Introduction
Esophageal cancer is a common malignant tumor of the
gastrointestinal tract, the eighth most common malignant tumor and
the sixth leading cause of cancer-related death worldwide (1). With the development of minimally
invasive techniques, minimally invasive surgery now occupies an
absolute position in esophageal cancer surgery. Esophageal squamous
cell carcinoma is dominant in East Asia, including China, while
esophageal adenocarcinoma is dominant in Europe, which may be
related to genetic susceptibility between different ethnicities and
dietary conditions among different populations (2). Patients with esophageal cancer often
only present with progressive dysphagia in the middle and late
stages of disease due to the lack of specific clinical
manifestations. Therefore, when patients present at the clinic, the
indication for direct surgery has typically past, and even if
surgery is possible, there is a risk of local and distant
metastasis, which greatly increases the tumor burden on patients
(3,4). With the development of neoadjuvant
therapy in recent years, neoadjuvant therapy to achieve tumor
shrinkage or even downstaging before surgery (thus increasing the
radical resection rate of tumors) has become the optimal treatment
option for patients with intermediate to advanced esophageal cancer
(5). However, neoadjuvant therapy
often causes adverse effects in patients, including anemia and
hypoproteinemia (6), and a study
has indicated that patients undergoing surgery following
neoadjuvant therapy have an increased risk of pulmonary infection
and anastomotic fistula (7).
Therefore, it is particularly important to find a low-cost modality
with few side effects to prevent or treat esophageal cancer.
Stromal interaction molecule 1 (STIM1) is a protein
mainly found in the endoplasmic reticulum membrane and has a highly
conserved protein structure (8).
STIM1 is one of the key proteins of the store-operated calcium
entry (SOCE) channel, which mediates the entry of extracellular
Ca2+ into the intracellular compartment to maintain the
relative stability of Ca2+ inside and outside the cell.
When the calcium pool is emptied for various reasons, such as drug
action and hypoxia (9),
Ca2+ dissociates from the EF-chiral structure
(structural domain of STIM1), which induces a multimerization
process in the N-terminal region of STIM1, resulting in a
conformational change of STIM1 and its transfer to the inner cell
membrane to couple with Orai1 and transient receptor potential
canonical channels, thereby activating SOCE to allow the entry of
extracellular Ca2+ into the intracellular compartment
(10,11). STIM1 has a close relationship with a
variety of tumorigenesis mechanisms; it can participate in the
recruitment of endothelial cells by the bone marrow, thus promoting
tumor angiogenesis (12), and
downregulation of the STIM1 gene blocks the cell cycle in the S or
G2 phase (13). It has also been
shown that STIM1 expression is related to the differentiation and
prognosis of esophageal malignant tumors, and the higher the
expression of STIM1, the worse the prognosis, while downregulation
of STIM1 expression significantly inhibits the proliferation and
migration of esophageal cancer cells (14).
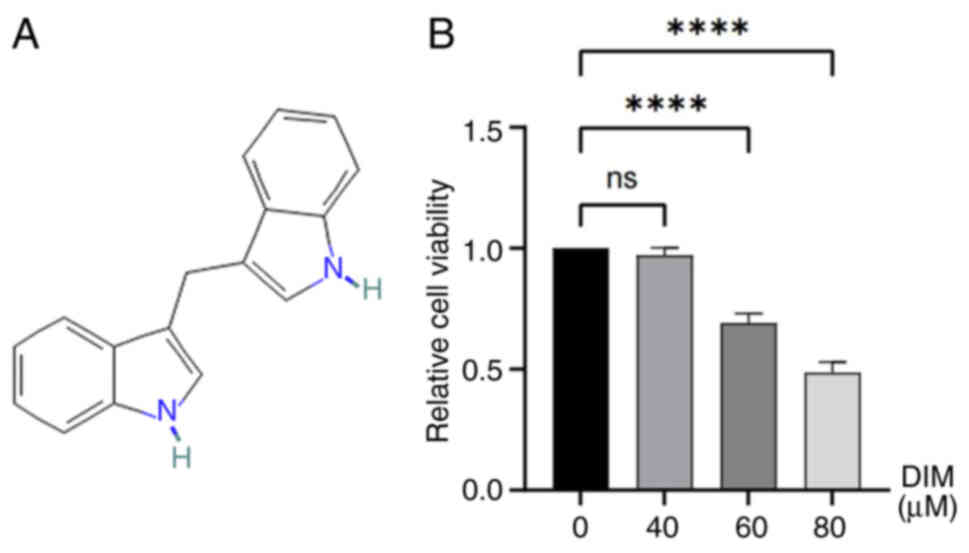
3,3′-Diindolylmethane (DIM; molecular formula,
C17H14N2; molecular weight,
246.3065) is a natural plant compound derived from cruciferous
plants, including cauliflower and broccoli, and is a metabolite of
indole-3-carbinol (I3C). After entering the digestive tract, I3C
from cruciferous plants is readily hydrolyzed to DIM under acidic
conditions to exert its biological effects (15). It has been demonstrated that DIM has
antitumor activity against breast, nasopharyngeal, gastric and
ovarian cancer (16,17). DIM can induce gastric cancer cell
death by upregulating the expression of STIM1 protein (18), and it has been reported that DIM
enhances sensitivity to radiotherapy in human esophageal cancer
cells (19). However, whether DIM
can directly inhibit the proliferation of esophageal cancer cells
and through what possible pathway, and whether it can be regulated
by STIM1 protein, has been rarely reported.
In the present study, the Cell Counting Kit-8 assay
was used to verify whether DIM could inhibit the variation of TE-1
cells, and western blotting was applied to detect the expression of
STIM1, Bcl-2 and Bax protein after DIM acted on TE-1 cells, to
preliminarily explore the possible mechanism behind this.
Materials and methods
Prediction of DIM and esophageal
cancer targets
‘3,3′-diindolylmethane’ was searched in the
Comparative Toxicogenomics Database (CTD; http://ctdbase.org/detail.go?type=chem&acc=C016392)
and the GeneCards database (https://www.genecards.org/Search/Keyword?queryString=3,3%27-diindolylmethane),
selecting ‘Homo sapiens’ as the species. The disease key words,
‘esophageal cancer’, were also searched in the CTD (https://ctdbase.org/detail.go?type=disease&acc=MESH%3AD004938)
and GeneCards database (https://www.genecards.org/Search/Keyword?queryString=esophagus%20cancer).
The results files were downloaded as Excel files. The DIM and
esophageal cancer targets were stored in two different Excel files
and any duplicated content within each file was deleted.
Construction and analysis of a target
network
The DIM and esophageal cancer targets were imported
into Venny 2.1.0 (https://bioinfogp.cnb.csic.es/tools/venny/) to obtain
the common targets. The common targets were then imported into
STRING V12.0 (https://cn.string-db.org/cgi/input.pl) for network
analysis, selecting ‘Homo sapiens’ as the species. The results were
downloaded and saved as a tsv file, which was imported into
Cytoscape 3.9.1 (Oracle Corporation) for visualization. Next, the
PPI results were imported into Cytoscape and the Centiscape 2.2
plug-in was used. ‘Degree’, ‘closeness’ and ‘betweenness’ were
selected to analyze the results, and 39 possible core targets were
obtained.
Gene ontology (GO) and Kyoto
encyclopedia of genes and genomes (KEGG) pathway enrichment
analyses
The common intersection targets of DIM and
esophageal cancer were entered into the Database for Annotation,
Visualization and Integrated Discovery (https://david.ncifcrf.gov/), selecting ‘Homo sapiens’
as the species, for GO enrichment [Molecular Function (MF),
Biological Process (BP) and Cell Composition (CC)] and KEGG pathway
analyses. P<0.05 was considered to indicate statistical
significance. The selected data were arranged in descending order,
and the first 20 results were imported into SRplot (https://www.bioinformatics.com.cn/) to produce
bubble diagrams.
Chemicals and reagents
DIM (cat. no. D9568; MilliporeSigma) was dissolved
in dimethylsulfoxide (99%) and prepared as a 100 mM stock solution,
which was stored at 4°C. Thapsigargin (Tg; HY-13433;
MedChemExpress),2-(2-Methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo-phenyl)-2H-tetrazole
monosodium salt [Cell Counting Kit-8 (CCK-8)] and bicinchoninic
acid (BCA) protein assay kit (cat. no. P0011) were purchased from
Beyotime Institute of Biotechnology. Primary antibodies against
STIM1 (1:1,000; cat. no. ab108994; Abcam), B-cell lymphoma-2
(Bcl-2; 1:1,000; cat. no. 3498S; CST Biological Reagents Co.,
Ltd.), Bax (1:1,000; cat. no. 50599-2-Ig; Proteintech Group, Inc.)
and GAPDH (1:10,000; cat. no. ab8245; Abcam) were also purchased.
Goat anti-rabbit HRP-conjugated (1:10,000; cat. no. ab6721)
secondary antibody was purchased from Abcam.
Cell culture and drug treatment
The human TE-1 esophageal cancer cell line
(esophageal squamous cell carcinoma; cat. no. TCHu 89) was
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. Cells were cultured in RPMI 1640
medium (Dalian Meilun Biology Technology Co., Ltd.), containing 20%
fetal bovine serum (Shanghai ExCell Biology, Inc.), at 37°C in a 5%
CO2 incubator. As according to the literature (18), TE-1 cells were treated with DIM at
concentrations of 0, 40, 60 and 80 µM for 24 h. In addition, the
STIM1 agonist toxic, Tg (1 µM), was used to pre-treat TE-1 cells
for 10 min prior to DIM exposure.
CCK-8 assay
TE-1 cells (10,000 cells/well) were inoculated into
96-well plates and exposed to different concentrations of DIM (0,
40, 60 and 80 µM) for 24 h, once cells had sufficiently adherent to
the wall of the plate. Then, 10 µl CCK-8 solution was incubated
with the cells for 1 h at 37°C, protected from the light. The cell
viability was evaluated by absorbance at 450 nm with a microplate
reader.
Scratch test
TE-1 cells in the logarithmic growth phase were
inoculated into 6-well plates. After 24 h of fully adherent cell
growth, 200 µl pipette tips were used to draw a straight line
through the cells with a width of ~1 mm, perpendicular to the plate
surface. After washing the 6-well plate with PBS to remove
non-adherent cells, different concentrations of DIM (0, 40, 60 and
80 µM) in serum-free medium were added to each well. The scratch
width in each well was recorded under a light microscope at 0 and
12 h. The cell migration rate (%) was calculated as follows: (0 h
scratch width-12 h scratch width)/0 h scratch width, and assessed
using ImageJ V1.8.0 (National Institutes of Health).
Protein extraction and
immunoblotting
After the TE-1 cells were washed 2–3 times with
pre-cooled PBS (cat. no. MA0015; Dalian Meilun Biology Technology
Co., Ltd.), the cells were lysed with cell lysis buffer (PMSF:RIPA,
1:100; PMSF cat. no. ST505; Beyotime Institute of Biotechnology;
RIPA cat. no. P0013B; Beyotime Institute of Biotechnology). The
resulting precipitate was scraped away and incubated on ice for 15
min, after which the supernatant was collected by centrifugation in
an Eppendorf microcentrifuge at 4,747 × g for 15 min at 4°C. The
protein content was detected by the BCA kit, and the total protein
amount in each group was adjusted to a consistent amount using 5X
loading buffer (cat. no. P0015L; Beyotime Institute of
Biotechnology). Equal amounts of protein (50 mg) were subjected to
12% SDS-PAGE, then transferred to polyvinylidene difluoride
membranes (cat. no. FFP22; Beyotime Institute of Biotechnology).
The membranes were incubated with 5% skimmed milk [prepared in
tris-buffered saline (TBS) containing 0.05% Tween-20] at room
temperature for 2 h, and then with STIM1, Bax, Bcl-2 and GAPDH
antibodies at 4°C overnight. The membranes were then incubated with
the corresponding secondary antibody for 1 h at 4°C. All primary
and secondary antibodies were diluted with TBS containing 0.1%
Tween-20 at 4°C. Protein bands were visualized using BeyoECL Plus
(cat. no. P0018S; Beyotime Institute of Biotechnology) and analyzed
by ImageJ V1.8.0 (National Institutes of Health).
Statistical analysis
Each experiment was repeated three times to obtain
more stable results. Statistical analysis was performed using
GraphPad Prism 9.0 (Dotmatics). The results are presented as the
mean ± SD. One-way ANOVA or Kruskal-Wallis followed by the Tukey or
Nemenyi test post hoc tests, respectively, were used to assess the
significant differences between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
DIM and esophageal cancer protein
interaction network
Through the retrieval of data from relevant
databases, a total of 289 potential targets of DIM and 18,441
potential esophageal cancer targets were obtained. Through
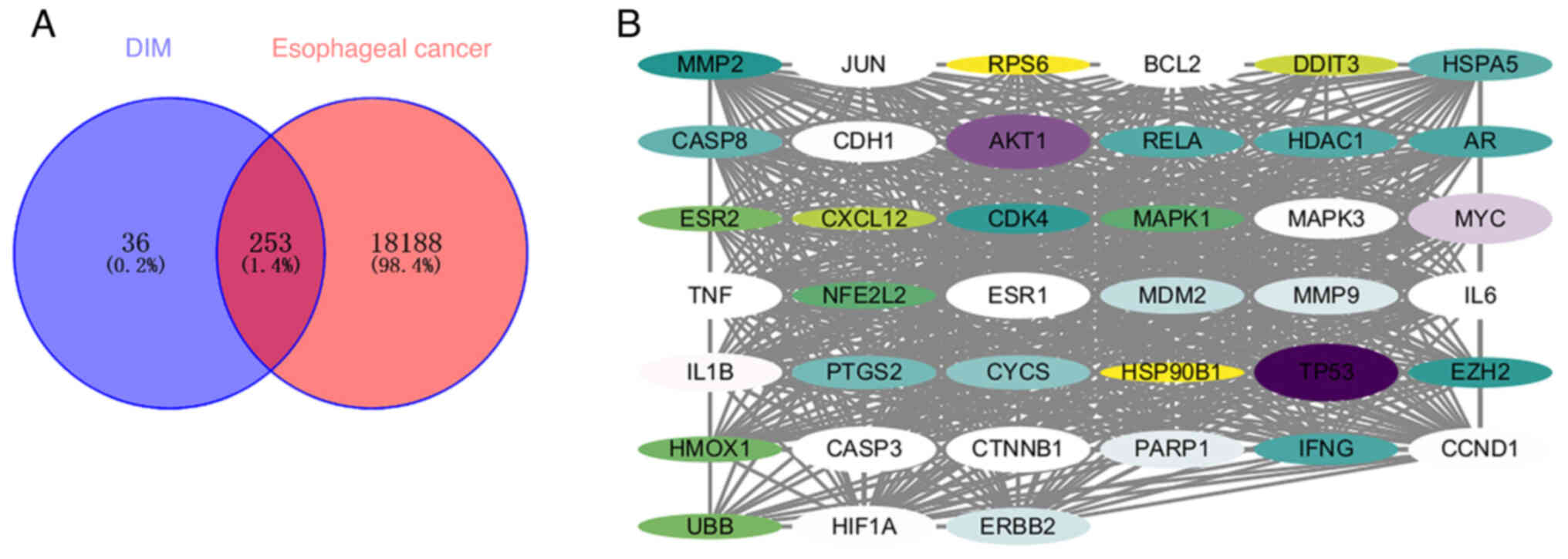
cross-analysis, 253 cross-targets were identified (Fig. 1A). To further determine the possible
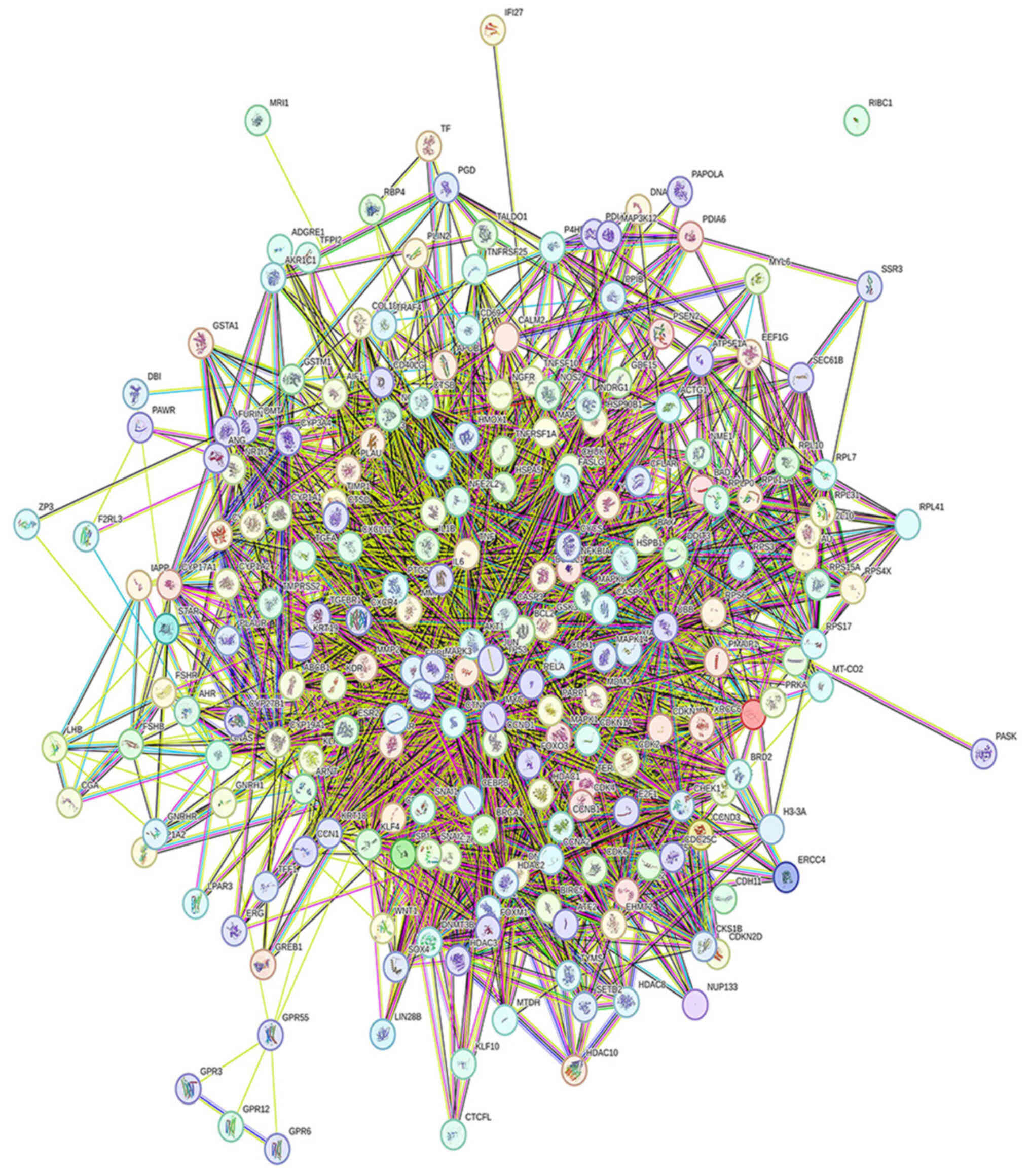
core targets of DIM acting on esophageal cancer cells, STRING was
used to construct a protein-protein interaction (PPI) network of
related cross-targets (Fig. 2).
Next, the PPI results were imported into Cytoscape and the
Centiscape 2.2 plug-in was used to analyze the results, and 39
possible core targets were obtained (Fig. 1B).
GO and KEGG pathway enrichment
analyses
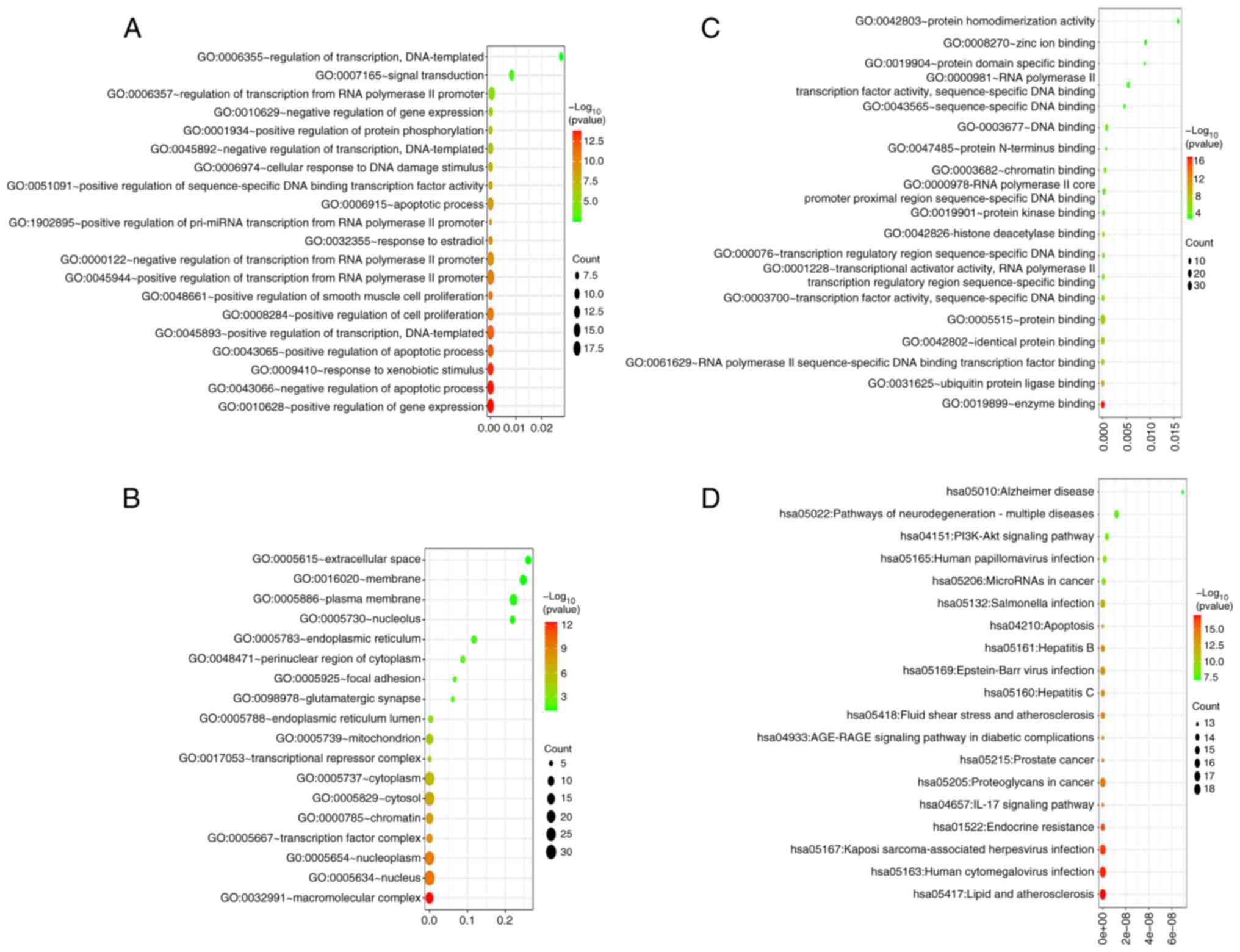
The GO-BP results showed that core targets mainly
involved biological processes such as ‘cell transcription’, ‘signal
transduction’, ‘RNA polymerase II regulation’, ‘apoptosis
regulation’ and ‘smooth muscle cell proliferation regulation’
(Fig. 3A). The GO-CC results showed
that core targets mainly involved cell components such as
‘nucleolus’, ‘endoplasmic reticulum’, ‘cytoplasm’ and ‘chromatin’
(Fig. 3B). The GO-MF results showed
that core targets mainly involved molecular functions such as
‘protein homodimerization activity’, ‘chromatin binding’, ‘protein
kinase binding’ and ‘ubiquitin protein ligase binding’ (Fig. 3C). The KEGG results showed that core
targets were involved in a number of neurodegenerative diseases,
‘PI3K-Akt signaling pathway’, ‘Prostate cancer’, ‘Apoptosis’,
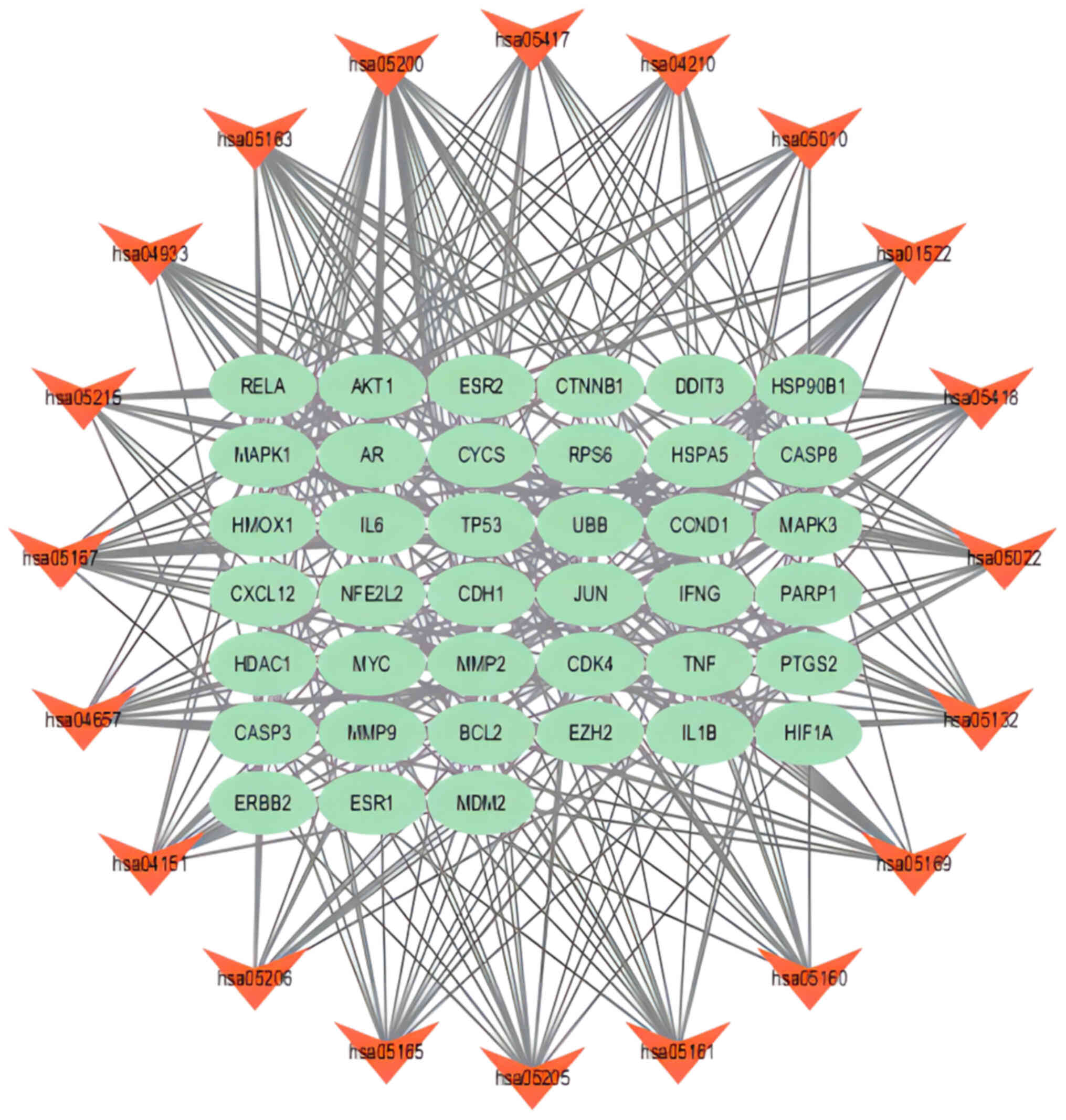
‘IL-17 signaling pathway’ and ‘Lipid and atherosclerosis’ (Fig. 3D). The network analysis of signaling
pathways and core targets showed the relationship between target
pathways and core targets. For example, the ‘PI3K-Akt signaling
pathway’ was related to the expression of ‘Bcl-2’ (Fig. 4).
DIM inhibits the viability of TE-1
esophageal cancer cells
Cells in the logarithmic growth phase were incubated
with different concentrations of DIM (0, 40, 60 and 80 µM) for 24
h, then the cell viability was detected by the CCK-8 method. The
results showed that DIM decreased the cell viability of TE-1
esophageal cancer cells in a concentration-dependent manner, which
was statistically significant at DIM concentrations of 60 and 80 µM
(P<0.05; Fig. 5B).
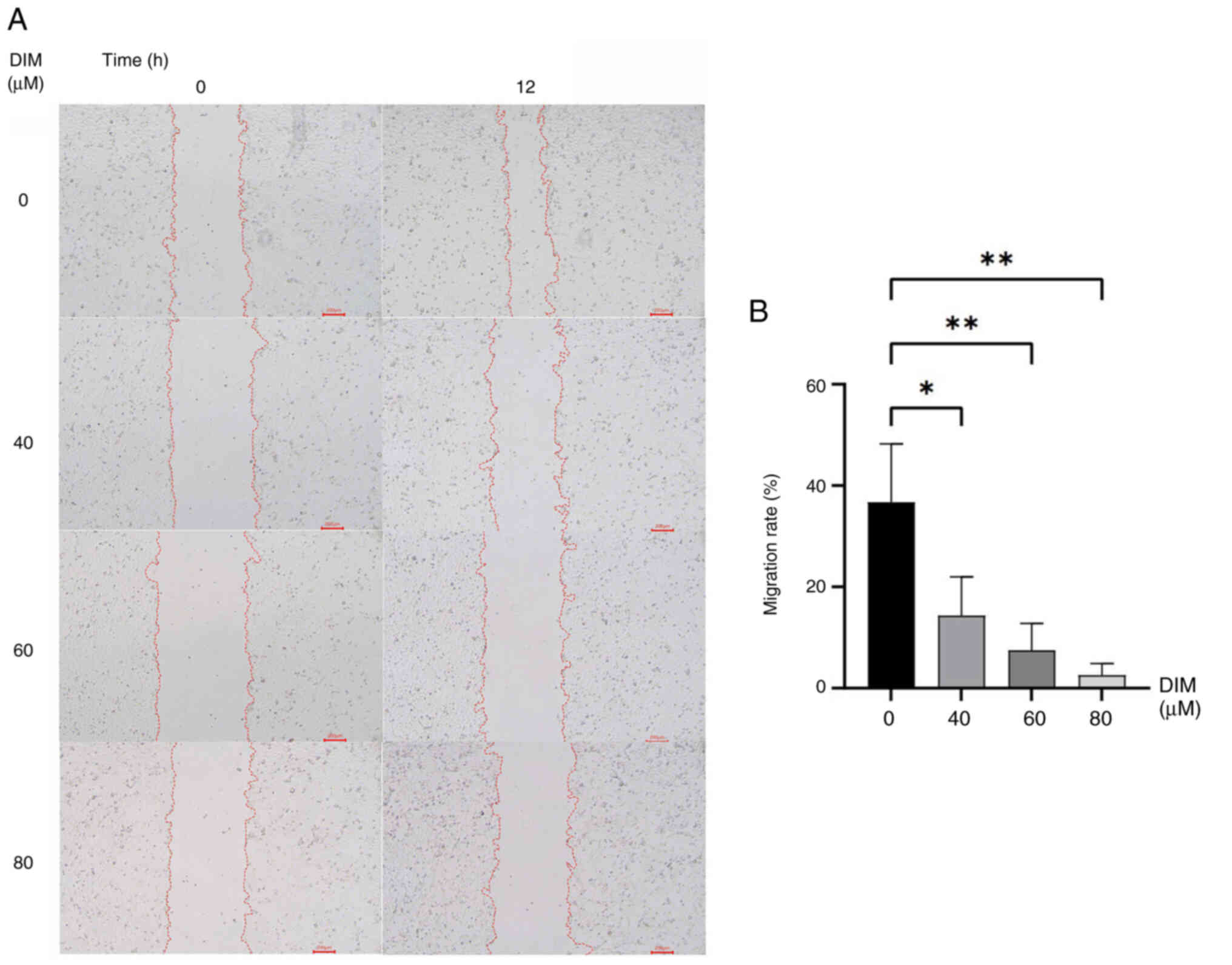
DIM inhibits the migration of TE-1
cells
To verify the effect of DIM on the migration of TE-1
cells, a scratch assay was used. The results showed that DIM
inhibited the migration of TE-1 cells in a concentration-dependent
manner, which was statistically significant at DIM concentrations
of 40, 60 and 80 µM (P<0.05; Fig. 6A
and B).
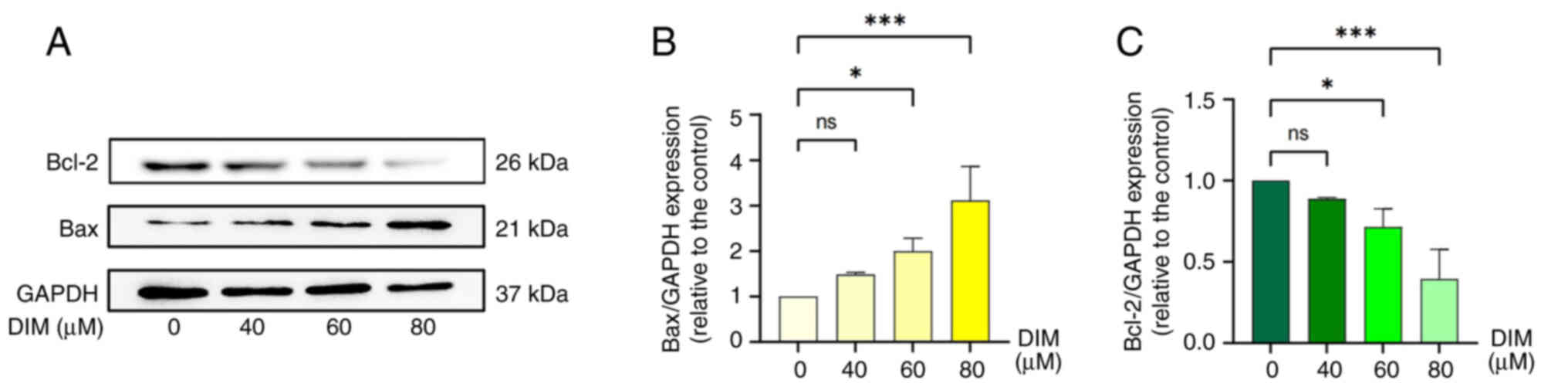
DIM promotes apoptosis in TE-1
cells
To investigate the way in which DIM affects TE-1
esophageal cancer cell viability, the cells were treated with
different concentrations of DIM (0, 40, 60 and 80 µM) for 24 h,
then the protein expression levels of Bcl-2 and Bax were analyzed
by western blotting. The results showed that DIM induced a
concentration-dependent decrease in Bcl-2 protein expression in
TE-1 cells, while it caused a concentration-dependent increase in
Bax protein expression, both of which were statistically
significant at DIM concentrations of 60 and 80 µM (P<0.05;
Fig. 7A-C).
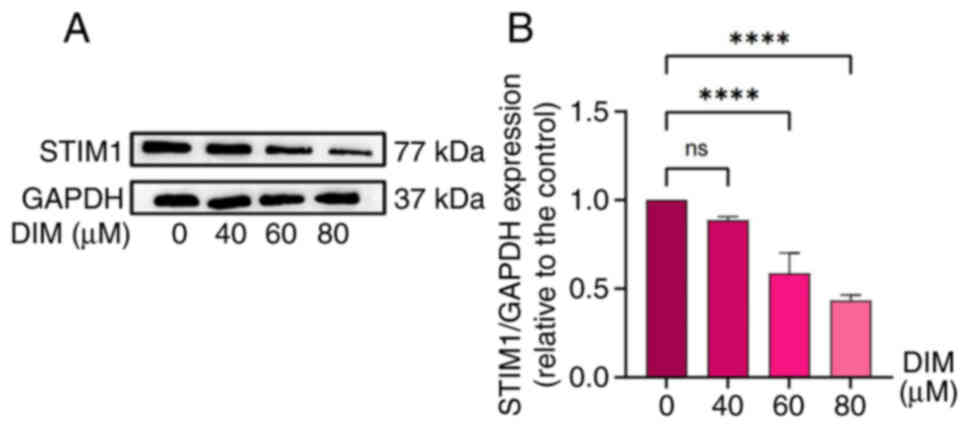
DIM upregulates the expression of
STIM1 protein
The results of network pharmacology suggested that
DIM may affect esophageal cancer cells by regulating the PI3K-Akt
pathway. Endoplasmic reticulum stress is the upstream pathway of
the PI3K-Akt pathway, and STIM1 is one of the key proteins of
endoplasmic reticulum stress (20).
Therefore, STIM1 was used as the next research object in the
present study. While determining whether DIM could promote
apoptosis, at the same time, the changes in STIM1 expression in
TE-1 human esophageal cancer cells treated with different
concentrations of DIM (0, 40, 60 and 80 µM) were also investigated
using western blotting. The results showed that DIM downregulated
the expression of STIM1 protein with a certain concentration
dependence, which was statistically significant at DIM
concentrations of 60 and 80 µM (Fig. 8A
and B).
Tg counteracts the changes to STIM1,
Bcl-2 and Bax protein levels included by DIM in TE-1 cells
Based on the aforementioned results, to further
verify whether DIM promotes apoptosis and inhibits the viability of
TE-1 cells by downregulating the expression of STIM1, cells were
co-treated with Tg (to upregulate the expression of STIM1) and 80
µM DIM. After detecting cell viability using CCK-8, it was found
that the inhibition effect of DIM on TE-1 cells was reverted, which
was statistically significant (P<0.05; Fig. 9A). Following upregulation of STIM1
using Tg, the downregulation of bcl-2 and STIM1 was alleviated,
while the role of bax expression was suppressed compared with that
in the DIM group, and the differences were statistically
significant (P<0.05; Fig. 9B and
C).
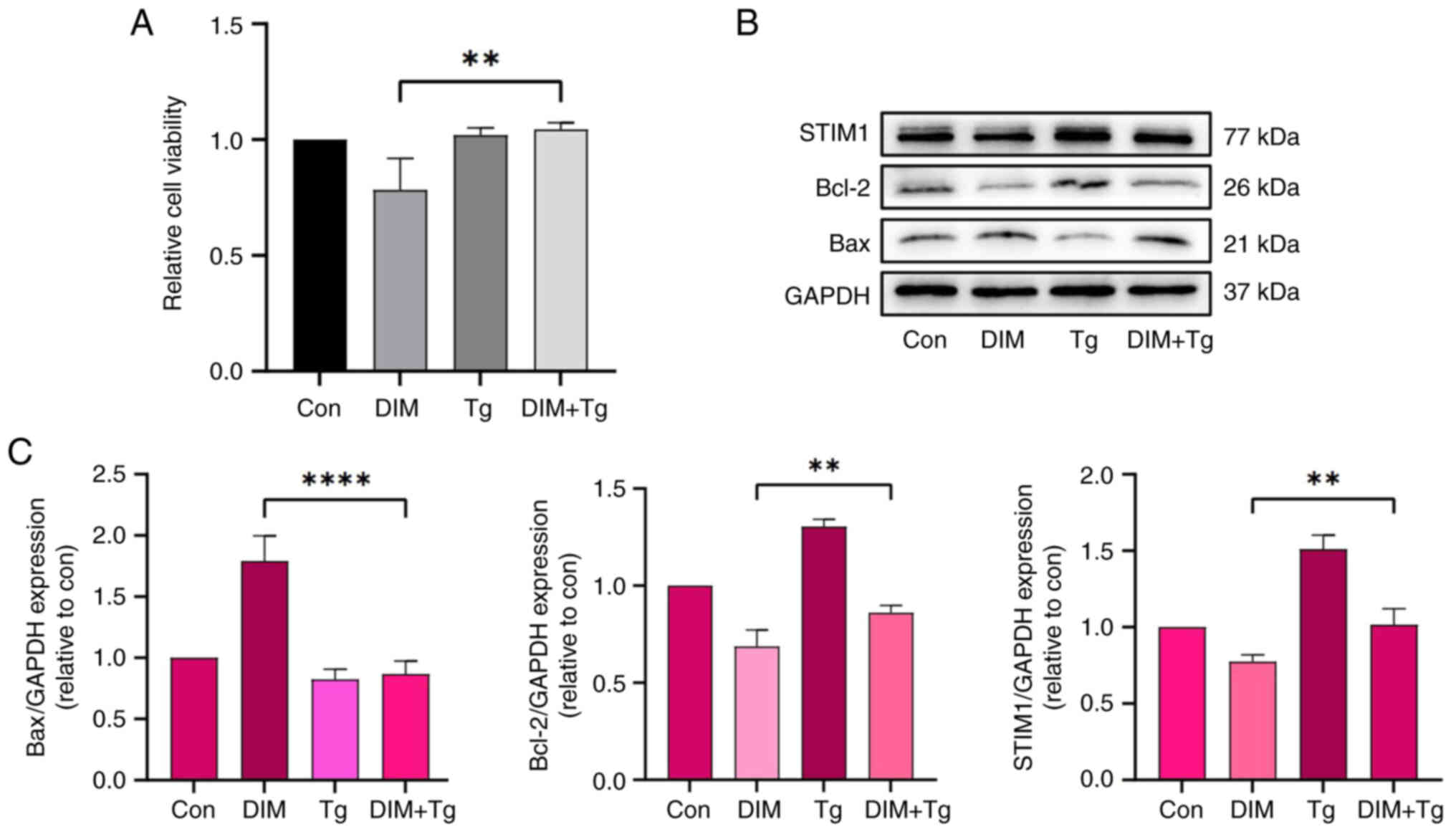 | Figure 9.Effect of Tg on STIM1, Bcl-2 and Bax
protein levels in TE-1 cells incubated with DIM (control group
cells cultured without DIM for 24 h). (A) Cells were treated with
DIM (80 µM) with or without Tg (1 µM) for 24 h, then cell viability
was measured using the CCK-8 assay. Data are presented as the mean
± SD of three independent experiments. **P<0.01 vs. con, by
Kruskal-Wallis followed by Nemenyi test. (B) The Bax, Bcl-2 and
STIM1 expression levels were evaluated by western blotting
following treatment with DIM and Tg. (C) Semi-quantitative analysis
of the Bax, Bcl-2 and STIM1 protein levels following treatment with
DIM and Tg. Data are presented as the mean ± SD of three
independent experiments. **P<0.05, ****P<0.0001 vs. con, by
one-way ANOVA followed by Tukey test. Bcl-2, B-cell lymphoma-2;
con, control; DIM, 3,3′-diindolylmethane; Tg, thapsigargin. |
Discussion
Esophageal cancer is a highly aggressive
gastrointestinal tumor and, since the clinical manifestations of
patients in the early stage of disease are not typical, patients
are often in the middle to late stages when they present with
obvious symptoms. This leads to a poor prognosis if treating with
surgery alone; therefore, comprehensive treatment of esophageal
cancer has become the standard treatment option (21). However, the adverse effects of
conventional neoadjuvant therapy may aggravate the tumor burden of
patients further (6,7). In the present study, it was found that
DIM, a natural phytochemical from cruciferous plants, induced
apoptosis and inhibited the viability of esophageal cancer cells by
downregulating the expression of STIM1, which provides a new
theoretical direction for the early prevention and treatment of
esophageal cancer using DIM.
Through KEGG analysis, it was found that DIM may be
involved in the apoptosis, IL-17 and PI3K-Akt signaling pathways. A
study has shown that TP53 and IL-17 can affect the proliferation
and migration of esophageal cancer cells (22). Furthermore, PI3K-Akt can affect the
proliferation, invasion and metastasis of a variety of tumor cells.
A number of mutations in PI3K-Akt are known to exist in patients
with esophageal cancer, and inhibition of the PI3K-Akt pathway can
reduce the resistance of esophageal cancer to chemotherapy drugs
(23–25). In addition, the PI3K-Akt pathway can
inhibit endoplasmic reticulum stress and induce cell death
(26). STIM1 participates in the
stability of Ca2+ in the endoplasmic reticulum and
participates in endoplasmic reticulum stress (27). Therefore, in the present study, the
upstream regulatory signal of the PI3K-Akt pathway-endoplasmic
reticulum stress signal, represented by STIM1, was studied to
determine the effect of DIM on esophageal cancer cells (20). As a powerful pharmacological
analysis tool, network pharmacology fully integrates the knowledge
of system biology, pharmacology, information networks and computer
science to explain the relationship between drugs, diseases, and
argets (28). However, there were
some limitations to the pharmacology network analysis performed in
the present study. Due to the different inclusion criteria of
studies, the comparability, integrity and reliability of data may
differ between different databases, which may lead to certain
differences in the relationship between data from different sources
and between drugs and disease targets. In addition, the
relationship between drug targets and disease targets is constantly
updated, which may lead to a certain deviation in the results of
the database. This may explain why STIM1 was not among the
identified core targets.
Apoptosis is a type of programmed cell death. The
Bcl-2 gene family is one of the important factors that causes
apoptosis through the mitochondrial pathway, and Bax is one of its
important members (29). Both Bcl-2
and Bax are located in the mitochondria and endoplasmic reticulum.
The enhanced expression of Bcl-2 can promote cell apoptosis, while
Bax has the opposite effect (29,30).
In the present study, western blotting was used to demonstrate that
DIM downregulated the expression of Bcl-2 and upregulated the
expression of Bax in a concentration-dependent manner. After
stimulation of STIM1 expression with Tg, this effect was
prevented.
Through GO analysis, it was found that DIM may act
on cellular components such as the endoplasmic reticulum. STIM1 is
a transmembrane Ca2+-binding phosphoprotein located on
the endoplasmic reticulum membrane. STIM1 acts as a Ca2+
sensor in the endoplasmic reticulum cavity and participates in the
regulation of the SOCE pathway (31). Studies have shown that SOCE has a
certain role in the occurrence and development of tumors. In breast
cancer cells, α-glucosidase inhibitors can significantly reduce the
expression level of STIM1, thereby weakening the expression of SOCE
to inhibit breast cancer cell viability (32). In addition, downregulation of STIM1
expression can inhibit the migration and invasion of thyroid cancer
cells, and it can restore the expression of thyroid-stimulating
hormone receptors (33). Tg is an
irreversible endoplasmic reticulum Ca2+-ATPase binder,
which can induce endoplasmic reticulum protein folding disorder,
leading to the accumulation of unprocessed proteins in the
endoplasmic reticulum. This causes dysfunction of the endoplasmic
reticulum and activation of SOCE, which is typically regarded as an
agonist of endoplasmic reticulum stress (34). STIM1 is one of the key proteins of
SOCE. Tg can empty the endoplasmic reticulum Ca2+ pool,
thereby indirectly activating the expression of STIM1, which leads
to extracellular Ca2+ entering the cell to maintain the
balance of intracellular and extracellular Ca2+
(35). Therefore, Tg can be
regarded as an agonist of STIM1 protein. In the present study, the
expression of STIM1 protein in TE-1 cells pretreated with Tg was
restored compared with that of DIM alone. Therefore, we hypothesize
that DIM can overload Ca2+ in the endoplasmic reticulum
of TE-1 human esophageal cancer cells, thus causing apoptosis of
these cells. However, further research is required to confirm
this.
However, there are still some shortcomings to the
present study. The aim of the present study was limited to the
examination of esophageal squamous cell carcinoma and did not
involve other pathological types of esophageal cancer. In addition,
the present study only included experiments at the cellular level
and was not extended to experimental mice and patients. The present
study preliminarily verified that DIM affected the oncology-related
characteristics of esophageal cancer cells by regulating the
expression of STIM1 protein, which is a transmembrane protein
located on the endoplasmic reticulum membrane that regulates
calcium homeostasis. However, whether there is a change in the
concentration of Ca2+ inside and outside of the
endoplasmic reticulum during the anti-esophageal cancer activity of
DIM requires further study. In addition, whether other tumor
suppression methods, such as ferroptosis and pyroptosis, are
influenced by DIM still needs further exploration. It has also been
demonstrated that DIM has reproductive toxicity in mice, that is,
it can inhibit the secretion of related sex hormones in male mice,
thereby reducing the quality of sperm (36). At present, study of the antitumor
activity of DIM remains in the cell and animal experiments stage,
and no drugs for human consumption have been developed. However,
DIM-related side effects still need to be further confirmed by a
large number of clinical experiments after careful evaluation by
pharmacologists and physicians.
In conclusion, the results of the present study
indicated that DIM promoted apoptosis and inhibited the viability
of esophageal cancer cells by downregulating the expression of
STIM1. Therefore, the natural phytochemical, DIM, may be a
potential substance for the early prevention and treatment of
esophageal cancer cells.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CX YY, FL and YT designed the study. CX, YT FL, YY
and XL assisted with the data analyses. CX, XL and JL performed
western blotting. CX and SD performed the CCK-8 assay. CX and SD
performed the scratch test. CX wrote the initial draft of the
manuscript. CX and XL contributed to the analysis and
interpretation of the data. FL and YY assisted in the preparation
and critical review of the manuscript. CX YY, FL and YT confirm the
authenticity of all the raw data. All authors agreed to be
accountable for all aspects of the work. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kamangar F, Nasrollahzadeh D, Safiri S,
Sepanlou SG, Fitzmaurice C, Ikuta KS, Bisignano C, Islami F,
Roshandel G, Lim SS, et al: The global, regional, and national
burden of oesophageal cancer and its attributable risk factors in
195 countries and territories, 1990–2017: A systematic analysis for
the global burden of disease study 2017. Lancet Gastroenterol
Hepatol. 5:582–597. 2020. View Article : Google Scholar
|
|
2
|
Chen W, Zheng R, Zhang S, Zeng H, Fan Y,
Qiao Y and Zhou Q: Esophageal cancer incidence and mortality in
China, 2010. Thorac Cancer. 5:343–348. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jung HK, Tae CH, Lee HA, Lee H, Don Choi
K, Park JC, Kwon JG, Choi YJ, Hong SJ, Sung J, et al: Treatment
pattern and overall survival in esophageal cancer during a 13-year
period: A nationwide cohort study of 6,354 Korean patients. PLoS
One. 15:e02314562020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uhlenhopp DJ, Then EO, Sunkara T and
Gaduputi V: Epidemiology of esophageal cancer: Update in global
trends, etiology and risk factors. Clin J Gastroenterol.
13:1010–1021. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shah MA, Kennedy EB, Catenacci DV,
Deighton DC, Goodman KA, Malhotra NK, Willett C, Stiles B, Sharma
P, Tang L, et al: Treatment of locally advanced esophageal
carcinoma: ASCO guideline. J Clin Oncol. 38:2677–2694. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Herzberg J, Strate T, Guraya SY and
Honarpisheh H: Risk factors for anastomotic leakage after surgical
resections for esophageal cancer. Langenbecks Arch Surg.
406:1859–1866. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schröder W, Raptis DA, Schmidt HM,
Gisbertz SS, Moons J, Asti E, Luyer MDP, Hölscher AH, Schneider PM,
Berge Henegouwen MI, et al: Anastomotic techniques and associated
morbidity in total minimally invasive transthoracic esophagectomy:
Results from the EsoBenchmark database. Ann Surg. 270:820–826.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Collins SR and Meyer T: Evolutionary
origins of STIM1 and STIM2 within ancient Ca2+ signaling systems.
Trends Cell Biol. 21:202–211. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stathopulos PB, Li GY, Plevin MJ, Ames JB
and Ikura M: Stored Ca2+ depletion-induced oligomerization of
stromal interaction molecule 1 (STIM1) via the EF-SAM region: An
initiation mechanism for capacitive Ca2+ entry. J Biol Chem.
281:35855–35862. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson JL and Shuttleworth TJ: Molecular
basis of activation of the arachidonate-regulated Ca2+ (ARC)
channel, a store-independent Orai channel, by plasma membrane
STIM1. J Physiol. 591:3507–3523. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng L, Stathopulos PB, Schindl R, Li GY,
Romanin C and Ikura M: Auto-inhibitory role of the EF-SAM domain of
STIM proteins in store-operated calcium entry. Proc Natl Acad Sci
USA. 108:1337–1342. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lodola F, Laforenza U, Bonetti E, Lim D,
Dragoni S, Bottino C, Ong HL, Guerra G, Ganini C, Massa M, et al:
Store-operated Ca2+ entry is remodelled and controls in vitro
angiogenesis in endothelial progenitor cells isolated from tumoral
patients. PLoS One. 7:e425412012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Abdullaev IF, Bisaillon JM, Potier M,
Gonzalez JC, Motiani RK and Trebak M: Stim1 and Orai1 mediate CRAC
currents and store-operated calcium entry important for endothelial
cell proliferation. Circ Res. 103:1289–1299. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang J, Ye SF, Wang MQ, Li J, Meng X and
Liu F: Stromal interaction molecule 1 promotes tumor growth in
esophageal squamous cell carcinoma. Genomics. 112:2146–2153. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim HW, Kim J, Kim J, Lee S, Choi BR, Han
JS, Lee KW and Lee HJ: 3,3′-Diindolylmethane inhibits
lipopolysaccharide-induced microglial hyperactivation and
attenuates brain inflammation. Toxicol Sci. 137:158–167. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee GA, Hwang KA and Choi KC: Inhibitory
effects of 3,3′-diindolylmethane on epithelial-mesenchymal
transition induced by endocrine disrupting chemicals in cellular
and xenograft mouse models of breast cancer. Food Chem Toxicol.
109:284–295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li F, Xu Y, Chen C, Chen SM, Xiao BK and
Tao ZZ: Pro-apoptotic and anti-proliferative effects of
3,3′-diindolylmethane in nasopharyngeal carcinoma cells via
downregulation of telomerase activity. Mol Med Rep. 12:3815–3820.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye Y, Li X, Wang Z, Ye F, Xu W, Lu R, Shen
H and Miao S: 3,3′-Diindolylmethane induces gastric cancer cells
death via STIM1 mediated store-operated calcium entry. Int J Biol
Sci. 17:1217–1233. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang G: Radiosensitizing effect of
3,3′-diindolylmethane on human esophageal cancer Eca109 cell line
and its mechanism (unpublished PhD thesis). Bengbu Medical College.
2014.
|
|
20
|
Huang Z: The apparent molecular mechanism
of artesunate regulating endoplasmic reticulum stress-PI3K-AKT
inhibiting colorectal cancer (unpublished PhD thesis). Guangzhou
University of Traditional Chinese Medicine. 2018.
|
|
21
|
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang
L, Wang B, Sun G, Ji Y, Cao G, et al: Sintilimab versus placebo in
combination with chemotherapy as first line treatment for locally
advanced or metastatic oesophageal squamous cell carcinoma
(ORIENT-15): Multicentre, randomised, double blind, phase 3 trial.
BMJ. 377:e0687142022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ye F, Song J, Wang Y, Xu X and Zhang K:
Proliferation potential-related protein promotes the esophageal
cancer cell proliferation, migration and suppresses apoptosis by
mediating the expression of p53 and interleukin-17. Pathobiology.
85:322–331. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fattahi S, Amjadi-Moheb F, Tabaripour R,
Ashrafi GH and Akhavan-Niaki H: PI3K/AKT/mTOR signaling in gastric
cancer: Epigenetics and beyond. Life Sci. 262:1185132020.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li B, Li J, Xu WW, Guan XY, Qin YR, Zhang
LY, Law S, Tsao SW and Cheung ALM: Suppression of esophageal tumor
growth and chemoresistance by directly targeting the PI3K/AKT
pathway. Oncotarget. 5:11576–11587. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Luo Q, Du R, Liu W, Huang G, Dong Z and Li
X: PI3K/Akt/mTOR signaling pathway: Role in esophageal squamous
cell carcinoma, regulatory mechanisms and opportunities for
targeted therapy. Front Oncol. 12:8523832022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu P, Han Z, Couvillon AD and Exton JH:
Critical role of endogenous Akt/IAPs and MEK1/ERK pathways in
counteracting endoplasmic reticulum stress-induced cell death. J
Biol Chem. 279:49420–49429. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Benson JC and Trebak M: Too much of a good
thing: The case of SOCE in cellular apoptosis. Cell Calcium.
111:1027162023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barabási AL, Gulbahce N and Loscalzo J:
Network medicine: A network-based approach to human disease. Nat
Rev Genet. 12:56–68. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paone S, Baxter AA, Hulett MD and Poon
IKH: Endothelial cell apoptosis and the role of endothelial
cell-derived extracellular vesicles in the progression of
atherosclerosis. Cell Mol Life Sci. 76:1093–1106. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao Y, Fu Y and Wang X: The effects of
Xuebijing injection on apoptosis and the expression of regulatory
factors including Bcl-2,Bax and Caspase-3 in the renal cells of
rats with acute paraquat poisoning. Hebei Med J. 40:2889–2894.
2018.
|
|
31
|
Leech CA, Kopp RF, Nelson HA, Nandi J and
Roe MW: Stromal interaction molecule 1 (STIM1) regulates
ATP-sensitive potassium (KATP) and store-operated
Ca2+ channels in MIN6 β-Cells. J Biol Chem.
292:2266–2277. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Jardin I, Lopez JJ, Salido GM and Rosado
JA: Store-operated Ca2+ entry in breast cancer cells:
Remodeling and functional role. Int J Mol Sci. 19:40532018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Asghar MY, Lassila T, Paatero I, Nguyen
VD, Kronqvist P, Zhang J, Slita A, Löf C, Zhou Y, Rosenholm J and
Törnquist K: Stromal interaction molecule 1 (STIM1) knock down
attenuates invasion and proliferation and enhances the expression
of thyroid-specific proteins in human follicular thyroid cancer
cells. Cell Mol Life Sci. 78:5827–5846. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sposito S, Secondo A, Romanelli AM,
Montefusco A, Nanayakkara M, Auricchio S, Barone MV, Caputo I and
Paolella G: Peculiar Ca2+ homeostasis, ER stress,
autophagy, and TG2 modulation in celiac disease patient-derived
cells. Int J Mol Sci. 24:14952023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Evinova A, Hatokova Z, Tatarkova Z,
Brodnanova M, Dibdiakova K and Racay P: Endoplasmic reticulum
stress induces mitochondrial dysfunction but not mitochondrial
unfolded protein response in SH-SY5Y cells. Mol Cell Biochem.
477:965–975. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aksu EH, Akman O, Ömür AD, Karakuş E, Can
I, Kandemir FM, Dorman E and Uçar Ö: 3,3 Diindolylmethane leads to
apoptosis, decreases sperm quality, affects blood estradiol 17 β
and testosterone, oestrogen (α and β) and androgen receptor levels
in the reproductive system in male rats. Andrologia. 48:1155–1165.
2016. View Article : Google Scholar : PubMed/NCBI
|