deparaffinization and dehydration. The samples were stained with
0.5% hematoxylin for 1 min, and with 0.5% eosin for a few seconds,
both at room temperature. Finally, the HE staining results were
observed under an optical microscope. The slices were then soaked
in sodium citrate solution and heated at 75°C for 15 min for
antigen retrieval. Sealing was performed with 10% sheep serum (cat.
no. ZLI-9022; OriGene Technologies, Inc.) at room temperature for 1
h before removing the agent. Subsequently, sections were incubated
with primary antibodies at 4°C overnight and then treated with
secondary antibodies at 4°C for 20 min the next day. The positive
signal was visualized with DAB reagents (OriGene Technologies,
Inc.) and slices were counterstained with hematoxylin. The
antibodies employed in the current study included CD117 (cat. no.
ZA-0523; 1:50; bone marrow sample; Thermo Fisher Scientific, Inc.),
MPO (cat. no. MA1-80878; 1:500; bone marrow sample; Thermo Fisher
Scientific, Inc.), CD20 (cat. no. TA800385; 1:150; lymph node
sample; OriGene Technologies, Inc.), CD3 (cat. no. TA800385; 1:150;
bone marrow sample; OriGene Technologies, Inc.); CD79a (cat. no.
TA800688; 1:150; lymph node sample; OriGene Technologies, Inc.),
PAX-5 (cat. no. 1TA801884; 1:150; lymph node sample; OriGene
Technologies, Inc.), Bcl-2 (cat. no. PA5-27094; 1:150; lymph node
sample; Thermo Fisher Scientific, Inc.) and goat anti-rabbit IgG
(H+L) (cat. no. A-11012; 2 µg/ml; Alexa Fluor™594; Thermo Fisher
Scientific, Inc.). Bone marrow gene mutation detection performed by
Shanghai Tissuebank Diagnostics Co., Ltd., showed the TP53 gene
missense mutation V173G.
The patient presented with repeated bouts of low
fever and chills, but the body temperature subsided without medical
intervention, and antibiotic anti-infection treatment (2 g
latamoxef, twice a day for 2 weeks and 2 g cefoperazone sodium,
once every 8 h for 4 weeks) was ineffective. Re-examination of bone
marrow routine (September and October 2019) showed that the
proportion of erythroid dysplasia was higher than the initial
result (10 days earlier in September 2019). The bone marrow
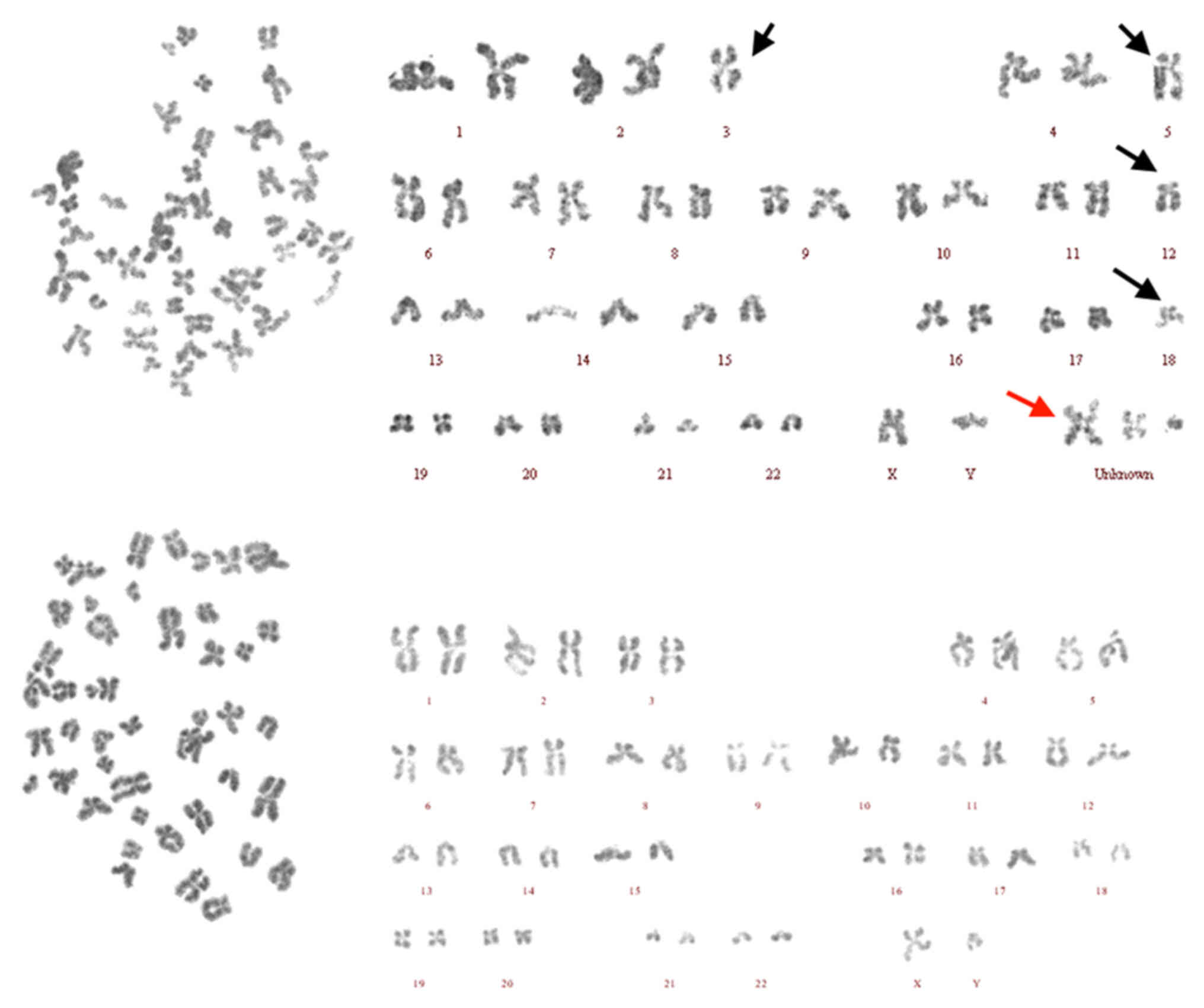
chromosome examination report showed that the patient karyotype was
45,XY,-3,-5,-12,-18,+Mar-3[17]/46,XY[3] (Fig. 5). Combined with the aforementioned
examination results, the patient was diagnosed with MDS-refractory
anemia. In November 2016, lymphoma was considered to be the source
of fever.10 mg lenalidomide and 10 mg prednisone twice a day were
used for treatment for 21 days. However, the number of primordial
cells increased after treatment, indicating that the disease was
developing.
For further diagnosis, the pathology of the left
cervical lymph node was sent to the Shanghai Cancer Center
(Shanghai, China) for further immunohistochemical analysis in
December 2016, performed as follows: Fresh tissue was cut into
small pieces with a diameter of 2 mm, fixed at room temperature
overnight with 4% paraformaldehyde (prepared with PBS and pre
cooled at 4°C) and dehydrated. Conventional paraffin sections (2-µm
thick) were baked for 15 min on the surface, and embedded blocks
made at 60°C for 4 h before deparaffinization and dehydration. The
samples were stained with 0.5% hematoxylin for 1 min and with 0.5%
eosin for a few seconds, both at room temperature. Finally, the HE
staining results were observed under an optical microscope. Slices
were then soaked in sodium citrate solution and heated at 60°C for
4 h for antigen retrieval. Sealing was performed with 2.5% goat
serum (cat. no. R37624) at room temperature for 45 min.
Subsequently, sections were incubated with primary antibodies at
4°C overnight and with secondary antibodies at 4°C for 20 min the
next day. The positive signal was visualized with DAB reagents
(OriGene Technologies, Inc.) and slices were counterstained with
hematoxylin at room temperature for 2 min. The antibodies employed
included CD20 (cat. no. PA5-16701; 1:300); CD3 (cat. no.
14-0032-82; 1:100); CD43 (cat. no. 14-0039-82; 20 µg/ml), KP1 (cat.
no. 14-0688-82; 1–5 µg/ml), CD117 (cat. no. 34-8800; 1:50), Ki-67
(cat. no. MA5-14520; 1:100), CD31 (cat. no. BMS137; 30 µg/ml) and
donkey anti-rabbit IgG (H+L) (cat. no. A-21206; 1–10 µg/ml; Alexa
Fluor™488) (all eBioscience; Thermo Fisher Scientific, Inc.). Due
to the invasion of the lymph nodes by myeloid tumors, in
combination with the medical history and results from the
immunohistochemical analysis [CD3+, CD43+,
CD20+, CD68/KP1+, CD117−/+,
CD31+ and Ki-67+ (~70%)], MDS was considered.
Furthermore, ~70% of lymph node proliferative changes showed a
large number of myeloid cells. Based on the aforementioned tests
and combined with the symptoms of the patient, a diagnosis of MS
with MDS was reached. Conventional chemotherapy regimens include
induction remission chemotherapy and 2–3 cycles of consolidation
chemotherapy for 2 months per cycle. In December 2016, the patient
began the first induction remission chemotherapy for MS. In the
first 3 days, 25 mg decitabine was used. From day 4 until day 11, 1
mg homoharringtonine and 12.5 mg cytarabine were administered twice
a day.
After one course of chemotherapy, the PLT, RBC and
WBC counts recovered, and analysis showed a WBC count of
4.3×109/l, Hb levels of 124 g/l and a PLT count of
139×109/l. Bone marrow routine showed active
proliferation of bone marrow cells, and active proliferation and
normal morphology of granulocytes and erythroid cells. A B-scan
showed extensive lymph node reduction, suggesting complete
remission (CR) after chemotherapy. In February 2017, the previous
protocol was improved upon by adding 25 mg etoposide on day 4,
followed by continuous use of homoharringtonine and cytarabine for
7 days. A PLT count of 43×109/l was reported after
chemotherapy. In April 2017, the use of decitabine was extended to
5 days, and the etoposide was changed to aclacinomycin at a dose of
20 mg once every other day for a total of 6 doses. The use of
aclacinomycin and cytarabine was extended to 11 days, and the dose
of homoharringtonine was increased to 2 mg. The PLT, Hb and WBC
counts decreased significantly after this cycle of
chemotherapy.
In April 2017, B-scan showed that the volume of
right clavicle lymph nodes was larger than that at admission. In
February and April 2017, bone marrow reexamination showed low bone
marrow cell proliferation and the peripheral blood showed 10%
primitive cells. This was considered to be the invasion of MS. The
WBC count was severely reduced, and agranulocytosis was reported.
The patient's inflammation index increased, a lung CT showed
interstitial pneumonia and the diagnosis of pulmonary infection was
clear. However, the pathogen was not identified by blood culture.
Starting from April 2017, meropenem (1 g; 8 h once a day for 2
weeks) was used for anti-infection treatment, and empirical
combination therapy with voriconazole (3 mg on the first day, and
200 mg orally thereafter, once every 12 h for 3 months) was used
for antifungal treatment. Starting in June, treatment with
vancomycin (1 g per day for 2 weeks) and amphotericin (20 mg per
day for 1 month) was administered. Starting from July, cefoperazone
sodium (2 g per day until death) and moxifloxacin (0.4 g per day
for 2 weeks) were used for anti-infection treatment. After
treatment, the inflammatory index did not decrease, but increased
once again. Finally, the patient died of respiratory failure due to
decreased oxygen partial pressure.
Discussion
As MS is mainly characterized by extramedullary
soft-tissue masses that can occur in any part of the body, it is
often misdiagnosed as lymphoma (14). A previous study reported that the
misdiagnosis rate of MS is as high as 47%, and that of primary MS
is even up to 75–86% (15). The
diagnosis of MS mainly depends on the results of the pathological
biopsy and immunohistochemistry. It has been reported that the most
commonly expressed antigens in MS include CD43, CD68, lysozyme, MPO
and CD117, with 66–69% of MS cases being MPO+ (16,17).
Since the clinical manifestations of MS are not
unique, most MS cases develop into acute leukemia soon after
diagnosis, with a median time of ~7 months (5). Moreover, TP53-targeted drugs are still
in clinical trials and have not yet shown much success due to
significant side effects, including bone marrow suppression
(18,19). Therefore, AML chemotherapy regimens,
including idarubicin and cytarabine, and fludarabine, cytarabine,
idarubicin and granulocyte colony-stimulating factor, are often the
preferred therapy for MS (5). AML
chemotherapy is required for both new-onset MS and other types of
MS. Studies have shown that early intervention with chemotherapy
can delay the progression of MS to AML (20,21).
Older patients are often unfit for intensive chemotherapy, and for
these cases, epigenetic therapy with hypomethylating agents offers
a small advantage over chemotherapy in TP53-mutant AML (22).
Decitabine is a deoxynucleotide analog that can
selectively inhibit DNA methyltransferase, causing DNA
hypomethylation and cell differentiation or apoptosis to exert
antitumor effects. Decitabine is often used to treat elderly
patients with AML or patients with high-risk chemoresistant
phenotypes (23). Some studies have
confirmed the efficacy of decitabine in the treatment of MS,
reporting that it can induce long-term remission (4,24). In
a Surveillance, Epidemiology and End Results database analysis,
Liang et al (13) found that
when using overall survival as an indicator of efficacy, patients
with non-isolated MS showed an improved response to
cytarabine-based AML chemotherapy regimens. It has also been
reported that a patient with bladder MS with TP53 mutation achieved
a significant improvement in symptoms and achieved complete CR in
the first cycle of 20 mg decitabine treatment combined with
induction chemotherapy. Due to the consideration of high-risk
cytogenetics and TP53 mutations, subsequent allo-HCT was performed
for consolidation (4). Similarly,
the patient reported in the present study was treated with
decitabine combined chemotherapy and achieved CR after the first
chemotherapy. This indicates that decitabine combined with
chemotherapy is effective in the induction therapy for MS with TP53
mutation. However, the treatment effect after CR varies between
patients (3). Consolidation therapy
after the induction of remission still requires future prospective
randomized controlled trials to clarify the optimal treatment
regimen.
In recent years, an increasing number of studies
have recognized the importance of TP53 in tumors (25–27).
p53 is a transcription factor that stabilizes genotoxic stress and
induces the transcription of genes involved in cell cycle arrest,
apoptosis and metabolism, thereby acting as a tumor suppressor
(22). In general, tumor
suppressors have a loss-of-function or deletion mutation in cancers
(28). However, most TP53 mutations
are missense mutations in the DNA binding domain, making mutant
TP53 lose tumor suppressor functions and gain carcinogenic
functioning independent of wild-type TP53 (29). TP53 is closely related to the
genomic and chromosomal instability of osteosarcoma (30,31).
However, no representative chromosome translocation has yet been
found (32). The frequency of
complex karyotypes in TP53-positive patients is significantly
higher than that in TP53-negative patients (33). At the molecular level, since TP53
mutations mostly occur in exons of the DNA binding domain, the
arginine residue of the p53 protein is considered to be a mutation
hot spot, which can affect DNA binding and change the activity of
the mutant protein (25,26,34).
TP53 is associated with chemosensitivity, mainly by affecting the
patient's chemical tolerance or overall survival rate (27). Middeke et al (35) showed that TP53-positive patients
have a high risk of recurrence after remission, and that allo-HCT
plays an important role in post-remission treatment. However, few
patients have access to this treatment. Therefore, TP53 mutation
means that compared with patients with TP53-negative MS, patients
with TP53-positive MS have low sensitivity to intensive
chemotherapy, poor tolerance, short CR duration, high risk of
recurrence after remission and complex cytogenetics.
The pathogenic V173G mutation of TP53 was first
reported in a 2015 article on non-small cell lung cancer (NSCLC)
(36). V173G is considered to make
up 15% of all TP53 mutations in NSCLC, and is expected to be
harmful and destructive. In 2021, the case of one young patient
with florid cemento-osseous dysplasia and Li-Fraumeni syndrome, who
eventually developed osteosarcoma, was reported. After biopsy, the
p.V173G TP53 mutation was identified, and the allele frequency was
high at 86% (37). It can be
speculated that TP53 (V173G) is a feature of concurrent diseases
that occur rarely and that it creates conditions for tumor
development. However, the correlation between TP53 (V173G) mutation
and the prognosis and development of tumors still needs to be
further explored with large sample data.
Patients with MS who harbor TP53 (V173G) mutations
and complex karyotypes are considered to have a poorer prognosis
(38). In the present study, the
patient had a TP53 mutation, an abnormal chromosome karyotype and
poor cytogenetic characteristics, which were associated with a
poorer prognosis. Disease recurrence occurred after the third
cycle. Due to severe neutropenia and uncontrolled inflammation, the
use of multiple antibiotics for treatment was ineffective. In the
end, the patient developed severe pneumonia and died. It has been
reported that the expression of TP53 is increased in drug-resistant
NSCLC (39). It can be speculated
that the refractory pneumonia of this patient may be related to the
positive expression of TP53. A previous study found that TP53
positivity was associated with tumor lymph node metastasis
(40). Patients with TP53-positive
tumors have a higher rate of lymph node metastasis. In addition to
genetics, the cause of recurrence may also be related to the
invasiveness of the MS. One study has shown that MS-derived cell
lines have type IV collagenase, which has been reported to be
associated with a poorer prognosis in gastric cancer (41).
In conclusion, MS has a low incidence rate, poor
prognosis and short survival time. The clinical manifestations of
MS are not specific and are frequently misdiagnosed. MS should be
considered in the differential diagnosis of suspicious masses or
atypical cell infiltration with or without bone marrow involvement.
If economic conditions permit, genetic testing is recommended while
performing immunohistochemistry, and next-generation sequencing is
a better choice than whole exome sequencing. TP53-positive MS
should be awarded more attention in clinical practice due to its
unsatisfactory prognosis and lower survival rate compared with that
of TP53-negative MS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MM and SD conceived the study, participated in the
design of the study and collected the data and the images. MM
drafted the manuscript, collected the clinical data and performed
the literature research. SD participated in the data acquisition
and interpretation, drafted the manuscript and critically revised
the manuscript. MM and SD confirm the authenticity of all the raw
data. Both authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent to
publish the medical data and images for this case.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arber DA, Orazi A, Hasserjian R, Thiele J,
Borowitz MJ, Le Beau MM, Bloomfield CD, Cazzola M and Vardiman JW:
The 2016 revision to the World Health Organization classification
of myeloid neoplasms and acute leukemia. Blood. 127:2391–2405.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhao H, Dong Z, Wan D, Cao W, Xing H, Liu
Z, Fan J, Wang H, Lu R, Zhang Y, et al: Clinical characteristics,
treatment, and prognosis of 118 cases of myeloid sarcoma. Sci Rep.
12:67522022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Almond LM, Charalampakis M, Ford SJ,
Gourevitch D and Desai A: Myeloid sarcoma: Presentation, diagnosis,
and treatment. Clin Lymphoma Myeloma Leuk. 17:263–267. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mandhan N, Yassine F, Li K and Badar T:
Bladder myeloid sarcoma with TP53 mutated myelodysplastic
syndrome/myeloproliferative neoplasm overlap syndrome: Response to
Decitabine-Venetoclax regimen. Leuk Res Rep.
17:1002862021.PubMed/NCBI
|
|
5
|
Bakst RL, Tallman MS, Douer D and Yahalom
J: How I treat extramedullary acute myeloid leukemia. Blood.
118:3785–3793. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kaur V, Swami A, Alapat D, Abdallah AO,
Motwani P, Hutchins LF and Jethava Y: Clinical characteristics,
molecular profile and outcomes of myeloid sarcoma: A single
institution experience over 13 years. Hematology. 23:17–24. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ganzel C, Manola J, Douer D, Rowe JM,
Fernandez HF, Paietta EM, Litzow MR, Lee JW, Luger SM, Lazarus HM,
et al: Tallman, Extramedullary disease in adult acute myeloid
leukemia is common but lacks independent significance: Analysis of
patients in ECOG-ACRIN cancer research group trials, 1980–2008. J
Clin Oncol. 34:3544–3553. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang HQ and Li J: Clinicopathological
features of myeloid sarcoma: Report of 39 cases and literature
review. Pathol Res Pract. 212:817–824. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Toguchida J, Yamaguchi T, Ritchie B,
Beauchamp RL, Dayton SH, Herrera GE, Yamamuro T, Kotoura Y, Sasaki
MS and Little JB: Mutation spectrum of the p53 gene in bone and
soft tissue sarcomas. Cancer Res. 52:6194–6199. 1993.PubMed/NCBI
|
|
10
|
Thoenen E, Curl A and Iwakuma T: TP53 in
bone and soft tissue sarcomas. Pharmacol Ther. 202:149–164. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ortiz-Cuaran S, Cox D, Villar S, Friesen
MD, Durand G, Chabrier A, Khuhaprema T, Sangrajrang S, Ognjanovic
S, Groopman JD, et al: Association between TP53 R249S mutation and
polymorphisms in TP53 intron 1 in hepatocellular carcinoma. Genes
Chromosomes Cancer. 52:912–919. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Welch JS, Petti AA, Miller CA, Fronick CC,
O'Laughlin M, Fulton RS, Wilson RK, Baty JD, Duncavage EJ, Tandon
B, et al: TP53 and Decitabine in acute myeloid leukemia and
Myelodysplastic Syndromes. N Engl J Med. 375:2023–2036. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liang J and Yang L, Yang B, Tian Y, Ren J
and Yang L: Clinical characteristics, treatment options, and
prognosis of myeloid sarcoma: Analysis using the SEER database.
Hematology. 28:22478982023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gajendra S, Goel S, Sharma R, Dhiman P and
Sachdev R: Myeloid sarcoma presented as generalized
lymphadenopathy: Mimicking malignant lymphoma. Indian J Hematol
Blood Transfus. 34:173–177. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang L, Hua C, Jie B, et al: A case of
gastric granulocytic sarcoma misdiagnosed as non Hodgkin's
lymphoma. Int J Lab Med. 37:3524–3526. 2016.(In Chinese).
|
|
16
|
Kawamoto K, Miyoshi H, Yoshida N, Takizawa
J, Sone H and Ohshima K: Clinicopathological, cytogenetic, and
prognostic analysis of 131 myeloid sarcoma patients. Am J Surg
Pathol. 40:1473–1483. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shallis RM, Gale RP, Lazarus HM, Roberts
KB, Xu ML, Seropian SE, Gore SD and Podoltsev NA: Myeloid sarcoma,
chloroma, or extramedullary acute myeloid leukemia tumor: A tale of
misnomers, controversy and the unresolved. Blood Rev.
47:1007732021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ray-Coquard I, Blay JY, Italiano A, Le
Cesne A, Penel N, Zhi J, Heil F, Rueger R, Graves B, Ding M, et al:
Effect of the MDM2 antagonist RG7112 on the P53 pathway in patients
with MDM2-amplified, well-differentiated or dedifferentiated
liposarcoma: An exploratory proof-of-mechanism study. Lancet Oncol.
13:1133–1140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gopalakrishnan V, Amini B, Wagner MJ,
Nowell EN, Lazar AJ, Lin PP, Benjamin RS and Araujo DM: Synovial
sarcoma of the head and neck: A single institution review. Sarcoma.
2017:20167522017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee JY, Chung H, Cho H, Jang JE, Kim Y,
Kim SJ, Kim JS, Hyun SY, Min YH and Cheong JW: Clinical
characteristics and treatment outcomes of isolated myeloid sarcoma
without bone marrow involvement: A single-institution experience.
Blood Res. 52:184–192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imrie KR, Kovacs MJ, Selby D, Lipton J,
Patterson BJ, Pantalony D, Poldre P, Ngan BY and Keating A:
Isolated chloroma: The effect of early antileukemic therapy. Ann
Intern Med. 123:351–353. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim K, Maiti A, Loghavi S, Pourebrahim R,
Kadia TM, Rausch CR, Furudate K, Daver NG, Alvarado Y, Ohanian M,
et al: Outcomes of TP53-mutant acute myeloid leukemia with
decitabine and venetoclax. Cancer. 127:3772–3781. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hackanson B and Daskalakis M: Decitabine.
Recent Results Cancer Res. 201:269–297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gornicec M, Wolfler A, Stanzel S, Sill H
and Zebisch A: Evidence for a role of decitabine in the treatment
of myeloid sarcoma. Ann Hematol. 96:505–506. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Soussi T and Lozano G: p53 mutation
heterogeneity in cancer. Biochem Biophys Res Commun. 331:834–842.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Goh AM, Xue Y, Leushacke M, Li L, Wong JS,
Chiam PC, Rahmat SA, Mann MB, Mann KM, Barker N, et al: Mutant p53
accumulates in cycling and proliferating cells in the normal
tissues of p53 R172H mutant mice. Oncotarget. 6:17968–17980. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Asada N, Tsuchiya H and Tomita K: De novo
deletions of p53 gene and wild-type p53 correlate with acquired
cisplatin-resistance in human osteosarcoma OST cell line.
Anticancer Res. 19:5131–5137. 1999.PubMed/NCBI
|
|
28
|
Liu Y, Hu X, Han C, Wang L, Zhang X, He X
and Lu X: Targeting tumor suppressor genes for cancer therapy.
Bioessays. 37:1277–1286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lane D and Levine A: p53 research: The
past thirty years and the next thirty years. Cold Spring Harb
Perspect Biol. 2:a0008932010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Al-Romaih K, Bayani J, Vorobyova J,
Karaskova J, Park PC, Zielenska M and Squire JA: Chromosomal
instability in osteosarcoma and its association with centrosome
abnormalities. Cancer Genet Cytogenet. 144:91–99. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zuffa E, Mancini M, Brusa G, Pagnotta E,
Hattinger CM, Serra M, Remondini D, Castellani G, Corrado P,
Barbieri E and Santucci MA: P53 oncosuppressor influences selection
of genomic imbalances in response to ionizing radiations in human
osteosarcoma cell line SAOS-2. Int J Radiat Biol. 84:591–601. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin JW, Squire JA and Zielenska M: The
genetics of osteosarcoma. Sarcoma. 2012:6272542012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Daver NG, Maiti A, Kadia TM, Vyas P,
Majeti R, Wei AH, Garcia-Manero G, Craddock C, Sallman DA and
Kantarjian HM: TP53-mutated myelodysplastic syndrome and acute
myeloid leukemia: Biology, current therapy, and future directions.
Cancer Discov. 12:2516–2529. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Olivier M, Hollstein M and Hainaut P: TP53
mutations in human cancers: Origins, consequences, and clinical
use. Cold Spring Harb Perspect Biol. 2:a0010082010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Middeke JM, Herold S, Rucker-Braun E,
Berdel WE, Stelljes M, Kaufmann M, Schäfer-Eckart K, Baldus CD,
Stuhlmann R, Ho AD, et al: TP53 mutation in patients with high-risk
acute myeloid leukaemia treated with allogeneic haematopoietic stem
cell transplantation. Br J Haematol. 172:914–922. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Veldore VH, Patil S, Satheesh CT,
Shashidhara HP, Tejaswi R, Prabhudesai SA, Krishnamoorthy N,
Hazarika D, Naik R, Rao RM and Kumar BS: Genomic profiling in a
homogeneous molecular subtype of non-small cell lung cancer: An
effort to explore new drug targets. Indian J Cancer. 52:243–248.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Haefliger S, Harder D, Kovac M,
Linkeschova K, Eufinger H and Baumhoer D: Osteosarcoma of the
mandible in a patient with Florid Cemento-Osseous Dysplasia and
Li-Fraumeni syndrome: A rare coincidence. Head Neck Pathol.
15:704–708. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pastoret C, Houot R, Llamas-Gutierrez F,
Boulland ML, Marchand T, Tas P, Ly-Sunnaram B, Gandemer V, Lamy T,
Roussel M and Fest T: Detection of clonal heterogeneity and
targetable mutations in myeloid sarcoma by high-throughput
sequencing. Leuk Lymphoma. 58:1008–1012. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kryczka J, Kryczka J, Czarnecka-Chrebelska
KH and Brzeziańska-Lasota E: Molecular mechanisms of
chemoresistance induced by cisplatin in NSCLC cancer therapy. Int J
Mol Sci. 22:88852021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xiangli L, Mingli Z, Jiao W, Jie C, Yu C,
Zhenyi N and Xuetao L: Expression and significance of PD-L1, Ki-67,
TP53, and EGFR in lung adenocarcinoma tissues. J Basic Clin Oncol.
36:324–327. 2023.(In Chinese).
|
|
41
|
Shen W, Xi H, Wei B and Chen L: The
prognostic role of matrix metalloproteinase 2 in gastric cancer: A
systematic review with meta-analysis. J Cancer Res Clin Oncol.
140:1003–1009. 2014.(In Chinese). View Article : Google Scholar : PubMed/NCBI
|